Primary Cardiac Sarcoma: Clinical Characteristics and Prognostic Factors over the Past 2 Decades
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Data Selection
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Study Variables
2.3.1. Main Exposure
2.3.2. Outcomes
2.3.3. Survival Months
2.3.4. Sociodemographic and Tumor Characteristics
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molina, J.E.; Edwards, J.E.; Ward, H.B. Primary cardiac tumors: Experience at the University of Minnesota. Thorac. Cardiovasc. Surg. 1990, 38 (Suppl. 2), 183–191. [Google Scholar] [CrossRef]
- Andrei, V.; Scheggi, V.; Stefàno, P.L.; Marchionni, N. Primary cardiac sarcoma: A case report of a therapeutic challenge. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Burke, A.P.; Cowan, D.; Virmani, R. Primary sarcomas of the heart. Cancer 1992, 69, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Donsbeck, A.V.; Ranchere, D.; Coindre, J.M.; Le Gall, F.; Cordier, J.F.; Loire, R. Primary cardiac sarcomas: An immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 1999, 34, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.; Kumar, S.K.; Okuno, S.H.; Schaff, H.V.; Porrata, L.F.; Buckner, J.C.; Moynihan, T.J. Malignant primary cardiac tumors: Review of a single institution experience. Cancer 2008, 112, 2440–2446. [Google Scholar] [CrossRef]
- Zhang, P.J.; Brooks, J.S.; Goldblum, J.R.; Yoder, B.; Seethala, R.; Pawel, B.; Gorman, J.H.; Gorman, R.C.; Huang, J.-H.; Acker, M.; et al. Primary cardiac sarcomas: A clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Hum. Pathol. 2008, 39, 1385–1395. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Goode, D.L.; Ray-Coquard, I.; James, P.A.; Mitchell, G.; Niedermayr, E.; Puri, A.; Schiffman, J.D.; Dite, G.S.; Cipponi, A.; et al. Monogenic and polygenic determinants of sarcoma risk: An international genetic study. Lancet Oncol. 2016, 17, 1261–1271. [Google Scholar] [CrossRef]
- Vander Salm, T.J. Unusual primary tumors of the heart. Semin. Thorac. Cardiovasc. Surg. 2000, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.; Connolly, H.M.; Brown, R.D., Jr. Central nervous system manifestations of cardiac myxoma. Arch Neurol. 2007, 64, 1115–1120. [Google Scholar] [CrossRef]
- Hoey, E.T.; Mankad, K.; Puppala, S.; Gopalan, D.; Sivananthan, M.U. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009, 64, 1214–1230. [Google Scholar] [CrossRef]
- Pearman, J.L.; Wall, S.L.; Chen, L.; Rogers, J.H. Intracardiac echocardiographic-guided right-sided cardiac biopsy: Case series and literature review. Catheter. Cardiovasc. Interv. 2021, 98, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Luo, R.; Wei, Y.; Wang, F.; Zhang, Y.; Karlson, K.J.; Zhang, Z.; Reardon, M.J.; Dobrilovic, N. Survival outcomes in patients with primary cardiac sarcoma in the United States. J. Thorac. Cardiovasc. Surg. 2021, 162, 107–115.e2. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94. [Google Scholar] [CrossRef]
- Homeister, J.W.; Kelly, K.L. The Cardiovascular System. In Pathology: A Modern Case Study, 2nd ed.; Reisner, H.M., Ed.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Schill, M.R.; Khiabani, A.J.; Kachroo, P.; Damiano, R.J., Jr. Acquired Heart Disease. In Schwartz;s Principles of Surgery, 11th ed.; Brunicardi, F.C., Andersen, D.K., Billiar, T.R., Dunn, D.L., Kao, L.S., Hunter, J.G., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Awtry, E.H. Atrial Myxoma and Other Cardiac Tumors. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Hammami, M.B.; Al-Wawi, M.Z.; Fazel, H.; Oudih, M. Incidence, prognostic significance, and survival outcomes of primary cardiac sarcoma: An updated population-based retrospective study. Anatol. J. Cardiol. 2021, 25, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Laskowski-Jones, L. Racism and healthcare disparities. Nursing 2020, 50, 6. [Google Scholar] [CrossRef]
- Tang, L.; Pan, Z.; Zhang, X. The effect of marital status on the survival of patients with multiple myeloma. Hematology 2022, 27, 187–197. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Chen, D.; Chao, J.; Shao, Y.; Tang, K.; Chen, W. Effect of Marital Status on the Survival of Patients with Adenocarcinoma of the Esophagogastric Junction: A Population-Based, Propensity-Matched Study. Cancer Control. 2021, 28, 10732748211066309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Hu, X.; Fang, M.; Xiao, S. The effect of marital and insurance status on the survival of elderly patients with stage M1b colon cancer: A SEER-based study. BMC Cancer 2021, 21, 891. [Google Scholar] [CrossRef]
- Alyabsi, M.; Ramadan, M.; Algarni, M.; Alshammari, K.; Jazieh, A.R. The effect of marital status on stage at diagnosis and survival in Saudis diagnosed with colorectal cancer: Cancer registry analysis. Sci. Rep. 2021, 11, 8603. [Google Scholar] [CrossRef]
- Xiao, K.; Zhao, Y.; Cai, Y.; Chen, P.; Chen, J.; Ye, R.; Liu, X.; Yuan, B.; Zhao, Y. The effect of marital status on the survival of patients with colorectal neuroendocrine neoplasms: An analysis of the SEER database. Rev. Esp. Enferm. Dig. 2020, 112, 109–117. [Google Scholar] [CrossRef]
- Dong, J.; Dai, Q.; Zhang, F. The effect of marital status on endometrial cancer-related diagnosis and prognosis: A Surveillance Epidemiology and End Results database analysis. Future Oncol. 2019, 15, 3963–3976. [Google Scholar] [CrossRef]
- Hinyard, L.; Wirth, L.S.; Clancy, J.M.; Schwartz, T. The effect of marital status on breast cancer-related outcomes in women under 65: A SEER database analysis. Breast 2017, 32, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.C.; Yang, S.; Liu, X.Y.; Zhao, Y.X. Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Med. 2018, 7, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, X.; Lu, C.; Xiao, F. Impact of marital status on the prognosis of liver cancer patients without surgery and the critical window. Ann. Palliat. Med. 2021, 10, 2990–2999. [Google Scholar] [CrossRef]
- Feng, Y.; Dai, W.; Li, Y.; Mo, S.; Li, Q.; Cai, S. The effect of marital status by age on patients with colorectal cancer over the past decades: A SEER-based analysis. Int. J. Colorectal. Dis. 2018, 33, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.J. Cardiac tumours: Diagnosis and management. Heart 2011, 97, 151–160. [Google Scholar] [CrossRef]
- Centofanti, P.; Di Rosa, E.; Deorsola, L.; Dato GM, A.; Patane, F.; La Torre, M.; Barbato, L.; Verzini, A.; Fortunato, G.; di Summa, M. Primary cardiac tumors: Early and late results of surgical treatment in 91 patients. Ann. Thorac. Surg. 1999, 68, 1236–1241. [Google Scholar] [CrossRef]
- Bakaeen, F.G.; Reardon, M.J.; Coselli, J.S.; Miller, C.C.; Howell, J.F.; Lawrie, G.M.; Espada, R.; Ramchandani, M.K.; Noon, G.P.; Weilbaecher, D.G.; et al. Surgical outcome in 85 patients with primary cardiac tumors. Am. J. Surg. 2003, 186, 641–647. [Google Scholar] [CrossRef]
- Ramlawi, B.; Leja, M.J.; Saleh WK, A.; Al Jabbari, O.; Benjamin, R.; Ravi, V.; Shapira, O.M.; Blackmon, S.H.; Bruckner, B.A.; Reardonet, M.J. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. Ann. Thorac. Surg. 2016, 101, 698–702. [Google Scholar] [CrossRef]
- Kosuga, T.; Fukunaga, S.; Kawara, T.; Yokose, S. Surgery for primary cardiac tumors. Clinical experience and surgical results in 60 patients. J. Cardiovasc. Surg. 2002, 43, 581–587. [Google Scholar]
- Raaf, H.N.; Raaf, J.H. Sarcomas related to the heart and vasculature. Semin. Surg. Oncol. 1994, 10, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Chimeric antigen receptor T (CAR-T) cell immunotherapy for sarcomas: From mechanisms to potential clinical applications. Cancer Treat. Rev. 2020, 82, 101934. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | ||
| N = | % | |
| Total | 362 | 100 |
| Gender | ||
| Female | 178 | 49.17 |
| Male | 184 | 50.83 |
| Age at diagnosis, y.o | ||
| 0–39 | 116 | 32.04 |
| 40–59 | 137 | 37.85 |
| 60–79 | 86 | 23.76 |
| 80+ | 23 | 6.35 |
| Race | ||
| Non-Hispanic white | 213 | 58.84 |
| Non-Hispanic black | 44 | 12.15 |
| Hispanic | 68 | 18.78 |
| Other | 37 | 10.22 |
| Histopathology | ||
| Angiosarcoma | 172 | 42.51 |
| Rhabdomyosarcoma | 12 | 3.31 |
| Malignant fibrous histiocytomas | 51 | 14.09 |
| Leiomyosarcomas/spindle cell sarcomas | 42 | 11.60 |
| Sarcoma, NOS | 38 | 10.50 |
| Synovial sarcomas | 14 | 3.87 |
| Osteosarcoma/Chondrosarcoma | 33 | 9.12 |
| Tumor stage | ||
| Localized | 96 | 26.52 |
| Regional | 110 | 30.39 |
| Distant | 140 | 38.67 |
| Unknown | 16 | 4.42 |
| Living area | ||
| Counties in metropolitan areas of 1 million persons | 215 | 59.39 |
| Counties in metropolitan areas of 250,000 to 1 million persons | 79 | 21.82 |
| Counties in metropolitan areas of 250,000 persons | 27 | 7.46 |
| Nonmetropolitan counties adjacent to a metropolitan area | 15 | 4.14 |
| Nonmetropolitan counties not adjacent to a metropolitan area | 26 | 7.18 |
| Income per year | ||
| $ <$45,000 | 18 | 4.97 |
| $45,000–54,999 | 57 | 15.75 |
| $55,000–64,999 | 81 | 22.38 |
| $65,000–74,999 | 90 | 24.86 |
| $75,000+ | 116 | 32.04 |
| Marital Status | ||
| Married | 197 | 54.42 |
| Single | 97 | 26.80 |
| Divorced/separated | 33 | 9.12 |
| Widowed | 21 | 5.80 |
| Unknown | 14 | 3.87 |
| Chemotherapy | ||
| No | 181 | 50.00 |
| Yes | 181 | 50.00 |
| Radiation | ||
| No | 285 | 79.83 |
| Yes | 72 | 20.17 |
| Surgery | ||
| No | 123 | 33.98 |
| Yes | 237 | 65.47 |
| Unknown | 2 | 0.55 |
| Year of diagnosis | ||
| 2000 | 17 | 4.70 |
| 2001 | 20 | 5.52 |
| 2002 | 13 | 3.59 |
| 2003 | 13 | 3.59 |
| 2004 | 26 | 7.18 |
| 2005 | 11 | 3.04 |
| 2006 | 13 | 3.59 |
| 2007 | 23 | 6.35 |
| 2008 | 9 | 2.49 |
| 2009 | 32 | 8.84 |
| 2010 | 22 | 6.08 |
| 2011 | 28 | 7.73 |
| 2012 | 14 | 3.87 |
| 2013 | 20 | 5.52 |
| 2014 | 28 | 7.73 |
| 2015 | 20 | 5.52 |
| 2016 | 23 | 6.35 |
| 2017 | 30 | 8.29 |
| Characteristics | Overall Mortality. Crude Proportional Hazard Ratio (95% Confidence Interval) | Primary Cardiac Sarcoma Mortality. Crude Proportional Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Gender | ||
| Female | 1.00 (reference) | 1.00 (reference) |
| Male | 0.964 (0.758–1.226) | 0.995 (0.766–1.293) |
| Age at diagnosis, y.o | ||
| 0–39 | 1.00 (reference) | 1.00 (reference) |
| 40–59 | 1.212 (0.911–1.615) | 1.258 (0.925–1.712) |
| 60–79 | 1.429 (1.028–1.986) ** | 1.35 (0.939–1.941) |
| 80+ | 5.958 (3.357–10.575) *** | 5.037 (2.606–9.736) *** |
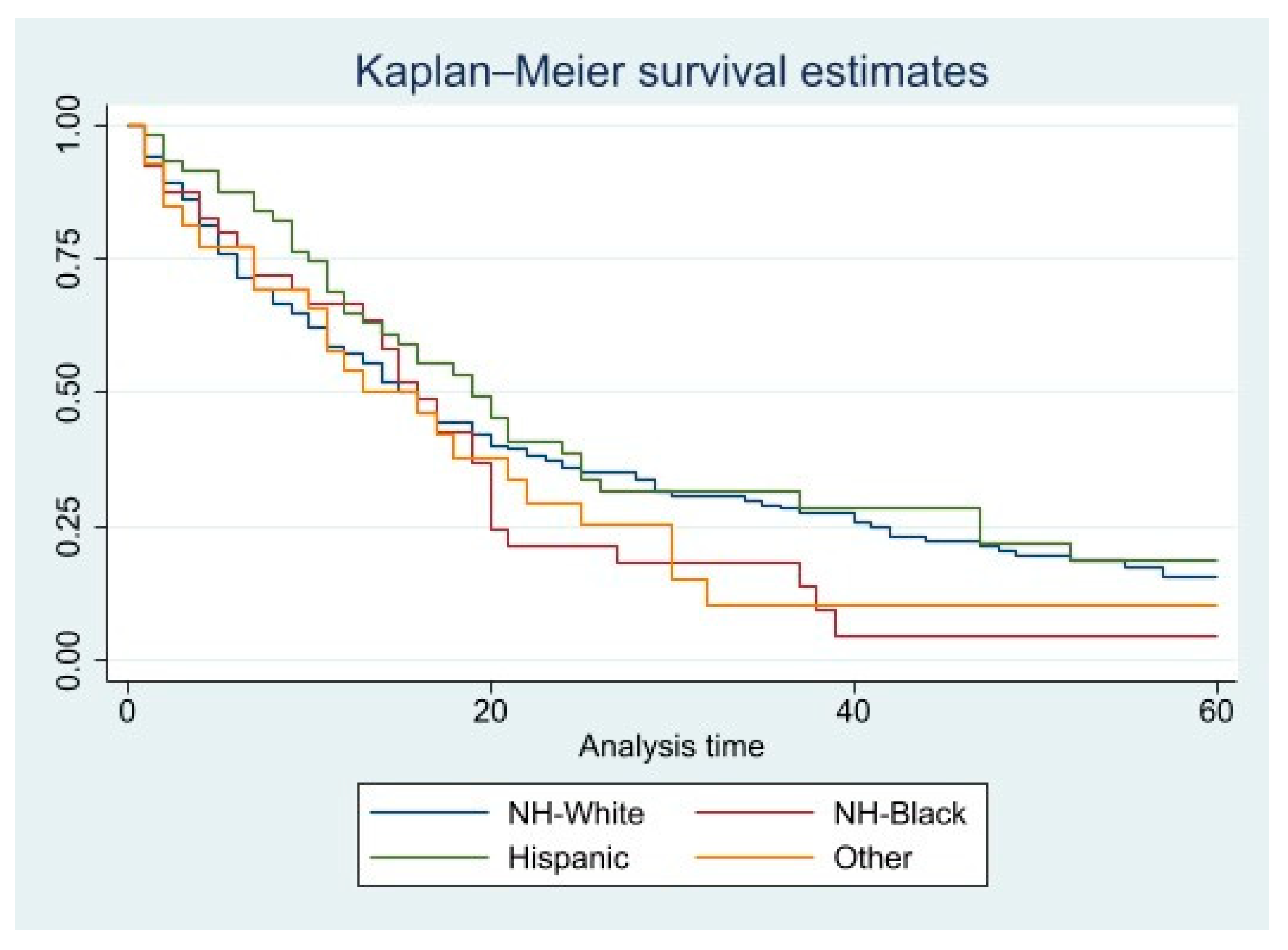
| Race | ||
| Non-Hispanic white | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic black | 1.222 (0.851–1.754) | 1.257 (0.851–1.857) |
| Hispanic | 0.833 (0.602–1.154) | 0.827 (0.578–1.182) |
| Other | 1.063 (0.685–1.648) | 1.212 (0.77–1.906) |
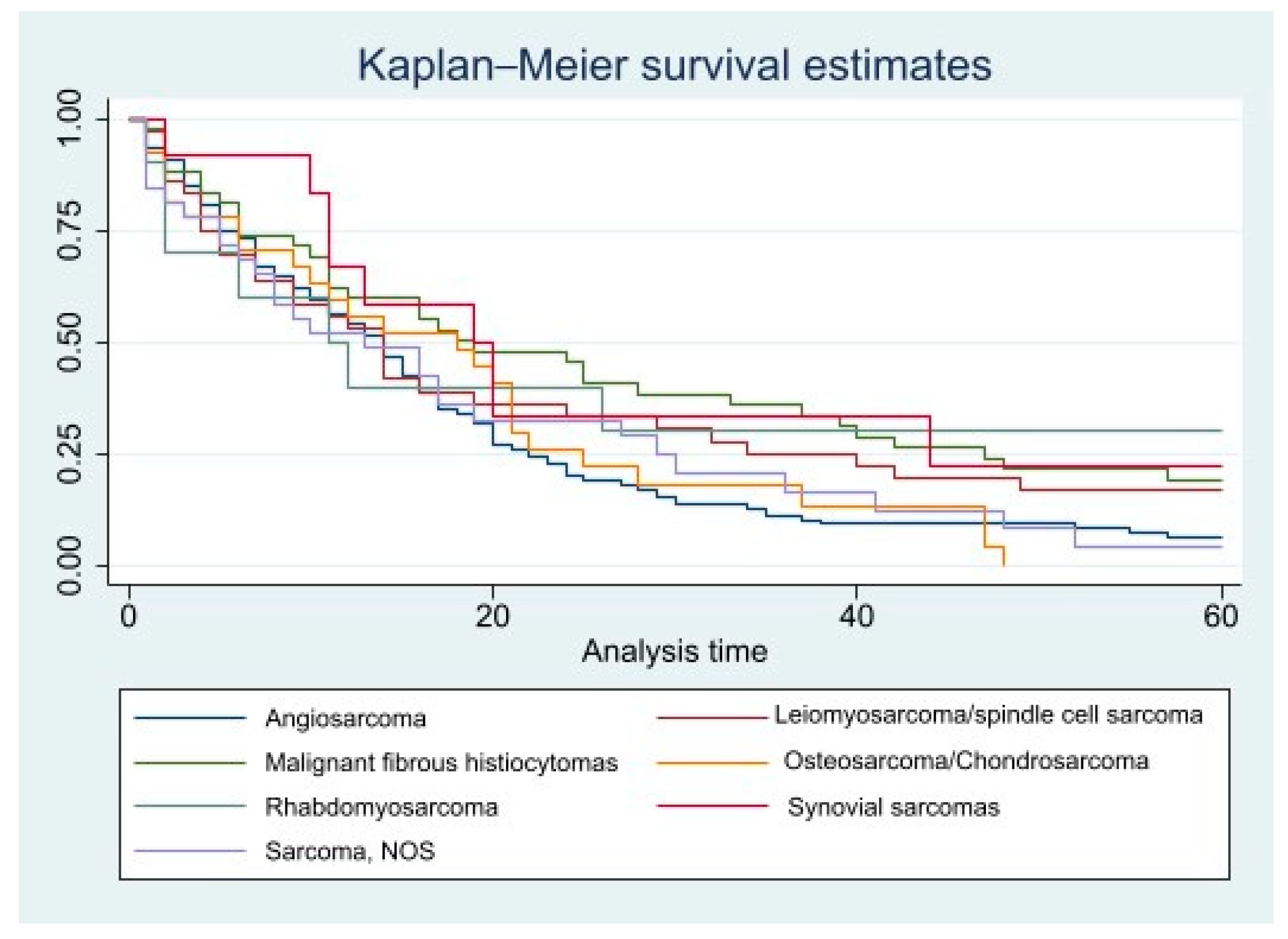
| Histopathology | ||
| Angiosarcoma | 1.00 (reference) | 1.00 (reference) |
| Rhabdomyosarcoma | 0.608 (0.283–1.307) | 0.501 (0.204–1.231) |
| Malignant fibrous histiocytomas | 0.657 (0.455–0.95) ** | 0.572 (0.378–0.865) *** |
| Leiomyosarcomas/spindle cell sarcomas | 0.787 (0.535–1.16) | 0.734 (0.48–1.123) |
| Sarcoma, NOS | 0.965 (0.64–1.456) | 0.853 (0.539–1.349) |
| Synovial sarcomas | 0.535 (0.271–1.056) | 0.608 (0.307–1.203) |
| Osteosarcoma/Chondrosarcoma | 0.987 (0.646–1.507) | 0.932 (0.589–1.474) |
| Tumor stage | ||
| Localized | 1.00 (reference) | 1.00 (reference) |
| Regional | 1.334 (0.964–1.846) | 1.386 (0.972–1.976) |
| Distant | 1.888 (1.389–2.566) *** | 1.953 (1.396–2.733) *** |
| Living area | ||
| Counties in metropolitan areas of 1 million persons | 1.00 (reference) | 1.00 (reference) |
| Counties in metropolitan areas of 250,000 to 1 million persons | 1.01 (0.747–1.366) | 0.993 (0.714–1.381) |
| Counties in metropolitan areas of 250,000 persons | 1.037 (0.662–1.623) | 1.051 (0.648–1.703) |
| Nonmetropolitan counties adjacent to a metropolitan area | 1.666 (0.923–3.007) | 1.614 (0.846–3.081) |
| Nonmetropolitan counties not adjacent to a metropolitan area | 1.128 (0.721–1.766) | 1.16 (0.716–1.88) |
| Income per year | ||
| $ <$45,000 | 1.00 (reference) | 1.00 (reference) |
| $45,000–54,999 | 0.72 (0.372–1.392) | 0.842 (0.392–1.805) |
| $55,000–64,999 | 0.689 (0.363–1.309) | 0.774 (0.367–1.632) |
| $65,000–74,999 | 0.601 (0.316–1.144) | 0.729 (0.346–1.534) |
| $75,000+ | 0.624 (0.332–1.172) | 0.755 (0.363–1.569) |
| Marital Status | ||
| Married | 1.00 (reference) | 1.00 (reference) |
| Single | 0.854 (0.643–1.135) | 0.783 (0.572–1.071) |
| Divorced/separated | 1.121 (0.728–1.726) | 1.121 (0.707–1.778) |
| Widowed | 1.203 (0.717–2.019) | 1.207 (0.694–2.099) |
| Chemotherapy | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.928 (0.724–1.19) | 0.956 (0.729–1.253) |
| Radiation | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.931 (0.7–1.24) | 0.937 (0.688–1.278) |
| Surgery | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.606 (0.465–0.791) *** | 0.581 (0.436–0.774) *** |
| Characteristics | Overall Mortality. Adjusted Proportional Hazard Ratio (95% Confidence Interval) | Primary Cardiac Sarcoma Mortality. Adjusted Proportional Hazard Ratio (95% Confidence Interval) |
|---|---|---|
| Gender | ||
| Female | 1.00 (reference) | 1.00 (reference) |
| Male | 0.952 (0.692–1.309) | 0.917 (0.649–1.296) |
| Age at diagnosis, y.o | ||
| 0–39 | 1.00 (reference) | 1.00 (reference) |
| 40–59 | 1.201 (0.847–1.703) | 1.173 (0.81–1.699) |
| 60–79 | 1.916 (1.213–3.025) *** | 1.61 (0.976–2.657) |
| 80+ | 13.261 (5.839–30.119) *** | 11.177 (4.449–28.08) *** |
| Race | ||
| Non-Hispanic white | 1.00 (reference) | 1.00 (reference) |
| Non-Hispanic black | 1.446 (0.919–2.276) | 1.557 (0.962–2.52) |
| Hispanic | 1.051 (0.691–1.597) | 1.015 (0.645–1.596) |
| Other | 1.174 (0.709–1.944) | 1.379 (0.814–2.336) |
| Histopathology | ||
| Angiosarcoma | 1.00 (reference) | 1.00 (reference) |
| Rhabdomyosarcoma | 0.364 (0.154–0.86) ** | 0.344 (0.128–0.929) ** |
| Malignant fibrous histiocytomas | 0.668 (0.422–1.056) | 0.62 (0.373–1.029) |
| Leiomyosarcomas/spindle cell sarcomas | 0.751 (0.458–1.229) | 0.705 (0.411–1.207) |
| Sarcoma, NOS | 1.376 (0.818–2.314) | 1.322 (0.75–2.33) |
| Synovial sarcomas | 0.603 (0.271–1.341) | 0.738 (0.329–1.657) |
| Osteosarcoma/Chondrosarcoma | 1.355 (0.792–2.316) | 1.37 (0.758–2.476) |
| Tumor stage | ||
| Localized | 1.00 (reference) | 1.00 (reference) |
| Regional | 1.518 (1.041–2.214) ** | 1.574 (1.042–2.379) ** |
| Distant | 2.013 (1.355–2.99) *** | 2.117 (1.37–3.271) *** |
| Living area | ||
| Counties in metropolitan areas of 1 million persons | 1.00 (reference) | 1.00 (reference) |
| Counties in metropolitan areas of 250,000 to 1 million persons | 0.999 (0.692–1.44) | 0.927 (0.624–1.377) |
| Counties in metropolitan areas of 250,000 persons | 0.654 (0.372–1.148) | 0.586 (0.318–1.082) |
| Nonmetropolitan counties adjacent to a metropolitan area | 1.16 (0.556–2.421) | 1.007 (0.444–2.283) |
| Nonmetropolitan counties not adjacent to a metropolitan area | 1.226 (0.663–2.268) | 1.434 (0.743–2.771) |
| Income per year | ||
| $ <$45,000 | 1.00 (reference) | 1.00 (reference) |
| $45,000–54,999 | 1.049 (0.48–2.292) | 1.531 (0.62–3.781) |
| $55,000–64,999 | 0.856 (0.379–1.93) | 1.207 (0.475–3.069) |
| $65,000–74,999 | 0.778 (0.339–1.787) | 1.158 (0.448–2.991) |
| $75,000+ | 0.812 (0.353–1.864) | 1.199 (0.463–3.104) |
| Marital Status | ||
| Married | 1.00 (reference) | 1.00 (reference) |
| Single | 0.834 (0.573–1.215) | 0.681 (0.451–1.028) |
| Divorced/separated | 1.27 (0.746–2.163) | 1.183 (0.664–2.108) |
| Widowed | 0.506 (0.263–0.977) ** | 0.576 (0.282–1.18) |
| Chemotherapy | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.97 (0.704–1.337) | 1.047 (0.739–1.484) |
| Radiation | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.94 (0.674–1.313) | 0.829 (0.578–1.188) |
| Surgery | ||
| No | 1.00 (reference) | 1.00 (reference) |
| Yes | 0.735 (0.508–1.062) | 0.724 (0.489–1.072) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bangolo, A.; Fwelo, P.; Iyer, K.M.; Klinger, S.; Tavares, L.; Dey, S.; Chacko, A.A.; Hein, M.; Gudena, S.; Lawal, G.; et al. Primary Cardiac Sarcoma: Clinical Characteristics and Prognostic Factors over the Past 2 Decades. Diseases 2023, 11, 74. https://doi.org/10.3390/diseases11020074
Bangolo A, Fwelo P, Iyer KM, Klinger S, Tavares L, Dey S, Chacko AA, Hein M, Gudena S, Lawal G, et al. Primary Cardiac Sarcoma: Clinical Characteristics and Prognostic Factors over the Past 2 Decades. Diseases. 2023; 11(2):74. https://doi.org/10.3390/diseases11020074
Chicago/Turabian StyleBangolo, Ayrton, Pierre Fwelo, Kritika M. Iyer, Sarah Klinger, Lorena Tavares, Shraboni Dey, Angel Ann Chacko, Myat Hein, Samyukta Gudena, Gbenga Lawal, and et al. 2023. "Primary Cardiac Sarcoma: Clinical Characteristics and Prognostic Factors over the Past 2 Decades" Diseases 11, no. 2: 74. https://doi.org/10.3390/diseases11020074
APA StyleBangolo, A., Fwelo, P., Iyer, K. M., Klinger, S., Tavares, L., Dey, S., Chacko, A. A., Hein, M., Gudena, S., Lawal, G., Sivasubramanian, B. P., Rimba, Z., Hirpara, K., Merajunnissa, M., Veliginti, S., Arana, G., Sathyarajan, D. T., Singh, S., Shetty, T., ... Weissman, S. (2023). Primary Cardiac Sarcoma: Clinical Characteristics and Prognostic Factors over the Past 2 Decades. Diseases, 11(2), 74. https://doi.org/10.3390/diseases11020074





