Abstract
The Haemophilus influenzae type B (Hib) conjugate vaccine is the most effective way to prevent Hib infection in infants and young children, and it is designed to induce the production of antibodies against polyribosylribitol phosphate (PRP) to protect babies from infection. However, the mechanism of immunity induced by the Hib vaccine is not fully understood. Recently, with the development of the combination diphtheria and tetanus toxoids and acellular pertussis vaccines (DTaP), increasing numbers of manufacturers have begun to develop DTaP-based combination vaccines, like the combination vaccine diphtheria and tetanus toxoids and acellular pertussis and Hib conjugate vaccine (DTaP-Hib), which contains adjuvants. However, the Hib vaccine does not contain adjuvants. It was theorized that the Hib antigen has poor compatibility with aluminum adjuvants for unclear reasons. Therefore, understanding the mechanism of the Hib-vaccine-induced immune response and the influence of adjuvants on the Hib vaccine is of great significance. In this paper, we immunized BalBc mice with either the Hib vaccine or the Hib vaccine that adsorbs aluminum adjuvants (Hib-Al). Here, we analyzed the anti-PRP antibody level and immune response of different cells using cell and cytokine levels. We found that the Hib vaccine could induce a humoral and cellular immune response, and the Hib-Al vaccine could induce greater quantities of IFN-γ, IL-4, and IL-6 and more antigen-specific antibodies through B cells, Th1, Th2, and ILC3s in the spleen. Together, our findings demonstrate the serologic responses and immune response in terms of cell and cytokine levels induced by the Hib vaccine, and they also imply that the addition of aluminum hydroxide adjuvant could enhance the function of the Hib vaccine, which preliminarily reveals the mechanism of immune response induced by the Hib-related vaccine.
1. Introduction
Hib is a common parasitic bacterium mainly causing pneumonia and meningitis in children. Early studies documented that children aged 3 months to 5 years are particularly susceptible to Hib meningitis due to their blood not possessing bactericidal activity [1]. The early Hib vaccine composed of the capsular polysaccharide of the organism, PRP, protects older children but does not induce antibodies or provide protection in infants under 18 months. This is because PRP is a T-cell-independent antigen with age-related immunogenicity. To enhance the immunogenicity of PRP, PRP is combined with a carrier (such as DT, TT, or CRM) to transform into a T-cell-dependent antigen, and clinical results have shown that Hib conjugate vaccines are more immunogenic for infants and young children than the unconjugated Hib vaccine [2]. The Hib vaccine can enhance the serum anti-PRP antibody titers through the T-cell-dependent B-cell response. However, whether the other immune cells are induced by the Hib vaccine is not clear. For the past few years, a multi-vaccine approach has become the preferred development direction for vaccines, as the use of combined vaccines could reduce the number of vaccinations required (especially in infants and young children), which is conducive to improving the vaccination rate, and it has great clinical demand and public health value. In recent years, many of these combined vaccines have been based on Hib, including DTaP-Hib, and, in combined vaccines, due to the complex antigenic components used, some components require adjuvants to improve the immunogenicity. Some studies have shown that the aluminum-adjuvant-adsorbed Hib has low immunogenicity compared with the Hib vaccine, and that the immunogenicity of the Hib vaccine is reduced in combined vaccines [3,4]. However, other studies have shown that Hib/DTwP combined vaccines have no significant reduction in anti-PRP antibody levels [5,6]. Therefore, in this study, we aim to determine whether the aluminum adjuvant can influence the immunogenicity of Hib.
The immunogenicity of Hib vaccines is thought to test the anti-PRR antibody level [7,8,9]. Clinically, anti-PRP antibodies in human serum reflect the degree of immunity against Hib infection and have been effectively linked to protection; for example, anti-PRP antibodies at concentrations of ≥0.15 g/mL are thought to provide short-term protection against invasive Hib disease, and the concentration of ≥1.0 g/mL one month after Hib vaccine immunization was considered to confer an immunoprotective effect. Because most studies have focused on serologic responses, the mechanisms underlying the immunogenicity of conjugate vaccines remain poorly understood. It is not clear how the Hib vaccine induces changes at the cell level, like the T-cell memory responses or cytokine production from the immune cells to anti-PRR antibodies, which could provide some information to guide the use of adjuvants to enhance the immunogenicity of the vaccines.
In this work, we immunized BalBc mice with Hib and Hib+Al vaccines and analyzed the different types of antibodies in serum. As the spleen is the largest secondary lymphoid organ with a high density of antigen-presenting cells and lymphocytes, and it is responsible for blood filtration and producing an immune response to pathogens [10], we also analyzed the immune response in the spleen. The humoral and cellular immune response to the Hib and Hib+Al vaccines could address the question of whether the addition of an aluminum adjuvant to the Hib vaccine could have a protective function. This study provides theoretical support for the study and development of combined vaccines.
2. Results
2.1. Hib and Aluminum-Adjuvant-Adsorbed Hib Vaccines Both Induce Humoral Immune Response
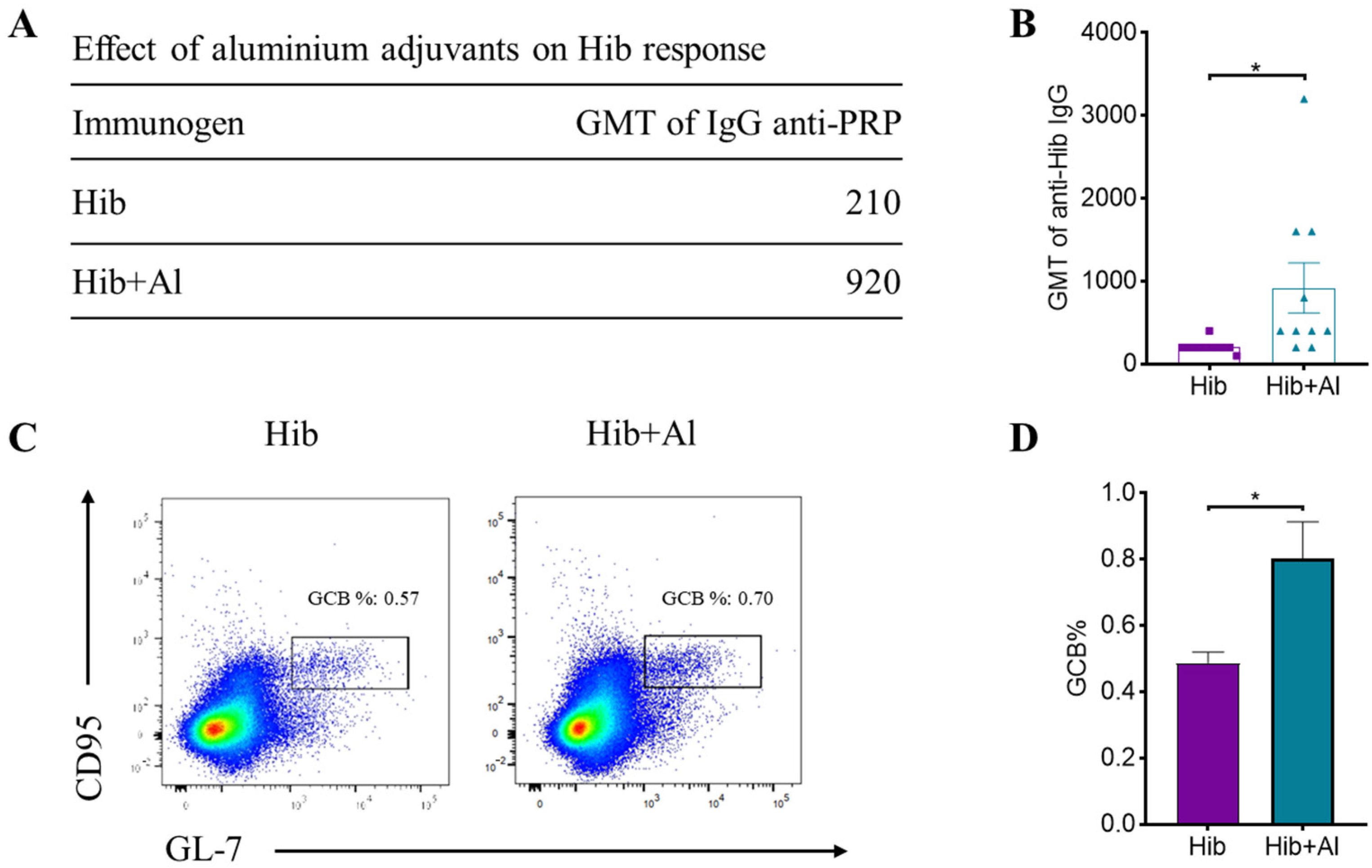
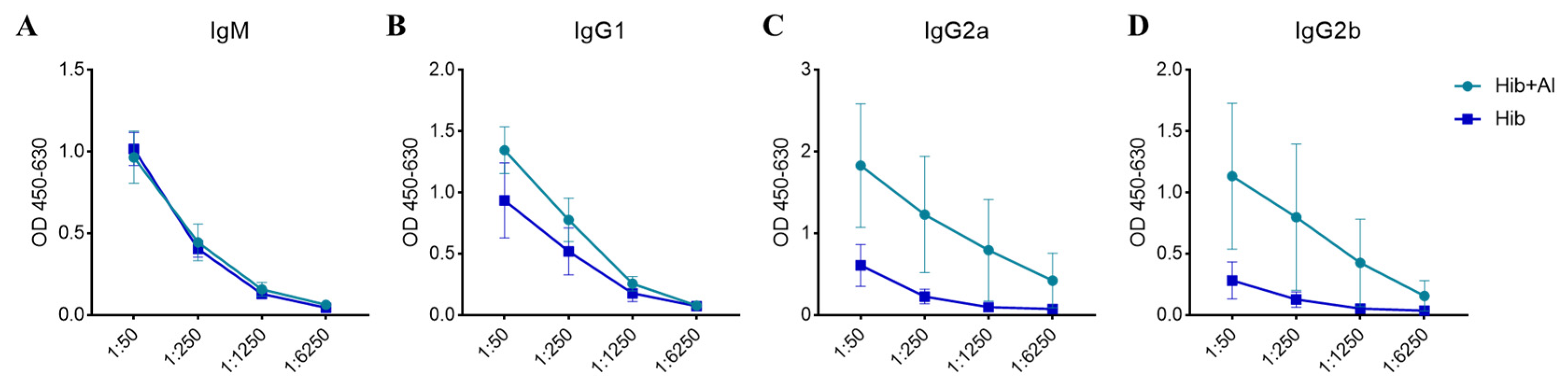
B lymphocytes need to undergo a germinal central response to have the ability to produce high-affinity antibodies specific to pathogens, thereby protecting the body from pathogen infection. To test whether aluminum hydroxide Al(OH)3 affects Hib immunogenicity, an Al(OH)3-adsorbed Hib vaccine (Hib+Al) and a Hib vaccine were administered to mice, and the germinal center B cells (GCBs) and the anti-PRP antibody level were analyzed. The results show that both the Hib and Hib+Al vaccines induce a humoral immune response, including anti-PRP antibody and GCBs. However, the Hib+Al vaccine induced a higher level of anti-PRP antibody (Figure 1A,B) and more GCB cells (Figure 1C,D) than the Hib vaccine, suggesting that the aluminum hydroxide adjuvant could enhance the humoral immune response in the Hib vaccine. Different antibodies were induced after the antigen stimulation. Here, we analyzed the antibodies of different isotypes, including IgM, IgG1, IgG2a, and IgG2b. The results show that IgM caused no difference in the Hib and Hib+Al groups (Figure 2A), and the aluminum adjuvant improved the expression of IgG1, IgG2a, and IgG2b (Figure 2B–D), which was consistent with the above results (Figure 1B). Together, these data confirm that the addition of aluminum adjuvant could improve the humoral immune response in mice.
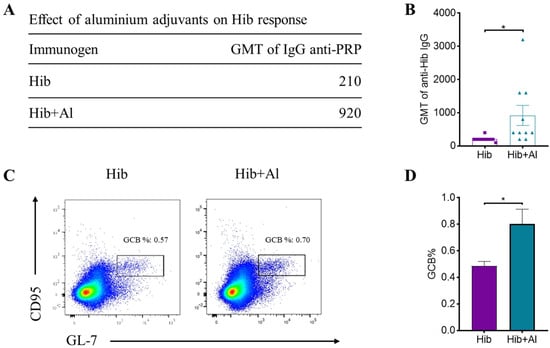
Figure 1.
Hib and Hib+Al vaccine-induced humoral immune response. (A) The GMT level of IgG anti-PRP in Hib- and Hib+Al-immunized mice; (B) comparison plot of GMT levels for each mouse in Hib and Hib+Al vaccines (n = 10); (C) the responses of GCBs in the spleen were analyzed at day 21, and the representative flow cytometry charts of GCBs are shown; (D) the percentages of the GCB cells are shown (n = 4). Error bars represent the SEM. * p < 0.05.
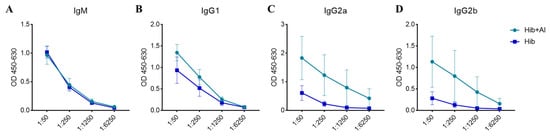
Figure 2.
The concentration of the anti-PRP antibodies in serum at day 28 post immunization. (A) The OD level of IgM anti-PRP in Hib- and Hib+Al-immunized mice; (B) the OD level of IgG1 anti-PRP in Hib- and Hib+Al-immunized mice; (C) the OD level of IgG2a anti-PRP in Hib- and Hib+Al-immunized mice; (D) the OD level of IgG2b anti-PRP in Hib- and Hib+Al-immunized mice; n = 5, error bars represent the SEM.
2.2. The Effect of Aluminum Adjuvant on Hib-Induced Cytokine Expression
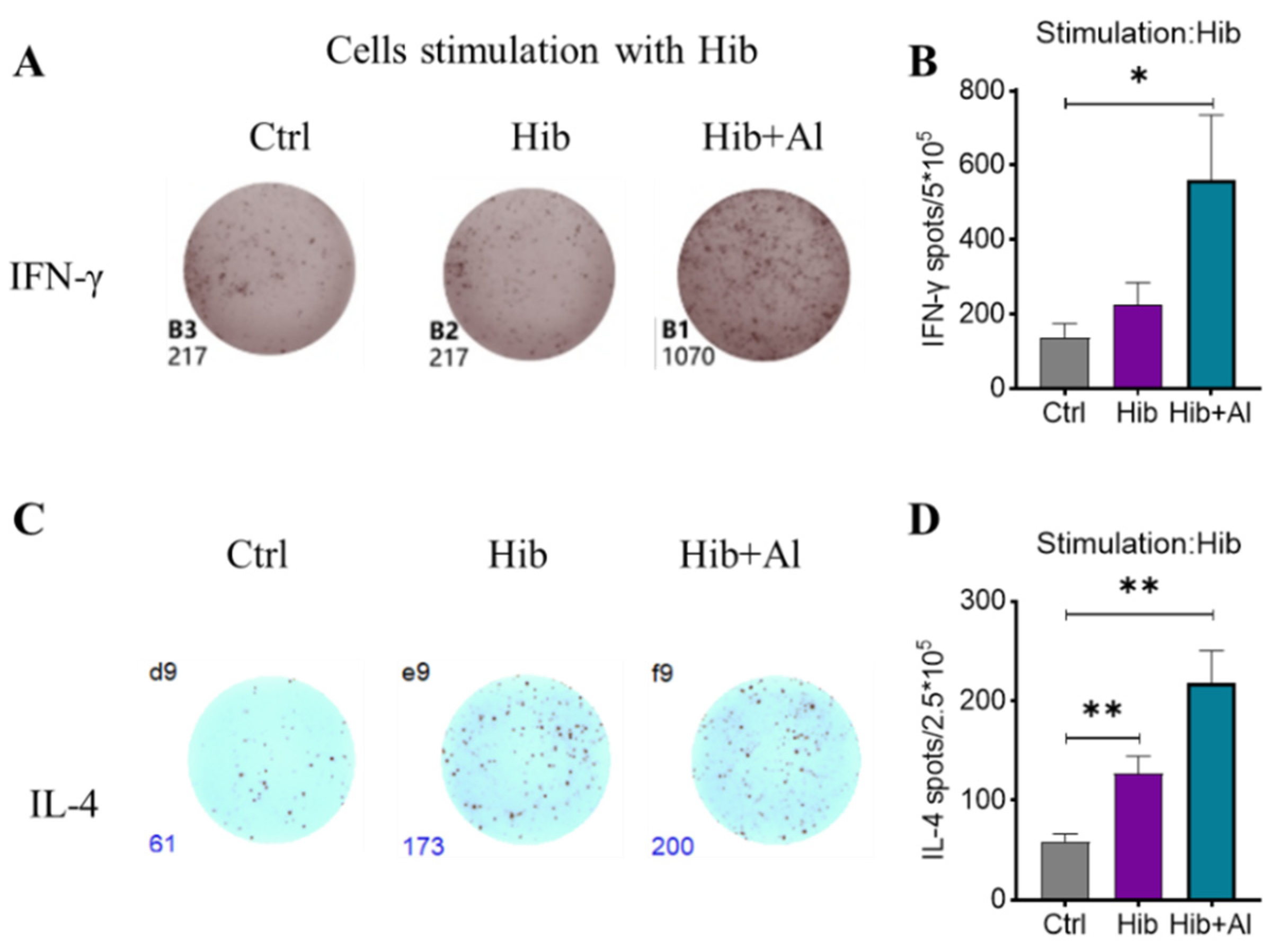
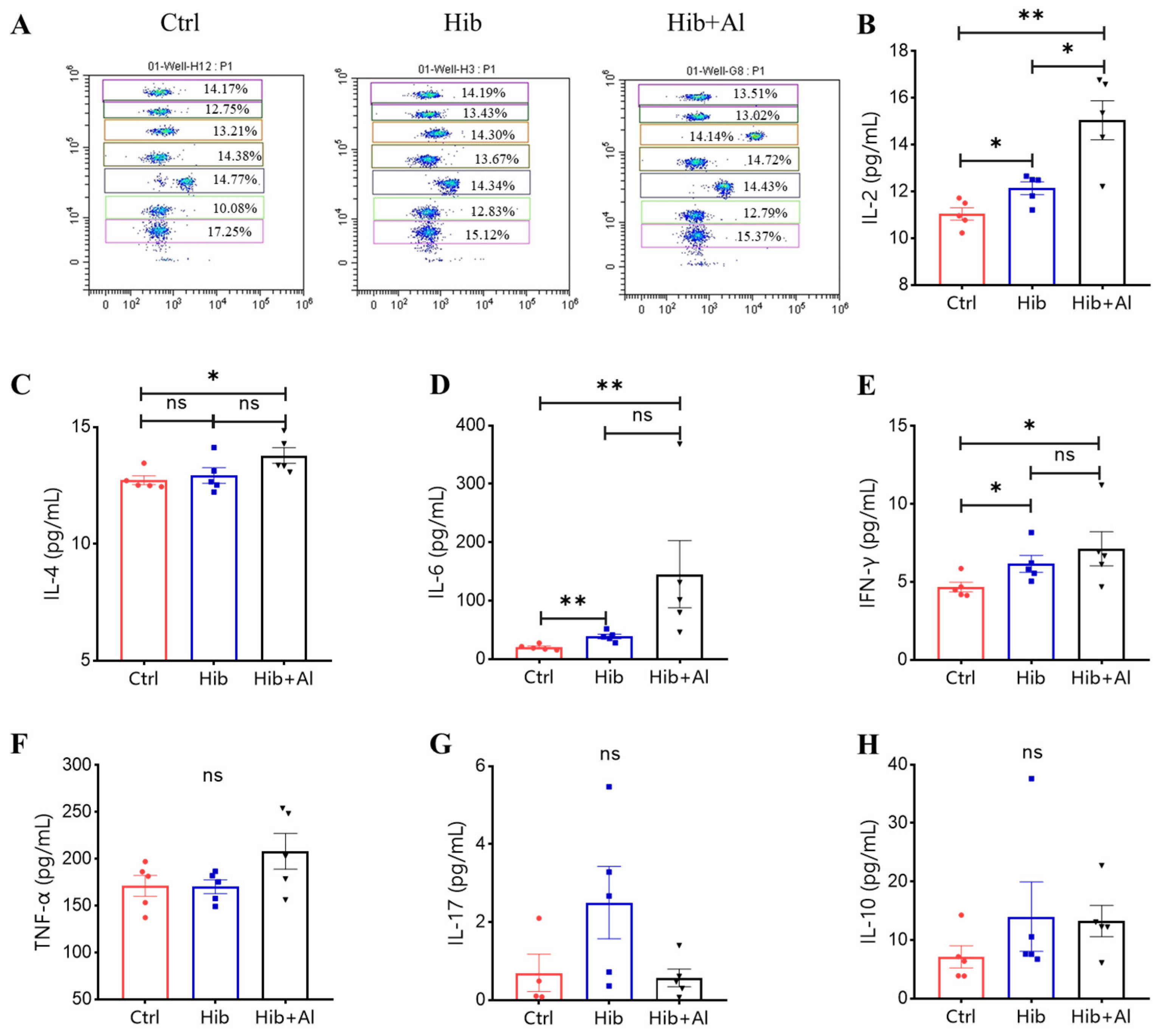
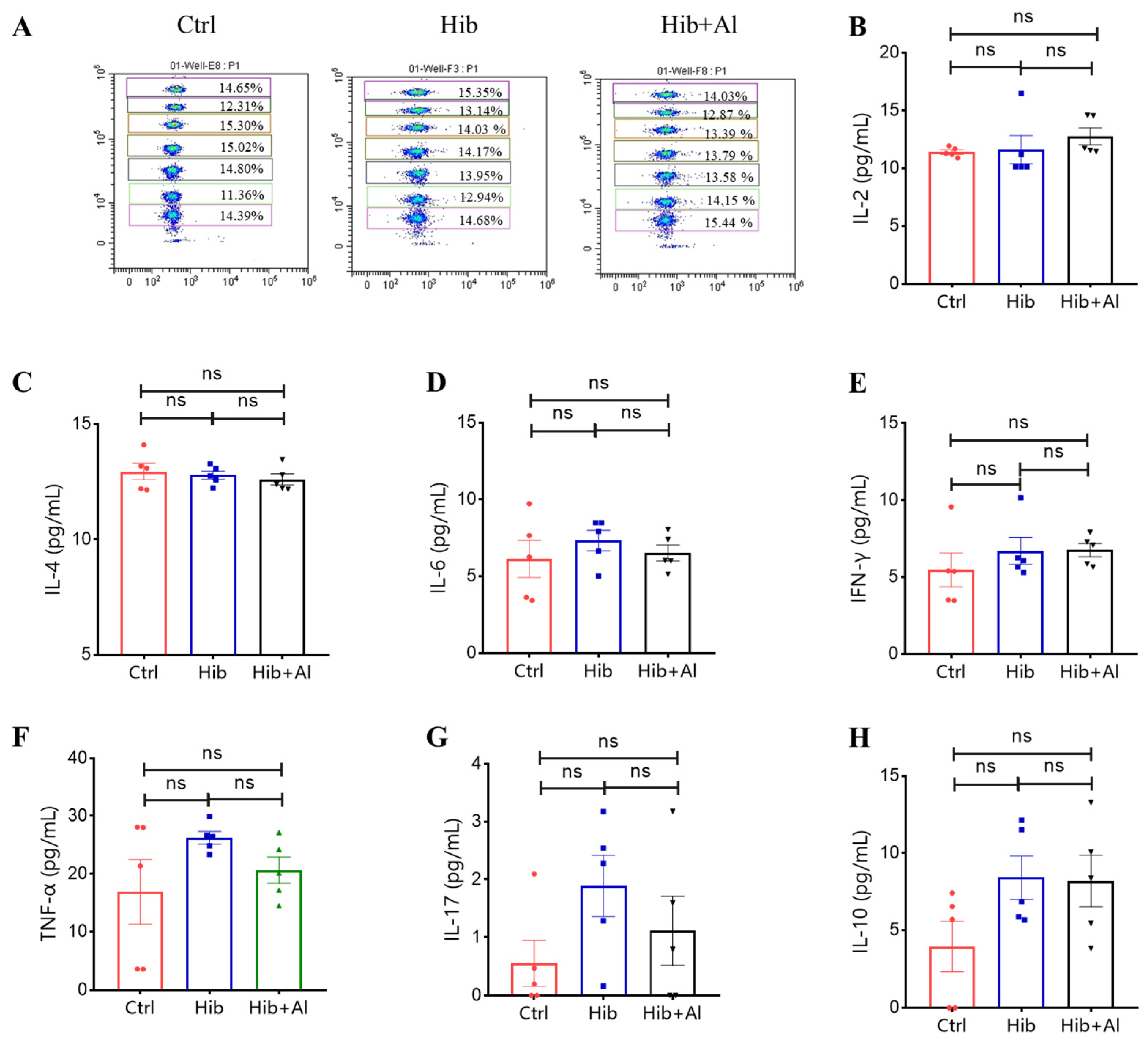
IgG2b was mainly induced by Th1 cells, which could produce IL-2 and IFN-γ, while IgG1 and IgG2a were mainly elicited by Th2 cells, which could produce IL-4. The ratio of IgG1/IgG2a could be used to evaluate whether Th1 or Th2 was biased in the vaccine-induced immune response. IgG2a was upregulated in the Hib-Al vaccine groups, which indicated that the aluminum hydroxide adjuvant may induce a Th1 response. To further explore the effect of the aluminum adjuvant on the Hib vaccine, the immune cells in the spleens of the immunized mice were stimulated with Hib. The cytokine expression levels were analyzed. Through ELISpot assay, we found that the Hib vaccine could induce IL-4 expression, which was consistent with the high expression of the antibody in Hib-immunized mice (Figure 3C,D). Also, the Hib vaccine showed an upward trend for inducing IFN-γ expression, although it showed no significant difference compared to the control (Figure 3A,B). However, the Hib+Al vaccine could induce both IL-4 and IFN-γ expression (Figure 3B,D), suggesting that the aluminum adjuvant could enhance both the humoral and cellular immune response. Furthermore, the immune cells in the spleen were stimulated with Hib for 24 h, and we tested different cytokine levels using a CBA kit (Figure 4). The results show that the Hib and Hib+Al vaccines induced IL-2, IL-6, and IFN-γ expression(Figure 4B,D,E), suggesting that the Hib vaccine could induce cellular immune response and antibody production. In addition, the Hib+Al vaccine could also induce IL-4 expression(Figure 4C), and it produced more IL-2 than the Hib vaccine. These results indicate that the aluminum adjuvant could induce a stronger immune response, including the cellular and humoral immune response.
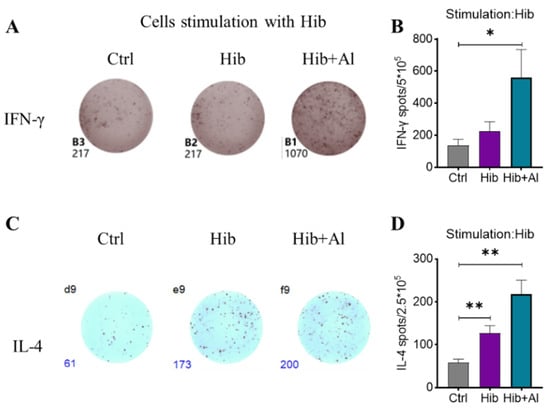
Figure 3.
ELISpot analysis of IFN-γ and IL-4 in Hib- and Hib+Al-immunized mice. Mice were immunized with different vaccines, and splenocytes were collected on day 21 post immunization (A,C). Hib-specific IFN-γ and IL-4 in spleens were measured using ELISpot assay (n = 5) after injection of the Hib or Hib+Al vaccines or in the control group. The representative plot is shown in A,C and the statistical analysis is shown in (B,D). Error bars represent SEM. * p < 0.05; ** p < 0.01; (Student’s t-test).
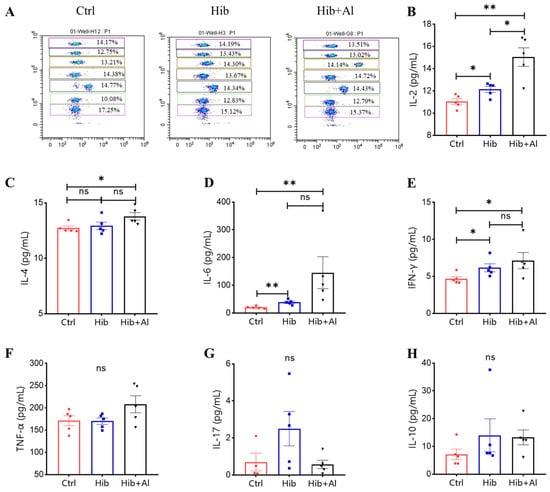
Figure 4.
Flow cytometry analysis of Hib-specific Th1/Th2/TH17 cytokine expression. (A) The representative flow cytometry chart of different cytokines. The APC level represents different cytokines, and the cytokines from top to bottom are IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17, and IL-10; (B) the IL-2 expression levels; (C) the IL-4 expression levels; (D) the IL-6 expression levels; (E) the IFN-γ expression levels; (F) the TNF-α expression levels; (G) the IL-17 expression levels; (H) the IL-10 expression levels; n = 5, error bars represent the SEM. * p < 0.05; ** p < 0.01(Student’s t-test); ns, no significant difference.
To determine whether the aluminum adjuvant could disrupt the immune system, we tested the Th1/Th2/Th17 cytokine levels in serum from the immunized mice. The results showed that there were no significant differences in Hib+Al- and Hib-vaccine-immunized mice (Scheme 1), indicating that the aluminum adjuvant in the Hib vaccine could also maintain the homeostasis of immune cells. Together, these results demonstrate that the aluminum adjuvant could improve Th1 and Th2 cytokine expression.
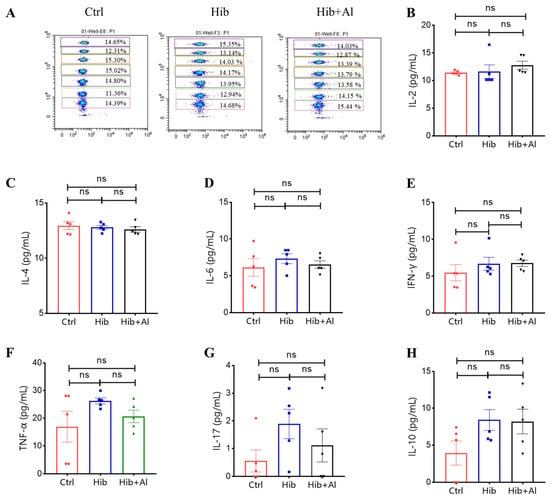
Scheme 1.
Flow cytometry analysis of Th1/Th2/TH17 cytokine expression in serum. (A) The representative flow cytometry chart of different cytokines. The APC level represents different cytokines, and the cytokines from top to bottom are IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17, and IL-10; (B) the IL-2 expression levels; (C) the IL-4 expression levels; (D) the IL-6 expression levels; (E) the IFN-γ expression levels; (F) the TNF-α expression levels; (G) the IL-17 expression levels; (H) the IL-10 expression levels; n = 5, error bars represent the SEM (Student’s t-test); ns, no significant difference.
2.3. The Distribution of Immune Cells following Administration of Hib and Hib+Al Vaccine
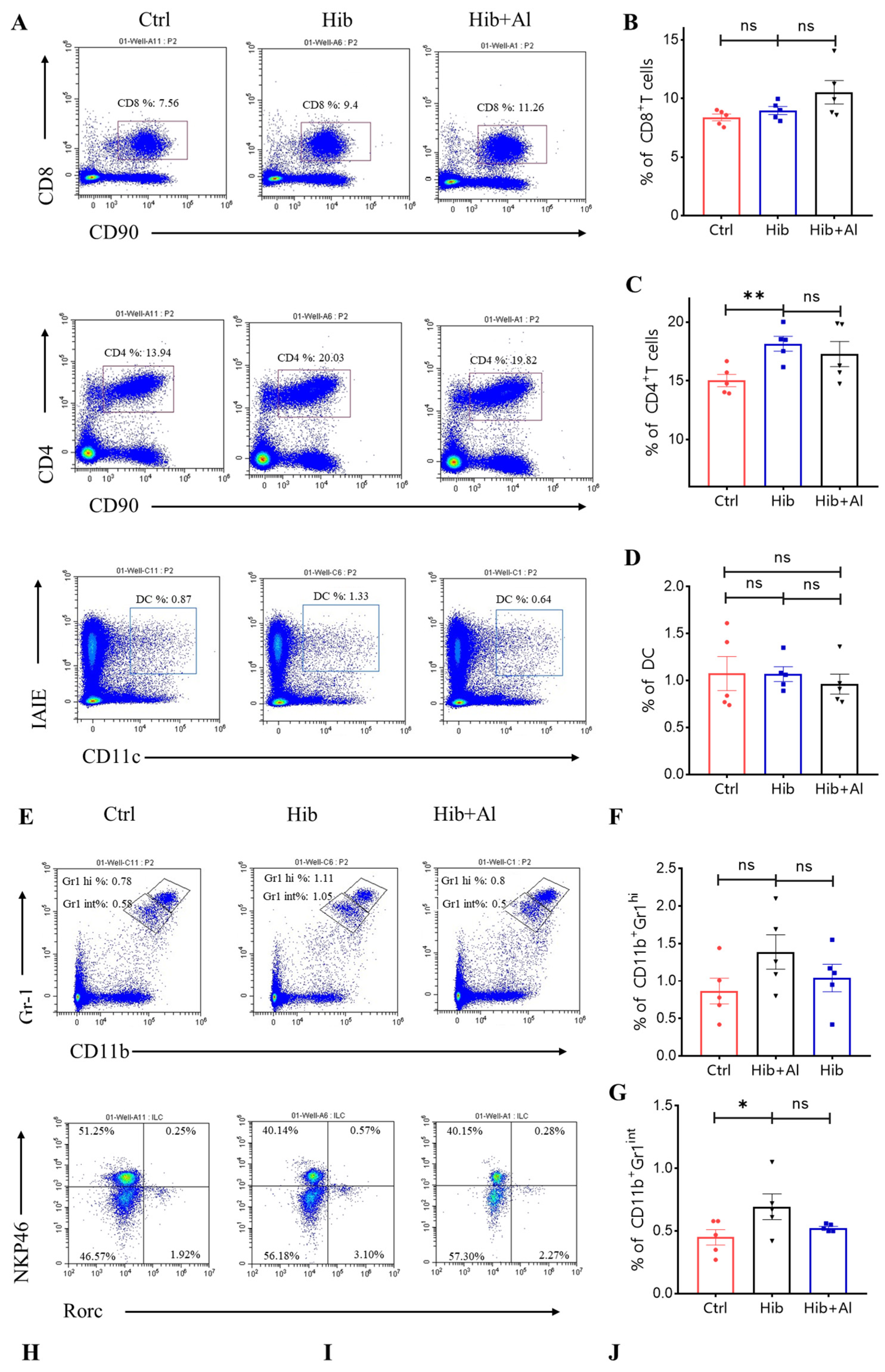
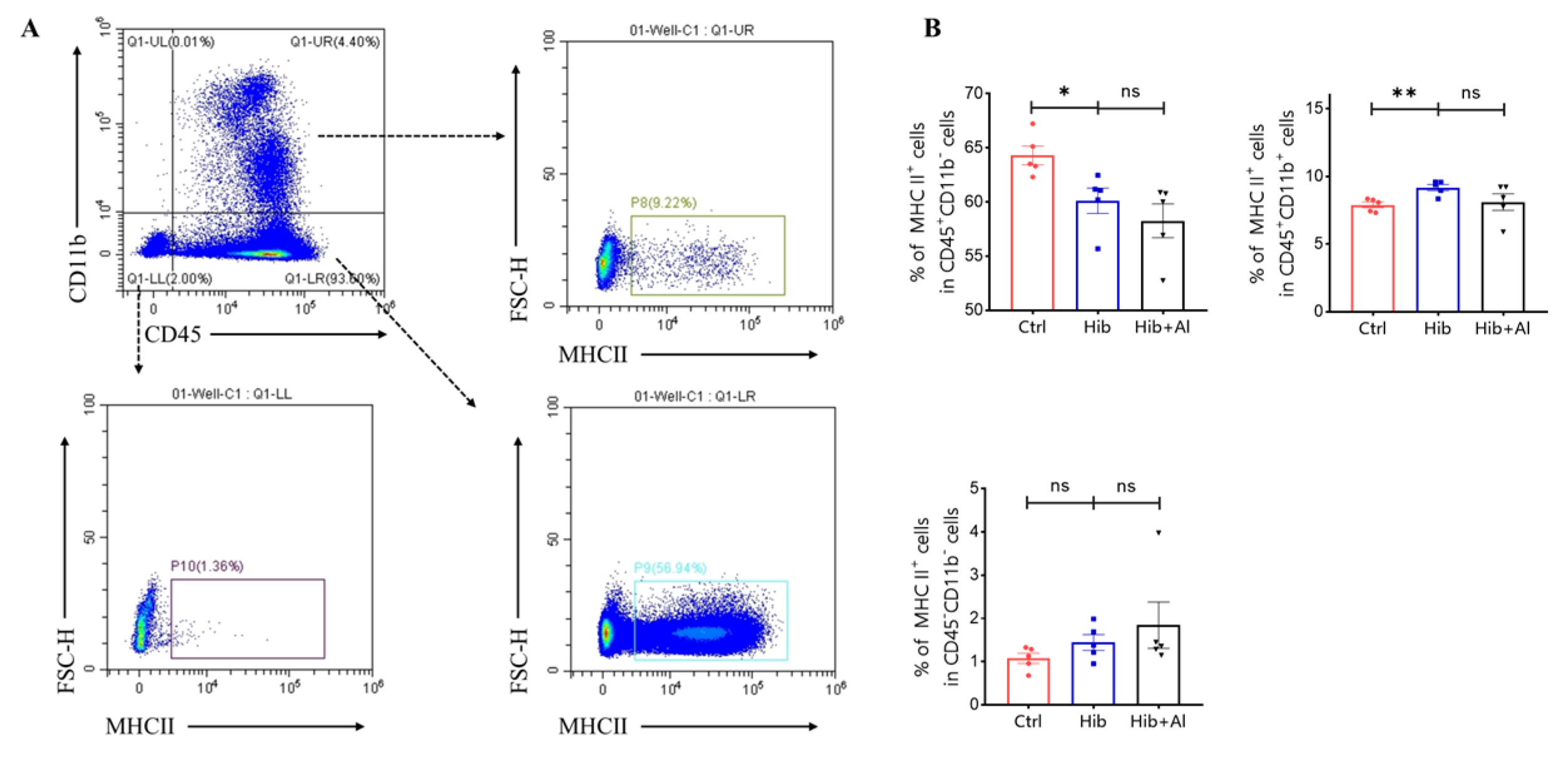
The Hib vaccine could enhance the anti-PRP antibody titers in serum. However, the distributions of other immune cells induced by the Hib vaccine were unclear. Our results above have proven that the addition of aluminum adjuvant was beneficial to the production of antibodies and the cellular immune response. Next, we explored which types of immune cells were changed after the mice were immunized with the Hib or Hib+Al vaccines(Figure 5). We found that the Hib vaccine could increase CD4+ T cells, neutrophil, and ILC2 cells and decrease ILC1 cells(Figure 5A,C,E,G). We also found that the Hib vaccine could decrease the MHC II expression in the CD11b-CD45+ population and increased the MHC II expression in the CD11b+CD45+ population, which suggests that the Hib vaccine could enhance the MHC II expression in myeloid cells (Scheme 2). CD4+ T cells were important both in humoral and cellular immune responses. The neutrophils played an important role in the innate immune responses, and it has been reported that they are required during vaccination for host protection and for promoting the antibody responses at immunization sites [11]. These results indicated that the Hib vaccines could induce a high level of antibodies. However, there were no differences in CD8+T, DC, monocytes, or ILC3s between the Hib and control groups (Figure 5A,B,D,E,F). In comparison with the Hib vaccine, the Hib+Al vaccine significantly decreased the percentage of ILC3s. It has been reported that ILC3 could influence the Tfh function and inhibit the antibody production of GCB cells through PDL1 and MHCII, suggesting that the aluminum adjuvant may also induce the antibody through inhibition of ILC3s. These results show that the Hib vaccines could induce the innate and adaptive immune responses, and the aluminum adjuvant could promote the immune response by regulating the ILC3s.
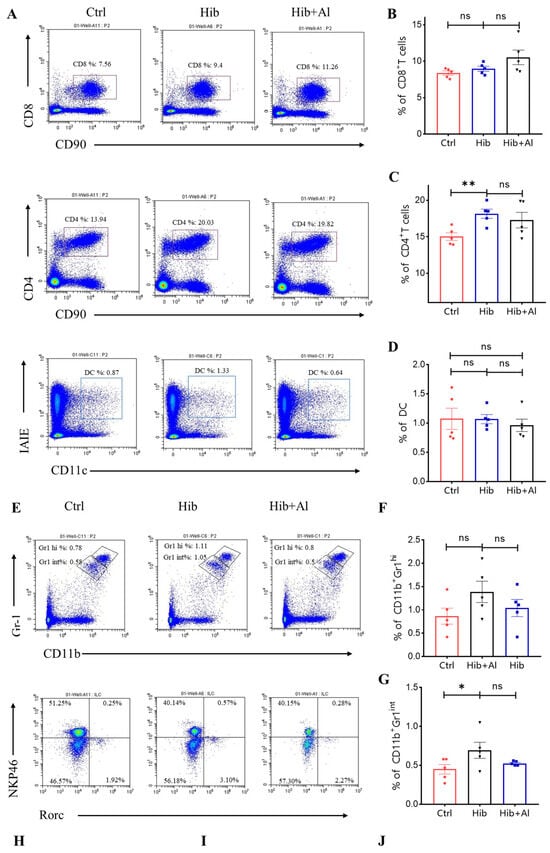
Figure 5.
Flow cytometry analysis of immune cells. (A,E) The representative flow cytometry charts of different cells. CD8+ T cells, CD4+ T cells, MDSC, DC: gated in live CD45+, ILCs: gated in live CD45+CD90+CD3−; (B) the percentage of the CD8+ T cells (n = 5); (C) the percentage of the CD4+ T cells (n = 5); (D) the percentage of the DC cells (n = 5); (F) the percentage of the monocytes (CD11b+Gr1hi) (n = 5); (G) the percentage of the neutrophil (CD11b+Gr1int) (n = 5); (H) the percentage of the ILC1 (n = 5); (I) the percentage of the ILC3 (n = 5); (J) the percentage of the ILC2 (n = 5); error bars represent the SEM. * p < 0.05; ** p < 0.01 (Student’s t-test); ns, no significant difference.
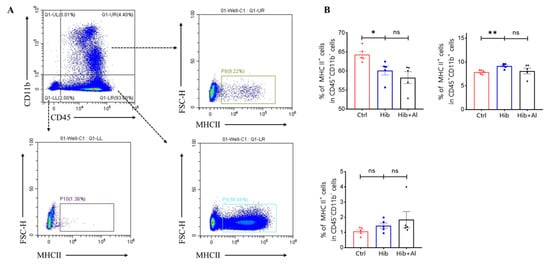
Scheme 2.
Flow cytometry analysis of MHC II expression in myeloid cells. (A) The representative flow cytometry chart of MHC II expression; (B) the percentage of MHC II expression. Error bars represent the SEM. * p < 0.05; ** p < 0.01 (Student’s t-test); ns, no significant difference.
3. Discussion
Hib is one of the common causes of pneumonia, meningitis, and other serious infections in children, and vaccination is one of the most effective means of preventing Hib disease worldwide [12]. With the development of DTaP-series combination vaccines [13], understanding the mechanisms of the Hib vaccine and the interaction of the adjuvant with Hib is of great significance to the development of combination vaccines. In this work, we analyzed the immune response induced by the Hib vaccine and found that the Hib vaccine induced the formation of GCBs, which are a core part of humoral immunity, and their differentiation to plasma cells and memory B cells determines the quantity and quality of final antibodies. Consistent with the formation of GCBs, the Hib vaccine also promotes the production of anti-PRP antibodies, including IgG1, IgG2a, IgG2b, and IgM. With the exception of humoral immunity, it can also improve cellular immunity. After immunization with the Hib vaccine, we evaluated the Th1 response (IL-2, IFN-γ). IL-2 promoted the differentiation of CD4+ T cells into Th1 and Th2 cells, promoted CD+4 and CD8+ T cell activation and memory phenotype formation, and could also inhibit the differentiation of CD4+ T cells into inflammatory Th17 cells [14]. Therefore, the cytokines could improve the function of the Hib vaccine. These results show that the Hib vaccine protects the body both through humoral and cellular immunity.
With the development of DTaP/Hib combination vaccines, a number of noteworthy problems have arisen. There are many components of the combined vaccine, and these different components have numerous properties. Additionally, the DTaP antigens require an adjuvant to activate the immune system, but the Hib vaccine does not contain adjuvants. Many reports have shown that the functions of Hib are decreased in DTaP/Hib combined vaccines [15]. For example, in trials with the Hib/DTaP3 combined vaccine, the results showed significant reduction in Hib antibodies, especially in pre-term infants, compared with the individual injection of Hib and DTaP3 vaccines. Also, some clinical studies show that there were no significant reductions in anti-PRP antibody levels using the Hib/DTwP combined vaccines [5,6,16]. The problem of reduced Hib antibodies in combination vaccines is believed to be due to interference between antigens or pre-existing immune responses to carrier proteins (conjugated to Hib antigens, such as TT) significantly reducing the production of anti-PRP antibodies in some studies. However, reducing the dose of TT did not influence the immunogenicity of Hib either [17], while some issues are believed to be caused by the incompatibility of Hib and adjuvants [18,19]. Therefore, whether adjuvants can influence the Hib vaccine is a question worth studying. Here, we used the Hib+Al vaccine to immunize mice. The efficacy of the vaccine was evaluated from different dimensions. The results show that the addition of the adjuvant promoted greater GCB and anti-PRP antibodies compared with the Hib vaccine. The different subtypes of IgG (IgG1, IgG2a, IgG2b) also demonstrated a rising trend in the Hib+Al group. The IgG1 was associated with a Th2-type immune response, and IgG2a was stimulated during Th1-type immune responses [20,21,22]. The Th1 subset could produce IFN-γ and IL-2 [23]. Therefore, the aluminum adjuvant may induce the Th1 and Th2 responses, which was proved by the increase in IL-2 and IFN-γ expression in the Hib+Al group. The aluminum adjuvant played an effective role in the Hib vaccine, which is inconsistent with previous reports [3]. Hib conjugate vaccines have different immunological profiles depending on the manufacturer, and the immunogenicity mainly depends on the carrier protein type, binding method, conjugate molecular size, and glycoprotein ratio, among which the conjugate molecular size is crucial for the immunogenicity of the vaccine. Therefore, the different effects of aluminum adjuvants on Hib vaccines may be influenced by animal models, aluminum adjuvant types, immune programs, and the antigenic components. The mechanisms of the effects of aluminum adjuvants on Hib vaccines by different manufacturers need to be further explored. The Hib vaccine demonstrates good safety and efficacy. However, it is still unclear which cells are induced by the Hib vaccine. In this work, we also found that CD4+ T cells, neutrophils, and ILC2 were upregulated after immunization with the Hib vaccine. CD4+ T cells can promote the antibody production of B cells, and they can also differentiate into Th17 or Treg cells, inhibiting or promoting inflammation. We also measured the IL-10 and IL-17 secretion in the serum and in the supernatants of cell secretions. There were no significant differences between the Hib and control groups, which suggests that the Hib vaccine may not induce high levels of Th17 or Treg cells. However, this point needs to be examined further. The neutrophil also plays an important role in the antibody response [24]. The addition of the aluminum adjuvant reduced the ILC3 levels in the spleen. It has been reported that ICL3 expresses the major histocompatibility complex class II and PD-1, which regulates the CD4+ T cell responses [25,26] and could inhibit the function of Tfh and then influence GCB formation [27]. Therefore, in this study, the aluminum adjuvant may have influenced ILC3 and promoted the production of antibodies via B cells.
In summary, our data illustrate the mechanism of Hib vaccine induction and that it can induce both the Th1 and Th2 cell responses while also promoting the neutrophil and ILC2 response. The addition of the aluminum adjuvant upregulated both the Th1 and Th2 cell response and decreased the ILC3 expression. These results reveal that the addition of the adjuvant in the Hib vaccine was beneficial for inducing a stronger immune response, which provides theoretical support for the development of subsequent combination vaccines. The interaction of Hib and DTaP components in the immune response should be further explored in order to develop better Hib-containing combination vaccines.
4. Materials and Methods
4.1. Vaccines
A Hib vaccine containing 10 μg of PRP/0.5 mL conjugated to TT, with a protein/polysaccharide ratio of 2–3, was used. The Hib vaccine was absorbed with aluminum hydroxide adjuvant.
4.2. Animals and Immunization
Mice were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd., Beijing, China and then maintained in a specific-pathogen-free (SPF) environment at the Laboratory Animal Center of Beijing Institute of Biological Products Co., Ltd., Beijing, China. All of the mice were female, Balb/C, and they weighed 12~14 g. The mice were immunized with vaccines (2.5 μg/dose) on day 0 and day 14, and the sera were extracted at day 21. The total number of animals per group is presented in Table 1.

Table 1.
Total number of animals per group.
4.3. Reagents
The reagent information is presented in Table 2.

Table 2.
The information of the reagents used.
4.4. ELISA
Antibodies to PRP were detected by ELISA. Briefly, plates were coated with PRP overnight at 4 °C (0.5 Lf/mL). After blocking for 1 h with PBS containing 1% BSA, the gradient-diluted serum samples were added to the plate, and, after 1 h, anti-mouse HRP-IgG (1:1000), HRP-IgG1 (1:5000), HRP-IgG2a (1:5000), HRP-IgG2b (1:5000), and HRP-IgM (1:5000) were added to the plates and incubated for 30 min, RT. After washing, TME and stop solution were added to the plates. The absorbance was measured at 450 nm and OD630.
4.5. FACS
The spleens were analyzed on day 28 after initial immunity. The spleen cells were lysed using RBC (Biolegend, San Diego, CA, USA, 420301), and then the cells were blocked with blocking antibody (CD16/32) for 30 min at 4–8 °C. After washing, the cells were stained with Fixable Viability Dye eFluor 506, which was added to exclude the dead cells, and then the cells were stained with other antibodies using the appropriate antibody on ice for 30 min. Flow cytometry was performed on a CytoFLEX S instrument (Beckman, Brea, CA, USA) and analyzed with CytoFLEX S FlowJo software (1.0). The detailed protocol was described by [28].
4.6. ELISpot
Immune cells in the spleen were seeded into the 96-well plates (5 × 105 cells per well) and incubated with PRP solution (4 mg/mL) in DMEM medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA, C11995500BT) containing 1% penicillin–streptomycin (Procell, PB180120) and 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). After 36 h of stimulation, the IFN-γ and IL-4 produced by immune cells were tested with the kits (Mouse IFN-γ precoated ELISpot kit and the Mouse IL-4 precoated ELISpot kit) (Dakewe Group, Shenzhen, China).
4.7. CBA Analysis
Immune cells in the spleen were seeded into the 96-well plates (5 × 105 cells per well) and incubated with PRP solution (4 mg/mL) in DMEM medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA, C11995500BT) containing 1% penicillin–streptomycin (Procell, PB180120) and 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). After 36 h of stimulation, the cytokines in the supernatant were tested with a CBA kit (BD biosciences, USA). Briefly, we mixed the capture beads and added 50 µL of the sample and detection reagent to the tubes followed by incubation for 2 h at room temperature. The samples were then tested using a CytoFLEX S instrument (Beckman, Brea, CA, USA).
4.8. Statistics
Statistical analysis was performed using GraphPad Prism 8. A two-tailed Mann–Whitney test was used to determine significance. Error bars represent SEM. * p < 0.05; ** p < 0.01; *** p < 0.001.
5. Conclusions
We systematically evaluated the distribution of immune cells after subcutaneous injection of Hib vaccine, and found that the Hib vaccine could induce both the Th1 and Th2 cell responses, also the neutrophil and ILC2 response. The addition of the aluminum adjuvant could promote the immune response of Hib vaccine which was beneficial for inducing a stronger immune response.
Author Contributions
H.W. was the corresponding author. H.W., Y.H., Y.Z. and H.L. (Hongyang Liang) conceived and designed this study. Y.H. prepared the manuscript. Y.H., X.W., H.L. (Haoyue Lan), D.T. and Y.L. performed all experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Beijing Institute of Biological Products Company Limited, Laboratory Animal Welfare Ethics ( Approval Code: BSYYF20230525007 Approval Date: 25 May 2023). All animals were housed and cared for in an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. All experimental procedures with mice were conducted according to Chinese animal use guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article. Limitations of the Study: Our study only evaluated the Hib-vaccine-induced humoral and cellular immunity in mice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gilsdorf, J.R. Hib Vaccines: Their Impact on Haemophilus influenzae Type b Disease. J. Infect. Dis. 2021, 224, S321–S330. [Google Scholar] [CrossRef]
- Decker, M.D.; Edwards, K.M. Haemophilus influenzae type b vaccines: History, choice and comparisons. Pediatr. Infect. Dis. J. 1998, 17, S113–S116. [Google Scholar] [CrossRef]
- Mawas, F.; Dickinson, R.; Douglasbardsley, A.; Xing, D.K.; Sesardic, D.; Corbel, M.J. Immune interaction between components of acellular pertussis-diphtheria-tetanus (DTaP) vaccine and Haemophilus influenzae b (Hib) conjugate vaccine in a rat model. Vaccine 2006, 24, 3505–3512. [Google Scholar] [CrossRef]
- Mawas, F.; Newman, G.; Burns, S.; Corbel, M.J. Suppression and modulation of cellular and humoral immune responses to Haemophilus influenzae type B (Hib) conjugate vaccine in hib–diphtheria-tetanus toxoids–acellular pertussis combination vaccines: A study in a rat model. J. Infect. Dis. 2005, 191, 58–64. [Google Scholar] [CrossRef]
- Mulholland, E.K.; Hoestermann, A.; Ward, J.I.; Maine, N.; Ethevenaux, C.; Greenwood, B.M. The use of Haemophilus influenzae type b-tetanus toxoid conjugate vaccine mixed with diphtheria-tetanus-pertussis vaccine in Gambian infants. Vaccine 1996, 14, 905–909. [Google Scholar] [CrossRef]
- Cherian, T.; Thomas, N.; Raghupathy, P.; Durot, I.; Dutta, A. Safety and immunogenicity of Haemophilus influenzae type B vaccine given in combination with DTwP at 6, 10 and 14 weeks of age. Indian Pediatr. 2002, 39, 427–436. [Google Scholar]
- Tavakoli, M.; Bouzari, S.; Jafari, A.; Oloomi, M.; Karam, M.R.A.; Najar-Peerayeh, S.; Siadat, S.D. Effect of nontypeable Haemophilus influenzae protein E (PE) as a microbial adjuvant on the amount of antibody against PRP of Haemophilus influenzae type b (Hib) in BALB/c mice. Microb. Pathog. 2019, 129, 78–81. [Google Scholar] [CrossRef]
- Gupta, R.K.; Anderson, R.; Cecchini, D.; Rost, B.; Xu, J.; Gendreau, K.; Saroff, D.L.; Marchant, C.; Siber, G.R. Evaluation of a guinea pig model to assess interference in the immunogenicity of different components of a combination vaccine comprising diphtheria, tetanus and acellular pertussis (DTaP) vaccine and haemophilus influenzae type b capsular polysaccharide conjugate vaccine. Biologicals 1999, 27, 167–176. [Google Scholar] [CrossRef]
- Gupta, R.; Anderson, R.; Cecchini, D.; Rost, B.; Griffin, P.; Benscoter, K.; Xu, J.; Montanez-Ortiz, L.; Siber, G.R. Development of a guinea-pig model for potency/immunogenicity evaluation of diphtheria, tetanus acellular pertussis (DTaP) and Haemophilus influenzae type b polysaccharide conjugate vaccines. Dev. Biol. Stand. 1996, 86, 283–296. [Google Scholar]
- He, X.; Wang, J.; Tang, Y.; Chiang, S.T.; Han, T.; Chen, Q.; Qian, C.; Shen, X.; Li, R.; Ai, X. Recent Advances of Emerging Spleen-Targeting Nanovaccines for Immunotherapy. Adv. Healthc. Mater. 2023, 12, e2300351. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, K.; Lu, W.; Zhao, P.; Wang, Z.; Cui, C.; Liu, Y.; Yang, M.; Yu, Y.; Wang, L. Neutrophils recruited to immunization sites initiating vaccine-induced antibody responses by locally expressing BAFF. iScience 2022, 25, 104453. [Google Scholar] [CrossRef]
- Anderson, P.; Smith, D.H.; Ingram, D.L.; Wilkins, J.; Wehrle, P.F.; Howie, V.M. Antibody of polyribophate of Haemophilus influenzae type b in infants and children: Effect of immunization with polyribophosphate. J. Infect. Dis. 1977, 136, S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Kalies, H.; Grote, V.; Verstraeten, T.; Hessel, L.; Schmitt, H.-J.; von Kries, R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr. Infect. Dis. J. 2006, 25, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Malek, T.R. The biology of interleukin-2. Annu. Rev. Immunol. 2008, 26, 453–479. [Google Scholar] [CrossRef]
- Poolman, J.; Kaufhold, A.; De Grave, D.; Goldblatt, D. Clinical relevance of lower Hib response in DTPa-based combination vaccines. Vaccine 2001, 19, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Slack, M.H.; Schapira, D.; Thwaites, R.J.; Burrage, M.; Southern, J.; Andrews, N.; Borrow, R.; Goldblatt, D.; Miller, E. Immune response of premature infants to meningococcal serogroup C and combined diphtheria-tetanus toxoids-acellular pertussis-Haemophilus influenzae type b conjugate vaccines. J. Infect. Dis. 2001, 184, 1617–1620. [Google Scholar] [CrossRef]
- Bernstein, H.H.; Seyferth, E.R. Hib antibody responses in infants following diphtheria, tetanus, acellular pertussis, and conjugated Haemophilus influenzae type b (Hib) combination vaccines with decreasing amounts of tetanus toxoid. Vaccine 2017, 35, 6707–6711. [Google Scholar] [CrossRef]
- Tafreshi, S.-Y.H. Efficacy, safety, and formulation issues of the combined vaccines. Expert Rev. Vaccines 2020, 19, 949–958. [Google Scholar] [CrossRef]
- Konini, A.; Kang, M.; Moghadas, S.M. Simulating Immune Interference on the Effect of a Bivalent Glycoconjugate Vaccine against Haemophilus influenzae Serotypes “a” and “b”. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 5486869. [Google Scholar] [CrossRef]
- Benne, C.; Harmsen, M.; van der Graaff, W.; Verheul, A.; Snippe, H.; Kraaijeveld, C. Influenza virus neutralizing antibodies and IgG isotype profiles after immunization of mice with influenza A subunit vaccine using various adjuvants. Vaccine 1997, 15, 1039–1044. [Google Scholar] [CrossRef]
- Hocart, M.J.; Mackenzie, J.S.; Stewart, G.A. The immunoglobulin G subclass responses of mice to influenza a virus: The effect of mouse strain, and the neutralizing abilities of individual protein a-purified subclass antibodies. J. Gen. Virol. 1989, 70 Pt 9, 2439–2448. [Google Scholar] [CrossRef]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef]
- Mbulaiteye, S.; Kemp, T.; Gage, J.; Ajenifuja, K.; Kiruthu, C.; Wentzensen, N.; Adepiti, C.; Wacholder, S.; Burk, R.; Schiffman, M.; et al. Plasma cytokine levels and human papillomavirus infection at the cervix in rural Nigerian women. Cytokine 2013, 64, 146–151. [Google Scholar] [CrossRef]
- Tchalla, E.Y.; Bhalla, M.; Wohlfert, E.A.; Ghanem, E.N.B. Neutrophils Are Required during Immunization with the Pneumococcal Conjugate Vaccine for Protective Antibody Responses and Host Defense Against Infection. J. Infect. Dis. 2020, 222, 1363–1370. [Google Scholar] [CrossRef]
- Hepworth, M.R.; Monticelli, L.A.; Fung, T.C.; Ziegler, C.G.K.; Grunberg, S.; Sinha, R.; Mantegazza, A.R.; Ma, H.-L.; Crawford, A.; Angelosanto, J.M.; et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 2013, 498, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Fung, T.C.; Goc, J.; Wang, X.; Hepworth, M.R. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. J. Immunol. 2015, 348, 1031–1035. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Hao, J.; He, Y.; Dong, X.; Fu, Y.-X.; Guo, X. The Interaction between Lymphoid Tissue Inducer-Like Cells and T Cells in the Mesenteric Lymph Node Restrains Intestinal Humoral Immunity. Cell Rep. 2020, 32, 107936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, W.; Lou, Z.; Huang, B.; Zhou, W.; Zhao, Y.; Zhang, J.; Liang, H.; Li, N.; Zhu, X.; et al. Immunogenicity Evaluating of the Multivalent COVID-19 Inactivated Vaccine against the SARS-CoV-2 Variants. Vaccines 2022, 10, 956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).