Abstract
Introduction: Non-small-cell lung cancer is still diagnosed at an inoperable stage and systematic treatment is the only option. Immunotherapy is currently considered to be the tip of the arrow as the first-line treatment for patients with a programmed death-ligand 1 ≥ 50. Sleep is known to be an essential part of our everyday life. Patients and Methods: We investigated, upon diagnosis and after nine months, 49 non-small-cell lung cancer patients undergoing immunotherapy treatment with nivolumab and pemprolisumab. A polysomnographic examination was conducted. Moreover, the patients completed the Epworth Sleepiness Scale (ESS), the Pittsburgh Sleep Quality Index (PSQI), the Fatigue Severity Scale (FSS) and the Medical Research Council (MRC) dyspnea scale. Results: Tukey mean-difference plots, summary statistics, and the results of paired t-test of five questionnaire responses in accordance with the PD-L1 test across groups were examined. The results indicated that, upon diagnosis, patients had sleep disturbances which were not associated with brain metastases or their PD-L1 expression status. However, the PD-L1 status and disease control were strongly associated, since a PD-L1 ≥80 improved the disease status within the first 4 months. All data from the sleep questionnaires and polysomnography reports indicated that the majority of patients with a partial response and complete response had their initial sleep disturbances improved. There was no connection between nivolumab or pembrolisumab and sleep disturbances. Conclusion: Upon diagnosis, lung cancer patients have sleep disorders such as anxiety, early morning wakening, late sleep onset, prolonged nocturnal waking periods, daytime sleepiness, and unrefreshing sleep. However, these symptoms tend to improve very quickly for patients with a PD-L1 expression ≥80, because disease status improves also very quickly within the first 4 months of treatment.
1. Introduction
Lung cancer patients are still diagnosed at an advanced, inoperable stage. An effort is currently being made by the pneumonology community to inform, educate, and urge people at a high risk of lung cancer to perform computed tomography scans as a method of early lung cancer detection [1]. We have novel techniques for a safe biopsy in small pulmonary nodules with radial-ebus and electromagnetic navigation systems [2,3,4,5,6]. Unfortunately, the diagnosis of lung cancer is made at an advanced, inoperable stage. It has been previously observed that these patients have severe sleep disturbances, which are associated with a poor quality of life [7]. The usual sleep disorders are anxiety, early morning wakening, late sleep onset, prolonged nocturnal waking periods, daytime sleepiness, and unrefreshing sleep [8]. It has been observed that insomnia symptoms have a variation between hospitalizations and between cancer treatments and side effects [9,10,11]. Unfortunately, sleep disturbances increase symptoms such as pain, fatigue, and depression [12,13]. In newly diagnosed cancer patients, sleep disturbances have been reported in up to 50% of patients for all cancer types. The importance of sleep disturbances has been underestimated [14]. In most studies with sleep disturbances and cancer patients, different types of cancer were included, usually breast cancer and prostate cancer [9] There are few studies published with lung cancer patients receiving treatment with chemotherapy regimens [15,16]. In our present pilot study, we evaluated a subgroup of lung cancer, non-small-cell lung cancer (NSCLC) patients, who exhibit a programmed death-ligand 1 ≥ 50. This is a special group in which we administer immunotherapy alone as a first-line treatment [17]. These patients have a different survival profile and restaging methodology [18]. Until ten years ago, we only had chemotherapy regimens and tyrosine kinase inhibitors (TKIs). In the past ten years, the novel treatment of immunotherapy was introduced with different medications. Moreover, combinations of chemotherapy and immunotherapy are administered [19]. In our pilot study, we evaluated this specific subgroup of NSCLC patients using polysomnography and sleep questionnaires. We report the characteristics of these patients upon diagnosis without treatment and the impact of treatment after the second re-staging.
2. Patients and Methods
Forty-nine patients (forty-four males and five females), with primary lung cancer diagnoses were included in the pilot study. All patients had non-small-cell lung cancer (NSCLC), either adenocarcinoma or squamous cell carcinoma; we did not include non-other specific (NOS) types in order to maintain a homogenous genetic sample. In total, 14 were Stage IIIb and 35 were Stage IV. We obtained demographic and clinical data regarding age, sex, painkillers, brain metastasis, and we finally recorded the Eastern Cooperative Oncology Group (ECOG) performance status. All patients included had programmed death-ligand 1 (PD-L1) status ≥ 50% and were stratified into three categories: 4 = 50–70, 5 = 71–90, and 6 = 91–100 in our statistics. Based on previous studies, a higher expression implies that survival is prolonged. Since they had a PD-L1 ≥ 50%, all patients received only immunotherapy, either with pembrolisumab or nivoslumab as the first-line treatment. We did not include patients receiving corticosteroids orally or intravenously, however, we did include patients with chronic obstructive pulmonary disease (COPD) under treatment with inhaled corticosteroids. All patients included did have chronic heart failure, NIHA ≤ III, or they had their heart disease under control. The main exclusion criteria included the inability to understand and answer the questionnaires that were distributed for their sleep evaluation (see next section). The study was approved by our IRB 29/2022 the “AHEPA” hospital. Written informed consent was obtained from each patient before study enrollment.
3. Sleep Evaluation Methodology
Patients completed self-report questionnaires one day before their first treatment and again at nine months after. In order to evaluate daytime sleepiness, we used the Greek version of the Epworth Sleepiness Scale (ESS) [20]. We used the ESS to assess daytime sleepiness over the last three months under eight usual circumstances. The ESS was evaluated for the Greek population and the cut-off point, indicating excessive daytime sleepiness, was set at 10. We used the Greek version of the Pittsburgh Sleep Quality Index (PSQI) in order to assess the sleep quality [21]. The PSQI is composed of 19 self-rated questions that are grouped into seven domains (subjective sleep quality [SSQ], sleep latency [SL], sleep duration [SDU], habitual sleep efficiency [HSE], sleep disturbances [SDI], use of sleeping medication [SM], and daytime dysfunction [DD]). In order to evaluate fatigue, we used the Greek version of the Fatigue Severity Scale (FSS) [22]. In order to objectively evaluate sleep quality, we used overnight polysomnography (PSG) (Alice 3, Respironics) with a standard montage of electroencephalogram (EEG), electrooculogram, electromyogram (EMG), and electrocardiogram (ECG) signals in combination with pulse oximetry and airflow, which were detected using combined oronasal thermistors. The thoracic cage and abdominal motion were recorded by inductive plethysmography. EEG recordings were manually scored according to standard criteria. We used the Medical Research Council (MRC) scale in order to evaluate the presence and grade of dyspnea [23].
4. Statistics and Results
We employed Tukey mean-difference plots, summary statistics, and the results of the paired t-test of five questionnaire responses in accordance with the PD-L1 test across groups. Horizontal and vertical lines determined the mean difference, plus the 95% confidence intervals and the mean of paired sums, accordingly (Supplementary Materials).
The obstructive sleep apnea syndrome was investigated via the responses derived the disease questionnaires and recorded at two consecutive time intervals, before and after immunotherapy treatment, in 49 patients. The matched-pairs statistical criterion was adopted because it compares individual differences and the means of sums using the paired t-test. The Tukey mean-difference plot was also employed graphically. Additionally, the effect of PD-L1 expression in the repeated two periods was tested across the groups, coded as 4 (50–70%), 5 (71–90%), and 6 (91–100%). Pain was coded as 1 (present) and 2 (absent), thus producing four combined categories for the two consecutive periods: 11, 12, 21, and 22.
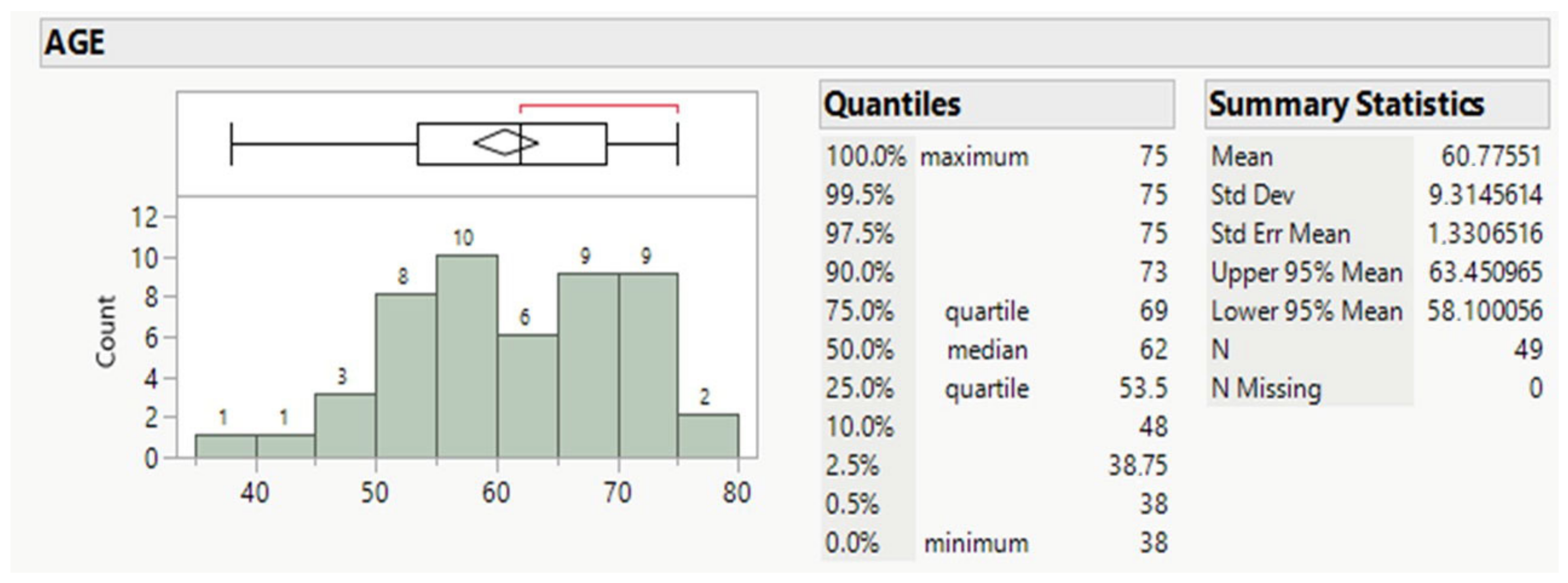
Forty-nine patients, averaging 61 years old, mostly males (89.8%, Table 1 and Table 2), and ranging for the 50% between 53.5 and 69 y.o., comprised the whole population (Figure 1).

Table 1.
Basic features of the study.

Table 2.
Descriptive statistics of the questionnaire responses in the two consecutive time intervals.
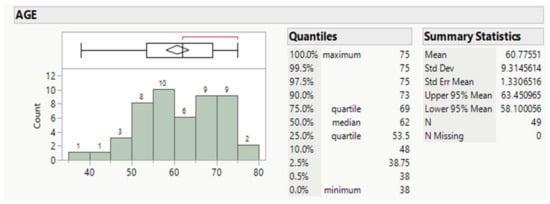
Figure 1.
Age distribution of patients.
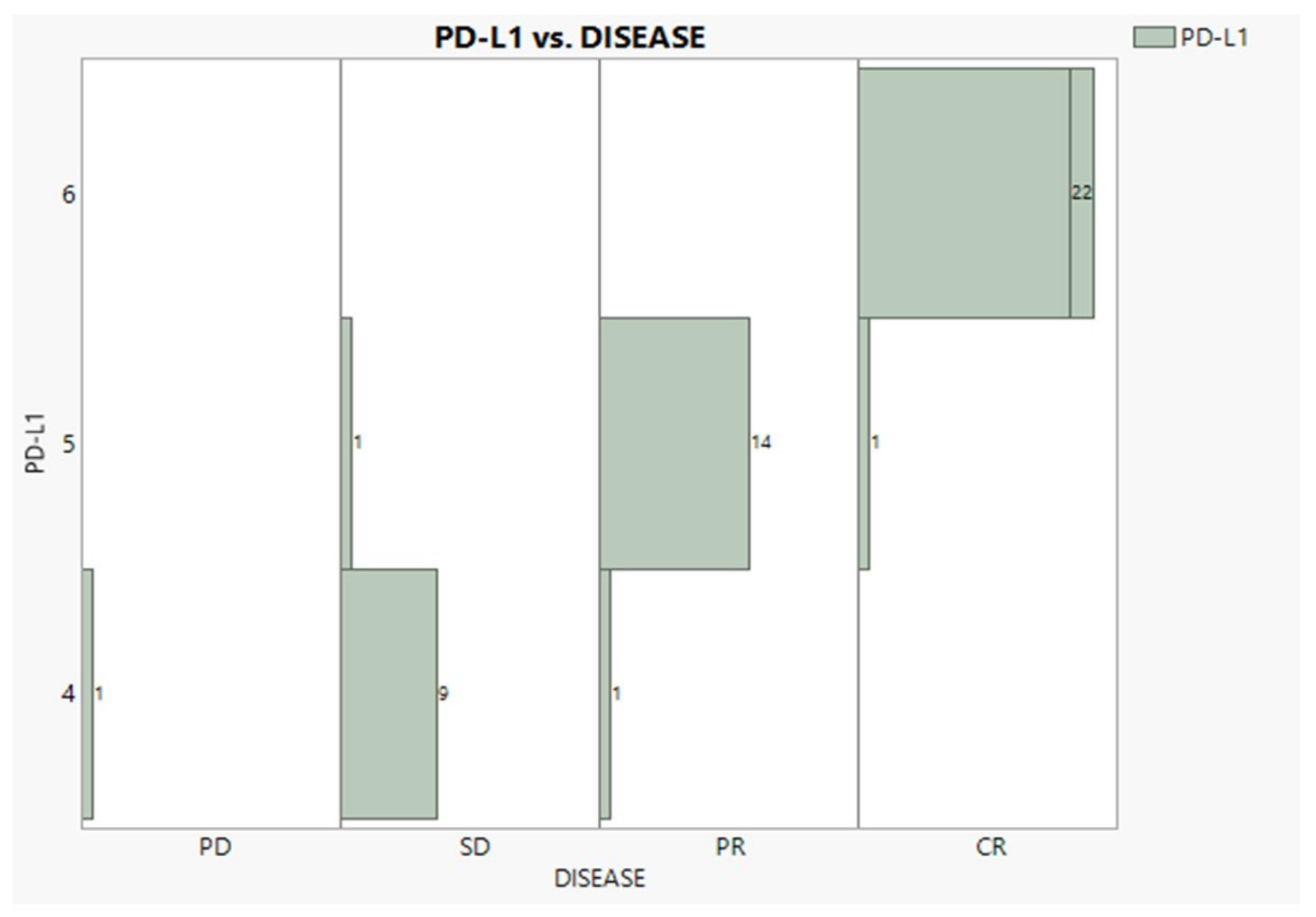
According to Table 1, cancer at Stages IIIb and IV was encountered nearly equally (47% and 43%). Adenocarcinoma was observed in a 2:1 proportion to squamous, while patients with no NSCLC BRAIN METS and no radiation exposure were recorded in the proportion of 3:1. Eleven patients suffered continuous pain in both time periods, twelve recuperated, and thirty six never suffered. Regarding the BMI condition, 22 individuals were overweight, 16 were normal, and 11 were obese. The PD-L1 expression was expectedly found in very good agreement with the disease progress (96.3%), as Figure 2 vividly demonstrates, showing only three mismatches and one outlier (PD).
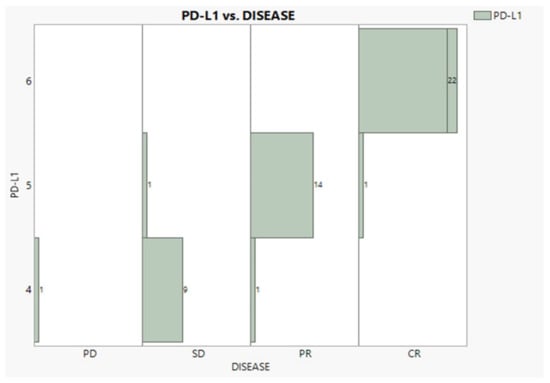
Figure 2.
Relationships between PD-L1 expression and disease progress.
No significant effect of any questionnaire response was found between the time intervals (before and after treatment with immunotherapy), as Figure S1 shows in detail for the paired t-test results. A significant effect of PD-L1 was not statistically documented (p-values greater than 0.05) across the expression groups, concerning either mean differences or the means of mean sums between pairs’ responses.
5. Discussion
All newly diagnosed patients had poor sleep quality, which was associated with daily fatigue. As previously observed in other immunotherapy studies, patients with high a PD-L1 status (≥80%) have a faster response and a prolonged response [24]. We stratified our patients, as in previous studies, into three categories in order to perform our analysis [25,26]. It was observed that patients in Groups 5 and 6, meaning ≥71% PD-L1 expression, indeed had faster responses and complete responses; hence, their sleep disorder symptoms were alleviated. Upon diagnoses, the most affected component was sleep latency, followed by sleep duration. During therapy, all patients who were using painkillers discontinued their use due to the efficiency of the therapy. Dyspnea is a major symptom for patients with chronic obstruction pulmonary disease (COPD); it affects both daytime function and sleep quality. However, in our study, all not all patients were smokers. Additionally, the mean MRC score upon diagnosis was low. Moreover, no relationship was observed between the MRC score and sleep quality.
Upon diagnosis, the reduced sleep duration was connected to poor sleep quality, and insomnia was the most common sleep disturbance observed in these lung cancer patients, with a major impact on their quality of life. In contrast to our study, in previous studies that investigated immunotherapy, insomnia remained even in patients who exhibited a partial response or a complete response [27]. Moreover, the sleep disturbance symptoms in these studies were indifferent to the PD-L1 expression status. Mild daytime sleepiness was reported, along with poor sleep duration and efficiency. In our study, no correlation was observed between daytime sleepiness and sleep quality, as assessed by the global PSQI score or any of its components. Fatigue was observed in our patients, which was associated with sleep quality, subjective sleep quality, sleep duration, and daily dysfunction. These findings are in accordance with previous studies [21,28,29,30]. After nine months of immunotherapy, these correlations changed dramatically, and all symptoms for those patients with a complete response were alleviated almost completely. It is known that cancer-related fatigue is a common symptom, and its prevalence reaches 80 to 90% of cancer patients under treatment [31,32]. Fatigue symptoms are often under-diagnosed and under-treated by health care practitioners. However, fatigue has a huge impact on the patients’ quality of life and daytime activities. Fatigue has an effect on the central nervous system, muscle metabolism, circadian rhythm, inflammatory and stress mediators, immune system activation, and hormonal alteration [29]. Therefore, the impact of treatment in these patients has a huge effect, as their effective therapy (treatment response) reduced the stress hormones (cortisol) and activated the immune system. We did not observe any differences between the two drugs, nivolumab or pembrolisumab. Patients with a partial response and mostly those with a complete response experienced a positive effect on ECOG status, pain status, and psychology status, with an improved sleep quality and reduced fatigue and insomnia, which again enhanced the treatment and response. The limitations of the present study are its small sample size and the small number of women in the sample. Furthermore, we did not keep record of the number of women in peri-menopausal or menopausal age, or the drugs that they were taking for these purposes. Women receiving drug medication for these conditions tend to have their symptoms ameliorated. The mean age of the five women in the study was 62 years of age. Additionally, different hormone levels were not measured to observe the impact and interaction of immunotherapy and body environment. Moreover, future studies should compare more patients with a PD-L1 status ≤50%; this category of patients receive chemotherapy along with immunotherapy.
6. Conclusions
The sleep quality of lung cancer patients, subjectively evaluated, was poor upon diagnosis. Sleep efficiency, objectively measured, increased during the course of immunotherapy for patients in all groups, but mostly for patients with a PD-L1 expression ≥91–100%. Fatigue was the major symptom associated with sleep quality in these patients upon diagnosis, and the first to be alleviated during treatment. Sleep quality in patients with lung cancer should be evaluated in large-scale, prospective studies, including PSG, in order to obtain a better understanding of the mechanisms of poor sleep and its contribution to daily fatigue. We should bear in mind that sleep impairment should be also one of our treatment targets, since immunotherapy is associated with hormones and PD-L1 status.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases11010026/s1, Figure S1: Turkey mean-difference plots, summary statistics and results of paired t-test of five questionnaire responses in accordance with PD-L1 test across groups. Horizontal and vertical lines determine the mean difference plus the 95% confidence intervals and the mean of paired sums accordingly.
Author Contributions
Conceptualization, formal analysis, writing—original draft preparation, writing—review and editing: P.Z., D.P., C.K., K.S., L.N., D.M., V.P., E.I.P., K.P., P.K. and P.S. wrote the manuscript and collected patient data. P.Z. and D.P. performed the statistics. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the investigational review board (IRB) of 3rd Surgery Department “AHEPA” hospital with ID 29/2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Any data can be provided by the corresponding author if requested.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Torrente, M.; Sousa, P.A.; Hernandez, R.; Blanco, M.; Calvo, V.; Collazo, A.; Guerreiro, G.R.; Nunez, B.; Pimentao, J.; Sanchez, J.C.; et al. An Artificial Intelligence-Based Tool for Data Analysis and Prognosis in Cancer Patients: Results from the Clarify Study. Cancers 2022, 14, 4041. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Huang, H.; Chen, W.; Petridis, D.; Matthaios, D.; Hohenforst-Schmidt, W.; Tolis, C.; Tsakiridis, K.; Baka, S.; Arnaoutoglou, C.; et al. Radial Endobronchial Ultrasound for Lung Cancer Diagnosis: Tips and Tricks. J. Cancer 2022, 13, 1307–1312. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, H.; Ning, Y.; Han, J.; Shen, Y.; Shi, H.; Wang, Q.; Bai, C.; Li, Q.; Michael, S.; et al. Radial probe endobronchial ultrasound assisted conventional transbronchial needle aspiration in the diagnosis of solitary peribronchial pulmonary lesion located in the segmental bronchi. J. Cancer 2019, 10, 634–642. [Google Scholar] [CrossRef]
- Zaric, B.; Stojsic, V.; Carapic, V.; Kovacevic, T.; Stojanovic, G.; Panjkovic, M.; Kioumis, I.; Darwiche, K.; Zarogoulidis, K.; Stratakos, G.; et al. Radial Endobronchial Ultrasound (EBUS) Guided Suction Catheter-Biopsy in Histological Diagnosis of Peripheral Pulmonary Lesions. J. Cancer 2016, 7, 7–13. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Kosmidis, C.S.; Hohenforst-Schmidt, W.; Matthaios, D.; Sapalidis, K.; Petridis, D.; Perdikouri, E.I.; Courcoutsakis, N.; Hatzibougias, D.; Arnaoutoglou, C.; et al. Radial-EBUS: CryoBiopsy Versus Conventional Biopsy: Time-Sample and C-Arm. Int. J. Environ. Res. Public Health 2022, 19, 3569. [Google Scholar] [CrossRef]
- Haidong, H.; Yunye, N.; Wei, Z.; Zarogoulidis, P.; Hohenforst-Schmidt, W.; Man, Y.G.; Yuguang, Y.; Yuchao, D.; Chong, B. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: A single-center study. J. Cancer 2017, 8, 3514–3521. [Google Scholar] [CrossRef]
- Imani, V.; Ahorsu, D.K.; Taghizadeh, N.; Parsapour, Z.; Nejati, B.; Chen, H.P.; Pakpour, A.H. The Mediating Roles of Anxiety, Depression, Sleepiness, Insomnia, and Sleep Quality in the Association between Problematic Social Media Use and Quality of Life among Patients with Cancer. Healthcare 2022, 10, 1745. [Google Scholar] [CrossRef]
- Spiegel, D. Losing sleep over cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 2431–2432. [Google Scholar] [CrossRef]
- Erickson, J.M.; Beck, S.L.; Christian, B.R.; Dudley, W.; Hollen, P.J.; Albritton, K.A.; Sennett, M.; Dillon, R.L.; Godder, K. Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. J. Pediatr. Hematol./Oncol. 2011, 33, e17–e25. [Google Scholar] [CrossRef]
- Osoba, D.; Zee, B.; Warr, D.; Latreille, J.; Kaizer, L.; Pater, J. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 1997, 5, 307–313. [Google Scholar] [CrossRef]
- Sheely, L.C. Sleep disturbances in hospitalized patients with cancer. Oncol. Nurs. Forum 1996, 23, 109–111. [Google Scholar]
- Baek, Y.; Jung, K.; Kim, H.; Lee, S. Association between fatigue, pain, digestive problems, and sleep disturbances and individuals’ health-related quality of life: A nationwide survey in South Korea. Health Qual. Life Outcomes 2020, 18, 159. [Google Scholar] [CrossRef]
- Lee, K.A. Sleep and fatigue. Annu. Rev. Nurs. Res. 2001, 19, 249–273. [Google Scholar] [CrossRef]
- Savard, J.; Morin, C.M. Insomnia in the context of cancer: A review of a neglected problem. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 895–908. [Google Scholar] [CrossRef]
- Pataka, A.; Kotoulas, S.; Kalamaras, G.; Schiza, S.; Sapalidis, K.; Giannakidis, D.; Michalopoulos, N.; Koulouris, C.; Aidoni, Z.; Amaniti, A.; et al. Gender Differences in Obstructive Sleep Apnea: The Value of Sleep Questionnaires with a Separate Analysis of Cardiovascular Patients. J. Clin. Med. 2020, 9, 130. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Steiropoulos, P.; Perantoni, E.; Archontogeorgis, K.; Eleftheriadou, E.; Porpodis, K.; Charpidou, A.G.; Angelopoulou, C.; Nena, E.; Zarogoulidis, K.; et al. Subjective sleep quality in lung cancer patients before and after chemotherapy. Thorac. Cancer 2013, 4, 138–142. [Google Scholar] [CrossRef]
- Lee, A.; Yuan, Y.; Eccles, L.; Chitkara, A.; Dalen, J.; Varol, N. Treatment patterns for advanced non-small cell lung cancer in the US: A systematic review of observational studies. Cancer Treat. Res. Commun. 2022, 33, 100648. [Google Scholar] [CrossRef]
- Shroff, G.S.; Strange, C.D.; Ahuja, J.; Altan, M.; Sheshadri, A.; Unlu, E.; Truong, M.T.; Vlahos, I. Imaging of Immune Checkpoint Inhibitor Immunotherapy for Non-Small Cell Lung Cancer. Radiographics 2022, 42, 220108. [Google Scholar] [CrossRef]
- Rittberg, R.; Leung, B.; Al-Hashami, Z.; Ho, C. Real-world eligibility for platinum doublet plus immune checkpoint inhibitors in extensive-stage small-cell lung cancer. Front. Oncol. 2022, 12, 1002385. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek version of the Epworth Sleepiness Scale. Sleep Breath. Schlaf Atm. 2004, 8, 91–95. [Google Scholar] [CrossRef]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef]
- Katsarou, Z.; Bostantjopoulou, S.; Hatzizisi, O.; Giza, E.; Soler-Cardona, A.; Kyriazis, G. Immune factors or depression? Fatigue correlates in Parkinson’s disease. Rev. Neurol. 2007, 45, 725–728. [Google Scholar]
- Williams, N. The MRC breathlessness scale. Occup. Med. 2017, 67, 496–497. [Google Scholar] [CrossRef]
- Liu, S.V.; Hu, X.; Li, Y.; Zhao, B.; Burke, T.; Velcheti, V. Pembrolizumab-combination therapy for previously untreated metastatic nonsquamous NSCLC: Real-world outcomes at US oncology practices. Front. Oncol. 2022, 12, 999343. [Google Scholar] [CrossRef]
- Petridis, D.; Matthaios, D.; Christakidis, V.; Sardeli, C.; Freitag, L.; Huang, H.; Zarogoulidis, P. A health condition index for assessing disease progression. Expert Rev. Respir. Med. 2022, 16, 867–873. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Sardeli, C.; Christakidis, V.; Hohenforst-Schmidt, W.; Huang, H.; Kosmidis, C.; Vagionas, A.; Baka, S.; Tsakiridis, K.; Perdikouri, E.I.; et al. PD-L1 and standardized uptake value expression in lung cancer: A possible connection for efficient early lung cancer treatment. Biomark. Med. 2021, 15, 463–466. [Google Scholar] [CrossRef]
- Kiss, I.; Kuhn, M.; Hrusak, K.; Buchler, B.; Boublikova, L.; Buchler, T. Insomnia in patients treated with checkpoint inhibitors for cancer: A meta-analysis. Front. Oncol. 2022, 12, 946307. [Google Scholar] [CrossRef]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Patiraki, E.; Galanos, A.; Vlahos, L. Sleep quality in advanced cancer patients. J. Psychosom. Res. 2007, 62, 527–533. [Google Scholar] [CrossRef]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Pathiaki, M.; Gennatas, K.; Smyrniotis, V.; Vassiliou, I. The relationship of subjective sleep quality, pain, and quality of life in advanced cancer patients. Sleep 2007, 30, 737–742. [Google Scholar] [CrossRef]
- Vena, C.; Parker, K.; Allen, R.; Bliwise, D.; Jain, S.; Kimble, L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncol. Nurs. Forum 2006, 33, 761–769. [Google Scholar] [CrossRef]
- Korenev, G.; Yakukhnov, S.; Druk, A.; Golovina, A.; Chasov, V.; Mirgayazova, R.; Ivanov, R.; Bulatov, E. USP7 Inhibitors in Cancer Immunotherapy: Current Status and Perspective. Cancers 2022, 14, 5539. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-related fatigue: The scale of the problem. Oncologist 2007, 12 (Suppl. 1), 4–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).