Racial Disparities and Common Respiratory Infectious Diseases in Children of the United States: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Guidelines
2.2. Screening Guidelines
2.3. Quality Appraisal
3. Results
3.1. Systematic Review
Racial Disparities
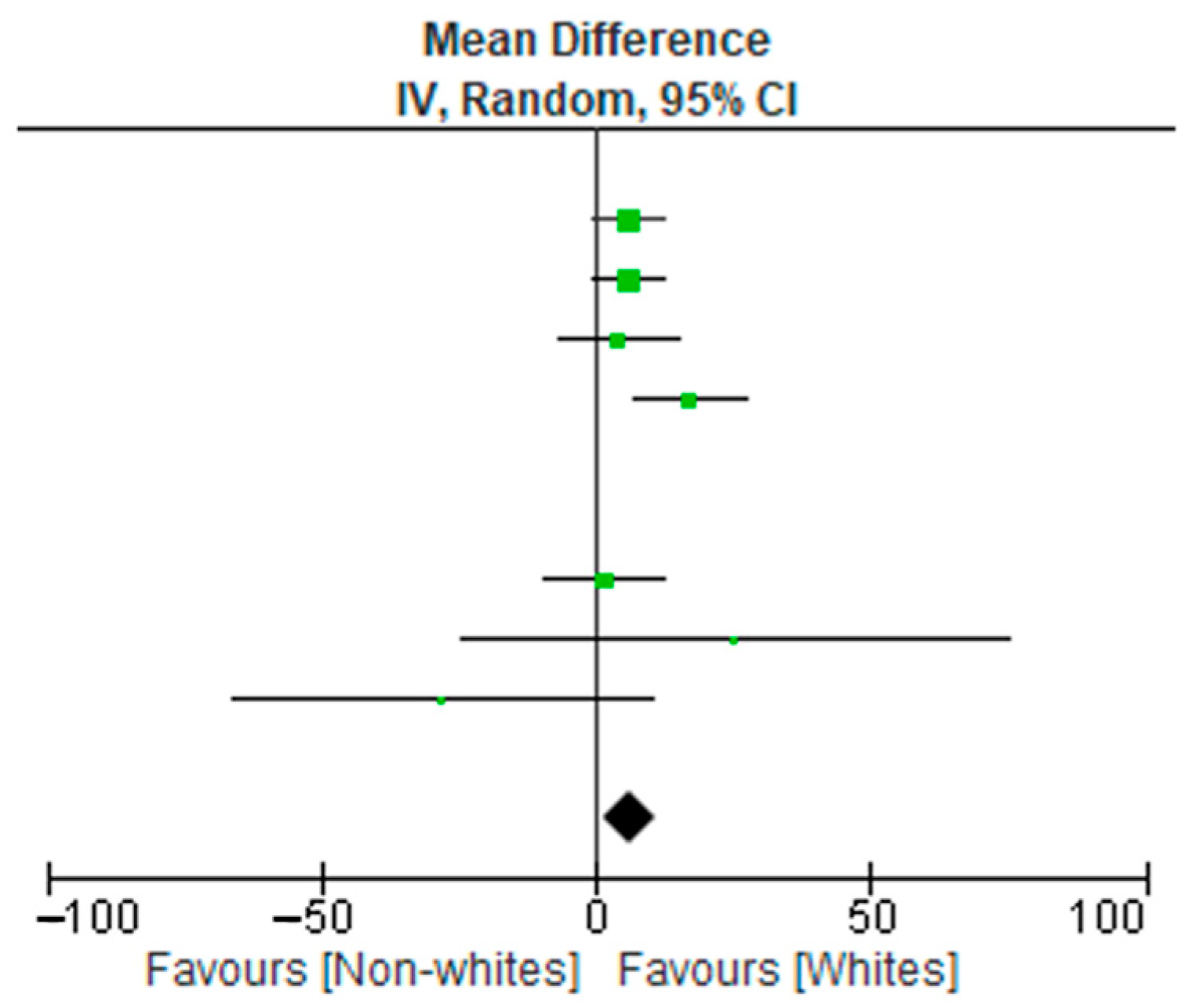
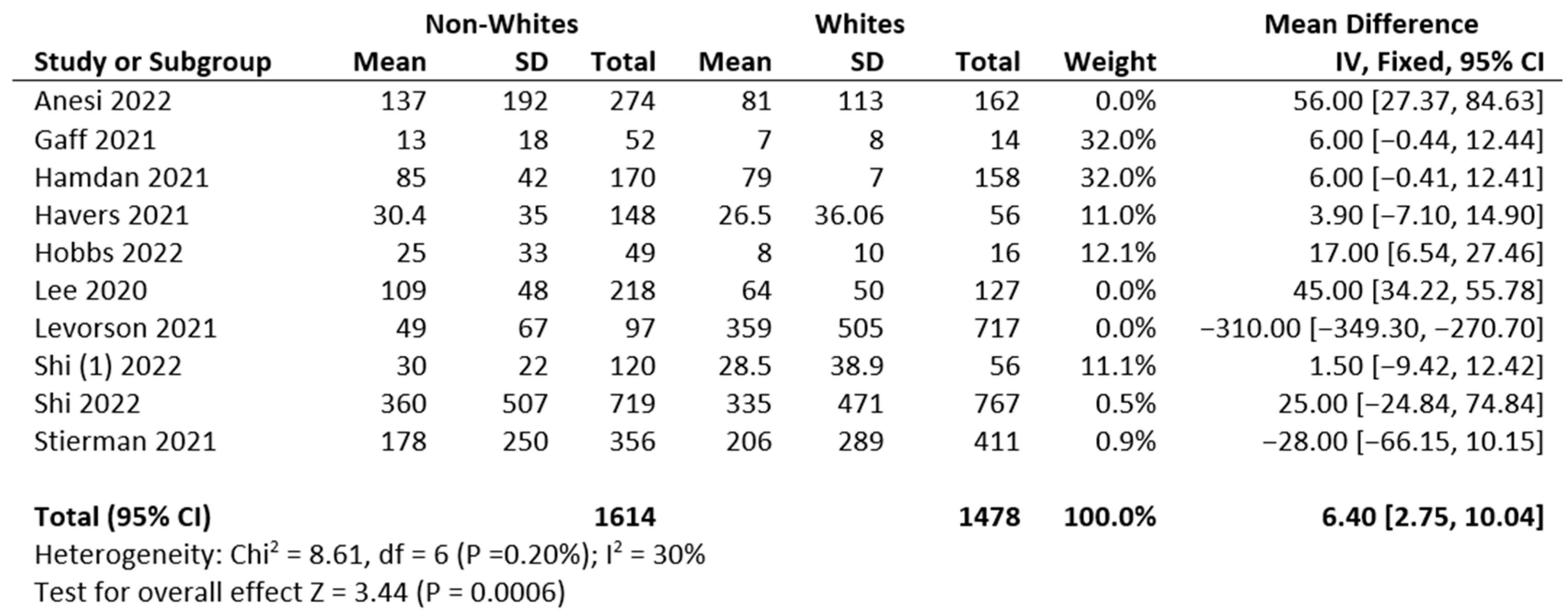
3.2. Meta-Analysis
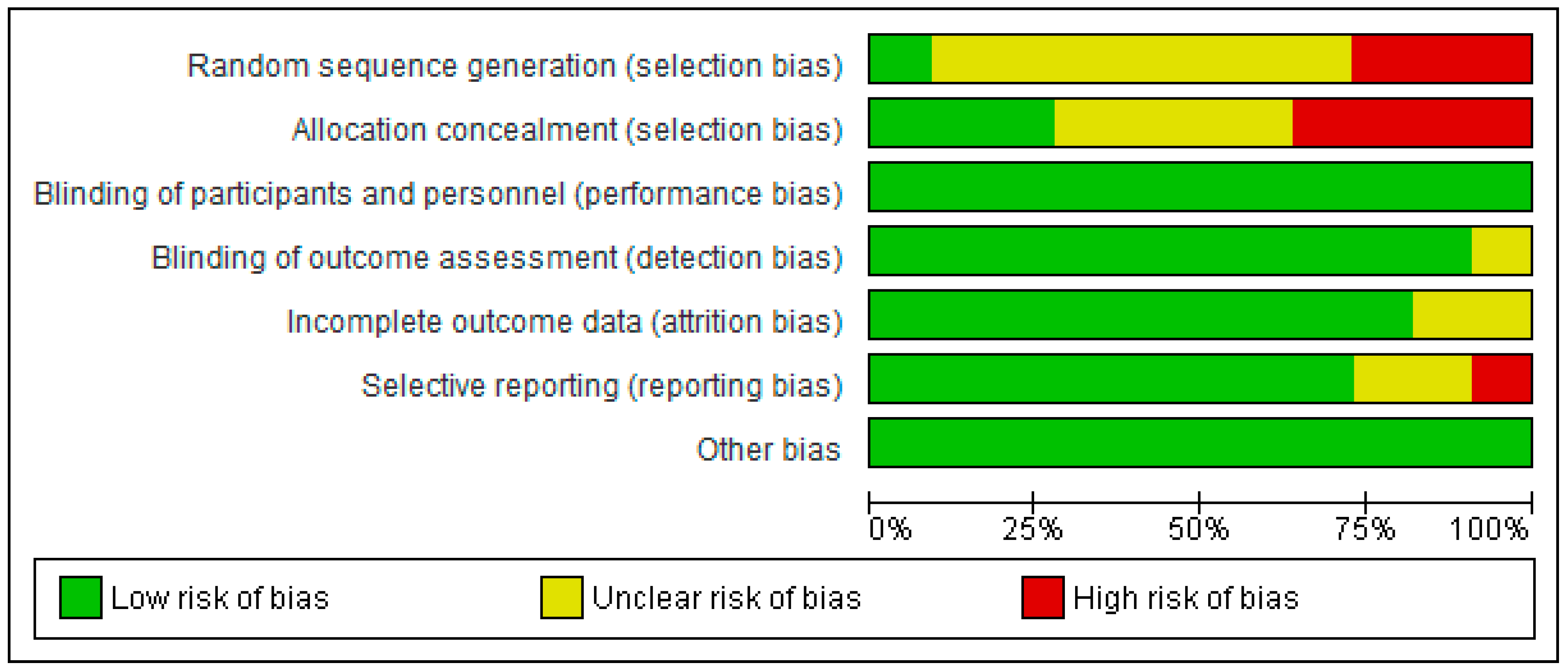
3.3. Risk of Bias
3.4. Risk of Bias Summary
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro, N. Tripledemic Update: RSV, Covid and Flu. Forbes 13 December 2022. Available online: https://www.forbes.com/sites/ninashapiro/article/whats-a-tripledemic-rsv-covid-and-flu/?sh=226354f77579 (accessed on 23 December 2022).
- Miguel, C.P.V.; Dasgupta-Tsinikas, S.; Lamb, G.S.; Olarte, L.; Santos, R.P. Race, Ethnicity, and Health Disparities in US Children With COVID-19: A Review of the Evidence and Recommendations for the Future. J. Pediatr. Infect. Dis. Soc. 2022, 11, S132–S140. [Google Scholar] [CrossRef] [PubMed]
- PIDS. What’s in the News. What’s in the News—Pediatric Infectious Diseases Society. Available online: https://pids.org/2021/05/07/whats-in-the-news-socioeconomic-racial-and-ethnic-disparities-in-mis-c/ (accessed on 23 December 2022).
- Thinking About Racial Disparities in COVID-19 Impacts. Center on the Developing Child at Harvard University. 27 April 2020. Available online: https://developingchild.harvard.edu/thinking-about-racial-disparities-in-covid-19-impacts-through-a-science-informed-early-childhood-lens/ (accessed on 23 December 2022).
- Smitherman, L.C.; Golden, W.C.; Walton, J.R. Health Disparities and Their Effects on Children and Their Caregivers During the Coronavirus Disease 2019 Pandemic. Pediatr. Clin. N. Am. 2021, 68, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; A Madhi, S.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Okomo, U.; Idoko, O.T.; Kampmann, B. The burden of viral respiratory infections in young children in low-resource settings. Lancet Glob. Health 2020, 8, e454–e455. [Google Scholar] [CrossRef]
- Schot, M.J.C.; Dekker, A.R.J.; Van Werkhoven, C.H.; Van Der Velden, A.W.; Cals, J.W.L.; Broekhuizen, B.D.L.; Hopstaken, R.M.; De Wit, N.J.; Verheij, T.J.M. Burden of disease in children with respiratory tract infections in primary care: Diary-based cohort study. Fam. Pract. 2019, 36, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Tsugawa, Y.; Mansbach, J.M.; Camargo, C.A.; Hasegawa, K. Trends in Infectious Disease Hospitalizations in US Children, 2000 to 2012. Pediatr. Infect. Dis. J. 2016, 35, e158–e163. [Google Scholar] [CrossRef]
- Severe Respiratory Illness Increasing in Children: What You Need to Know. RWJBarnabas Health. Available online: https://www.rwjbh.org/blog/2022/november/severe-respiratory-illness-increasing-in-childre/ (accessed on 23 December 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- JBI Critical Appraisal Tools. Joanna Briggs Institute, the University of Adelaide, South Australia. Available online: https://joannabriggs.org/criticalappraisal-tools (accessed on 5 February 2021).
- Perez, A.; Lively, J.Y.; Curns, A.; Weinberg, G.A.; Halasa, N.B.; Staat, M.A.; Szilagyi, P.G.; Stewart, L.S.; McNeal, M.M.; Clopper, B.; et al. Respiratory Virus Surveillance Among Children with Acute Respiratory Illnesses—New Vaccine Surveillance Network, United States, 2016–2021. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1253–1259. [Google Scholar] [CrossRef]
- Marks, K.J.; Whitaker, M.; Agathis, N.T.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Kirley, P.D.; Kawasaki, B.; Meek, J.; et al. Hospitalization of Infants and Children Aged 0–4 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 429–436. [Google Scholar] [CrossRef]
- Shi, D.S.; Whitaker, M.; Marks, K.J.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kawasaki, B.; Meek, J.; et al. Hospitalizations of Children Aged 5–11 Years with Laboratory-Confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 574–581. [Google Scholar] [CrossRef]
- Anesi, J. Commentary: Effectiveness of two-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults—Nine states, January–September 2021. Am. J. Transplant. 2021, 22, 304–305. [Google Scholar] [CrossRef]
- Shi, D.S.; Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; et al. COVID-19 Vaccine Safety in adolescents aged 12–17 years—United States, December 14, 2020–July 16, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1053–1058. [Google Scholar] [CrossRef]
- Levorson, R.E.; Christian, E.; Hunter, B.; Sayal, J.; Sun, J.; Bruce, S.A.; Garofalo, S.; Southerland, M.; Ho, S.; Levy, S.; et al. A cross-sectional investigation of SARS-CoV-2 seroprevalence and associated risk factors in children and adolescents in the United States. PLoS ONE 2021, 16, e0259823. [Google Scholar] [CrossRef]
- Lee, E.H.; Kepler, K.L.; Geevarughese, A.; Paneth-Pollak, R.; Dorsinville, M.S.; Ngai, S.; Reilly, K.H. Race/Ethnicity Among Children With COVID-19–Associated Multisystem Inflammatory Syndrome. JAMA Netw. Open 2020, 3, e2030280. [Google Scholar] [CrossRef] [PubMed]
- Kurup, S.; Burgess, R.; Tine, F.; Chahroudi, A.; Lee, D.L. SARS-CoV-2 Infection and Racial Disparities in Children: Protective Mechanisms and Severe Complications Related to MIS-C. J. Racial Ethn. Health Disparities 2022, 9, 1536–1542. [Google Scholar] [CrossRef]
- Stierman, B.M.; Abrams, J.Y.P.; Godfred-Cato, S.E.D.; Oster, M.E.M.; Meng, L.; Yip, L.; Patel, P.M.; Balachandran, N.M.; Prezzato, E.M.; Pierce, T.M.; et al. Racial and Ethnic Disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pediatr. Infect. Dis. J. 2021, 40, e400–e406. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Ly, K.N.; Link-Gelles, R.; Newhams, M.M.; Akande, M.; Wu, M.J.; Feldstein, L.R.; Tarquinio, K.M.; Sahni, L.C.; Riggs, B.J.; et al. Investigating Health Disparities Associated With Multisystem Inflammatory Syndrome in Children After SARS-CoV-2 Infection. Pediatr. Infect. Dis. J. 2022, 41, 891–898. [Google Scholar] [CrossRef]
- Hobbs, C.V.; Kim, S.S.; Vemula, P.; Inagaki, K.; Harrison, V.A.; Malloch, L.; Martin, L.M.; Singh, G.; Agana, U.; Williams, J.M.; et al. Active Surveillance With Seroprevalence-based Infection Rates Indicates Racial Disparities With Pediatric SARS-CoV-2 Requiring Hospitalization in Mississippi, March 2020–February 2021. Pediatr. Infect. Dis. J. 2022, 41, 736–741. [Google Scholar] [CrossRef]
- Mody, A.; Pfeifauf, K.; Bradley, C.; Fox, B.; Hlatshwayo, M.G.; Ross, W.; Sanders-Thompson, V.; Maddox, K.J.; Reidhead, M.; Schootman, M.; et al. Understanding Drivers of Coronavirus Disease 2019 (COVID-19) Racial Disparities: A Population-Level Analysis of COVID-19 Testing Among Black and White Populations. Clin. Infect. Dis. 2020, 73, e2921–e2931. [Google Scholar] [CrossRef]
- Hamdan, L.; Vandekar, S.; Spieker, A.J.; Rahman, H.; Ndi, D.; Shekarabi, E.S.; Thota, J.; A Rankin, D.; Haddadin, Z.; Markus, T.; et al. Epidemiological Trends of Racial Differences in Early- and Late-onset Group B Streptococcus Disease in Tennessee. Clin. Infect. Dis. 2021, 73, e3634–e3640. [Google Scholar] [CrossRef]
- Graff, K.; Smith, C.; Silveira, L.; Jung, S.; Curran-Hays, S.M.; Jarjour, J.; Carpenter, L.B.; Pickard, K.B.; Mattiucci, M.; Fresia, J.B.; et al. Risk Factors for Severe COVID-19 in Children. Pediatr. Infect. Dis. J. 2021, 40, e137–e145. [Google Scholar] [CrossRef]
- Mannheim, J.; Konda, S.; Logan, L.K. Racial, ethnic and socioeconomic disparities in SARS-CoV-2 infection amongst children. Paediatr. Périnat. Epidemiol. 2022, 36, 337–346. [Google Scholar] [CrossRef]
- Brandt, K.; Goel, V.; Keeler, C.; Bell, G.J.; Aiello, A.E.; Corbie-Smith, G.; Wilson, E.; Fleischauer, A.; Emch, M.; Boyce, R.M. SARS-CoV-2 testing in North Carolina: Racial, ethnic, and geographic disparities. Health Place 2021, 69, 102576. [Google Scholar] [CrossRef]
- Gualandi, N.; Mu, Y.; Bamberg, W.M.; Dumyati, G.; Harrison, L.H.; Lesher, L.; Nadle, J.; Petit, S.; Ray, S.M.; Schaffner, W.; et al. Racial Disparities in Invasive Methicillin-resistant Staphylococcus aureus Infections, 2005–2014. Clin. Infect. Dis. 2018, 67, 1175–1181. [Google Scholar] [CrossRef]
- Hansen, C.; Perofsky, A.C.; Burstein, R.; Famulare, M.; Boyle, S.; Prentice, R.; Marshall, C.; McCormick, B.J.J.; Reinhart, D.; Capodanno, B.; et al. Trends in Risk Factors and Symptoms Associated With SARS-CoV-2 and Rhinovirus Test Positivity in King County, Washington, June 2020 to July 2022. JAMA Netw. Open 2022, 5, e2245861. [Google Scholar] [CrossRef]
- O’Halloran, A.C.; Holstein, R.; Cummings, C.; Kirley, P.D.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; Ryan, P.; Kim, S.; Lynfield, R.; et al. Rates of Influenza-Associated Hospitalization, Intensive Care Unit Admission, and In-Hospital Death by Race and Ethnicity in the United States From 2009 to 2019. JAMA Netw. Open 2021, 4, e2121880. [Google Scholar] [CrossRef]
- Artiga, S.; Hill, L. Racial Disparities in COVID-19 Impacts and Vaccinations for Children. KFF. 22 September 2021. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/racial-disparities-in-covid-19-impacts-and-vaccinations-for-children/ (accessed on 16 January 2023).
- Havers, F.P.; Delahoy, M.J.; Ujamaa, D.; Taylor, C.A.; Cummings, C.; Anglin, O.; Holstein, R.; Milucky, J.; O’Halloran, A.; Patel, K.; et al. Comparison of influenza and COVID-19-associated hospitalizations among children < 18 years old in the United States-FluSurv-NET (October-April 2017-2021) and COVID-NET (October 2020-September 2021). Clin. Infect. Dis. 2022, ciac388. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Sharma, S.; Tasnim, N.; Agadi, K.; Asfeen, U.; Kanda, J. Vulnerability for Respiratory Infections in Asthma Patients: A Systematic Review. Cureus 2022, 14, 28839. [Google Scholar] [CrossRef]
- Homeschooling Statistics. ThinkImpact.com. 13 July 2022. Available online: https://www.thinkimpact.com/homeschooling-statistics/ (accessed on 23 December 2022).
- Structural Racism and Health Inequities in the USA: Evidence and… (n.d.). Available online: https://www.med.emory.edu/departments/human-genetics/dei/documents_images/documents/lancet_2017_structural-racism-and-health-inequities.pdf (accessed on 16 January 2023).
- Cavicchiolo, M.E.; Trevisanuto, D.; Lolli, E.; Mardegan, V.; Saieva, A.M.; Franchin, E.; Plebani, M.; Donato, D.; Baraldi, E. Universal screening of high-risk neonates, parents, and staff at a neonatal intensive care unit during the SARS-CoV-2 pandemic. Eur. J. Pediatr. 2020, 179, 1949–1955. [Google Scholar] [CrossRef]
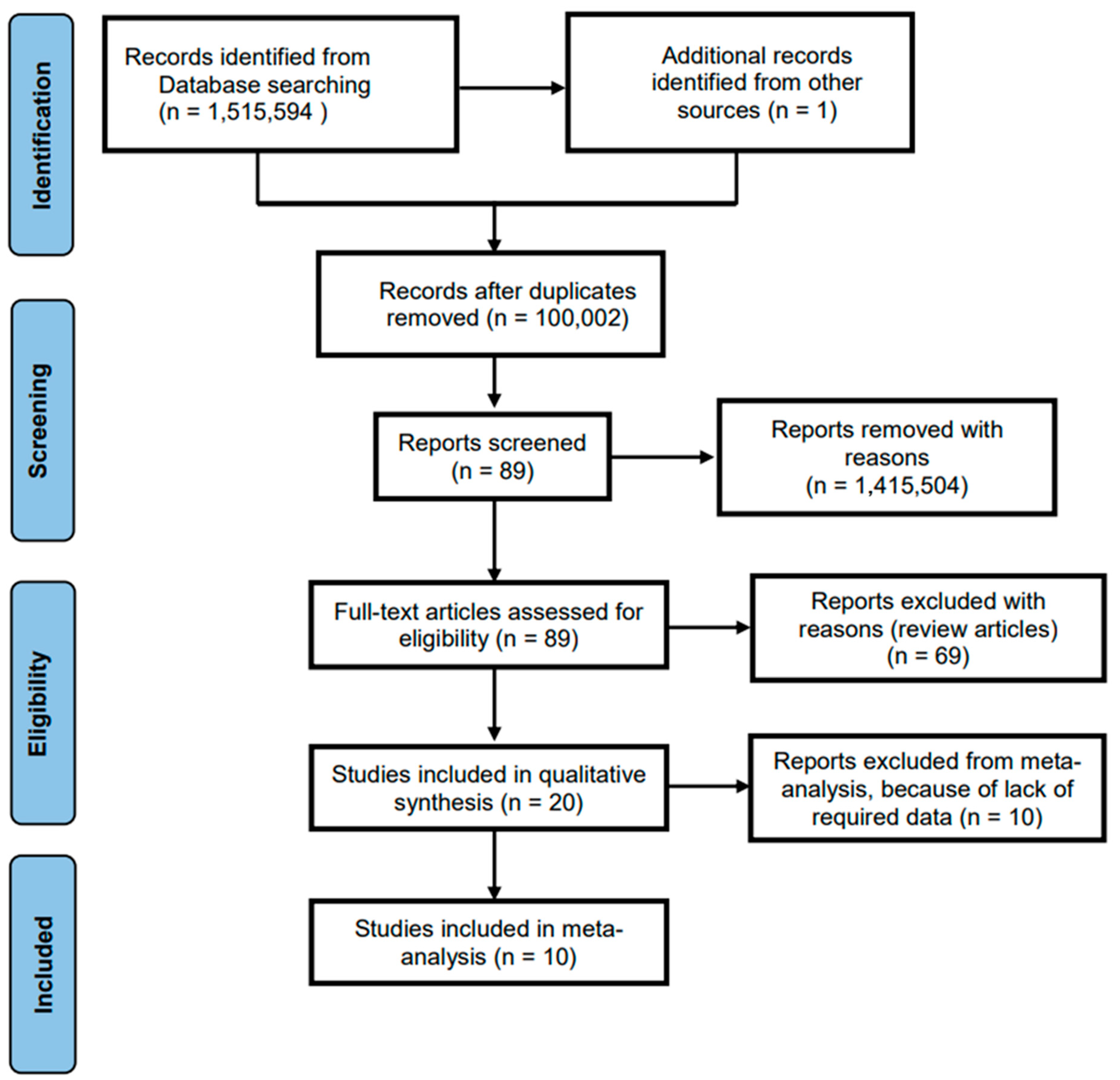
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Quantitative studies | Studies published in languages other than English |
| Human studies | Studies that only involved participants aged 18 and older |
| Scholarly papers | Review articles |
| Age group: 0–17 | Not human studies |
| Infectious respiratory diseases | Not in the United States |
| Research associated with racial disparities |
| Search Strategy | No. of Studies |
|---|---|
| Search terms used: ‘infectious diseases’ OR ‘communicable diseases’ OR ‘contagious diseases’ ‘ethnic differences’ OR ‘racial discrimination’ OR ‘children’ OR ‘kids’ | 1,515,595 |
| Total number of studies excluded based on eligibility criteria | 1,515,506 |
| Total number of studies excluded because either they were review articles or they did not provide full articles | 69 |
| Total number of studies accepted and reviewed | 20 |
| Author [Ref] | Country of Study | Type of Study | Major Findings | Racial Disparities Present | Quality Appraisal (Out of 4) |
|---|---|---|---|---|---|
| Perez et al., 2022 [13] | United States | Cohort | n = 51,441; 16,582 (32.3%) Black or African American children and 13,771 (26.8%) Hispanic children entered clinical settings with acute respiratory viruses, such as the influenza virus, respiratory syncytial virus, human metapneumovirus (HMPV), human parainfluenza (HPIVs), or SARS-CoV-2 (COVID-19), from 2016–2021. | Yes | 4 (Excellent) |
| Marks et al., 2022 [14] | United States | Cohort | n = 2562; 719 (26.7%) Black or African Americans, 710 (28.8%) Hispanics, and 154 Asian Americans (6.0%) between the ages of 0 and 4 were hospitalized for SARS-CoV-2 (COVID-19) during the periods dominated by the pre-delta, delta, and omicron variants in 14 states from March 2020 to February 2022. | Yes | 4 (Excellent) |
| Shi et al., 2022 [15] | United States | Cohort | n = 2100; 463 (21.8%) Hispanics and 736 (35.8%) Blacks or African Americans were hospitalized for SARS-CoV-2 (COVID-19) in 14 states from 1 July to 31 December 2021. | Yes | 4 (Excellent) |
| Anesi et al., 2022 [16] | United States | Cohort | n = 176; 60 (34.1%) Hispanics and 32 (18.2%) Blacks or African Americans <6 months were hospitalized with SARS-CoV-2 (COVID-19) in 17 states from July 2021 to January 2022. | Yes | 4 (Excellent) |
| Shi et al., 2022 [17] | United States | Cohort | n = 204; 117 Blacks or African Americans and 115 (30.6%) Hispanics were hospitalized for SARS-CoV-2 (COVID-19) in 14 states from January 2021 to March 2021. | Yes | 4 (Excellent) |
| Levorson et al., 2021 [18] | United States | Cross-sectional | n = 207 (Hispanics); Hispanic children (26.8%, 55/207) had an especially high prevalence rate of SARS-CoV-2 (COVID-19). | Yes | 4 (Excellent) |
| Lee et al., 2020 [19] | United States | Cohort | n = 223; Black or African American children constitute 22.2% of the NYC population. However, 19.9% of COVID-19 hospitalizations occurred among Black patients younger than 20; 75/223 (34%) patients in this study with COVID-19 associated multisystem inflammatory syndrome were Black. | Yes | 3 (Good) |
| Kurup et al., 2022 [20] | United States | Cross-sectional | n = 1000; of 207 positive cases of COVID-19, 46.6% were Hispanic and 30.0% were Black. | Yes | 4 (Excellent) |
| Stierman et al., 2021 [21] | United States | Case–control | n = 1,090,302; 473,785 (43.5%) Hispanics and 107,470 (9.9%) Blacks or African Americans were diagnosed with COVID-19 in 31 states. | Yes | 4 (Excellent) |
| Zambrano et al., 2022 [22] | United States | Case–control | n = 241; Black or African American children had a higher likelihood of severe COVID-19 outcomes. | Yes | 4 (Excellent) |
| Hobbs et al., 2022 [23] | United States | Cohort | n = 74; 49 (66%) Blacks or African Americans were suddenly hospitalized for COVID-19 from March 2020 to February 2021 at the University of Mississippi Medical Center in Jackson, MS. | Yes | 4 (Excellent) |
| Mody et al., 2020 [24] | United States | Cohort | n = 934,929; Blacks had a higher likelihood of testing positive for COVID-19. | Yes | 4 (Excellent) |
| Hamdan et al., 2021 [25] | United States | Cohort | n = 356; Whites had a higher rate of early-onset Group B streptococcus disease; Blacks had a higher rate of late-onset Group B streptococcus. | Yes/No | 4 (Excellent) |
| Graff et al., 2021 [26] | United States | Cohort | n = 454; 23 Blacks or African Americans and 248 Hispanics tested positive for SARS-CoV-2 (COVID-19) according to admission status. | Yes | 4 (Excellent) |
| Mannheim et al., 2021 [27] | United States | Case–control | n = 1, 302; 324 (25%) Blacks or African Americans and 695 (53%) Hispanics/Latinos tested positive for COVID-19; both Blacks or African Americans and Hispanics/ Latinos have a higher likelihood of being infected with COVID-19 than non-minority racial groups. | Yes | 4 (Excellent) |
| Brandt et al., 2021 [28] | United States | Cohort | n = 295,642; n = 19,408 (Under 18); 6.04% of children within the study population tested positive for COVID-19. Black and Hispanic children accounted for 60–70% of COVID-19 cases among children younger than 18 years of age. | Yes | 4 (Excellent) |
| Gualandi et al., 2018 [29] | United States | Cohort | n = 45,550; Black children had higher rates methicillin-resistant Staphylococcus aureus infections than White children. | Yes | 4 (Excellent) |
| Hansen et al., 2022 [30] | United States | Cross-sectional | n = 16,106; Black children under 12 years of age had signigicantly higher rates of rhinovirus than non-minority racial groups. | Yes | 3 (Good) |
| O’Halloran et al., 2021 [31] | United States | Cross-sectional | n = 15,114; Black, American Indian or Alaska Native, and Asian or Pacific Islanders had higher rates of ICU admission and hospitalization from influenza than non-minority racial groups. | Yes | 4 (Excellent) |
| Artiga and Hill, 2021 [32] | United States | Cohort | n = 2658; American Indian or Alaska Native, Native Hawaiian and other Pacific Islander, and Hispanic children experienced the highest rates of COVID-19 infection. American Indian or Alaska Native, Hispanic, Native Hawaiian and other Pacific Islander, and Black children were two or three times more likely to be hospitalized due to a COVID-19 infection than White children; death rates among American Indian or Alaska Native and Black children were 3.5 or 2.7 times higher than among White children. | Yes | 4 (Excellent) |
| Authors/Studies | Comments |
|---|---|
| Anesi et al. 2022 [16] | Self-reported data for a few participants were included in this analysis, a few infants may have been misclassified due to maternal vaccination status, or the mothers’ recollection of complete COVID-19 vaccination may be imperfect, as reported by the author as limitations. The small sample sizes mentioned by the authors resulted in wide confidence intervals. |
| Levorson et al. 2021 [18] | The regional population representativeness may have been affected by selection bias because the methodology focused on self-referral, and subjects in the study had blood labs completed for other clinical purposes. The authors made adjustments for these factors. |
| Shi et al. 2022 [15] | As mentioned by the authors, COVID-19 hospitalizations may have been overlooked because of testing practices and availability. Detailed clinical data are limited for the period during which the omicron variant was predominant (19–31 December 2021). The data do not include the peak of hospitalizations during this period; the delta variant was prevalent in late December. |
| Perez et al. 2022 [13] | New Vaccine Surveillance Network data are limited to enrolled consenting participants, who may not be representative of all children receiving health care, as mentioned by the authors as limitations. |
| Stierman et al. 2021 [21] | The proportion of positive SARS-CoV-2 test results recorded in drive-through clinics increased during the COVID-19 pandemic, as reported in the paper. This study focused on counties with complete ethnicity and race data. The results may not be generalizable to larger populations, as suggested by the authors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, E.A.K.; Mitra, A.K.; Malone, S. Racial Disparities and Common Respiratory Infectious Diseases in Children of the United States: A Systematic Review and Meta-Analysis. Diseases 2023, 11, 23. https://doi.org/10.3390/diseases11010023
Jones EAK, Mitra AK, Malone S. Racial Disparities and Common Respiratory Infectious Diseases in Children of the United States: A Systematic Review and Meta-Analysis. Diseases. 2023; 11(1):23. https://doi.org/10.3390/diseases11010023
Chicago/Turabian StyleJones, Elizabeth A. K., Amal K. Mitra, and Shelia Malone. 2023. "Racial Disparities and Common Respiratory Infectious Diseases in Children of the United States: A Systematic Review and Meta-Analysis" Diseases 11, no. 1: 23. https://doi.org/10.3390/diseases11010023
APA StyleJones, E. A. K., Mitra, A. K., & Malone, S. (2023). Racial Disparities and Common Respiratory Infectious Diseases in Children of the United States: A Systematic Review and Meta-Analysis. Diseases, 11(1), 23. https://doi.org/10.3390/diseases11010023








