Remote-Controlled Fully Implantable Neural Stimulator for Freely Moving Small Animal
Abstract
1. Introduction
2. Materials and Methods
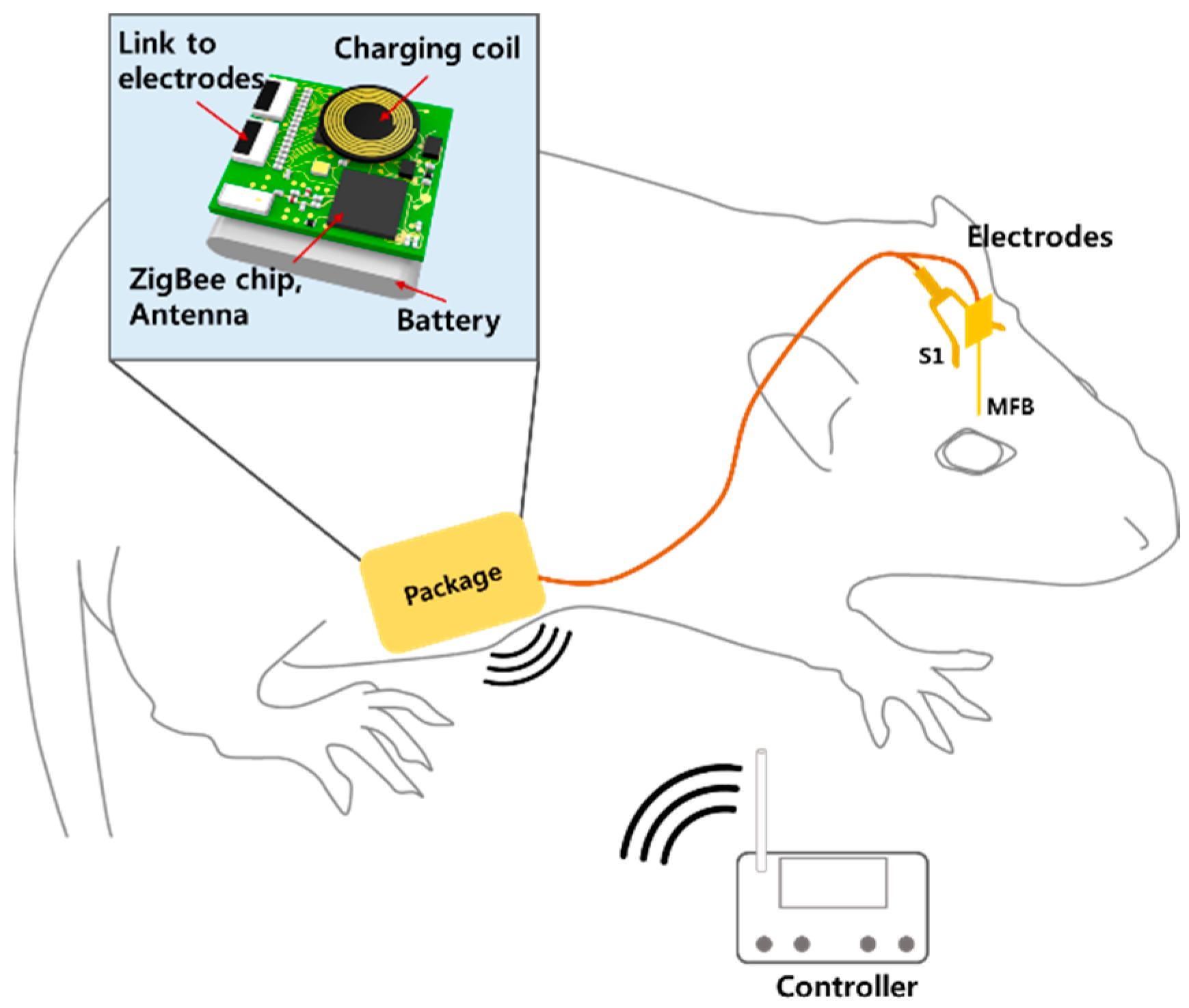
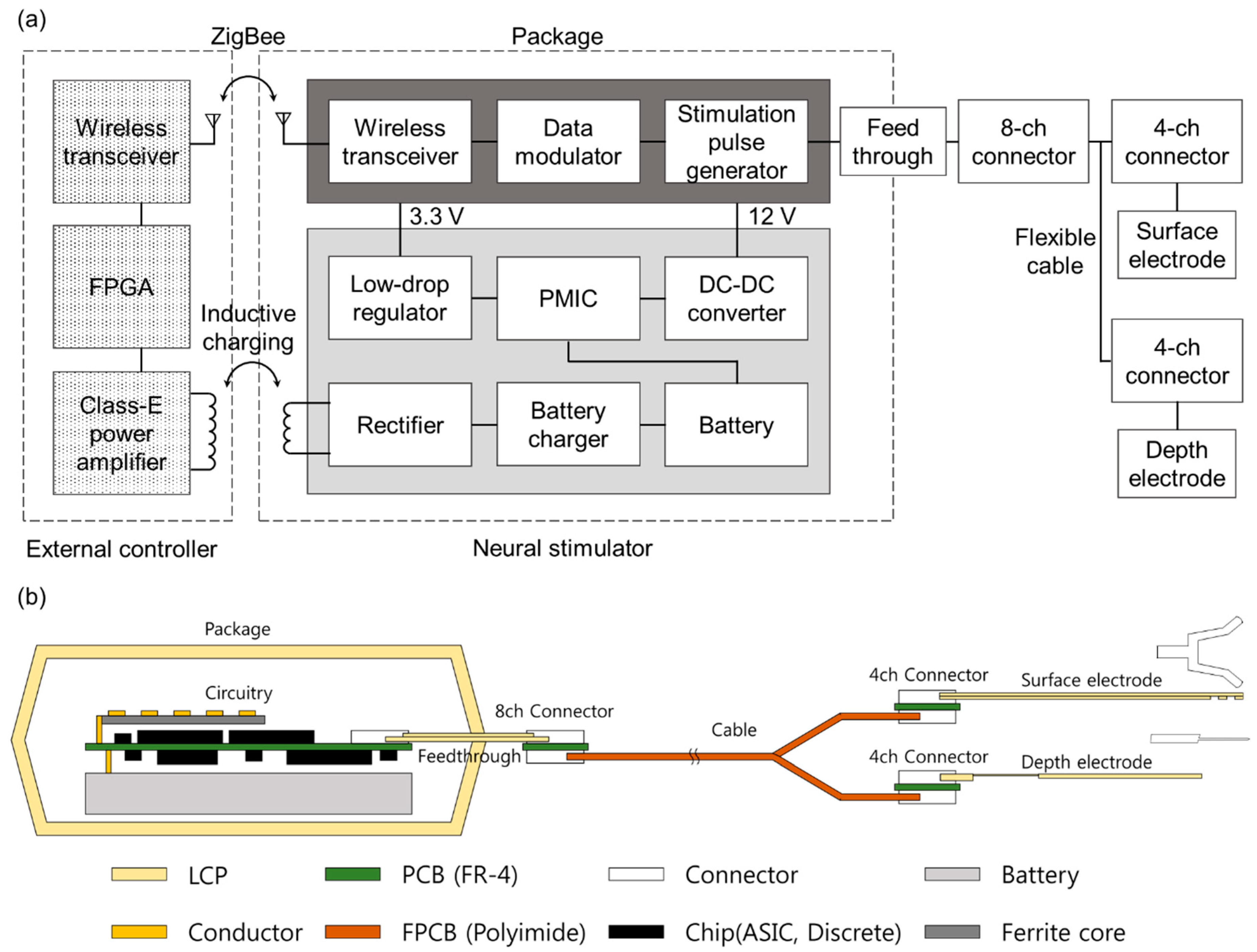
2.1. System Overview
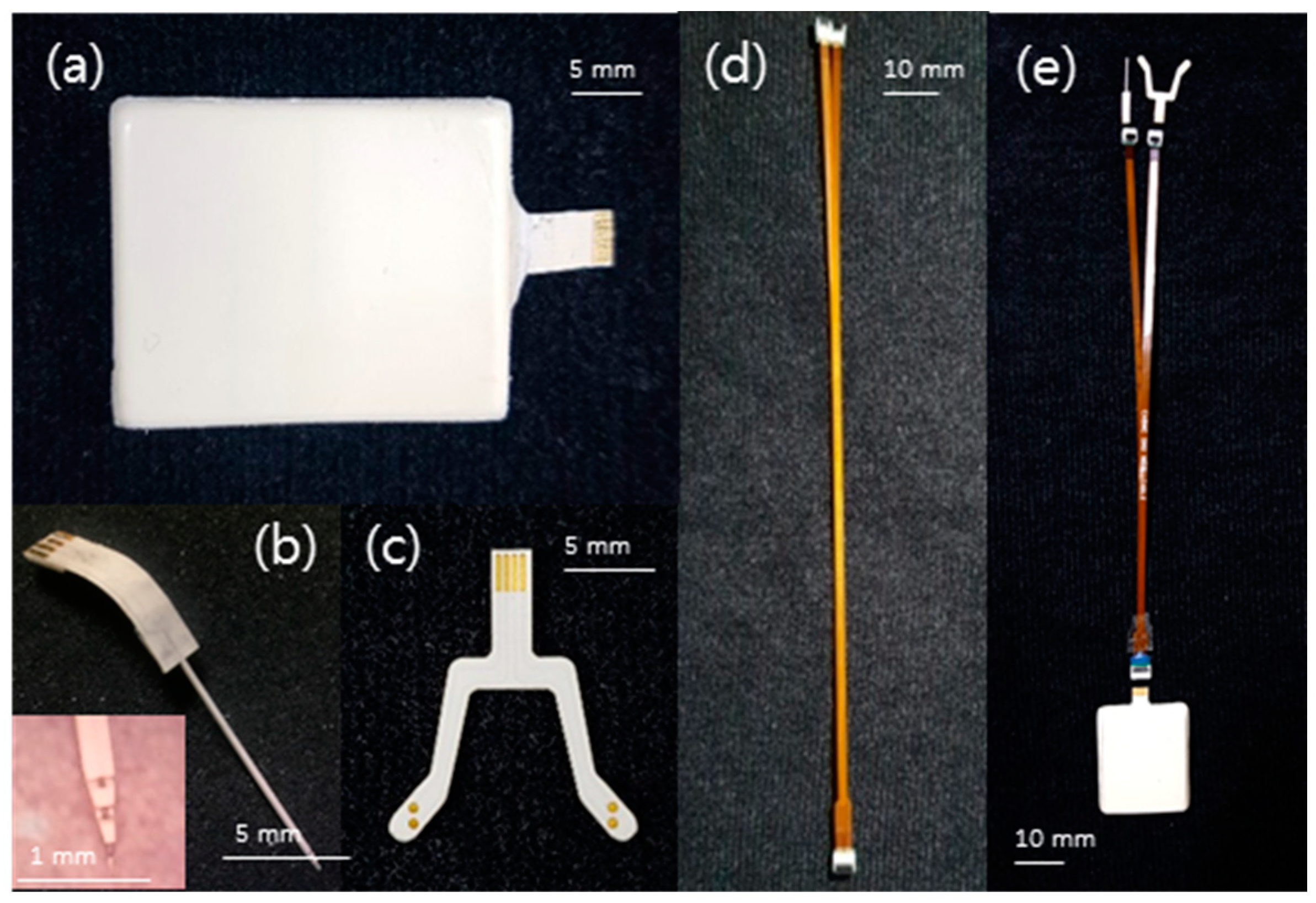
2.2. Fabrication
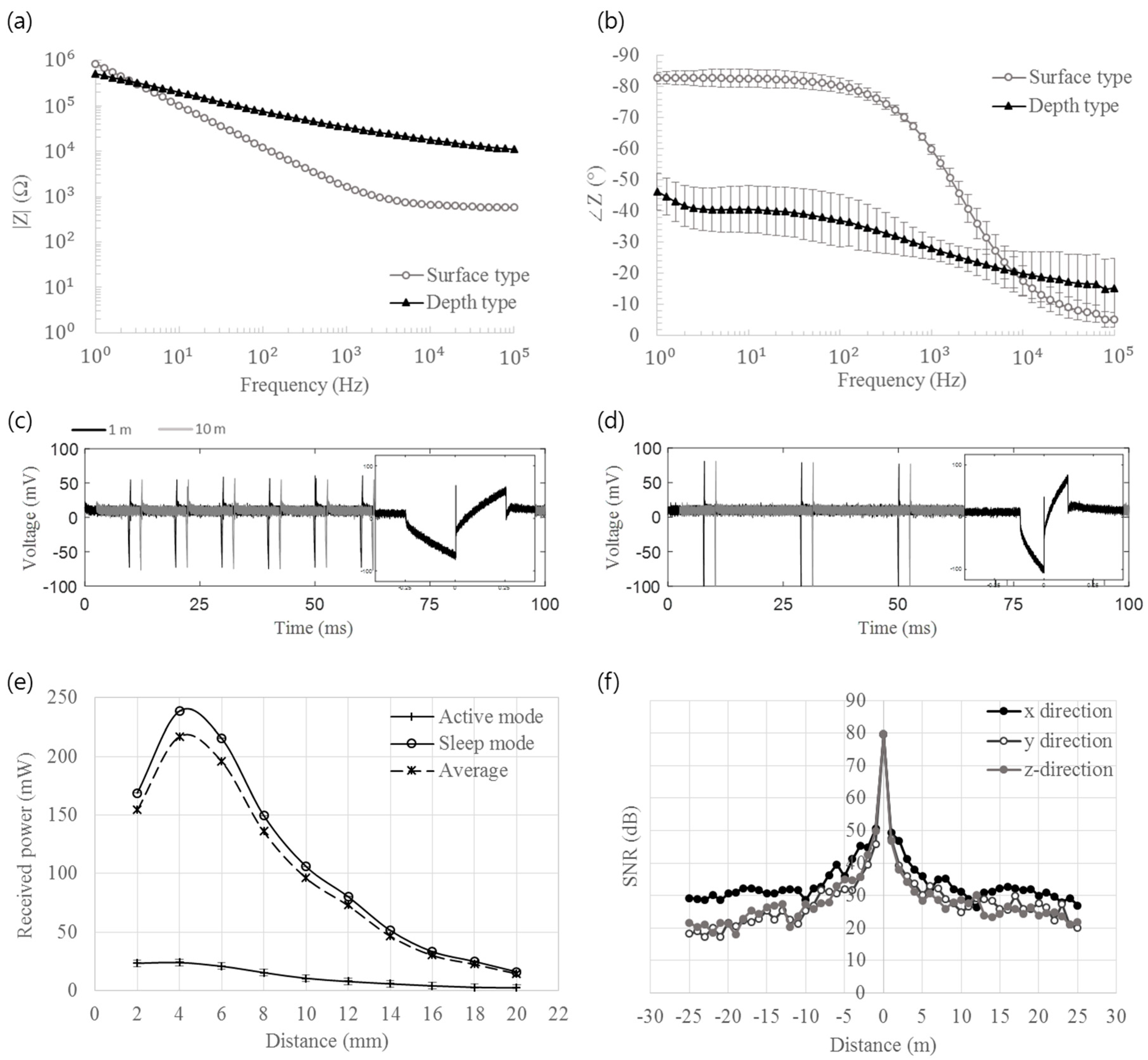
2.3. Bench Top Evaluation
2.4. In Vivo Evaluation
2.4.1. Animal Care
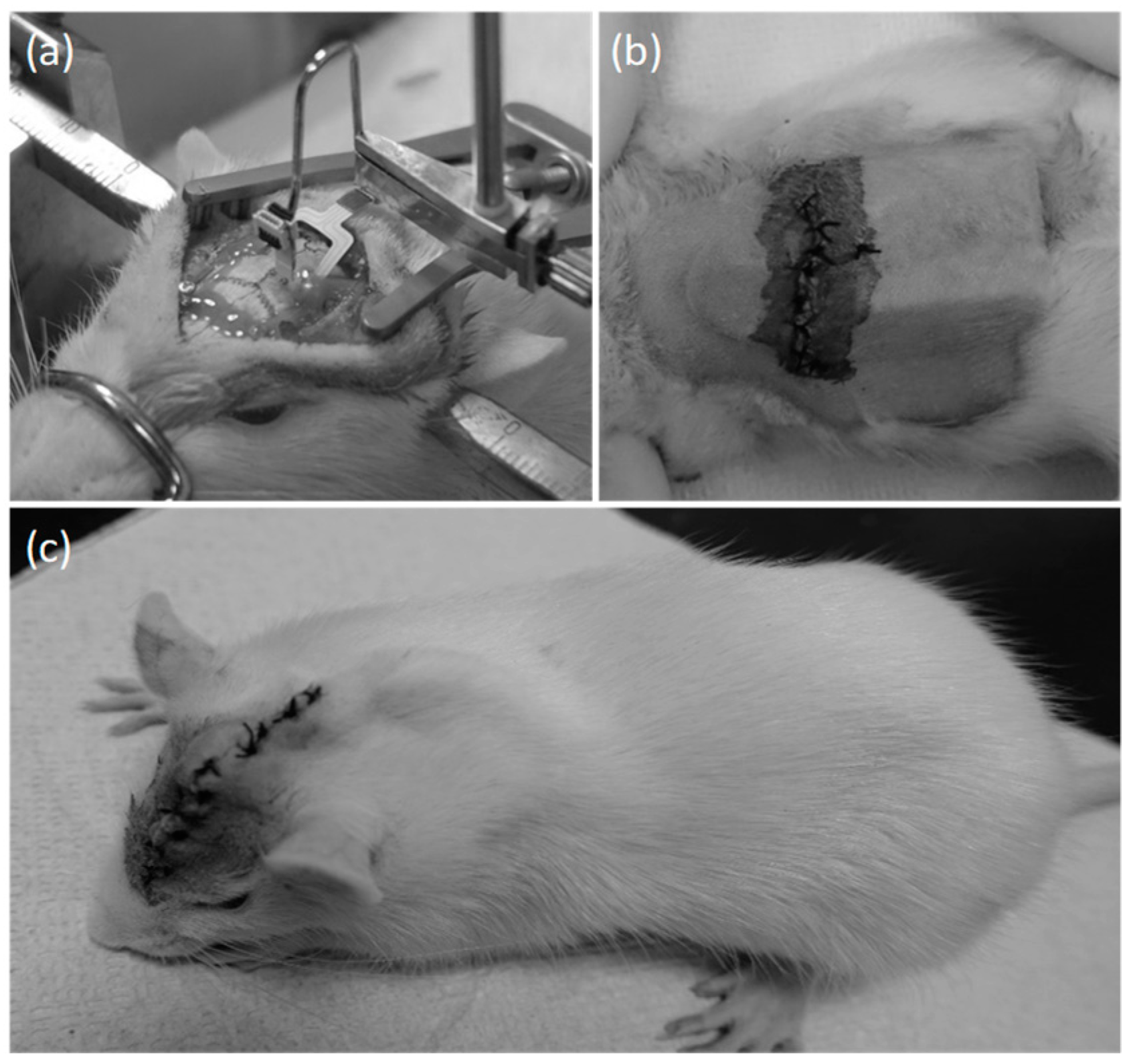
2.4.2. Surgical Procedure
2.4.3. Wired Test for Determination of the Stimulus Parameters
2.4.4. Wireless Stimulation and Training
2.4.5. Control Test
3. Results
3.1. Fabrication
3.2. Bench Top Evaluation
3.3. In Vivo Evaluation
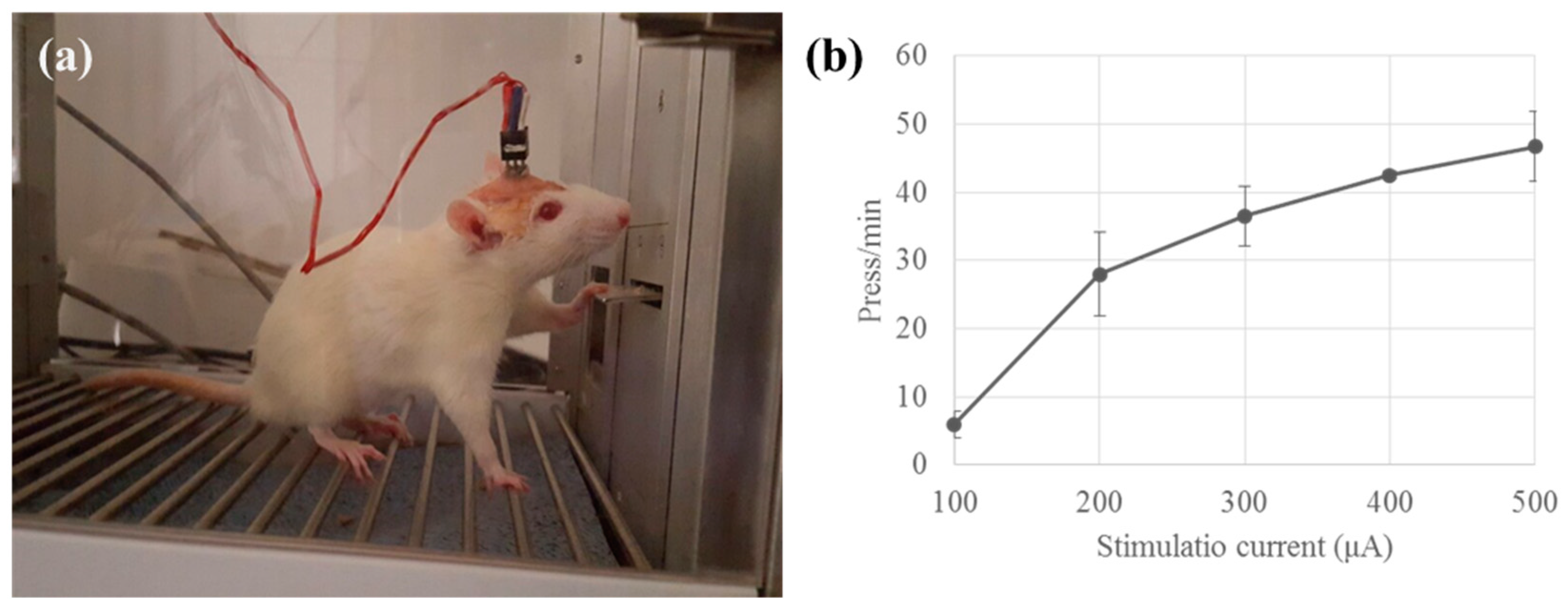
3.3.1. Wired Test for Determination of the Stimulus Parameters
3.3.2. Wireless Test in Freely Moving Condition
3.3.3. Control Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Birbaumer, N. Slow cortical potentials: Plasticity, operant control, and behavioral effects. Neuroscientist 1999, 5, 74–78. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Oliviero, A.; Dileone, M.; Saturno, E.; Mazzone, P.; Insola, A.; Profice, P.; Ranieri, F.; Capone, F.; et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: Direct recording of epidural activity in conscious humans. J. Neurophysiol. 2006, 96, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Moll, C.K.; Fried, I.; Ojemann, G.A. Invasive recordings from the human brain: Clinical insights and beyond. Nat. Rev. Neurosci. 2005, 6, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-G.; Jun, G.; Choi, H.-S.; Jang, H.S.; Bae, Y.C.; Suk, K.; Jang, I.-S.; Choi, B.-J. Operant conditioning of rat navigation using electrical stimulation for directional cues and rewards. Behav. Process. 2010, 84, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Talwar, S.K.; Xu, S.; Hawley, E.S.; Weiss, S.A.; Moxon, K.A.; Chapin, J.K. Behavioural neuroscience: Rat navigation guided by remote control. Nature 2002, 417, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.V.; Tyler-Kabara, E.C.; Wheeler, J.J.; Moran, D.W.; Gaunt, R.A.; Collinger, J.L.; Foldes, S.T.; Weber, D.J.; Chen, W.; Boninger, M.L.; et al. Human perception of electrical stimulation on the surface of somatosensory cortex. PLoS ONE 2017, 12, e0176020. [Google Scholar] [CrossRef] [PubMed]
- Libet, B.; Alberts, W.W.; Wright, E.; Delattre, L.; Levin, G.; Feinstein, B. Production of threshold levels of conscious sensation by electrical stimulation of human somatosensory cortex. In Neurophysiology of Consciousness; Springer: Boston, MA, USA, 1993; pp. 1–34. [Google Scholar]
- Romo, R.; Hernández, A.; Zainos, A.; Brody, C.D.; Lemus, L. Sensing without touching: Psychophysical performance based on cortical microstimulation. Neuron 2000, 26, 273–278. [Google Scholar] [CrossRef]
- Wickersham, I.; Groh, J.M. Neurophysiology: Electrically evoking sensory experience. Curr. Biol. 1998, 8, R412–R414. [Google Scholar] [CrossRef]
- Gratton, A.; Hoffer, B.J.; Gerhardt, G.A. Effects of electrical stimulation of brain reward sites on release of dopamine in rat: An in vivo electrochemical study. Brain Res. Bull. 1988, 21, 319–324. [Google Scholar] [CrossRef]
- Hermer-Vazquez, L.; Hermer-Vazquez, R.; Rybinnik, I.; Greebel, G.; Keller, R.; Xu, S.; Chapin, J.K. Rapid learning and flexible memory in “habit” tasks in rats trained with brain stimulation reward. Physiol. Behav. 2005, 84, 753–759. [Google Scholar] [CrossRef]
- Kuhr, W.G.; Wightman, R.M.; Rebec, G.V. Dopaminergic neurons: Simultaneous measurements of dopamine release and single-unit activity during stimulation of the medial forebrain bundle. Brain Res. 1987, 418, 122–128. [Google Scholar] [CrossRef]
- Adkins-Muir, D.L.; Jones, T.A. Cortical electrical stimulation combined with rehabilitative training: Enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol. Res. 2003, 25, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Berényi, A.; Belluscio, M.; Mao, D.; Buzsáki, G. Closed-loop control of epilepsy by transcranial electrical stimulation. Science 2012, 337, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Harris, W.; Broton, J.G.; Alexeeva, N.; Green, B.A. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J. Neurosurg. 1998, 88, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kozák, G.; Földi, T.; Berényi, A. Chronic Transcranial Electrical Stimulation and Intracortical Recording in Rats. JOVE (J. Vis. Exp.) 2018, 135, e56669. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, N.; Uwano, T.; Hori, E.; Ono, T.; Nishijo, H. Reward contingency modulates neuronal activity in rat septal nuclei during elemental and configural association tasks. Front. Behav. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, K.; Ye, S.; Guo, S.; Zheng, X. A remote constant current stimulator designed for rat-robot navigation. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2168–2171. [Google Scholar]
- Xu, S.; Talwar, S.K.; Hawley, E.S.; Li, L.; Chapin, J.K. A multi-channel telemetry system for brain microstimulation in freely roaming animals. J. Neurosci. Methods 2004, 133, 57–63. [Google Scholar] [CrossRef]
- Yang, J.; Huai, R.; Wang, H.; Lv, C.; Su, X. A robo-pigeon based on an innovative multi-mode telestimulation system. Bio-Med. Mater. Eng. 2015, 26, S357–S363. [Google Scholar] [CrossRef]
- Ye, X.; Wang, P.; Liu, J.; Zhang, S.; Jiang, J.; Wang, Q.; Chen, W.; Zheng, X. A portable telemetry system for brain stimulation and neuronal activity recording in freely behaving small animals. J. Neurosci. Methods 2008, 174, 186–193. [Google Scholar] [CrossRef]
- Millard, R.E.; Shepherd, R.K. A fully implantable stimulator for use in small laboratory animals. J. Neurosci. Methods 2007, 166, 168–177. [Google Scholar] [CrossRef]
- Perry, D.; Grayden, D.; Shepherd, R.; Fallon, J. A fully implantable rodent neural stimulator. J. Neural Eng. 2012, 9, 014001. [Google Scholar] [CrossRef] [PubMed]
- Baker, N. ZigBee and Bluetooth: Strengths and weaknesses for industrial applications. Comput. Control Eng. 2005, 16, 20–25. [Google Scholar] [CrossRef]
- Fadlullah, Z.M.; Fouda, M.M.; Kato, N.; Takeuchi, A.; Iwasaki, N.; Nozaki, Y. Toward intelligent machine-to-machine communications in smart grid. IEEE Commun. Mag. 2011, 49, 60–65. [Google Scholar] [CrossRef]
- Kinney, P. Zigbee technology: Wireless control that simply works. In Proceedings of the Communications Design Conference, San Jose, CA, USA, 30 September–2 October 2003; pp. 1–7. [Google Scholar]
- Lee, J.-S.; Su, Y.-W.; Shen, C.-C. A comparative study of wireless protocols: Bluetooth, UWB, ZigBee, and Wi-Fi. Ind. Electron. Soc. 2007, 5, 46–51. [Google Scholar]
- Rosello, V.; Portilla, J.; Riesgo, T. Ultra low power FPGA-based architecture for wake-up radio in wireless sensor networks. In Proceedings of the IECON 2011-37th Annual Conference of the IEEE Industrial Electronics Society, Melbourne, Australia, 7–10 November 2011; pp. 3826–3831. [Google Scholar]
- Qin, Y.; Howlader, M.M.; Deen, M.J.; Haddara, Y.M.; Selvaganapathy, P.R. Polymer integration for packaging of implantable sensors. Sens. Actuators B Chem. 2014, 202, 758–778. [Google Scholar] [CrossRef]
- Jeong, J.; Bae, S.H.; Min, K.S.; Seo, J.-M.; Chung, H.; Kim, S.J. A miniaturized, eye-conformable, and long-term reliable retinal prosthesis using monolithic fabrication of liquid crystal polymer (LCP). IEEE Trans. Biomed. Eng. 2015, 62, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.; Jeong, J.; Gwon, T.M.; Choi, G.J.; Lee, S.E.; Kim, J.; Jun, S.B.; Chang, J.W.; Kim, S.J. High charge storage capacity electrodeposited iridium oxide film on liquid crystal polymer-based neural electrodes. Sens. Mater. 2016, 28, 243–260. [Google Scholar]
- Seo, J.; Wee, J.H.; Park, J.H.; Park, P.; Kim, J.-W.; Kim, S.J. Nerve cuff electrode using embedded magnets and its application to hypoglossal nerve stimulation. J. Neural Eng. 2016, 13, 066014. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, W.; Ye, X.; Zhang, S.; Zheng, X.; Wang, P.; Jiang, J.; Jin, L.; Xu, Z.; Liu, C.; et al. A remote control training system for rat navigation in complicated environment. J. Zhejiang Univ. Sci. A 2007, 8, 323–330. [Google Scholar] [CrossRef]
- Bosman, L.W.; Houweling, A.R.; Owens, C.B.; Tanke, N.; Shevchouk, O.T.; Rahmati, N.; Teunissen, W.H.; Ju, C.; Gong, W.; Koekkoek, S.K.; et al. Anatomical pathways involved in generating and sensing rhythmic whisker movements. Front. Integr. Neurosci. 2011, 5, 53. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kim, S.; Jung, H.H.; Chang, J.W.; Kim, Y.-J.; Shin, H.-C.; Jun, S.B. Neuromodulation methods for animal locomotion control. Biomed. Eng. Lett. 2016, 6, 134–147. [Google Scholar] [CrossRef]
- Harrison, R.R. Designing efficient inductive power links for implantable devices. In Proceedings of the 2007 IEEE International Symposium on Circuits and Systems, New Orleans, LA, USA, 27–30 May 2007; pp. 2080–2083. [Google Scholar]
- Silay, K.M.; Dondi, D.; Larcher, L.; Declercq, M.; Benini, L.; Leblebici, Y.; Dehollain, C. Load optimization of an inductive power link for remote powering of biomedical implants. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems, Taipei, Taiwan, 24–27 May 2009; pp. 533–536. [Google Scholar]
- Ciampa, M. CWNA Guide to Wireless LANs; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Moran, T.H.; Schwartz, G.J.; Blass, E.M. Organized behavioral responses to lateral hypothalamic electrical stimulation in infant rats. J. Neurosci. 1983, 3, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.-T.; Im, D.; Lee, S.E.; Lee, J.; Koo, M.; Park, S.Y.; Kim, S.; Yang, K.; Kim, S.J.; Lee, K.; et al. In vivo silicon-based flexible radio frequency integrated circuits monolithically encapsulated with biocompatible liquid crystal polymers. ACS Nano 2013, 7, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Bae, S.H.; Seo, J.-M.; Chung, H.; Kim, S.J. Long-term evaluation of a liquid crystal polymer (LCP)-based retinal prosthesis. J. Neural Eng. 2016, 13, 025004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Woo, S.J.; Park, S.; Kim, E.; Seo, J.; Chung, H.; Kim, S. A suprachoroidal electrical retinal stimulator design for long-term animal experiments and in vivo assessment of its feasibility and biocompatibility in rabbits. BioMed Res. Int. 2008, 2008, 547428. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.C.; Renaud, P.; Tanila, H.; Djupsund, K. Flexible polyimide microelectrode array for in vivo recordings and current source density analysis. Biosens. Bioelectron. 2007, 22, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Seo, J.-M.; Ha, S.; Kim, E.T.; Chung, H.; Kim, S.J. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5859–5866. [Google Scholar] [CrossRef]
- Woods, V.; Trumpis, M.; Bent, B.; Palopoli-Trojani, K.; Chiang, C.-H.; Wang, C.; Yu, C.; Insanally, M.N.; Froemke, R.C.; Viventi, J. Long-term recording reliability of liquid crystal polymer µECoG arrays. J. Neural Eng. 2018, 15, 066024. [Google Scholar] [CrossRef]
- Lee, S.W.; Min, K.S.; Jeong, J.; Kim, J.; Kim, S.J. Monolithic encapsulation of implantable neuroprosthetic devices using liquid crystal polymers. IEEE Trans. Biomed. Eng. 2011, 58, 2255–2263. [Google Scholar]
- Hannan, M.A.; Mutashar, S.; Samad, S.A.; Hussain, A. Energy harvesting for the implantable biomedical devices: Issues and challenges. Biomed. Eng. Online 2014, 13, 79. [Google Scholar] [CrossRef]
- Hwang, G.-T.; Kim, Y.; Lee, J.-H.; Oh, S.; Jeong, C.K.; Park, D.Y.; Ryu, J.; Kwon, H.; Lee, S.-G.; Joung, B.; et al. Self-powered deep brain stimulation via a flexible PIMNT energy harvester. Energy Environ. Sci. 2015, 8, 2677–2684. [Google Scholar] [CrossRef]
- Olivo, J.; Carrara, S.; De Micheli, G. Energy harvesting and remote powering for implantable biosensors. IEEE Sens. J. 2011, 11, 1573–1586. [Google Scholar] [CrossRef]
- Zebda, A.; Cosnier, S.; Alcaraz, J.-P.; Holzinger, M.; Le Goff, A.; Gondran, C.; Boucher, F.; Giroud, F.; Gorgy, K.; Lamraoui, H. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 2013, 3, 1516. [Google Scholar] [CrossRef] [PubMed]

| [19] | [21] | [18] | [20] | [22] | [23] | This Work | |
|---|---|---|---|---|---|---|---|
| Size (mm3) | 48 × 23 × 19 | 36 × 22 × 7.5 | 32 × 25 × 6 | 26 × 16 × 9 | 14 × 12 × 6 | N/A | 29 × 26 × 8 |
| Weight (g) | 28 | 40 | 20 | 9 | 2.5 | N/A | 5.9 |
| Packaging | None | None | None | None | Silicone | Silicone/Parylene | LCP |
| Stimulation Electrode | Stainless steel wire | Stainless steel wire | Unknown | Stainless steel wire | Pt electrode array, Pt/Ir wires | Pt electrode array, Pt/Ir wires | Microfabricated LCP array |
| Stimulation Method | Constant voltage | Constant voltage/current | Constant current | Constant voltage | Constant current | Constant current | Constant current |
| Stimulation Target | MFB, S1 | MFB, S1 | dlPAG, S1, VTA | PAG, DIVA | Cochlea | Cochlea | MFB, S1 |
| Configuration | Head-mounted, percutaneous connection | Head-mounted, percutaneous connection | Head-mounted, percutaneous connection | Head-mounted, percutaneous connection, | Fully implanted, monolithic | Fully implanted, monolithic | Fully implanted, Modular |
| Communication | FM | Bluetooth | Bluetooth | FSK | N/A | 2.4 GHz radio link | ZigBee |
| Powering | Li battery (160 mAh) | Li battery (120 mAh × 2) | Li battery (80 mAh) | Li battery (120 mAh) | 3-axis Inductive coils on cage | 3-axis Inductive coils on cage | Li battery (90 mAh), Inductive charging |
| Avg. Power Consumption (Calculated) | 82.5 mW | 222 mW | 148 mW | 51.8 mW | N/A | N/A | 15.2 mW |
| Target Animal | Long–Evans rat | Sprague–Dawley rat | Sprague–Dawley rat | Pigeon | Mouse, rat | Hooded Wistar rat | Sprague–Dawley rat |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.; Koh, C.S.; Jeong, J.; Seo, J.; Ahn, S.-H.; Choi, G.J.; Shim, S.; Shin, J.; Jung, H.H.; Chang, J.W.; et al. Remote-Controlled Fully Implantable Neural Stimulator for Freely Moving Small Animal. Electronics 2019, 8, 706. https://doi.org/10.3390/electronics8060706
Yun S, Koh CS, Jeong J, Seo J, Ahn S-H, Choi GJ, Shim S, Shin J, Jung HH, Chang JW, et al. Remote-Controlled Fully Implantable Neural Stimulator for Freely Moving Small Animal. Electronics. 2019; 8(6):706. https://doi.org/10.3390/electronics8060706
Chicago/Turabian StyleYun, Seunghyeon, Chin Su Koh, Joonsoo Jeong, Jungmin Seo, Seung-Hee Ahn, Gwang Jin Choi, Shinyong Shim, Jaewoo Shin, Hyun Ho Jung, Jin Woo Chang, and et al. 2019. "Remote-Controlled Fully Implantable Neural Stimulator for Freely Moving Small Animal" Electronics 8, no. 6: 706. https://doi.org/10.3390/electronics8060706
APA StyleYun, S., Koh, C. S., Jeong, J., Seo, J., Ahn, S.-H., Choi, G. J., Shim, S., Shin, J., Jung, H. H., Chang, J. W., & Kim, S. J. (2019). Remote-Controlled Fully Implantable Neural Stimulator for Freely Moving Small Animal. Electronics, 8(6), 706. https://doi.org/10.3390/electronics8060706






