Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report
Abstract
:1. Introduction
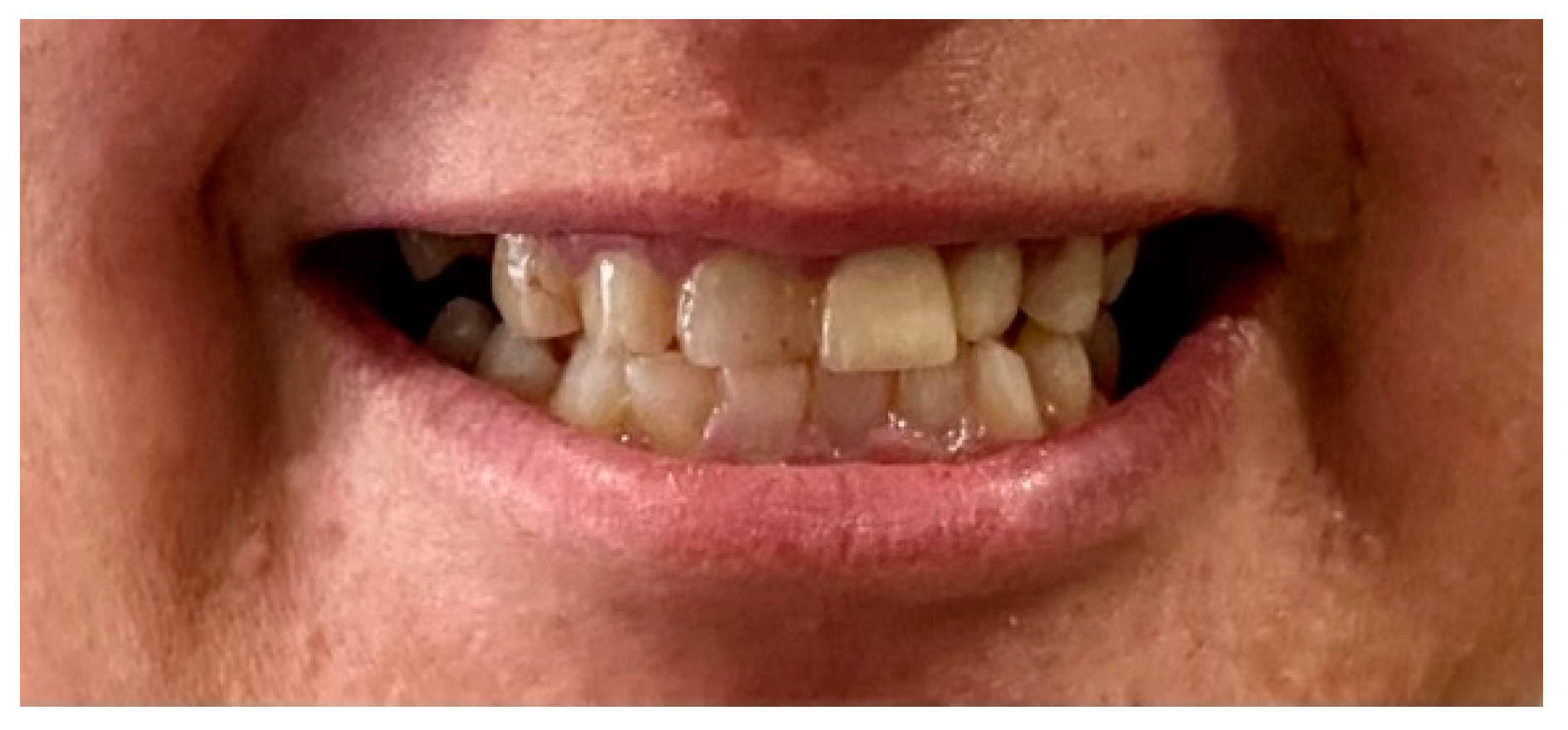
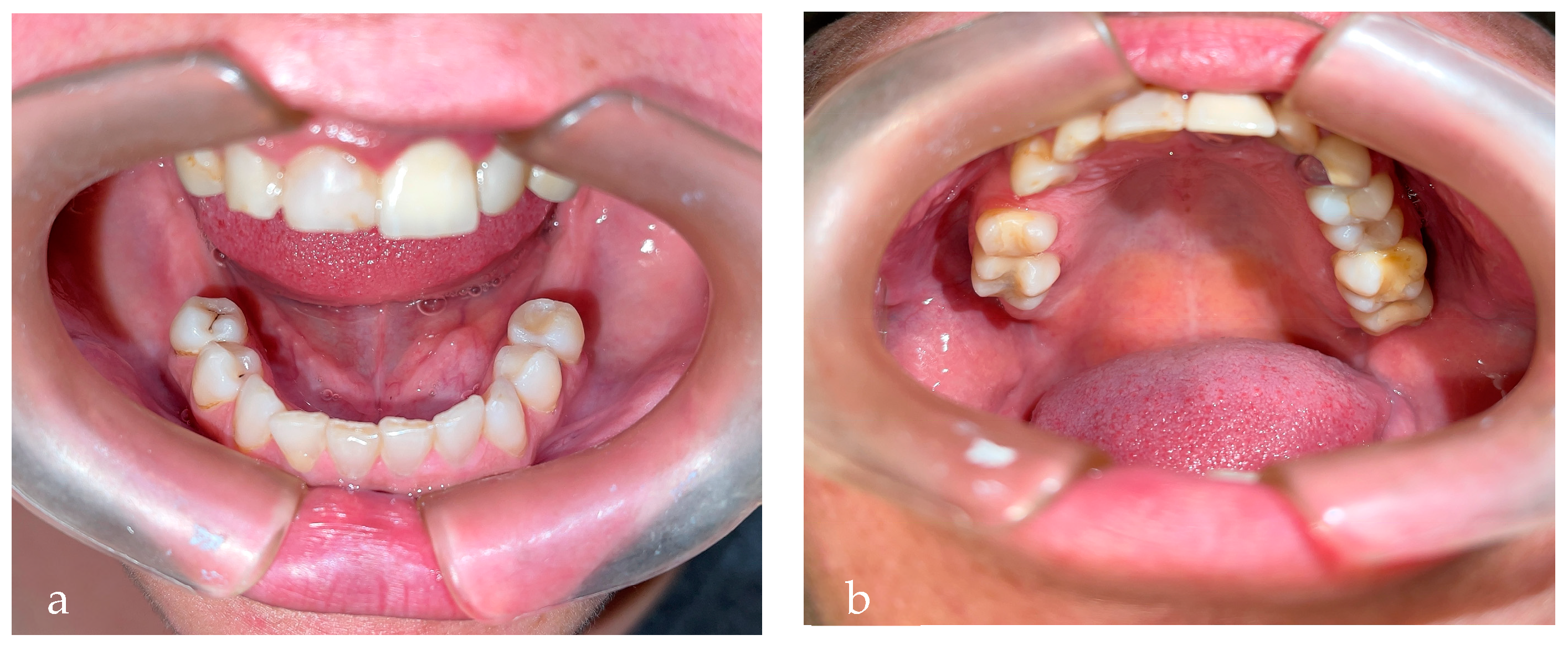
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, J.M. Multiple sclerosis: Diagnosis, differential diagnosis, and clinical presentation. Handb. Clin. Neurol. 2014, 122, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Sesenna, G.; Calzolari, C.; Gruppi, M.P.; Ciardi, G. Walking with UAN.GO Exoskeleton: Training and Compliance in a Multiple Sclerosis Patient. Neurol. Int. 2021, 13, 428–438. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Savic, D.; Lavrnja, I. Multiple Sclerosis and Neuroinflammation: The Overview of Current and Prospective Therapies. Curr. Pharm. Des. 2017, 23, 693–730. [Google Scholar] [CrossRef]
- Gentile, M.T.; Muto, G.; Lus, G.; Lövblad, K.-O.; Svenningsen, Å.F.; Colucci-D’Amato, L. Angiogenesis and Multiple Sclerosis Pathogenesis: A Glance at New Pharmaceutical Approaches. J. Clin. Med. 2022, 11, 4643. [Google Scholar] [CrossRef]
- Chemaly, D.; Lefrançois, A.; Pérusse, R. Oral and Maxillofacial Manifestations of Multiple Sclerosis. J. Can. Dent. Assoc. 2000, 66, 600–605. [Google Scholar]
- Fischer, D.J.; Epstein, J.B.; Klasser, G. Multiple sclerosis: An update for oral health care providers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2009, 108, 318–327. [Google Scholar] [CrossRef]
- Sheu, J.J.; Lin, H.C. Association between multiple sclerosis and chronic periodontitis: A population-based pilot study. Eur. J. Neurol. 2013, 20, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kovac, Z.; Uhac, I.; Bukovi, D.; Cabov, T.; Kovacevi, D.; Grzi, R. Oral health status and temporomandibular disorders in multiple sclerosis patients. Coll. Antropol. 2005, 29, 441–444. [Google Scholar] [PubMed]
- Manchery, N.; Henry, J.D.; Nangle, M.R. A systematic review of oral health in people with multiple sclerosis. Community Dent. Oral Epidemiol. 2020, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, N.; Pateman, K.; Taing, M.W.; Pradhan, A.; Ford, P.J. Managing the oral side effects of medications used to treat multiple sclerosis. Aust. Dent. J. 2017, 62, 331–336. [Google Scholar] [CrossRef]
- Covello, F.; Ruoppolo, G.; Carissimo, C.; Zumbo, G.; Ferrara, C.; Polimeni, A.; Vozza, I. Multiple Sclerosis: Impact on Oral Hygiene, Dysphagia, and Quality of Life. Int. J. Environ. Res. Public Health 2020, 17, 3979. [Google Scholar] [CrossRef]
- Priyanka, K.; Sudhir, K.M.; Reddy, V.C.S.; Kumar, R.K.; Srinivasulu, G. Impact of Alcohol Dependency on Oral Health—A Cross-sectional Comparative Study. J. Clin. Diagn Res. 2017, 11, ZC43–ZC46. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; He, B.; Huang, R.; Li, M. Effect of tobacco on periodontal disease and oral cancer. Tob. Induc. Dis. 2019, 9, 17–40. [Google Scholar] [CrossRef]
- Labuz-Roszakm, B.; Niewiadomska, E.; Starostka-Tatar, A.; Kubicka-Bączyk, K.; Krzystanek, E.; Arkuszewski, M.; Tyrpień-Golder, K.; Rybus-Kalinowska, B.; Pierzchała, B.; Pierzchała, K. Multiple sclerosis: Oral health, behaviours and limitations of daily oral hygiene—A questionnaire study. Pol. J. Neurol. Neurosurg. 2019, 53, 271–276. [Google Scholar] [CrossRef]
- Speight, P.M.; Kaul, A.; Melsom, R.D. Measurement of whole unstimulated salivary flow in the diagnosis of Sjögren’s syndrome. Ann. Rheum. Dis. 1992, 51, 499–502. [Google Scholar] [CrossRef]
- Pearce, J.M.S. Historical descriptions of multiple sclerosis. Eur. Neurol. 2005, 54, 49–53. [Google Scholar] [CrossRef]
- Patriarca, L.; Torlone, S.; Ferrari, F.; Di Carmine, C.; Totaro, R.; di Cesare, E.; Splendiani, A. Is size an essential criterion to define tumefactive plaque? MR features and clinical correlation in multiple sclerosis. Neuroradiol. J. 2016, 29, 384–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar] [PubMed]
- Dimitroulis, G. Management of temporomandibular joint disorders: A surgeon’s perspective. Aust. Dent. J. 2018, 63, S79–S90. [Google Scholar] [CrossRef] [PubMed]
- Sexton, C.; Lalloo, R.; Stormon, N.; Pateman, K.; van der Mei Campbell, J.; Ford, P. Oral health and behaviours of people living with Multiple Sclerosis in Australia. Community Dent. Oral Epidemiol. 2019, 47, 201–209. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Alves, H.H.C.; Alves, L.C.; Alves, N.C.; Siqueira de Andrade, C.M.; Finkelsztejn, A. Dental care in multiple sclerosis: An overlooked and under-assessed condition. J. Disabil. Oral Health 2010, 11, 53–56. [Google Scholar]
- Orthlieb, J.D.; Ré, J.P.; Jeany, M.; Giraudeau, A. Articulação temporo-mandibular, oclusão e bruxismo [articulação temporomandibular, oclusão e bruxismo]. Revue de Stomatologie Chirurgie Maxillo-faciale et de Chirurgie Orale 2016, 117, 207–211. [Google Scholar] [CrossRef]
- Williams, D.E.; Lynch, J.E.; Doshi, V.; Singh, G.D.; Hargens, A.R. Bruxism and temporal bone hypermobility in patients with multiple sclerosis. Cranio 2011, 29, 178–186. [Google Scholar] [CrossRef]
- Dionyssiotis, Y. Bone loss and fractures in multiple sclerosis: Focus on epidemiologic and physiopathological features. Int. J. Gen. Med. 2011, 4, 505–509. [Google Scholar] [CrossRef]
- Al-Ansari, A. Is there an association between multiple sclerosis and oral health? Evid. Based Dent. 2021, 22, 44–45. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef]
- Toth, C. Pregabalin: Latest safety evidence and clinical implications for the management of neuropathic pain. Ther. Adv. Drug Saf. 2014, 5, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Catalán, M.A.; Peña-Munzenmayer, G.; Melvin, J.E. Ca2+-dependent K+ Channels in Exocrine Salivary Glands. Cell Calcium 2014, 55, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Teare, J.P.; Spedding, C.; Whitehead, M.W.; Greenfield, S.M.; Challacombe, S.J.; Thompson, R.P.H. Omeprazole and Dry Mouth. Scand. J. Gastroenterol. 1995, 30, 216–218. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Meng, Y. Oral and craniofacial manifestations of multiple sclerosis: Implications for the oral health care provider. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4610–4620. [Google Scholar] [PubMed]
- Feys, P.; Giovannoni, G.; Dijsselbloem, N.; Elen, P.; Andersen, L. The importance of a multi-disciplinary perspective and patient activation programmes in MS. Mult Scler. 2016, 22 (Suppl. S2), 34–46. [Google Scholar] [CrossRef]
- Wallin, M.T.; Campea, S.; Haselkorn, J.K. Multidisciplinary Management of a Patient with Multiple Sclerosis. Fed. Pract. 2015, 32 (Suppl. 3), 14S–17S. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Santiago, H.; Pereira, S.; Castro, A.R.; Soares, S.C. Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases 2022, 10, 62. https://doi.org/10.3390/diseases10030062
Costa C, Santiago H, Pereira S, Castro AR, Soares SC. Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases. 2022; 10(3):62. https://doi.org/10.3390/diseases10030062
Chicago/Turabian StyleCosta, Céu, Hugo Santiago, Sofia Pereira, Ana Rita Castro, and Sandra Clara Soares. 2022. "Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report" Diseases 10, no. 3: 62. https://doi.org/10.3390/diseases10030062
APA StyleCosta, C., Santiago, H., Pereira, S., Castro, A. R., & Soares, S. C. (2022). Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases, 10(3), 62. https://doi.org/10.3390/diseases10030062







