Contact Force-Guided versus Contact Force-Blinded Cavo-Tricuspid Isthmus Ablation for Atrial Flutter: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias and Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results and Study Selection
3.2. Characteristics of Included Studies
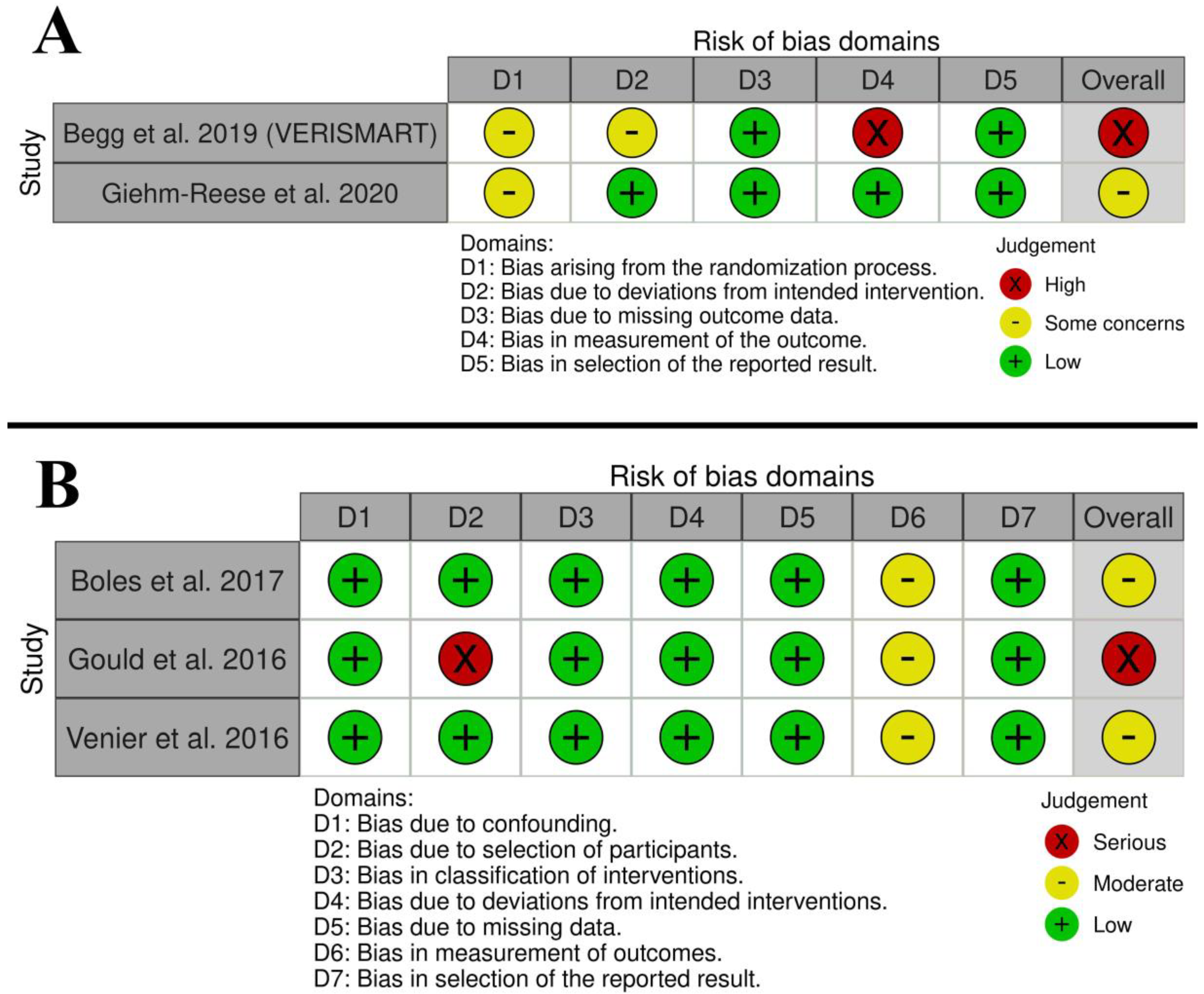
3.3. Risk of Bias and Quality of Evidence
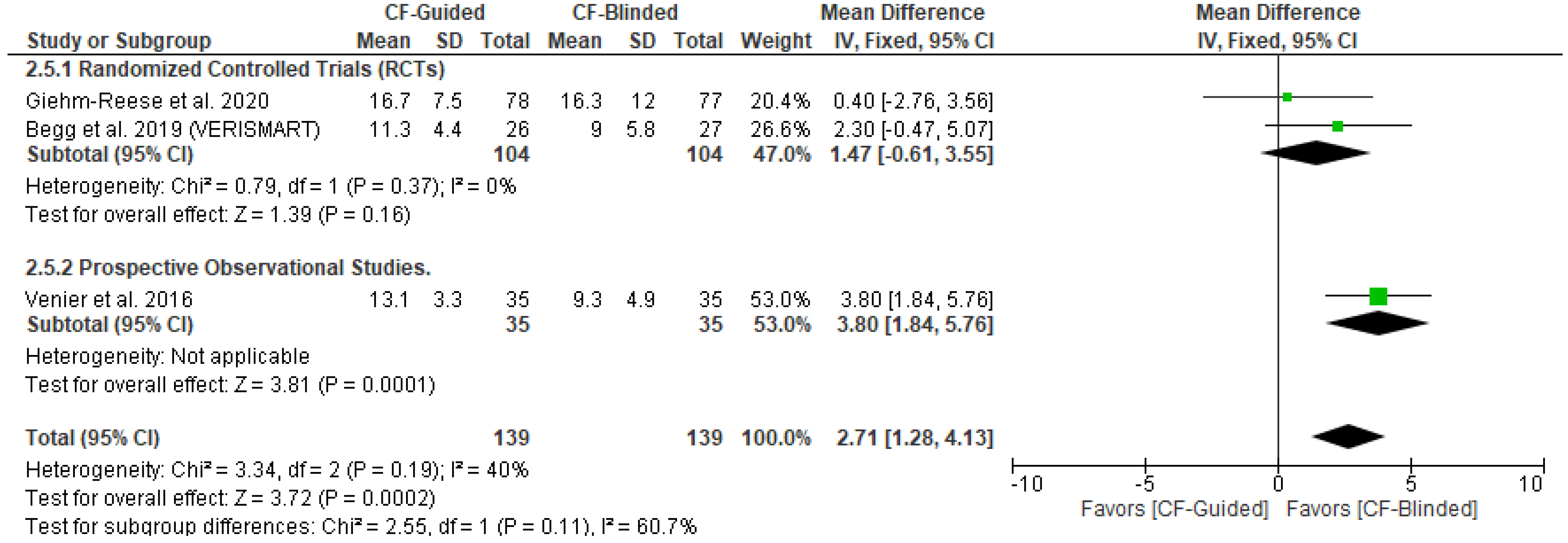
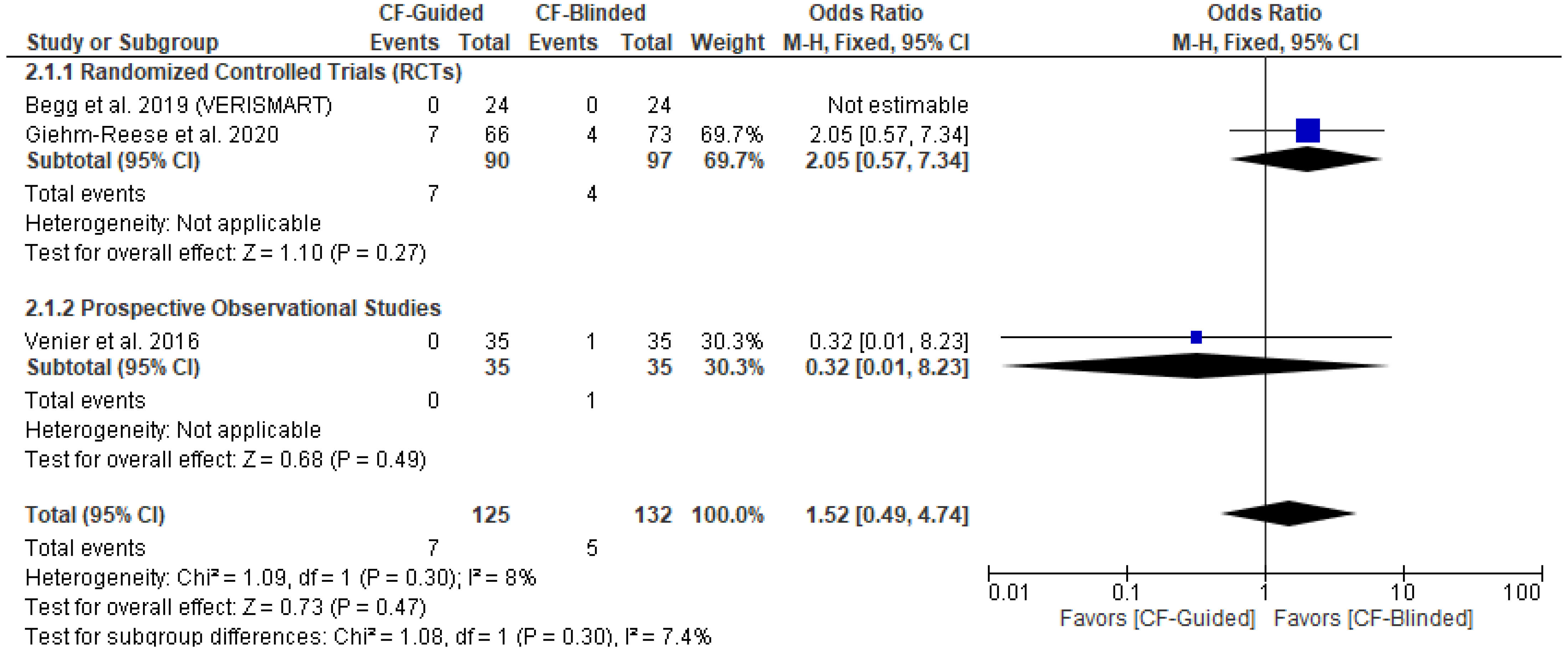
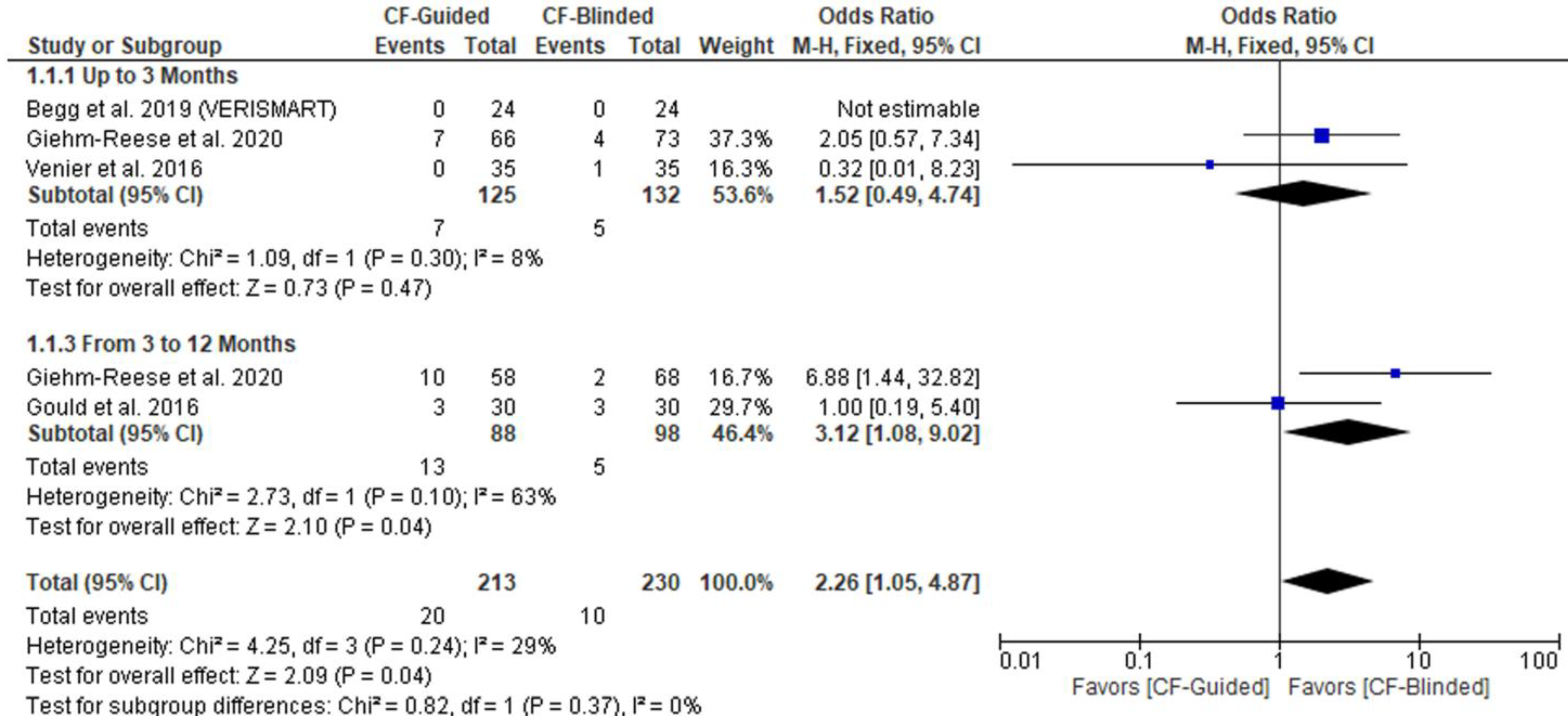
3.4. Primary Outcome (AFL Recurrence)
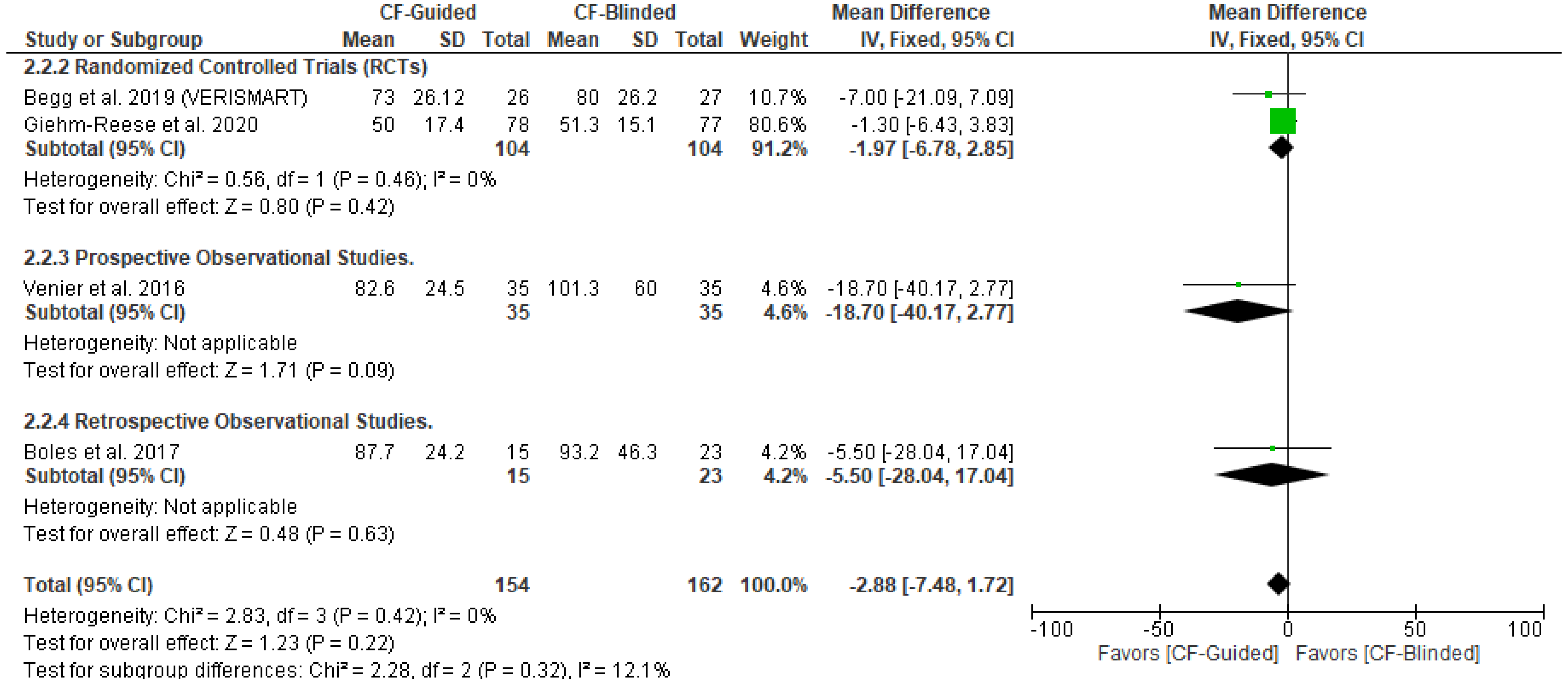
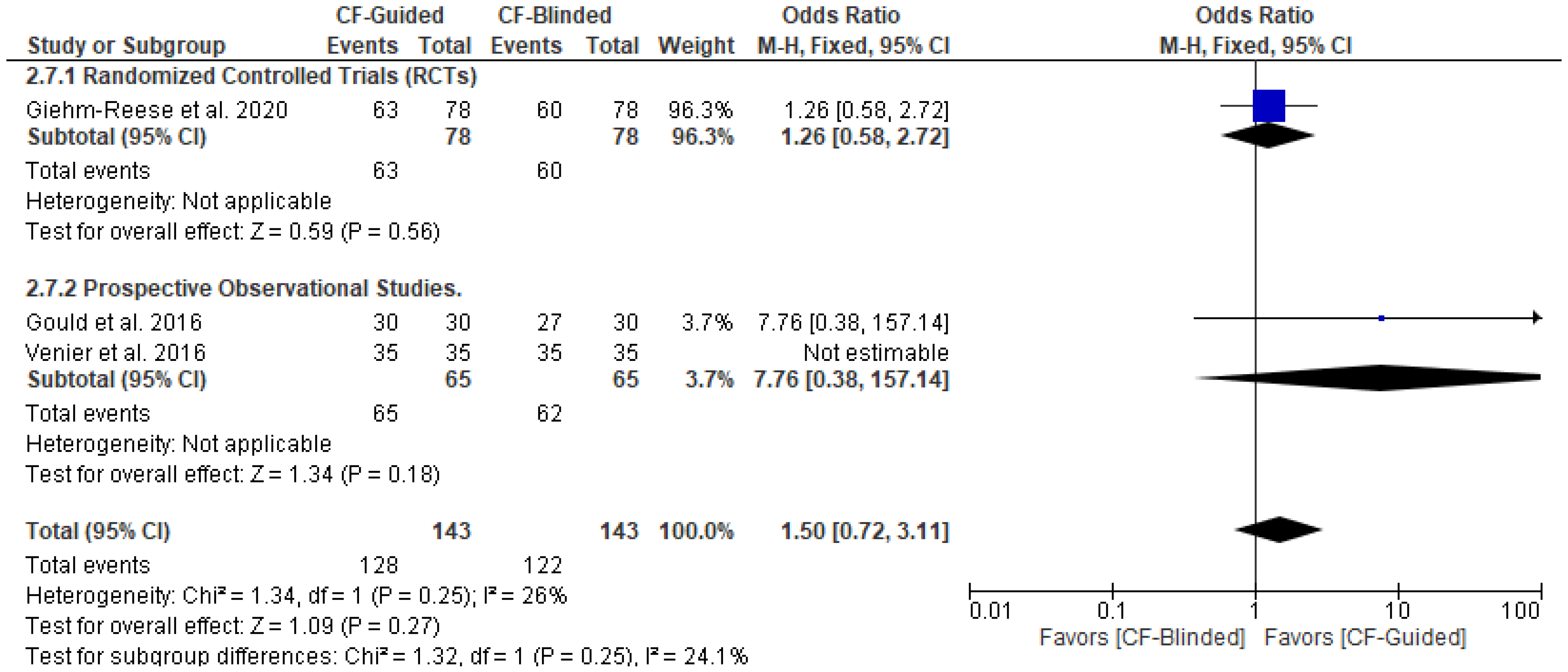
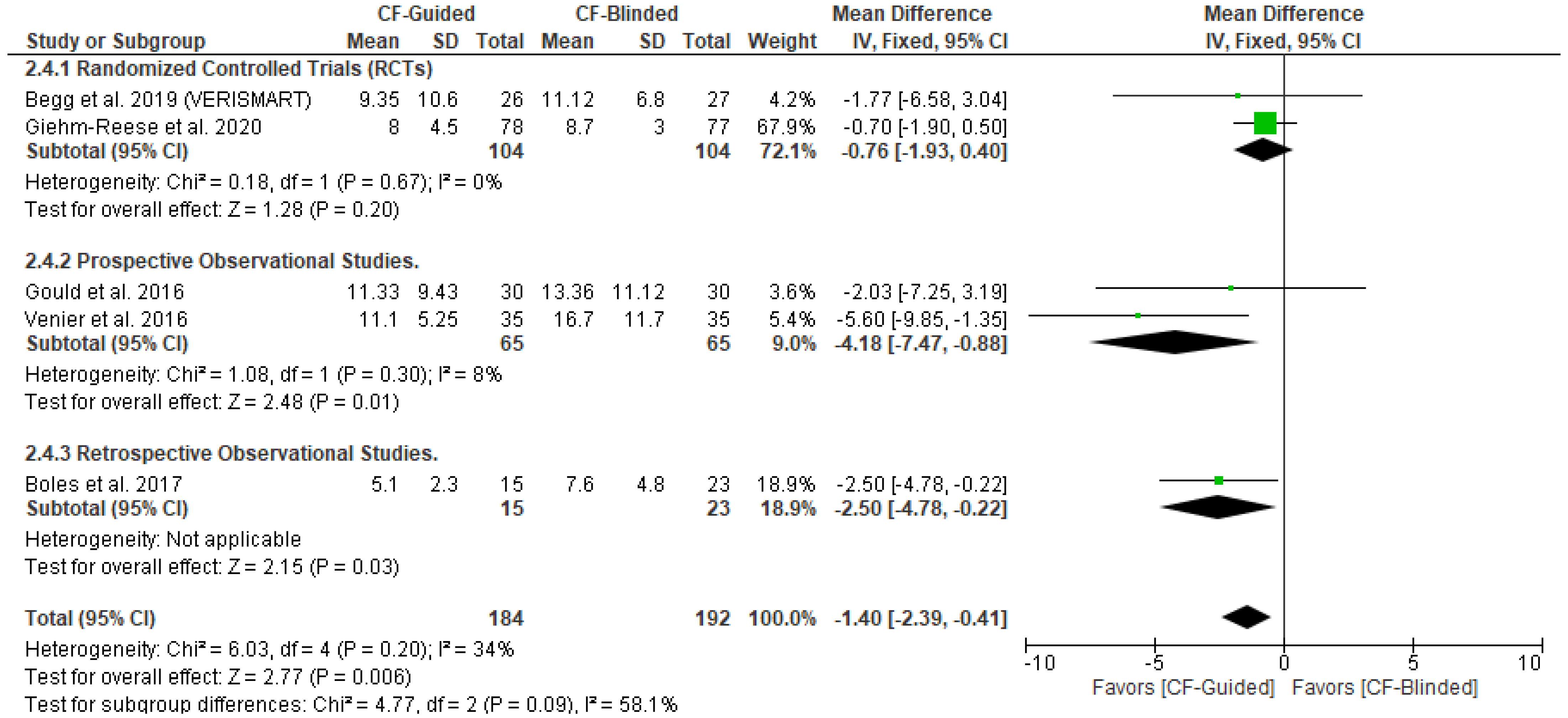
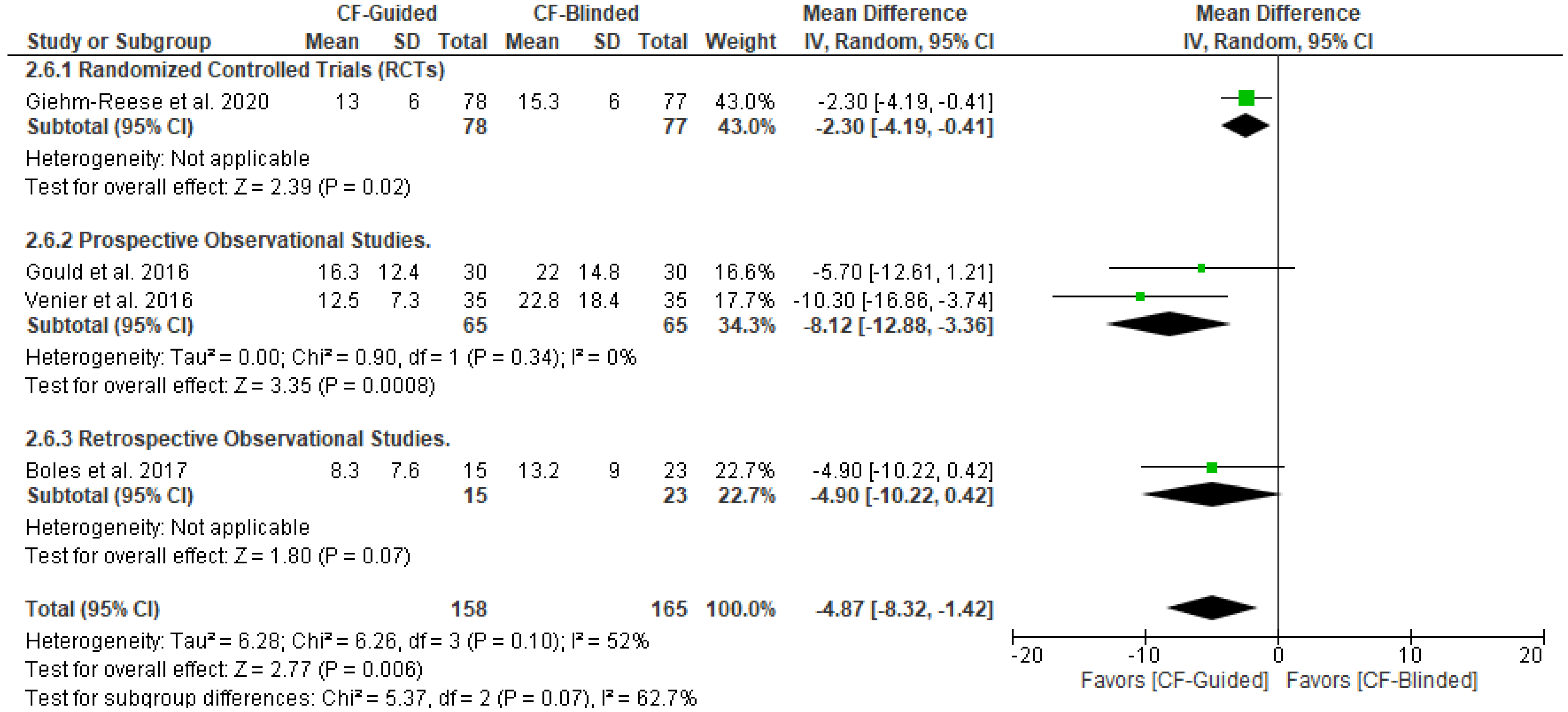
3.5. Secondary Outcomes
4. Discussion
4.1. Limitations
4.2. Future Research Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AFL | Atrial flutter |
| BDIB | Bidirectional isthmus block |
| CF | Contact force |
| CHA2DS-VASc | Congestive heart failure, hypertension, age > 75, diabetes mellitus, and prior stroke or transient ischemic attack [10] |
| CI | Confidence interval, the lower and upper limits of significance |
| CTI | Cavo-tricuspid isthmus |
| CTIA | Cavo-tricuspid isthmus ablation |
| DM | Diabetes mellitus |
| ECG | Electrocardiogram |
| EPS | Electrophysiology study |
| HF | Heart failure |
| HTN | Hypertension |
| ICH | Intracranial hemorrhage |
| ID | Identification |
| IHD | Ischemic heart disease |
| LSI | Lesion size index |
| LVEF | Left ventricular ejection fraction |
| MD | Mean difference |
| N | Number |
| N/A | Not available |
| NOS | Newcastle–Ottawa Scale [23] |
| OR | Odds ratio |
| OS | Observation study |
| p | Probability |
| PICO | Population intervention control outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses [8] |
| PV | Pulmonary vein |
| QUADAS | Quality Assessment of Diagnostic Accuracy Studies [22] |
| RCT | Randomized controlled trial |
| RF | Radiofrequency |
| RoB | Risk of bias |
| RoB 2 | Risk of bias 2 [11] |
| ROBINS-1 | Risk Of Bias In Non-randomized Studies—of Interventions [12] |
| SD | Standard deviation |
| SP | Steam pop |
| TIA | Transient ischemic attack |
| WOS | Web of Science |
PRISMA 2020 Checklist [8]
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 line 2 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | page 2 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 3 Section 2.2 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 3 Section 2.1 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Page 2,3 Table 1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Page 3 Section 2.3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Page 3 Section 2.3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Page 3 Section 2.4 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Page 3 Section 2.4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Page 3 Section 2.5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Page 4 Section 2.6 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 4 Section 2.6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Page 4 Section 2.6 | |
| 13c | Describe any methods used to tabulate or visually display the results of individual studies and syntheses. | Page 4 Section 2.6 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Page 4 Section 2.6 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | Not applicable | |
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results. | Not applicable | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | Page 3 Section 2.5 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Page 4 Section 2.6 |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 4 Section 3.1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Not applicable | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Page 5 Section 3.2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Page 8 Section 3.3 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Pages 9, 10 Section 3.4 and Section 3.5 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Page 5 Section 3.2 and Section 3.3 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was conducted, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Pages 9, 10 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Not applicable | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Not applicable | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Page 8 Section 3.3 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Not available |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 11 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 12 | |
| 23c | Discuss any limitations of the review processes used. | Page 12 | |
| 23d | Discuss the implications of the results for practice, policy, and future research. | Page 12 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including the register name and registration number, or state that the review was not registered. | Not available |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Not available | |
| 24c | Describe and explain any amendments to the information provided at registration or in the protocol. | Not available | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Page 12 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 12 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Page 12 |
Subgroup Analysis Based on the Study Design
References
- Giehm-Reese, M.; Kronborg, M.B.; Lukac, P.; Kristiansen, S.B.; Jensen, H.K.; Gerdes, C.; Kristensen, J.; Nielsen, J.M.; Nielsen, J.C. A Randomized Trial of Contact Force in Atrial Flutter Ablation. Europace 2020, 22, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Natale, A.; Newby, K.H.; Pisanó, E.; Leonelli, F.; Fanelli, R.; Potenza, D.; Beheiry, S.; Tomassoni, G. Prospective Randomized Comparison of Antiarrhythmic Therapy versus First-Line Radiofrequency Ablation in Patients with Atrial Flutter. J. Am. Coll. Cardiol. 2000, 35, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Nakagawa, H.; Shah, D.C.; Lambert, H.; Leo, G.; Aeby, N.; Ikeda, A.; Pitha, J.V.; Sharma, T.; Lazzara, R.; et al. Novel Contact Force Sensor Incorporated in Irrigated Radiofrequency Ablation Catheter Predicts Lesion Size and Incidence of Steam Pop and Thrombus. Circ. Arrhythm. Electrophysiol. 2008, 1, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Foley, L.; Leo, G.; D’Avila, A.; Reddy, V.Y.; Thiagalingam, A.; Ruskin, J.N.; Lambert, H.; Guerrero, J.L. Importance of Catheter Contact Force during Irrigated Radiofrequency Ablation: Evaluation in a Porcine Ex Vivo Model Using a Force-Sensing Catheter. J. Cardiovasc. Electrophysiol. 2010, 21, 806–811. [Google Scholar]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Natale, A.; Albenque, J.-P.; Kautzner, J.; Shah, D.; Michaud, G.; Wharton, M.; Harari, D.; et al. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015, 132, 907–915. [Google Scholar]
- Kautzner, J.; Neuzil, P.; Lambert, H.; Peichl, P.; Petru, J.; Cihak, R.; Skoda, J.; Wichterle, D.; Wissner, E.; Yulzari, A.; et al. EFFICAS II: Optimization of Catheter Contact Force Improves Outcome of Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation. Europace 2015, 17, 1229–1235. [Google Scholar] [CrossRef]
- Virk, S.A.; Ariyaratnam, J.; Bennett, R.G.; Kumar, S. Updated Systematic Review and Meta-Analysis of the Impact of Contact Force Sensing on the Safety and Efficacy of Atrial Fibrillation Ablation: Discrepancy between Observational Studies and Randomized Control Trial Data. Europace 2019, 21, 239–249. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, N71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley Online Library: Hoboken, NJ, USA, 2016; 694p. [Google Scholar] [CrossRef]
- Gage, B.F.; Van Walraven, C.; Pearce, L.; Hart, R.G.; Koudstaal, P.J.; Boode, B.S.P.; Petersen, P. Selecting Patients with Atrial Fibrillation for Anticoagulation: Stroke Risk Stratification in Patients Taking Aspirin. Circulation 2004, 110, 2287–2292. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, L4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- RevMan. Cochrane Training. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 3 August 2021).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, G.A.; O’Neill, J.; Sohaib, A.; McLean, A.; Pepper, C.B.; Graham, L.N.; Hogarth, A.J.; Page, S.P.; Gillott, R.G.; Hill, N.; et al. Multicentre Randomised Trial Comparing Contact Force with Electrical Coupling Index in Atrial Flutter Ablation (VERISMART Trial). PLoS ONE 2019, 14, e0212903. [Google Scholar] [CrossRef]
- Boles, U.; Gul, E.; Fitzpatrick, N.; Enriquez, A.; Conroy, J.; Ghassemian, A.; David, S.; Baranchuk, A.; Simpson, C.; Redfearn, D.; et al. Lesion Size Index in Maximum Voltage-Guided Cavotricuspid Ablation for Atrial Flutter. J. Innov. Card. Rhythm Manag. 2017, 8, 2732–2738. [Google Scholar] [CrossRef]
- Gould, P.A.; Booth, C.; Dauber, K.; Ng, K.; Claughton, A.; Kaye, G.C. Characteristics of Cavotricuspid Isthmus Ablation for Atrial Flutter Guided by Novel Parameters Using a Contact Force Catheter. J. Cardiovasc. Electrophysiol. 2016, 27, 1429–1436. [Google Scholar] [CrossRef]
- Venier, S.; Andrade, J.G.; Khairy, P.; Mondésert, B.; Dyrda, K.; Rivard, L.; Guerra, P.G.; Dubuc, M.; Thibault, B.; Talajic, M.; et al. Contact-Force-Guided vs. Contact-Force-Blinded Catheter Ablation of Typical Atrial Flutter: A Prospective Study. Europace 2017, 19, 1043–1048. [Google Scholar] [CrossRef]
- Pang, N.; Gao, J.; Zhang, N.; Guo, M.; Wang, R. Cavotricuspid Isthmus Ablation for Atrial Flutter Guided by Contact Force Related Parameters: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2023, 9, 1060542. [Google Scholar] [CrossRef]
- Sakama, S.; Yagishita, A.; Sakai, T.; Morise, M.; Ayabe, K.; Amino, M.; Ikari, Y.; Yoshioka, K. Ablation Index-Guided Cavotricuspid Isthmus Ablation with Contiguous Lesions Using Fluoroscopy Integrated 3D Mapping in Atrial Flutter. J. Interv. Card. Electrophysiol. 2022, 64, 217–222. [Google Scholar] [CrossRef]
- Whiting, P.F.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; Rutjes, A.W.S.S.; Westwood, M.E.; Mallet, S.; Deeks, J.J.; Reitsma, J.B.; et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Studies in Meta-Analyses; Oxford Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2015. [Google Scholar]
- Stabile, G.; Solimene, F.; Calò, L.; Anselmino, M.; Castro, A.; Pratola, C.; Golia, P.; Bottoni, N.; Grandinetti, G.; De Simone, A.; et al. Catheter-Tissue Contact Force Values Do Not Impact Mid-Term Clinical Outcome Following Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation. J. Interv. Card. Electrophysiol. 2015, 42, 21–26. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Shah, D.; Kautzner, J.; Schmidt, B.; Saoudi, N.; Herrera, C.; Jaïs, P.; Hindricks, G.; Peichl, P.; Yulzari, A.; et al. The Relationship between Contact Force and Clinical Outcome during Radiofrequency Catheter Ablation of Atrial Fibrillation in the TOCCATA Study. Heart Rhythm 2012, 9, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Monir, G.; Pollak, S.J.; Khairy, P.; Dubuc, M.; Roy, D.; Talajic, M.; Deyell, M.; Rivard, L.; Thibault, B.; et al. Pulmonary Vein Isolation Using “Contact Force” Ablation: The Effect on Dormant Conduction and Long-Term Freedom from Recurrent Atrial Fibrillation—A Prospective Study. Heart Rhythm 2014, 11, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Jarman, J.W.E.; Panikker, S.; Jones, D.G.; Salukhe, T.; Gupta, D.; Wynn, G.; Hussain, W.; Markides, V.; Wong, T. Contact Force Sensing Technology Identifies Sites of Inadequate Contact and Reduces Acute Pulmonary Vein Reconnection: A Prospective Case Control Study. Int. J. Cardiol. 2013, 168, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Chik, W.W.B.; Kosobrodov, R.; Bhaskaran, A.; Barry, M.A.; Nguyen, D.T.; Pouliopoulos, J.; Byth, K.; Sivagangabalan, G.; Thomas, S.P.; Ross, D.L.; et al. Acoustic Signal Emission Monitoring as a Novel Method to Predict Steam Pops during Radiofrequency Ablation: Preliminary Observations. J. Cardiovasc. Electrophysiol. 2015, 26, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Cochet, H.; Sacher, F.; Chaumeil, A.; Jais, P. Steam Pop during Radiofrequency Ablation: Imaging Features on Magnetic Resonance Imaging and Multidetector Computed Tomography. Circ. Arrhythmia Electrophysiol. 2014, 7, 559–560. [Google Scholar] [CrossRef]
- Francés, R.; Madrid, A.H.; Delgado, A.; Carrizo, L.; Pindado, C.; Serrano, C.M.; Gómez, J.L.Z. Characterization of the Impact of Catheter-Tissue Contact Force in Lesion Formation during Cavo-Tricuspid Isthmus Ablation in an Experimental Swine Model. Europace 2014, 16, 1679–1683. [Google Scholar] [CrossRef]
- Jiang, J.B.; Li, J.Y.; Jiang, Z.Y.; Wang, A.; Huang, Z.; Xu, H.Y.; Shu, C.L.; Li, G.J.; Zheng, Y.S.; He, Y.; et al. Impact of Catheter-Tissue Contact Force on Lesion Size during Right Ventricular Outflow Tract Ablation in a Swine Model. Chin. Med. J. 2020, 133, 1680–1687. [Google Scholar] [CrossRef]
- Kerst, G.; Weig, H.J.; Weretka, S.; Seizer, P.; Hofbeck, M.; Gawaz, M.; Schreieck, J. Contact Force-Controlled Zero-Fluoroscopy Catheter Ablation of Right-Sided and Left Atrial Arrhythmia Substrates. Heart Rhythm 2012, 9, 709–714. [Google Scholar] [CrossRef]
- Borlich, M.; Iden, L.; Kuhnhardt, K.; Paetsch, I.; Hindricks, G.; Sommer, P. 3D Mapping for PVI- Geometry, Image Integration and Incorporation of Contact Force into Work Flow. J. Atr. Fibrillation 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Picano, E.; Vañó, E.; Rehani, M.M.; Cuocolo, A.; Mont, L.; Bodi, V.; Bar, O.; Maccia, C.; Pierard, L.; Sicari, R.; et al. The Appropriate and Justified Use of Medical Radiation in Cardiovascular Imaging: A Position Document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur. Heart J. 2014, 35, 665–672. [Google Scholar] [CrossRef]
- Giaccardi, M.; Anselmino, M.; Del Greco, M.; Mascia, G.; Paoletti Perini, A.; Mascia, P.; De Ferrari, G.M.; Picano, E. Radiation Awareness in an Italian Multispecialist Sample Assessed with a Web-Based Survey. Acta Cardiol. 2021, 76, 307–311. [Google Scholar] [CrossRef]


| Database | Search Terms | Search Field | Search Results |
|---|---|---|---|
| PubMed | (“Contact force” OR contact force-sens* OR “Cavo-tricuspid isthmus ablation” OR CTIA) AND (“Atrial flutter” OR AFL OR “Auricular Flutter”) | All Fields | 75 |
| Cochrane | (“Contact force” OR contact force-sens* OR “Cavo-tricuspid isthmus ablation” OR CTIA) AND (“Atrial flutter” OR AFL OR “Auricular Flutter”) | All Fields | 36 |
| WOS | (“Contact force” OR contact force-sens* OR “Cavo-tricuspid isthmus ablation” OR CTIA) AND (“Atrial flutter” OR AFL OR “Auricular Flutter”) | All Fields | 84 |
| SCOPUS | TITLE-ABS-KEY ((“Contact force” OR contact AND force-sens* OR “Cavo-tricuspid isthmus ablation” OR ctia ) AND (“Atrial flutter” OR afl OR “Auricular Flutter”)) | Title, Abstract, Keyword | 19 |
| EMBASE | #3. #1 AND #2 #2. ‘atrial flutter’:ti, ab, kw OR afl:ti, ab, kw OR ‘auricular flutter’:ti, ab, kw #1. ‘contact force’:ti, ab, kw OR ‘contact force-sens*’:ti, ab, kw OR ‘cavo-tricuspid isthmus ablation’:ti, ab, kw OR ctia:ti, ab, kw | All Fields | 205 |
| Study ID | Study Design | C | T | Main Inclusion Criteria | Method of AFL Recurrence Detection | Primary Outcome | Follow-Up Duration | Ablation Catheter | CF Target (g) | Mean CF (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Begg et al. 2019 (VERISMART) [15] | Multicenter RCT | UK | 53 | Persistent or paroxysmal AFL. | Seven days of ECG monitoring | Time to BDIB | Six months | Thermocool Smart Touch | 5–40 | N/A |
| Boles et al. 2017 [16] | Retrospective single-center observational study | CA | 38 | Persistent or paroxysmal AFL | N/A | Complete BDIB | N/A | TactiCath Quartz (CF) CoolFlex (non-CF) | 10–30 | 13.9 |
| Giehm-Reese et al. 2020 [1] | Multicenter double-blinded superiority RCT | DK | 155 | Typical AFL undergoing first-time CTIA | Five days Holter ECG at one month and invasive EPS study at three months | Recurrent isthmus conduction measured with invasive EPS three months after ablation | Three months | TactiCathTM Quartz | 10–30 | 16.3 |
| Gould et al. 2016 [17] | Prospective single-center observational study with retrospective historical control | AU | 60 | Typical AFL | ECG and Holter monitor | BDIB | 12 months | Tacti-Cath, Quartz (CF) 8 mm F-Curve Biosense Webster Thermocouple catheter (non-CF) | 10–40 | 17 |
| Venier et al. 2016 [18] | Prospective single-center observational study | CA | 70 | Typical AFL undergoing first-time CTIA | 24 h Holter monitor and 12-lead ECG | BDIB | Six months | Thermocool Smart Touch | 10–25 | 11.5 |
| Study ID | Number of Patients in Each Group | Age (Years) Mean (SD) | Gender (Male) N (%) | CHA2DS-VASc Score Mean (SD) | LVEF (%) Mean (SD) | AFL Duration (Months) Mean (SD) | Comorbidities N (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | AF | HTN | HF | DM | IHD | Stroke/ TIA | |||||||
| CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | CFG | CFB | |||||||||||||
| Begg et al. 2019 (VERISMART) [15] | 26 | 27 | 62.7 (21.2) | 65.3 (16.5) | 24, (923) | 21 (77.8) | 2 (1.7) | 1.9 (1.5) | N/A | N/A | 9 (9.8) | 17 (24.4) | N/A | N/A | 12 (46.2) | 11 (40.7) | 6 (23.1) | 4 (14.8) | 7 (26.9) | 5 (8.5) | 8 (30.8) | 3 (11.1) | (1 3.8) | 0 |
| Boles et al. 2017 [16] | 15 | 23 | 69 (7.9) | 66.3 (10.4) | 10 (66.6) | 16 (69.6) | 2.6 (1.6) | 2.5, (1.6) | 53.5 (15.9) | 51.4 (22) | N/A | N/A | N/A | N/A | 10 (66.6) | 14 (61) | N/A | N/A | 5 (33.3) | 5 (21.7) | 5 (33.3) | 7 (30.4) | N/A | N/A |
| Giehm-Reese et al. 2020 [1] | 79 | 77 | 69.3 (9.8) | 65.7 (12.1) | 55 (70) | 65 (84) | 3 (1.5) | 2 (1.5) | 53.3 (11.3) | 55 (11.3) | N/A | N/A | 4 (5) | 2 (3) | 46 (58) | 36 (47) | 26 (33) | 23 (30) | 13 (16) | 18 (23) | 19 (24) | 10 (13) | 8 (10) | 8 (10) |
| Gould et al. 2016 [17] | 30 | 30 | 64 (8) | 64 (11) | 23 (76.7) | 24 (80) | N/A | N/A | 57 (6) | 56 (7) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/a | N/A | N/A | N/A | N/A |
| Venier et al. 2016 [18] | 35 | 35 | 63.9 (12.4) | 61.5 (9.2) | 32 (91) | 29 (83) | 1.2 (0.9) | 0.8 (0.7) | 55 (11.1) | 56.4 (7.2) | N/A | N/A | 17 (49) | 20 (57) | 15 (43) | 18 (51) | N/A | N/A | 10 (29) | 9 (26) | 7 (20) | 5 (14) | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuelazm, M.; Mohamed, I.; Seri, A.R.; Almaadawy, O.; Abdelazeem, B.; Brašić, J.R. Contact Force-Guided versus Contact Force-Blinded Cavo-Tricuspid Isthmus Ablation for Atrial Flutter: A Systematic Review and Meta-Analysis. Diseases 2023, 11, 98. https://doi.org/10.3390/diseases11030098
Abuelazm M, Mohamed I, Seri AR, Almaadawy O, Abdelazeem B, Brašić JR. Contact Force-Guided versus Contact Force-Blinded Cavo-Tricuspid Isthmus Ablation for Atrial Flutter: A Systematic Review and Meta-Analysis. Diseases. 2023; 11(3):98. https://doi.org/10.3390/diseases11030098
Chicago/Turabian StyleAbuelazm, Mohamed, Islam Mohamed, Amith Reddy Seri, Omar Almaadawy, Basel Abdelazeem, and James Robert Brašić. 2023. "Contact Force-Guided versus Contact Force-Blinded Cavo-Tricuspid Isthmus Ablation for Atrial Flutter: A Systematic Review and Meta-Analysis" Diseases 11, no. 3: 98. https://doi.org/10.3390/diseases11030098
APA StyleAbuelazm, M., Mohamed, I., Seri, A. R., Almaadawy, O., Abdelazeem, B., & Brašić, J. R. (2023). Contact Force-Guided versus Contact Force-Blinded Cavo-Tricuspid Isthmus Ablation for Atrial Flutter: A Systematic Review and Meta-Analysis. Diseases, 11(3), 98. https://doi.org/10.3390/diseases11030098