Learning-Based Screening of Endothelial Dysfunction From Photoplethysmographic Signals
Abstract
1. Introduction
2. Methods
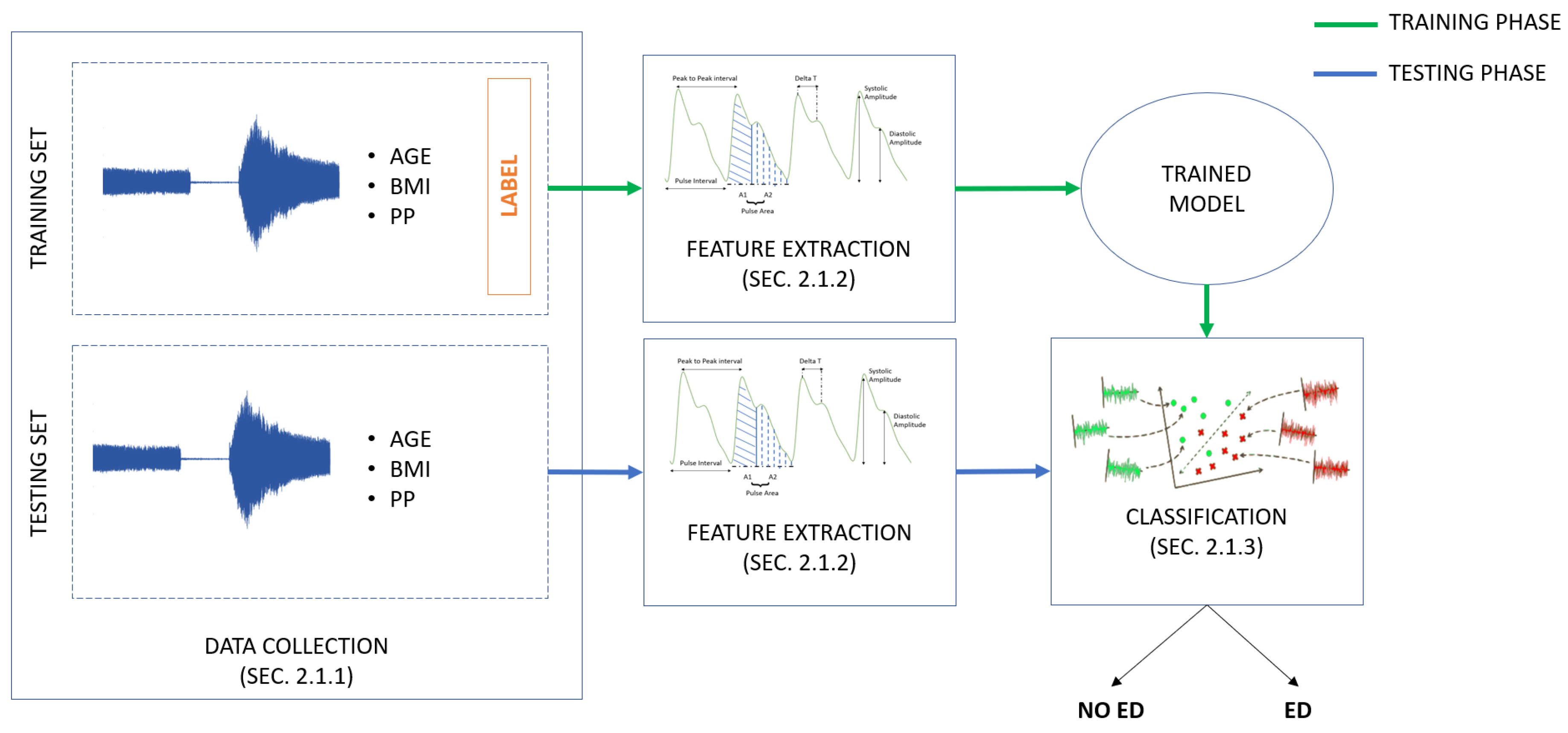
2.1. Endothelial-Dysfunction Screening Methodology
2.1.1. PPG Endothelial-Dysfunction-Dataset Collection
- -
- Fasting for at least 8 h
- -
- No drug consumption in the previous 6 h
- -
- No smoking in the previous 6 h
- -
- No intense physical activity in the hours immediately preceding the exam
- -
- No nail polish
- -
- VenoScreen® (medis). VenoScreen was connected via a USB interface to a computer equipped with the CardioVascular Lab software package (MEDIS company, Ilmenau). The software verified, evaluated and displayed the measured PPG signals.
- -
- Prakticus II aneroid sphygmomanometer® (Friedrich Bosch GmbH & Co. KG). The sphygmomanometer, applied above the elbow on the subject’s left arm, was used to induce blood-flow blockage while measuring the blood pressure.
- Noise: Inevitably, the PPG signal contained high-frequency noise, which resulted from ambient light, thermal noise and other unclassified noise. The power line represented another noise source characterized by 50 Hz sinusoidal interference, probably accompanied by a number of harmonics [18]. To remove this noise, a simple filtering approach was applied, i.e., low-pass filter with 20 dB attenuation at 8 Hz [19].
- Baseline wander: Baseline wander filtering was required in order to minimize changes in beat morphology, which did not have cardiac origin [18]. The technique used for baseline wander filtering consisted in down-sampling the PPG signal to 2 Hz, followed by forward/backwards filtering using a second-order low-pass Butterworth filter with a cut-off frequency of 0.5 Hz [20]. After that, the signal was unsampled and subtracted from the original PPG signal.
- Outliers: To remove outliers, the “isoutlier” function (The MathWorks, Inc., Natick, MA, USA), was applied to the PPG signal. A point was considered outlier when its value was more than three scaled median absolute deviations (MAD) away from the PPG signal median. The outliers were detected every 10 s and they were replaced by the mean value calculated in the same interval.
2.1.2. Feature Extraction
- Systolic Amplitude ():where and are the PPG signal amplitude in (systolic peak) and (dicrotic point before the systolic peak).
- Inflection Point Area ratio ():where area A1 and area A2 are obtained by dividing the pulse area into two areas at the dicrotic notch.
- Pulse Interval ():
- Hearth Rate ():where is the time at which the second systolic peak occurred.
- , which is the time between the systolic and diastolic peaks:where is the time of diastolic peak.
- Stiffness Index ():where is the subject’s height.
- Augmentation Index ():where and are the PPG signal amplitude in (systolic peak) and (dicrotic point before the systolic peak), and is the PPG signal amplitude in (diastolic peak).
- Recovery Time (). indicates how many seconds, from the maximum value of the PPG during the post-occlusion phase, are required to return to PPG pre-occlusion condition (Figure 3a).
2.1.3. Classification
2.2. Experimental Protocol
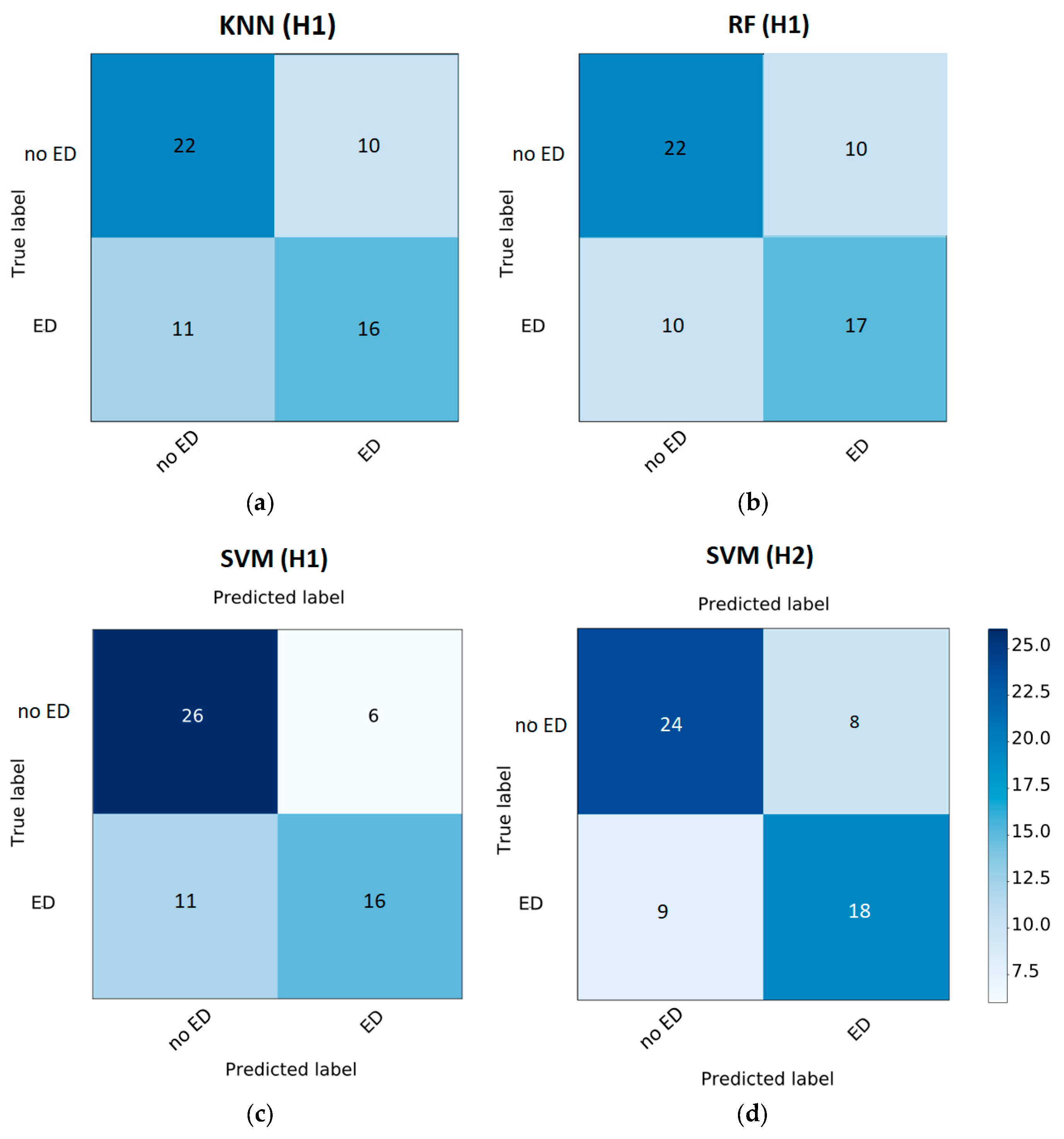
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Lüscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can. J. Cardiol. 2006, 22, 72B–80B. [Google Scholar] [CrossRef]
- Félétou, M. The endothelium, Part I: Multiple functions of the endothelial cells—Focus on endothelium-derived vasoactive mediators. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. Morgan Claypool Life Sci. 2011, 3, 1–306. [Google Scholar]
- van Hinsbergh, V.W. Endothelium role in regulation of coagulation and inflammation. In Seminars in Immunopathology; Springer: Berlin, Germany, 2012; Volume 34, pp. 93–106. [Google Scholar]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Zahedi, E.; Jaafar, R.; Ali, M.M.; Mohamed, A.; Maskon, O. Finger photoplethysmogram pulse amplitude changes induced by flow-mediated dilation. Physiol. Meas. 2008, 29, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Van Vlierberghe, E.; Knez, J.; Szczesny, G.; Thijs, L.; Jozeau, D.; Balestra, C.; Dhooge, J.; Staessen, J.A. Association of digital vascular function with cardiovascular risk factors: A population study. Br. Med J. Open 2014, 4, e004399. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, G.; Zahedi, E.; Attar, H.M.; Sharifi, F. Flow mediated dilation with photoplethysmography as a substitute for ultrasonic imaging. Physiol. Meas. 2015, 36, 1551–1571. [Google Scholar] [CrossRef] [PubMed]
- Moerland, M.; Kales, A.; Schrier, L.; Van Dongen, M.; Bradnock, D.; Burggraaf, J. Evaluation of the EndoPAT as a tool to assess endothelial function. Int. J. Vasc. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Rocha, M.; Vasconcelos, G.; Vasconcelos Filho, J.; de Albuquerque, V. Advances in photopletysmography signal analysis for biomedical applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE 2017, 12, e0174944. [Google Scholar] [CrossRef] [PubMed]
- Boursalie, O.; Samavi, R.; Doyle, T.E. M4CVD: Mobile machine learning model for monitoring cardiovascular disease. Procedia Comput. Sci. 2015, 63, 384–391. [Google Scholar] [CrossRef]
- Calamanti, C.; Paolanti, M.; Romeo, L.; Bernardini, M.; Frontoni, E. Machine learning-based approaches to analyse and improve the diagnosis of endothelial dysfunction. In Proceedings of the IEEE/ASME International Conference on Mechatronic and Embedded Systems and Applications, New York, NY, USA, 2–4 July 2018; pp. 1–6. [Google Scholar]
- Sörnmo, L.; Laguna, P. Bioelectrical Signal Processing in Cardiac and Neurological Applications; Academic Press: Cambridge, MA, USA, 2005; Volume 8. [Google Scholar]
- Peng, F.; Zhang, Z.; Gou, X.; Liu, H.; Wang, W. Motion artifact removal from photoplethysmographic signals by combining temporally constrained independent component analysis and adaptive filter. Biomed. Eng. Online 2014, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Solem, K.; Olde, B.; Sornmo, L. Prediction of intradialytic hypotension using photoplethysmography. IEEE Trans. Biomed. Eng. 2010, 57, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Maio, R.; Miceli, S.; Sciacqua, A.; Leone, G.G.; Bruni, R.; Naccarato, P.; Martino, F.; Sesti, G.; Perticone, F. Heart rate affects endothelial function in essential hypertension. Intern. Emerg. Med. 2013, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Wallace, S.; Mackenzie, I.S.; McDonnell, B.; Yasmin; Newby, D.E.; Cockcroft, J.R.; Wilkinson, I.B. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006, 48, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M. On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Beigel, R.; Dvir, D.; Arbel, Y.; Shechter, A.; Feinberg, M.S.; Shechter, M. Pulse pressure is a predictor of vascular endothelial function in middle-aged subjects with no apparent heart disease. Vasc. Med. 2010, 15, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Burges, C.J. A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Discov. 1998, 2, 121–167. [Google Scholar] [CrossRef]
- Keller, J.M.; Gray, M.R.; Givens, J.A. A fuzzy k-nearest neighbor algorithm. IEEE Trans. Syst. Man Cybern. 1985, SMC-15, 580–585. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Wong, T.T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar] [CrossRef]
- Nasrabadi, N.M. Pattern recognition and machine learning. J. Electron. Imaging 2007, 16, 049901. [Google Scholar]
- Rundo, F.; Ortis, A.; Battiato, S.; Conoci, S. Advanced bio-inspired system for noninvasive cuff-less blood pressure estimation from physiological signal analysis. Computation 2018, 6, 46. [Google Scholar] [CrossRef]
- Rundo, F.; Conoci, S.; Ortis, A.; Battiato, S. An advanced bio-inspired PhotoPlethysmoGraphy (PPG) and ECG pattern recognition system for medical assessment. Sensors 2018, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Dutt, D.N.; Shruthi, S. Digital processing of ECG and PPG signals for study of arterial parameters for cardiovascular risk assessment. In Proceedings of the 2015 International Conference on Communications and Signal Processing (ICCSP), Melmaruvathur, India, 2–4 April 2015; pp. 1506–1510. [Google Scholar]


| Features | Mean ± SD |
|---|---|
| SA | |
| IPA | |
| PI | |
| HR | |
| SI | |
| AI | |
| RT |
| Features | Mean ± SD |
|---|---|
| Age (years) | |
| BMI (Kg/m) | |
| PP (mmHg) |
| Classifier | |||
|---|---|---|---|
| KNN | 0.64 | 0.59 | 0.62 |
| RF | 0.66 | 0.63 | 0.63 |
| SVM | 0.71 | 0.59 | 0.73 |
| Classifier | |||
|---|---|---|---|
| KNN | 0.64 | 0.52 | 0.64 |
| RF | 0.49 | 0.44 | 0.44 |
| SVM | 0.71 | 0.67 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calamanti, C.; Moccia, S.; Migliorelli, L.; Paolanti, M.; Frontoni, E. Learning-Based Screening of Endothelial Dysfunction From Photoplethysmographic Signals. Electronics 2019, 8, 271. https://doi.org/10.3390/electronics8030271
Calamanti C, Moccia S, Migliorelli L, Paolanti M, Frontoni E. Learning-Based Screening of Endothelial Dysfunction From Photoplethysmographic Signals. Electronics. 2019; 8(3):271. https://doi.org/10.3390/electronics8030271
Chicago/Turabian StyleCalamanti, Chiara, Sara Moccia, Lucia Migliorelli, Marina Paolanti, and Emanuele Frontoni. 2019. "Learning-Based Screening of Endothelial Dysfunction From Photoplethysmographic Signals" Electronics 8, no. 3: 271. https://doi.org/10.3390/electronics8030271
APA StyleCalamanti, C., Moccia, S., Migliorelli, L., Paolanti, M., & Frontoni, E. (2019). Learning-Based Screening of Endothelial Dysfunction From Photoplethysmographic Signals. Electronics, 8(3), 271. https://doi.org/10.3390/electronics8030271







