Post-Infection Oscillometry and Pulmonary Metrics in SARS-CoV-2 Patients: A 40-Day Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethics
2.2. Inclusion and Exclusion Criteria
2.3. Definitions and Methods
2.4. Statistical Analysis
3. Results
3.1. Patients’ Background
3.2. Pulmonary Function Tests
3.3. Regression Analysis
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Al-Jahdhami, I.; Al-Naamani, K.; Al-Mawali, A.; Bennji, S.M. Respiratory Complications after COVID-19. Oman Med. J. 2022, 37, e343. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Luppi, F.; Rebora, P.; D’andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-year pulmonary impairment after severe COVID-19: A prospective, multicenter follow-up study. Respir. Res. 2022, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Ranu, H.; Wilde, M.; Madden, B. Pulmonary function tests. Ulst. Med. J. 2011, 80, 84–90. [Google Scholar]
- Boeriu, E.; Borda, A.; Vulcanescu, D.D.; Sarbu, V.; Arghirescu, S.T.; Ciorica, O.; Bratosin, F.; Marincu, I.; Horhat, F.G. Diagnosis and Management of Febrile Neutropenia in Pediatric Oncology Patients—A Systematic Review. Diagnostics 2022, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Dediu, M.; Ciuca, I.M.; Marc, M.S.; Boeriu, E.; Pop, L.L. Factors Influencing Lung Function in Patients with Cystic Fibrosis in Western Romania. J. Multidiscip. Healthc. 2021, 14, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Flesch, J.D.; Dine, C.J. Lung volumes: Measurement, clinical use, and coding. Chest 2012, 142, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, D.; Timar, B.; Bratosin, F.; Rosca, O.; Citu, C.; Oancea, C. Predictors for COVID-19 Complete Remission with HRCT Pattern Evolution: A Monocentric, Prospective Study. Diagnostics 2022, 12, 1397. [Google Scholar] [CrossRef]
- Lopes, A.J.; Litrento, P.F.; Provenzano, B.C.; Carneiro, A.S.; Monnerat, L.B.; da Cal, M.S.; Ghetti, A.T.A.; Mafort, T.T. Small airway dysfunction on impulse oscillometry and pathological signs on lung ultrasound are frequent in post-COVID-19 patients with persistent respiratory symptoms. PLoS ONE 2021, 16, e0260679. [Google Scholar] [CrossRef]
- Torregiani, C.; Veneroni, C.; Confalonieri, P.; Citton, G.M.; Salton, F.; Jaber, M.; Confalonieri, M.; Dellaca’, R.L. Monitoring respiratory mechanics by oscillometry in COVID-19 patients receiving non-invasive respiratory support. PLoS ONE 2022, 17, e0265202. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.E.; Gökçek, A.; Satar, S.; Ergün, P. Relation of impulse oscillometry and spirometry with quantitative thorax computed tomography after COVID-19 pneumonia. Rev. Assoc. Médica Bras. 2023, 69, e20221427. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, C.; Saadeh, C.; Cross, B.; Gaylor, M.; Griffith, M. Advantage of impulse oscillometry over spirometry to diagnose chronic obstructive pulmonary disease and monitor pulmonary responses to bronchodilators: An observational study. SAGE Open Med. 2015, 3, 2050312115578957. [Google Scholar] [CrossRef]
- Kakavas, S.; Kotsiou, O.S.; Perlikos, F.; Mermiri, M.; Mavrovounis, G.; Gourgoulianis, K.; Pantazopoulos, I. Pulmonary function testing in COPD: Looking beyond the curtain of FEV1. Npj Prim. Care Respir. Med. 2021, 31, 23. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Fericean, R.M.; Citu, C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Tudorache, E.; Oancea, C. Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves. J. Clin. Med. 2022, 11, 2916. [Google Scholar] [CrossRef]
- WHO. Living Guidance for Clinical Management of COVID-19; World Health Organization: Geneva, Switzerland, 2021; p. 63. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 21 June 2023).
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicentre trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. Eclinicalmedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Pakhale, S.; Visentin, C.; Tariq, S.; Kaur, T.; Florence, K.; Bignell, T.; Jama, S.; Huynh, N.; Boyd, R.; Haddad, J.; et al. Lung disease burden assessment by oscillometry in a systematically disadvantaged urban population experiencing homelessness or at-risk for homelessness in Ottawa, Canada from a prospective observational study. BMC Pulm. Med. 2022, 22, 235. [Google Scholar] [CrossRef]
- Frija-Masson, J.; Debray, M.-P.; Gilbert, M.; Lescure, F.-X.; Travert, F.; Borie, R.; Khalil, A.; Crestani, B.; D’Ortho, M.-P.; Bancal, C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020, 56, 2001754. [Google Scholar] [CrossRef]
- Thomas, R.E. Reducing Morbidity and Mortality Rates from COVID-19, Influenza and Pneumococcal Illness in Nursing Homes and Long-Term Care Facilities by Vaccination and Comprehensive Infection Control Interventions. Geriatrics 2021, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.; Mitchell, L.; Stokes, D.; Lacey, E.; Crowley, E.; Kelleher, C.C. A rapid systematic review of measures to protect older people in long-term care facilities from COVID-19. BMJ Open 2021, 11, e047012. [Google Scholar] [CrossRef]
- Bouza, E.; Navarro, J.A.G.; Alonso, S.; Alonso, J.C.D.; Escobar, C.; Gómez, B.J.F.; Borrás, M.I.G.; Rojas, A.J.G.; Pavón, F.J.G.; Gracia, D.; et al. Infection control in long term care institutions for the elderly: A reflection document on the situation in Spain. Rev. Española Quimioter. 2023, 36, 346–379. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Müller, M.; Schmehl, C.; Gasteyer, S.; Steul, K. COVID-19 in long-term care facilities in Frankfurt am Main, Germany: Incidence, case reports, and lessons learned. GMS Hyg. Infect. Control. 2020, 15, Doc26. [Google Scholar] [PubMed]
- Yilmaz, F.K.; Cakir, M.; Ikiisik, H.; Maral, I. The Effect of Pneumococcal, Influenza, and COVID-19 Vaccinations on COVID-19 Hospitalization and Progression in People over 65 Years Old Living in Nursing Homes. Vaccines 2023, 11, 943. [Google Scholar] [CrossRef]
- Blasi, F.; Di Pasquale, M.; Gramegna, A.; Viale, P.; Iacobello, C.; Gori, A.; Tumbarello, M.; Esposito, S.; Richeldi, L.; Bassetti, M. A new call for influenza and pneumococcal vaccinations during COVID-19 pandemic in Italy: A SIP/IRS (Italian Respiratory Society) and SITA (Italian Society of Antiinfective therapy) statement. Respir. Med. 2021, 190, 106674. [Google Scholar] [CrossRef]
| Variables (Mean ± SD) | Mild (n = 22) | Moderate (n = 22) | Severe (n = 22) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 54.1 ± 9.8 | 57.2 ± 8.6 | 58.3 ± 11.0 | 0.347 |
| Sex, male (n, %) | 11 (50.0%) | 14 (63.6%) | 13 (59.1%) | 0.647 |
| Place of residence, urban (n, %) | 9 (40.9%) | 12 (54.5%) | 10 (45.5%) | 0.653 |
| BMI, kg/m2 (mean ± SD) | 24.4 ± 7.5 | 26.1 ± 8.3 | 25.8 ± 7.9 | 0.749 |
| Smoking, current/former (n, %) | 7 (31.8%) | 6 (27.3%) | 10 (45.5%) | 0.419 |
| CCI ≥ 2 (n, %) * | 8 (36.4%) | 8 (36.4%) | 12 (54.5%) | 0.370 |
| Variables (Mean ± SD) | Mild (n = 22) | Moderate (n = 22) | Severe (n = 22) | p-Value |
|---|---|---|---|---|
| FVC | 106.0 ± 18.9 | 93.8 ± 21.2 | 86.8 ± 25.5 | 0.018 * |
| FEV1 | 98.7 ± 22.0 | 92.6 ± 20.3 | 87.4 ± 24.2 | 0.248 |
| FEV1/FVC | 80.6 ± 7.3 | 82.5 ± 5.0 | 84.0 ± 9.3 | 0.319 |
| FEF25–75 | 82.9 ± 5.6 | 79.2 ± 6.4 | 77.7 ± 6.0 | 0.017 * |
| AMPI | 85.9 ± 14.3 | 90.6 ± 11.9 | 92.4 ± 13.3 | 0.249 |
| Result (n, %) | ||||
| Ventilatory dysfunction | 5 (22.7%) | 9 (40.9%) | 13 (59.1%) | 0.049 |
| Post-COVID-19 fibrosis | 3 (13.6%) | 5 (22.7%) | 11 (50.0%) | 0.021 |
| Normal | 14 (63.6%) | 9 (40.9%) | 6 (27.3%) | 0.049 |
| Variables (Mean ± SD) | Mild (n = 22) | Moderate (n = 22) | Severe (n = 22) | p-Value |
|---|---|---|---|---|
| TLC | 119.1 ± 29.0 | 109.4 ± 40.6 | 100.8 ± 44.6 | 0.297 |
| RV | 204.7 ± 88.9 | 186.4 ± 82.2 | 171.7 ± 92.8 | 0.465 |
| sRAW | 58.2 ± 38.0 | 54.2 ± 24.2 | 40.5 ± 21.2 | 0.109 |
| RAW | 77.4 ± 40.0 | 108.6 ± 80.0 | 183.8 ± 168.2 | 0.007 * |
| GAW | 185.6 ± 150.2 | 125.3 ± 129.4 | 113.9 ± 126.5 | 0.178 |
| RV/TLC (%) | 35.0 ± 8.1 | 39.3 ± 8.3 | 41.5 ± 9.6 | 0.048 * |
| Result (n, %) | ||||
| Ventilatory dysfunction | 8 (36.4%) | 13 (59.1%) | 18 (81.8%) | 0.009 |
| Post-COVID-19 fibrosis | 4 (18.2%) | 7 (31.8%) | 12 (54.5%) | 0.038 |
| Normal | 12 (54.5%) | 8 (36.4%) | 3 (13.6%) | 0.017 |
| Variables (Mean ± SD) | Mild (n = 22) | Moderate (n = 22) | Severe (n = 22) | p-Value |
|---|---|---|---|---|
| RF | 14.3 ± 4.5 | 15.6 ± 2.9 | 17.4 ± 4.4 | 0.042 * |
| R4 | 4.6 ± 2.1 | 4.6 ± 1.4 | 3.5 ± 1.1 | 0.035 |
| R6 | 3.5 ± 1.6 | 3.8 ± 0.8 | 4.0 ± 1.0 | 0.375 |
| X4 | −2.2 ± 1.4 | −1.8 ± 1.1 | −2.8 ± 1.4 | 0.045 * |
| X6 | −1.5 ± 0.7 | −1.3 ± 0.5 | −1.2 ± 0.5 | 0.219 |
| R20 | 3.6 ± 1.5 | 3.0 ± 0.9 | 3.4 ± 1.5 | 0.320 |
| Result (n, %) | ||||
| Ventilatory dysfunction | 7 (31.8%) | 10 (45.5%) | 16 (72.7%) | 0.021 |
| Post-COVID-19 fibrosis | 10 (45.5%) | 12 (54.5%) | 15 (68.2%) | 0.310 |
| Normal | 9 (40.9%) | 4 (18.2%) | 2 (9.1%) | 0.034 |
| Variables (Mean ± SD) | Mild (n = 22) | Moderate (n = 22) | Severe (n = 22) | p-Value |
|---|---|---|---|---|
| Total lung volume (liters) | 4.3 ± 1.3 | 4.0 ± 0.9 | 4.1 ± 1.1 | 0.662 |
| Left lung volume (liters) | 1.9 ± 0.6 | 1.6 ± 0.8 | 1.4 ± 0.9 | 0.108 |
| Right lung volume (liters) | 2.1 ± 0.8 | 2.4 ± 1.0 | 2.0 ± 1.2 | 0.325 |
| Emphysema | 5 (22.7%) | 6 (27.3%) | 9 (40.9%) | 0.393 |
| Ground glass | 10 (45.5%) | 12 (54.5%) | 18 (81.8%) | 0.037 |
| Crazy paving | 2 (9.1%) | 4 (18.2%) | 7 (31.8%) | 0.162 |
| Consolidation | 1 (4.5%) | 2 (9.1%) | 5 (22.7%) | 0.157 |
| Vascular calcifications | 0 (0.0%) | 2 (9.1%) | 3 (13.6%) | 0.219 |
| Normal (R/L or both) | 9 (40.9%) | 4 (18.2%) | 2 (9.1%) | 0.034 |
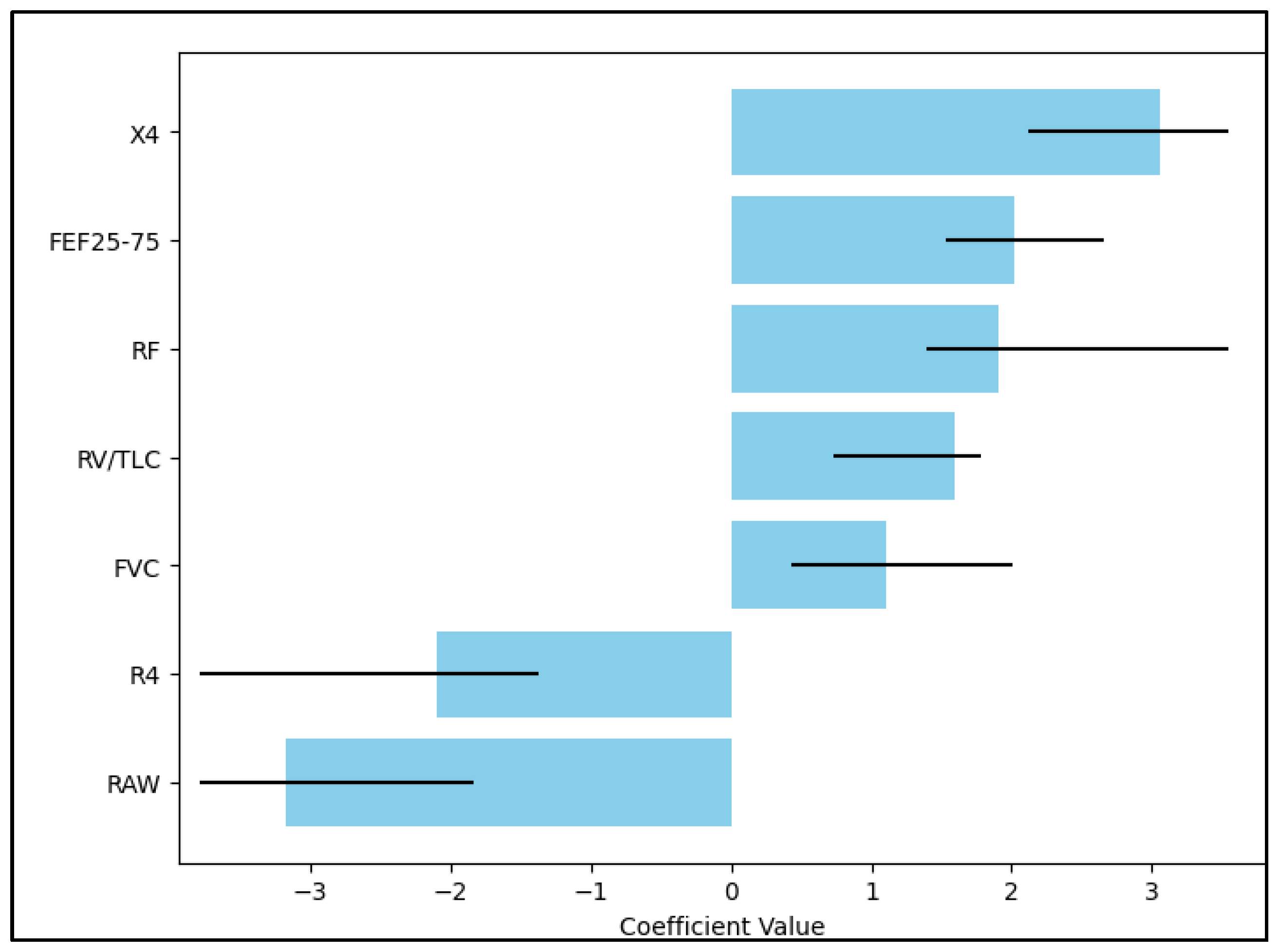
| Independent Variables | HR—Exp(B) | 95% CI | p-Value |
|---|---|---|---|
| X4 | 3.16 | 2.15–7.39 | <0.001 |
| FEF25–75 | 2.09 | 1.48–2.70 | 0.001 |
| RF | 1.90 | 1.31–4.26 | 0.036 |
| RV/TLC | 1.66 | 0.68–1.92 | 0.219 |
| FVC | 1.15 | 0.42–2.03 | 0.440 |
| R4 | −2.18 | −1.59–(−4.80) | <0.001 |
| RAW | −3.27 | −1.93–(−6.16) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suppini, N.; Oancea, C.; Fira-Mladinescu, O.; Traila, D.; Pescaru, C.; Marc, M.S.; Manolescu, D.; Vastag, E.; Ali, A.; Hogea, E.; et al. Post-Infection Oscillometry and Pulmonary Metrics in SARS-CoV-2 Patients: A 40-Day Follow-Up Study. Diseases 2023, 11, 102. https://doi.org/10.3390/diseases11030102
Suppini N, Oancea C, Fira-Mladinescu O, Traila D, Pescaru C, Marc MS, Manolescu D, Vastag E, Ali A, Hogea E, et al. Post-Infection Oscillometry and Pulmonary Metrics in SARS-CoV-2 Patients: A 40-Day Follow-Up Study. Diseases. 2023; 11(3):102. https://doi.org/10.3390/diseases11030102
Chicago/Turabian StyleSuppini, Noemi, Cristian Oancea, Ovidiu Fira-Mladinescu, Daniel Traila, Camelia Pescaru, Monica Steluta Marc, Diana Manolescu, Emanuela Vastag, Ayesha Ali, Elena Hogea, and et al. 2023. "Post-Infection Oscillometry and Pulmonary Metrics in SARS-CoV-2 Patients: A 40-Day Follow-Up Study" Diseases 11, no. 3: 102. https://doi.org/10.3390/diseases11030102
APA StyleSuppini, N., Oancea, C., Fira-Mladinescu, O., Traila, D., Pescaru, C., Marc, M. S., Manolescu, D., Vastag, E., Ali, A., Hogea, E., & Pilut, C. N. (2023). Post-Infection Oscillometry and Pulmonary Metrics in SARS-CoV-2 Patients: A 40-Day Follow-Up Study. Diseases, 11(3), 102. https://doi.org/10.3390/diseases11030102







