A Development Study of a New Bi-directional Solenoid Actuator for Active Locomotion Capsule Robots
Abstract
1. Introduction
2. New Bi-directional Locomotion CR
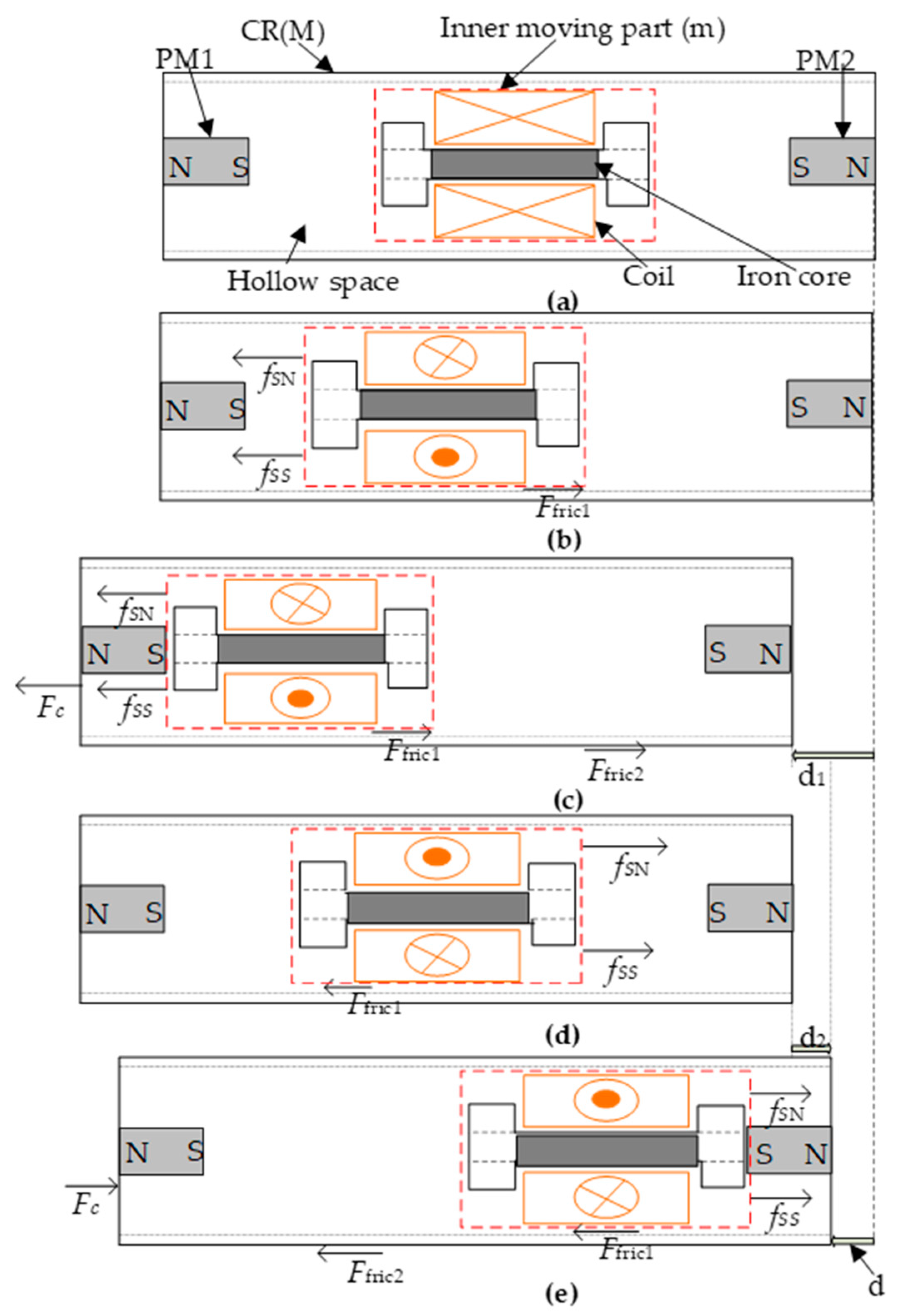
2.1. Mechanical Structure and Components
2.2. Active Actuation Principle
- Step 1. The coil is originally located at the center of the CR (Figure 2a), and meanwhile the supplied current to the coil is zero. Since a balanced force is acting on the coil by the two PMs, the coil as well as the CR body remain still.
- Step 2. When a positive rectangular shape current is supplied to the small coil, both the attractive force fSN between PM1 and the energized coil and the repelling force fSS between PM2 and the coil are in the same direction. The total force overcomes the frictional force Ffric1 and drives the small coil to move towards PM1 (Figure 2b). During this process, the reaction force acting on the CR body is insufficient to overcome the external frictional force between the CR body and its contact surface, and therefore the CR remains still as shown in Figure 2b.
- Step 3. Since the small coil is kept accelerated when moving towards PM1, it slams into PM1 with a large velocity. As a consequence, the instantaneous collision force Fc, which is generated by the collision action, acts on the CR body and drives the CR to move forward, overcoming the external frictional force Ffric2 (Figure 2c). Finally, the CR as well as the inner mass stop at a new position and thus the forward displacement d1 of the CR is achieved (Figure 2c).
- Step 4. Upon reversing the current direction, and comparison with the forces described in Figure 2b, the directions of the interaction forces between the small coil and the two inserted PMs as well as the friction force Ffric1 are reversed, as illustrated in Figure 2d. Consequently, the locomotion direction of the small coil is also reversed.
- Step 5. Similar to the descripted procedure in step 3, the opposite collision force then drives the CR to move backward and stop at another new position. The backward displacement of the CR in this process is denoted as d2 (Figure 2e), which is smaller than d1 since the negative current waveform is chosen to be sinusoidal instead of rectangular. Therefore, the net CR displacement in one cycle can be depicted as the displacement difference between its forward and backward displacements, namely:d = d1 − d2,
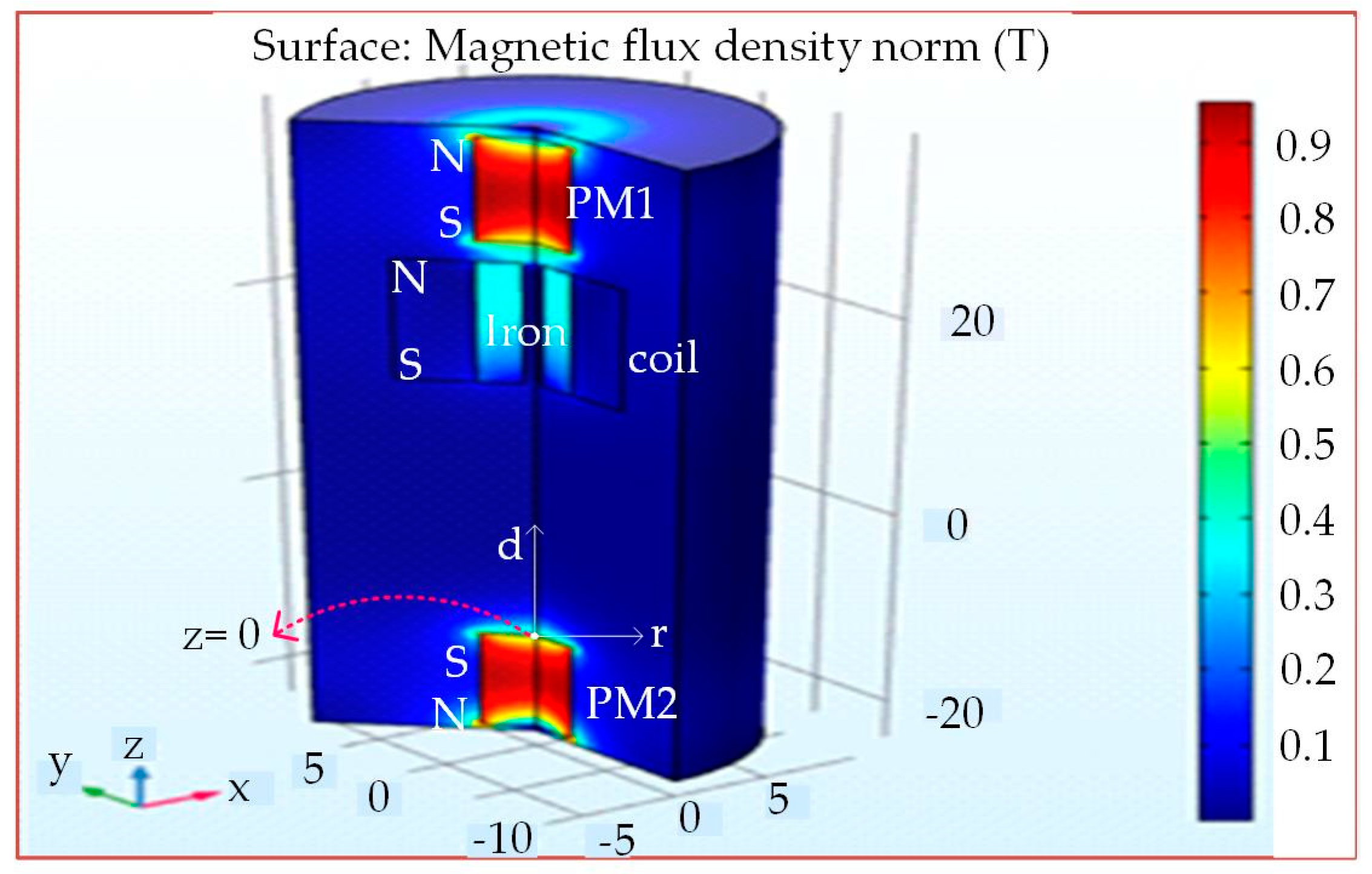
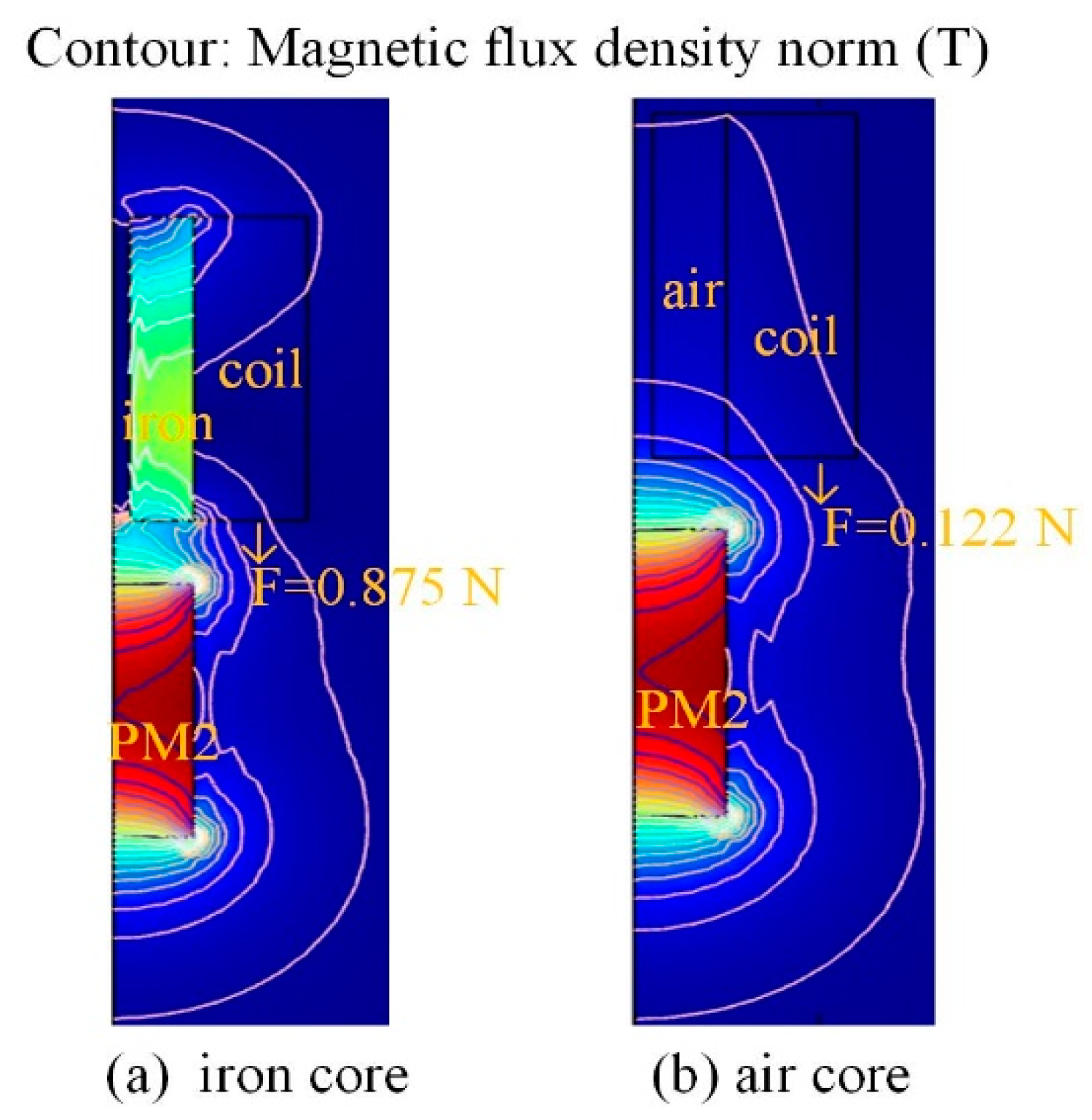
3. Finite Element Modelling and Analysis
4. Experimental Setup and Results
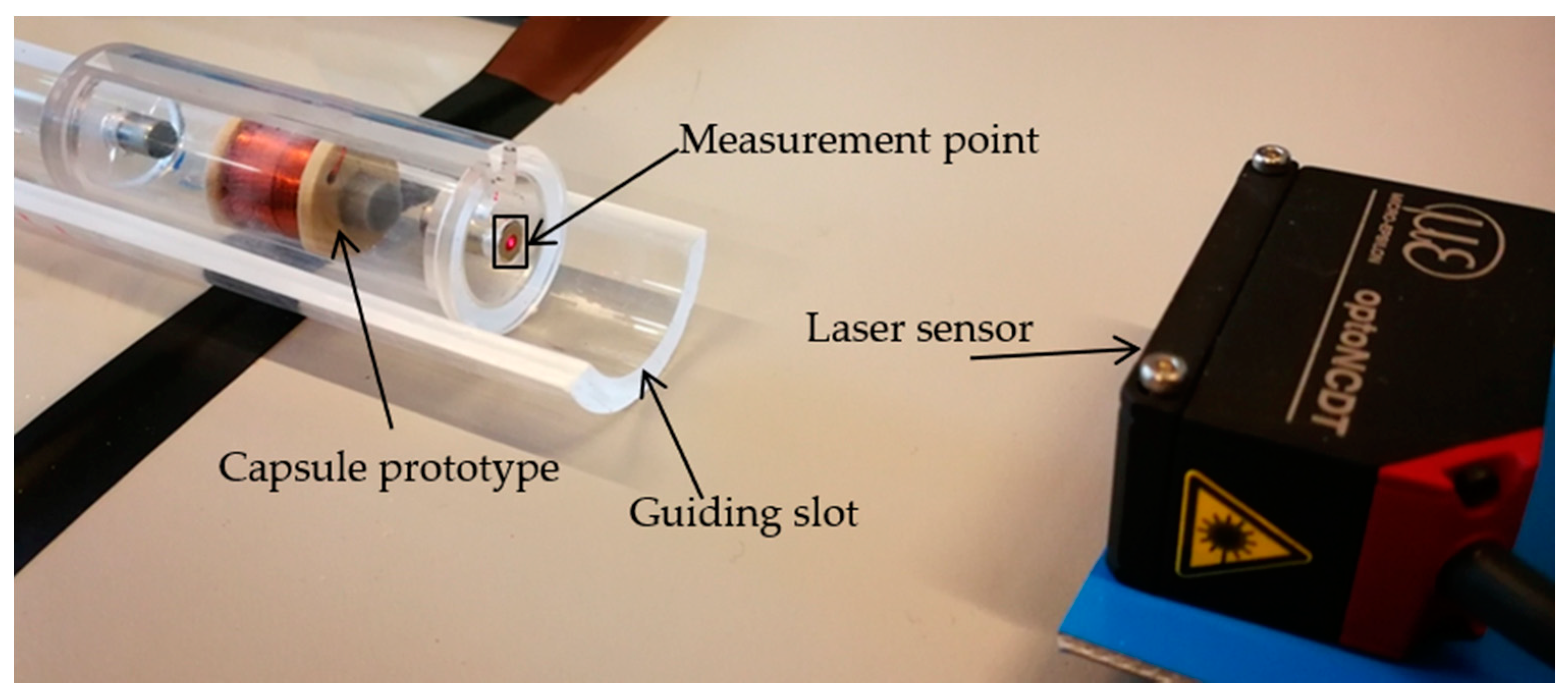
4.1. Experimental Setup
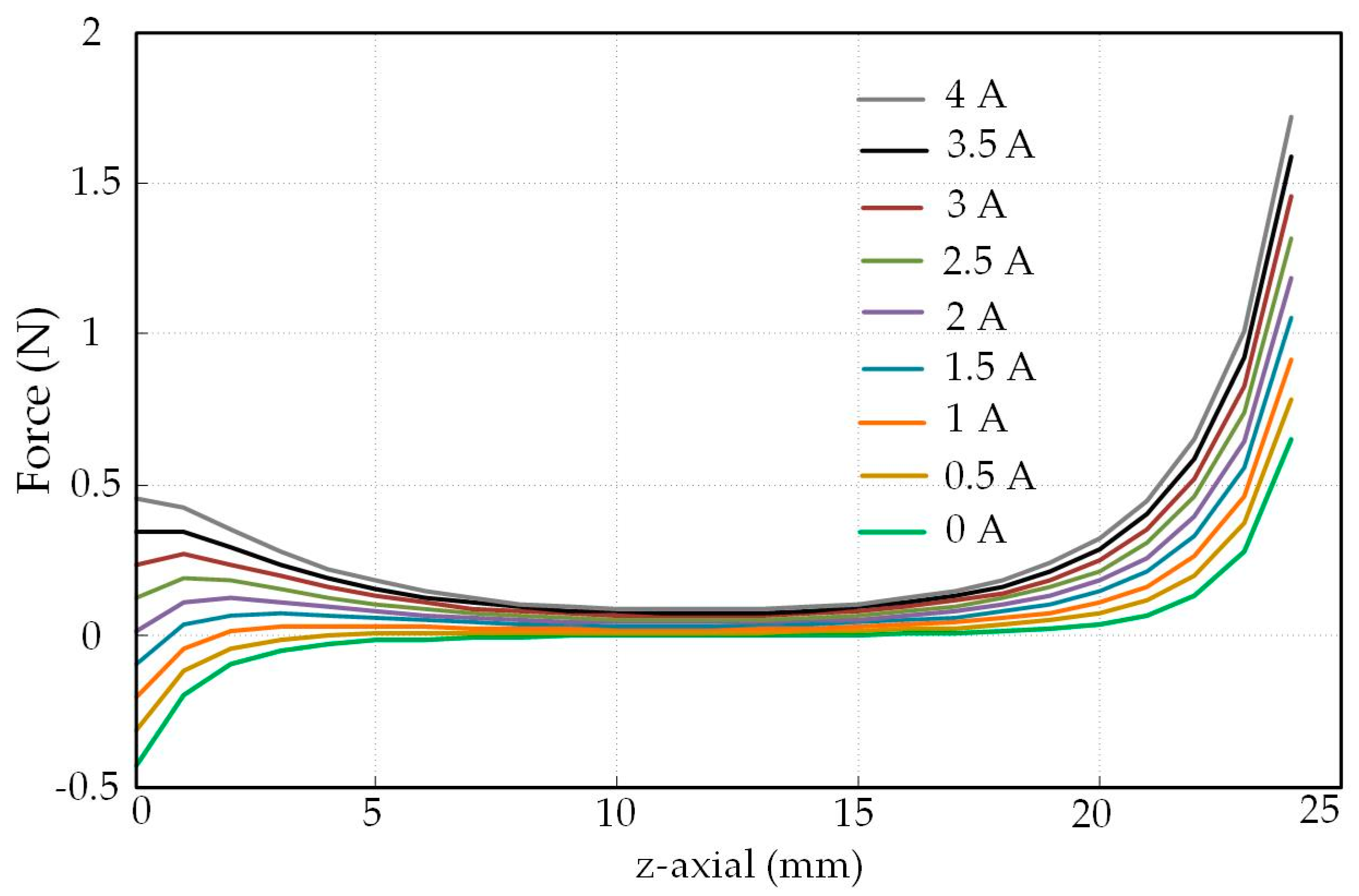
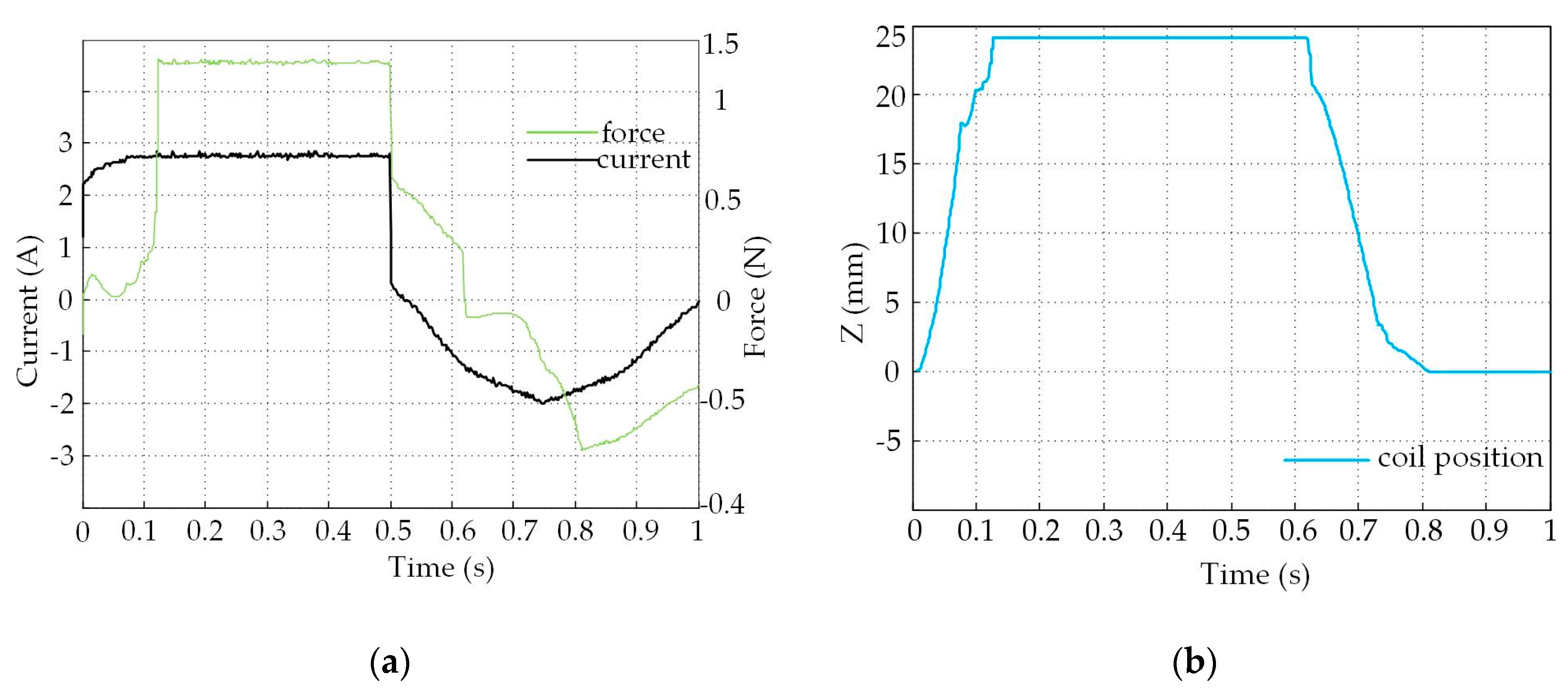
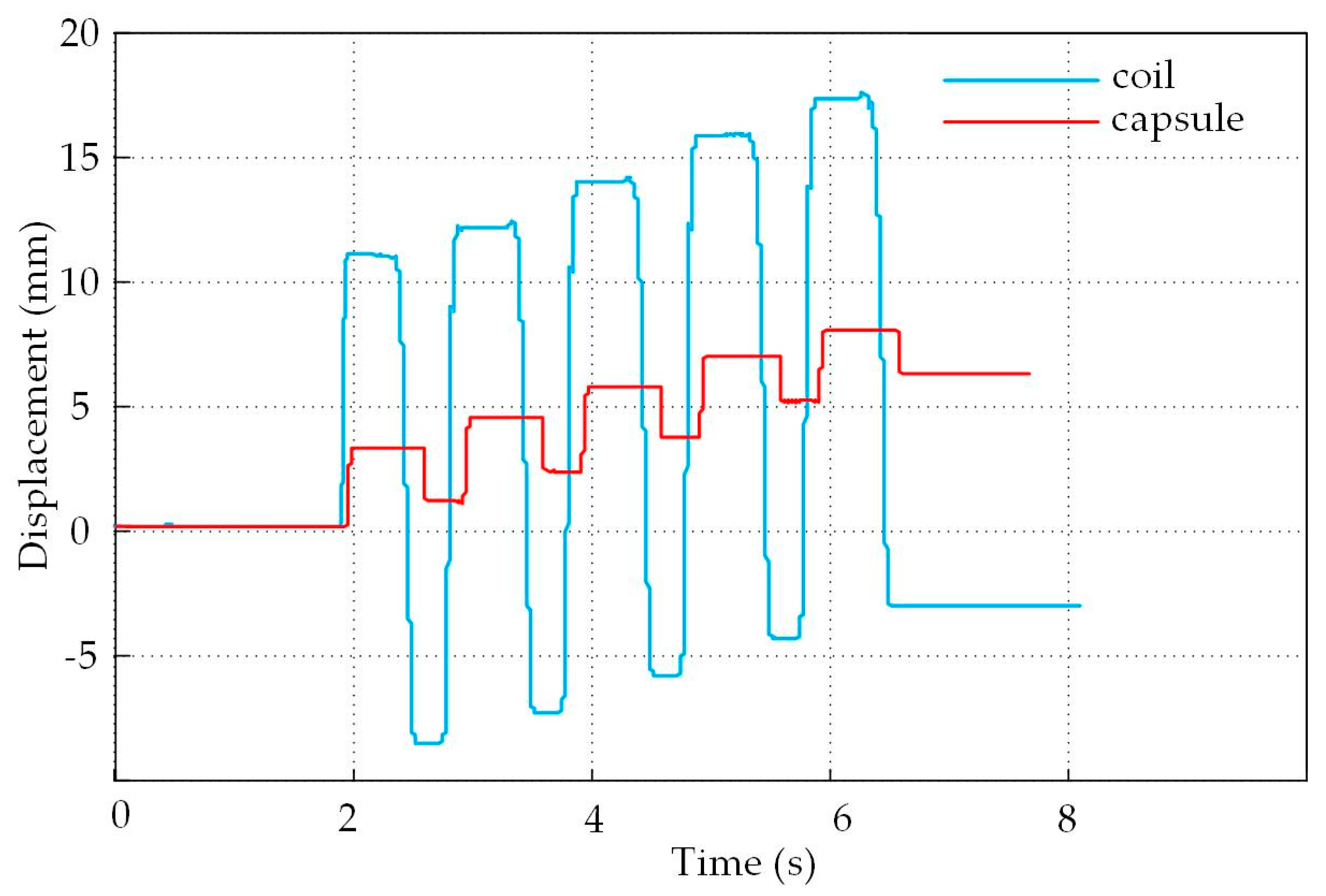
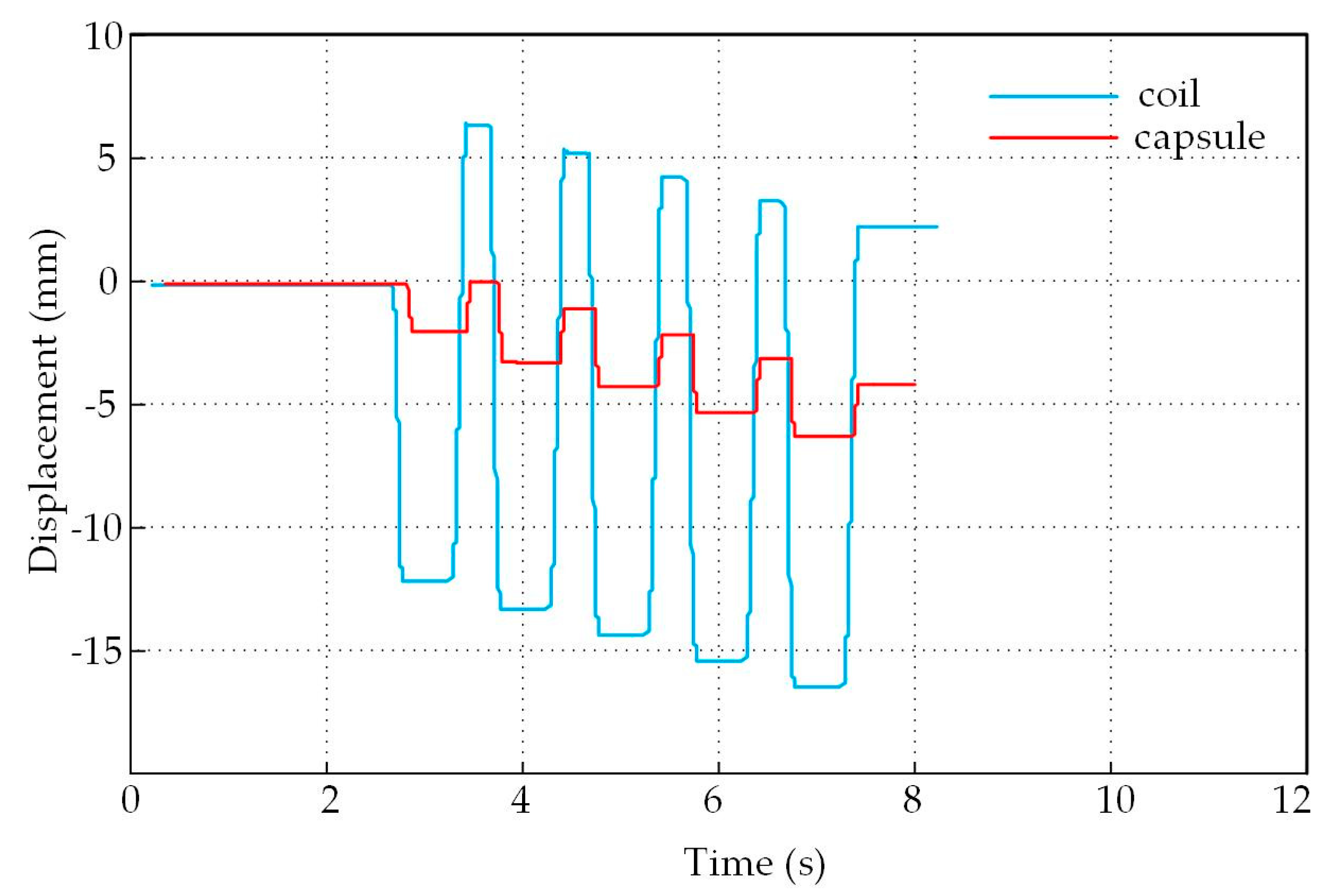
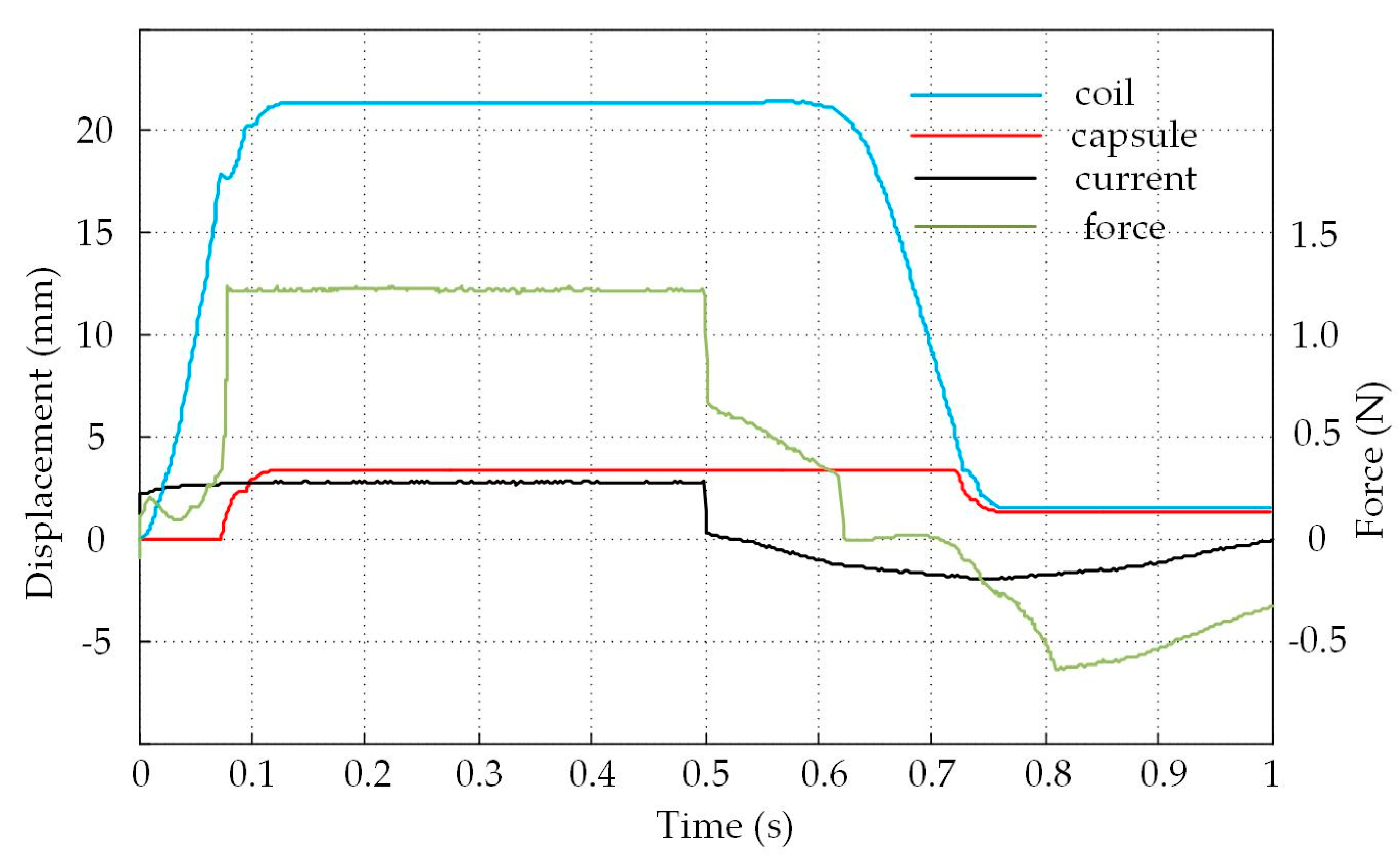
4.2. Experimental Results and Analysis
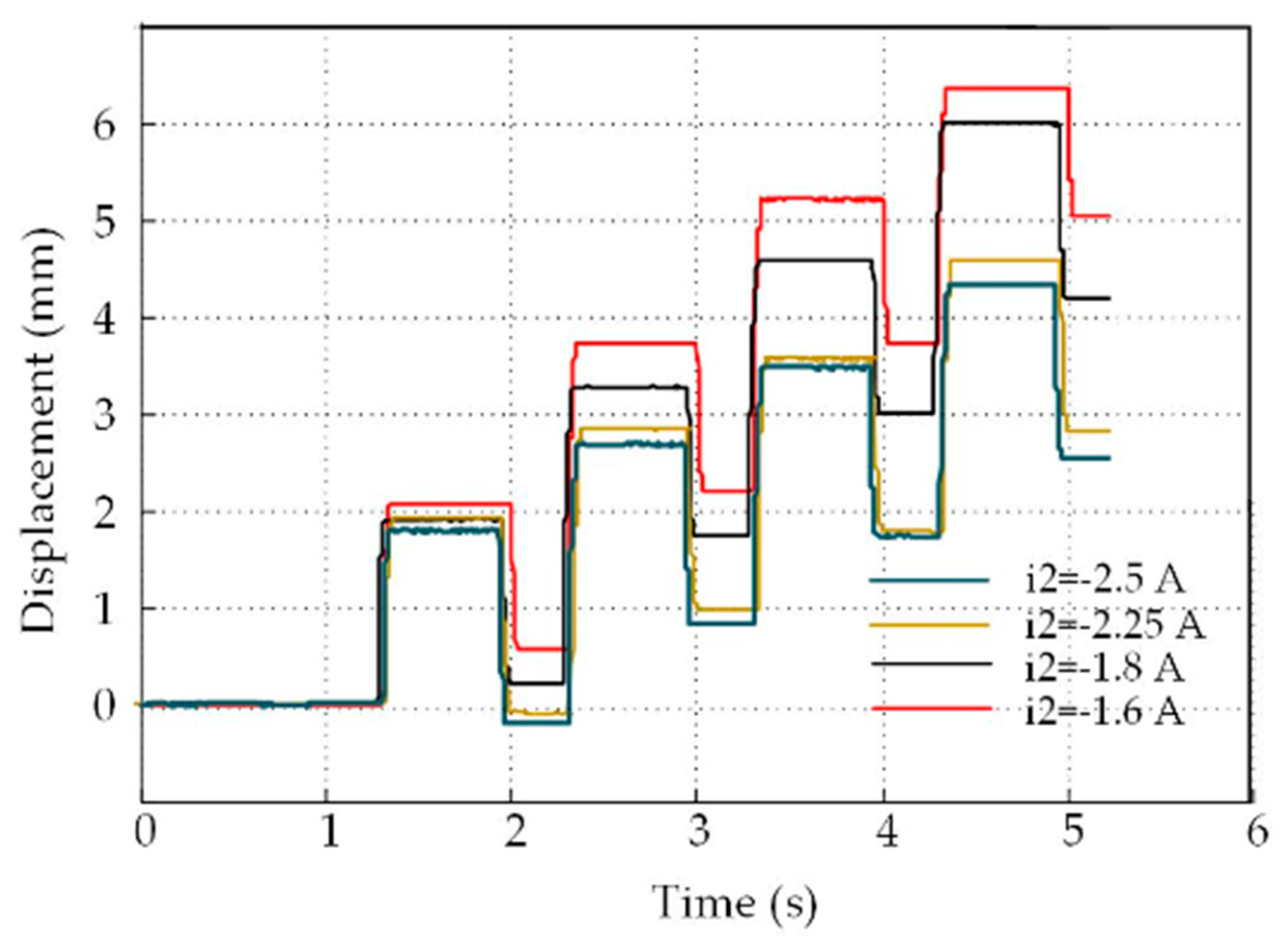
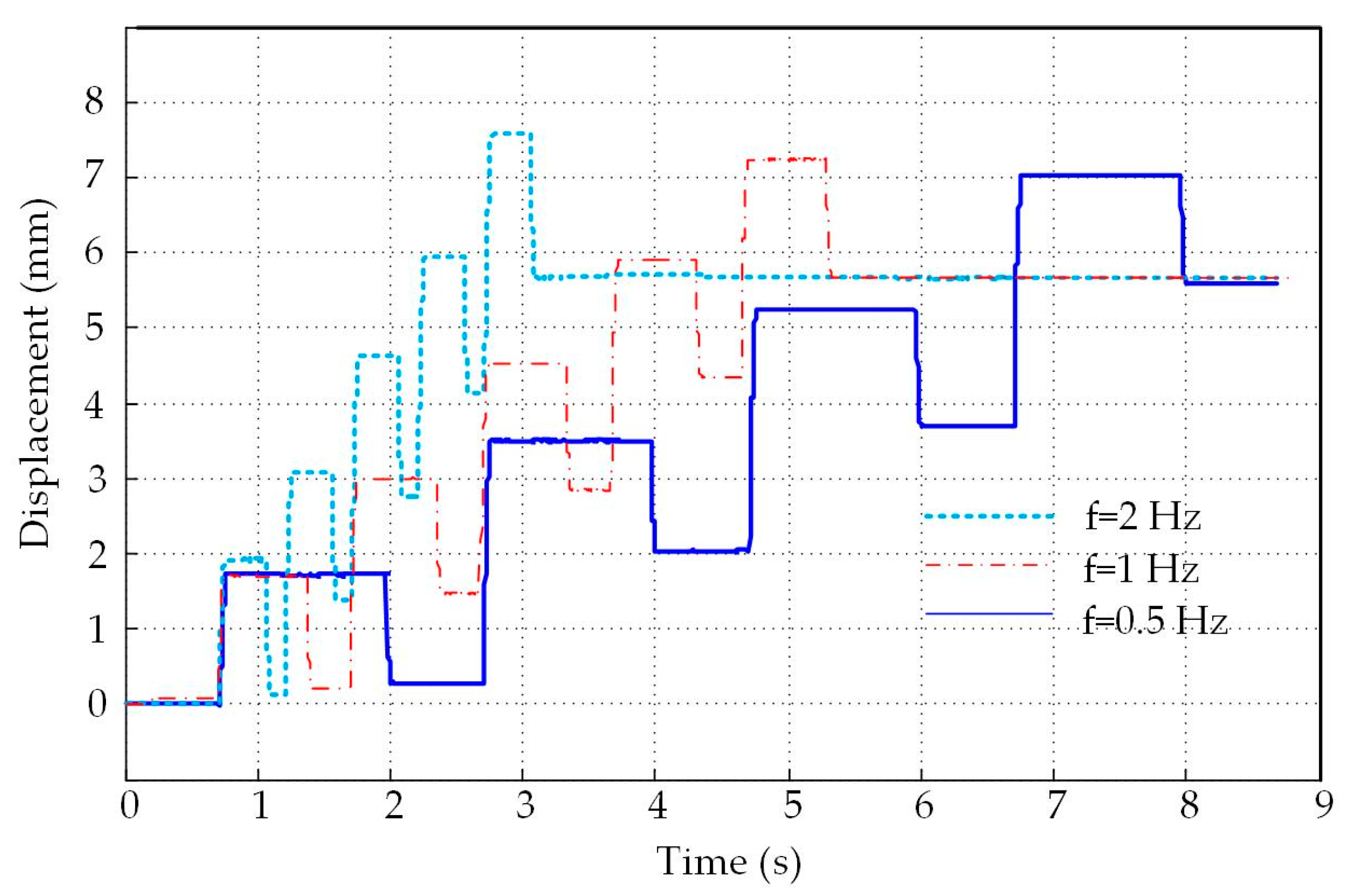
4.3. Analysis of The Displacement and Speed Control Abilities
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Basar, R.; Ahmad, Y.; Cho, J.; Ibrahim, F. An Improved Wearable Resonant Wireless Power Transfer System for Biomedical Capsule Endoscope. IEEE Trans. Ind. Electron. 2018, 65, 7772–7781. [Google Scholar] [CrossRef]
- Ciuti, G.; Menciassi, A.; Dario, P. Capsule endoscopy: From current achievements to open challenges. IEEE Rev. Biomed. Eng. 2011, 4, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Hu, C.; Chen, Z.; Zhang, H.; Liu, S. Design and fabrication of a magnetic propulsion system for self-propelled capsule endoscope. IEEE Trans. Biomed. Eng. 2010, 57, 2891–2902. [Google Scholar] [PubMed]
- Liu, L.; Towfighian, S.; Hila, A. A review of locomotion systems for capsule endoscopy. IEEE Rev. Biomed. Eng. 2015, 8, 138–151. [Google Scholar] [CrossRef]
- Kim, B.; Park, S.; Park, J. Microrobots for a capsule endoscope. In Proceedings of the IEEE/ASME 2009 International Conference on Advanced Intelligent Mechatronics, Singapore, 14–17 July 2009; pp. 729–734. [Google Scholar]
- Rondonotti, E.; Herrerias, J.M.; Pennazio, M.; Caunedo, A.; Mascarenhas-Saraiva, M.; de Franchis, R. Complications, limitations, and failures of capsule endoscopy: A review of 733 cases. Gastrointest. Endosc. 2005, 62, 712–716. [Google Scholar] [CrossRef]
- Meng Max, Q.H.; Mei, T.; Pu, J.; Hu, C.; Wang, X.; Chan, Y. Wireless robotic capsule endoscopy: State-of-the-art and challenges. In Proceedings of the Fifth World Congress on Intelligent Control and Automation, Hangzhou, China, 5–19 June 2004; pp. 5561–5565. [Google Scholar]
- Sliker, L.J.; Ciuti, G. Flexible and capsule endoscopy for screening, diagnosis and treatment. Expert Rev. Med. Devices 2014, 11, 649–666. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, N.; Yoon, E.S.; Cho, I.J. MEMS devices for drug delivery. Adv. Drug Deliv. Rev. 2018, 128, 132–147. [Google Scholar] [CrossRef]
- Le, H.; Do, T.; Phee, S. A survey on actuators-driven surgical robots. Sens. Actuators A Phys. 2016, 247, 323–354. [Google Scholar] [CrossRef]
- Catherine, J.; Rotinat-Libersa, C.; Micaelli, A. Comparative review of endoscopic devices articulations technologies developed for minimally invasive medical procedures. Appl. Bionics Biomech. 2011, 8, 151–171. [Google Scholar] [CrossRef]
- Cisse, C.; Zaki, W.; Ben Zineb, T. A review of constitutive models and modelling techniques for shape memory alloys. Int. J. Plast. 2016, 76, 244–284. [Google Scholar] [CrossRef]
- De Greef, A.; Lambert, P.; Delchambre, A. Towards flexible medical instruments: Review of flexible fluidic actuators. Precis. Eng. 2009, 33, 311–321. [Google Scholar] [CrossRef]
- Sliker, L.; Ciuti, G.; Rentschler, M.; Menciassi, A. Magnetically driven medical devices: A review. Expert Rev. Med. Devices 2015, 12, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guo, Y.; Peng, C. Review of the active locomotion system for capsule endoscope. J. Biomed. Eng. 2010, 27, 215–218. [Google Scholar]
- Do, T.; Seah, T.; Yu, H.; Phee, S. Development and Testing of a Magnetically Actuated Capsule Endoscopy for Obesity Treatment. PLoS ONE 2016, 11, e0151711. [Google Scholar]
- Do, T.; Ho, K.; Phee, S. A Magnetic Soft Endoscopic Capsule-Inflated Intragastric Balloon for Weight Management. Sci. Rep. 2016, 6, 39486. [Google Scholar] [CrossRef]
- Son, D.; Dogan, M.; Sitti, M. Magnetically actuated soft capsule endoscope for fine-needle aspiration biopsy. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; pp. 1132–1139. [Google Scholar]
- Valdastri, P.; Webster, R.; Quaglia, C.; Quirini, M.; Menciassi, A.; Dario, P. A New Mechanism for Mesoscale Legged Locomotion in Compliant Tubular Environments. IEEE Trans. Robot. 2009, 25, 1047–1057. [Google Scholar] [CrossRef]
- Quirini, M.; Menciassi, A.; Scapellato, S.; Dario, P.; Rieber, F.; Ho, C.-N.; Schostek, S.; Schurr, M. Feasibility proof of a legged locomotion capsule for the GI tract. Gastrointest. Endos. 2008, 67, 1153–1158. [Google Scholar] [CrossRef]
- Carta, R.; Sfakiotakis, M.; Pateromichelakis, N.; Thone, J.; Tsakiris, D.P.; Puers, R. A multi-coil inductive powering system for an endoscopic capsule with vibratory actuation. Sens. Actuators A Phys. 2011, 172, 253–258. [Google Scholar] [CrossRef]
- Sliker, L.J.; Kern, M.D.; Rentschler, M.E. An automated traction measurement platform and empirical model for evaluation of rolling micropatterned wheels. IEEE/ASME Trans. Mechatron. 2015, 20, 1854–1862. [Google Scholar] [CrossRef]
- Sliker, L.; Kern, M.; Schoen, J.A.; Rentschler, M.E. Surgical evaluation of a novel tethered robotic capsule endoscope using micro-patterned treads. Surg. Endosc. 2012, 26, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, D.; Kim, B.; Lee, B.-I. A self-propelled robotic colonoscope using elastic caterpillars. In Proceedings of the 44th International Symposium on Robotics (ISR), Seoul, Korea, 24–26 October 2013; pp. 1–4. [Google Scholar]
- Kim, Y.T.; Kim, D.E. Novel propelling mechanisms based on frictional interaction for endoscope robot. Adv. Tribol. 2010, 53, 203–211. [Google Scholar] [CrossRef]
- Lin, W.; Shi, Y.; Jia, Z.; Yan, G. Design of a wireless anchoring and extending micro robot system for gastrointestinal tract. Int. J. Med. Robot. Comput. Assisted Surg. MRCAS 2013, 9, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yan, G.; Jiang, P.; Ye, D. A wireless robotic endoscope for gastrointestine. IEEE Trans. Robot. 2008, 24, 206–210. [Google Scholar] [CrossRef]
- Bernth, J.E.; Arezzo, A.; Liu, H. A novel robotic mesh-worm with segment-bending anchoring for colonoscopy. IEEE Robot. Autom. Lett. 2017, 2, 1718–1724. [Google Scholar] [CrossRef]
- Gao, J.; Yan, G. Locomotion analysis of an inchworm-like capsule robot in the intestinal tract. IEEE Trans. Biomed. Eng. 2016, 63, 300–310. [Google Scholar] [CrossRef]
- Zarrouk, D.; Sharf, I.; Shoham, M. Conditions for worm-robot locomotion in a flexible environment: Theory and experiments. IEEE Trans. Biomed. Eng. 2012, 59, 1057–1067. [Google Scholar] [CrossRef]
- Wang, X.; Meng, M.Q.H. An experimental study of resistant properties of the small intestine for an active capsule endoscope. Proc. Inst. Mech. Eng. 2010, 224, 107–118. [Google Scholar] [CrossRef]



| Parameter | Value | Unit |
|---|---|---|
| coil inner radius | 2 | mm |
| coil thickness | 3.5 | mm |
| coil length | 12 | mm |
| magnet radius | 2.5 | mm |
| magnet length | 10 | mm |
| magnet remanence | 1.074 | T |
| iron mass | 0.362 | g |
| iron diameter | 3 | mm |
| iron height | 12 | mm |
| inner mass (m) | 9.36 | g |
| total capsule mass (M) | 27.15 | g |
| capsule length | 60 | mm |
| capsule inner diameter | 12 | mm |
| capsule outer diameter | 14 | mm |
| Contact Material | Acrylic | Paper | Sandpaper |
|---|---|---|---|
| Net displacement (mm) | 0.58 | 0.49 | 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Lu, K. A Development Study of a New Bi-directional Solenoid Actuator for Active Locomotion Capsule Robots. Electronics 2020, 9, 736. https://doi.org/10.3390/electronics9050736
Wu L, Lu K. A Development Study of a New Bi-directional Solenoid Actuator for Active Locomotion Capsule Robots. Electronics. 2020; 9(5):736. https://doi.org/10.3390/electronics9050736
Chicago/Turabian StyleWu, Linlin, and Kaiyuan Lu. 2020. "A Development Study of a New Bi-directional Solenoid Actuator for Active Locomotion Capsule Robots" Electronics 9, no. 5: 736. https://doi.org/10.3390/electronics9050736
APA StyleWu, L., & Lu, K. (2020). A Development Study of a New Bi-directional Solenoid Actuator for Active Locomotion Capsule Robots. Electronics, 9(5), 736. https://doi.org/10.3390/electronics9050736






