A Pulse Generation Circuit for Studying Waveform Effects on Neurostimulation
Abstract
1. Introduction
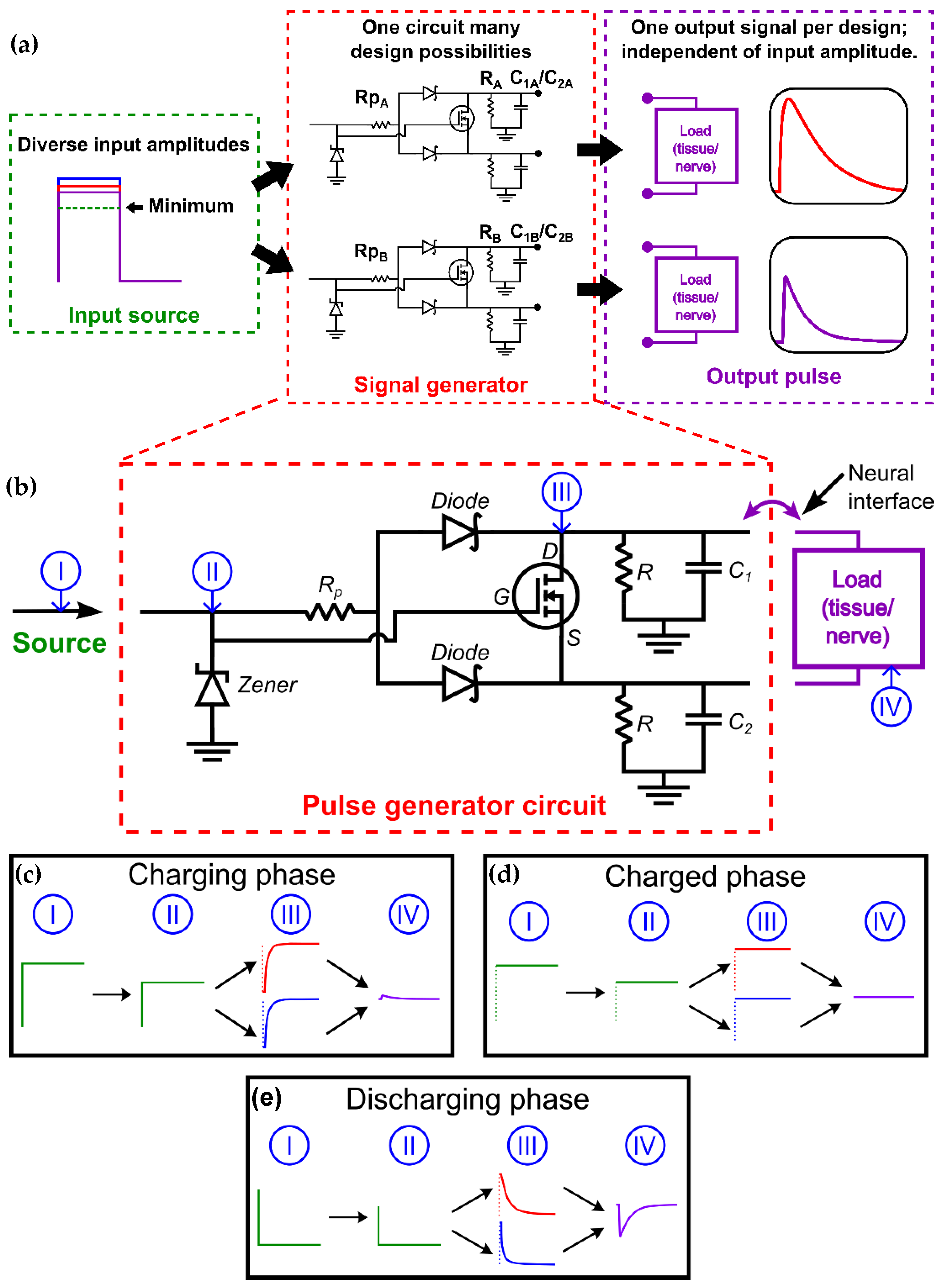
2. Design of Pulse Generation System
2.1. Pulse Generator Circuit Development
2.2. Safety and Failure Modes
2.3. Signal Generator Operation
2.4. Pulse Design Considerations
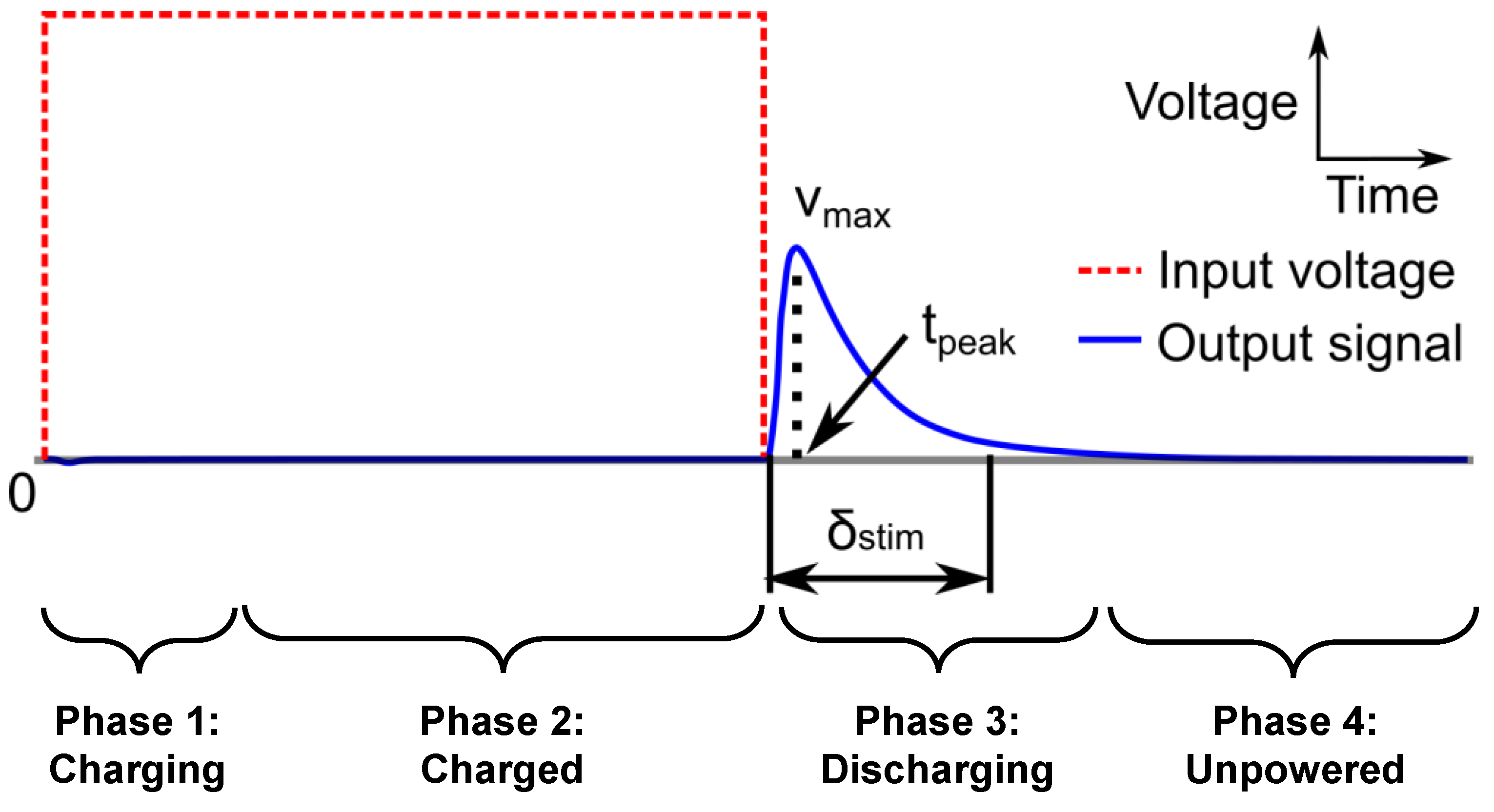
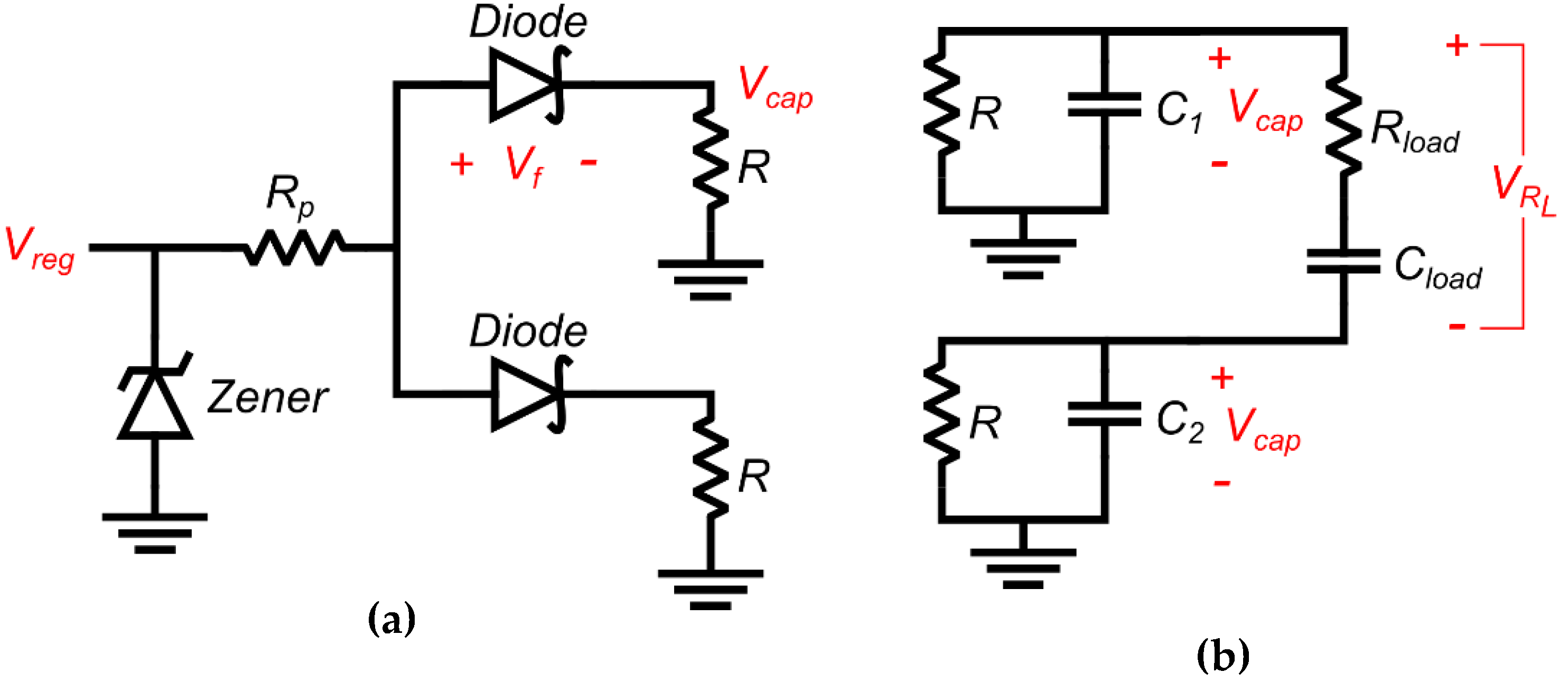
3. Circuit Analysis
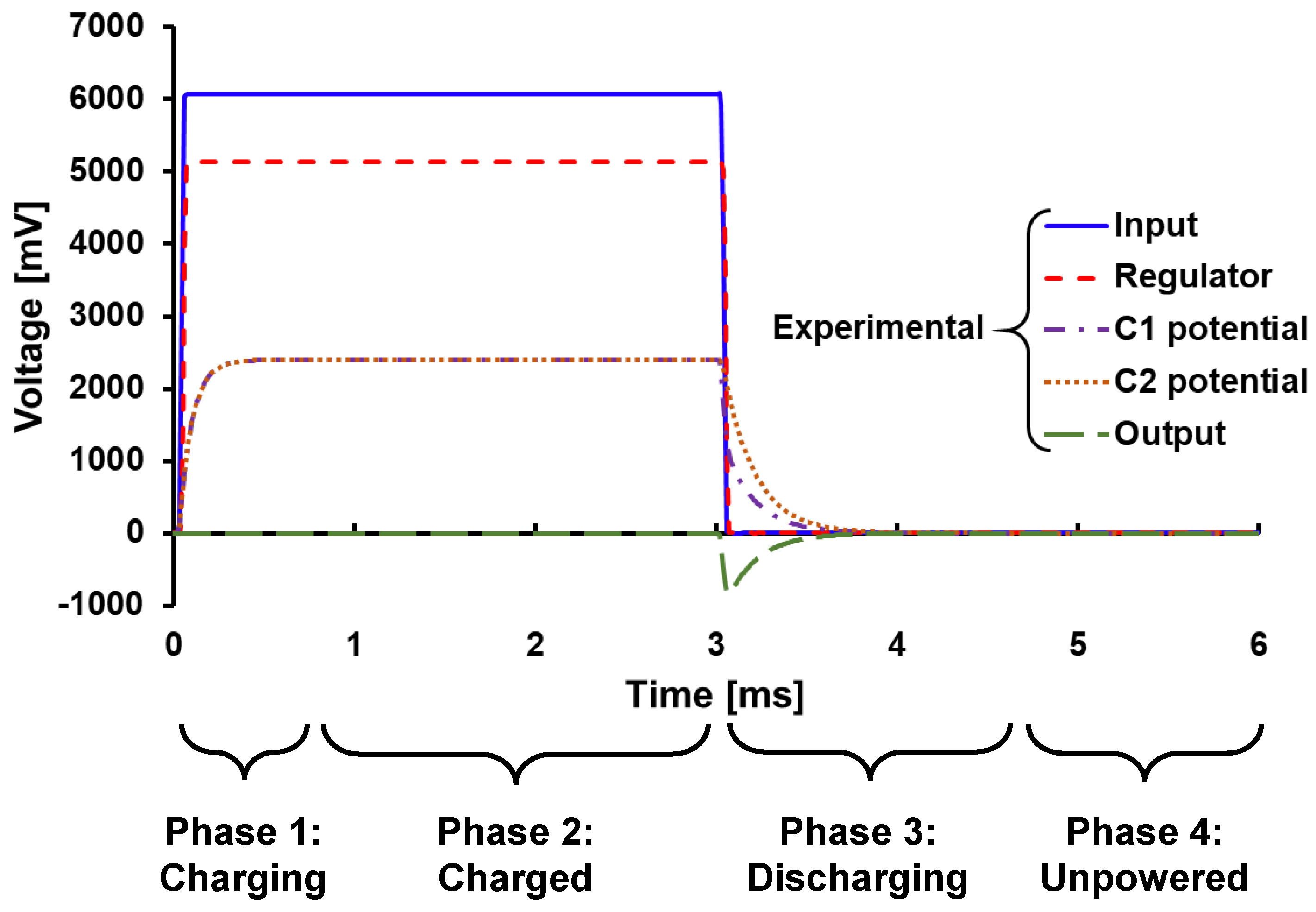
3.1. Overview of Circuit Analysis
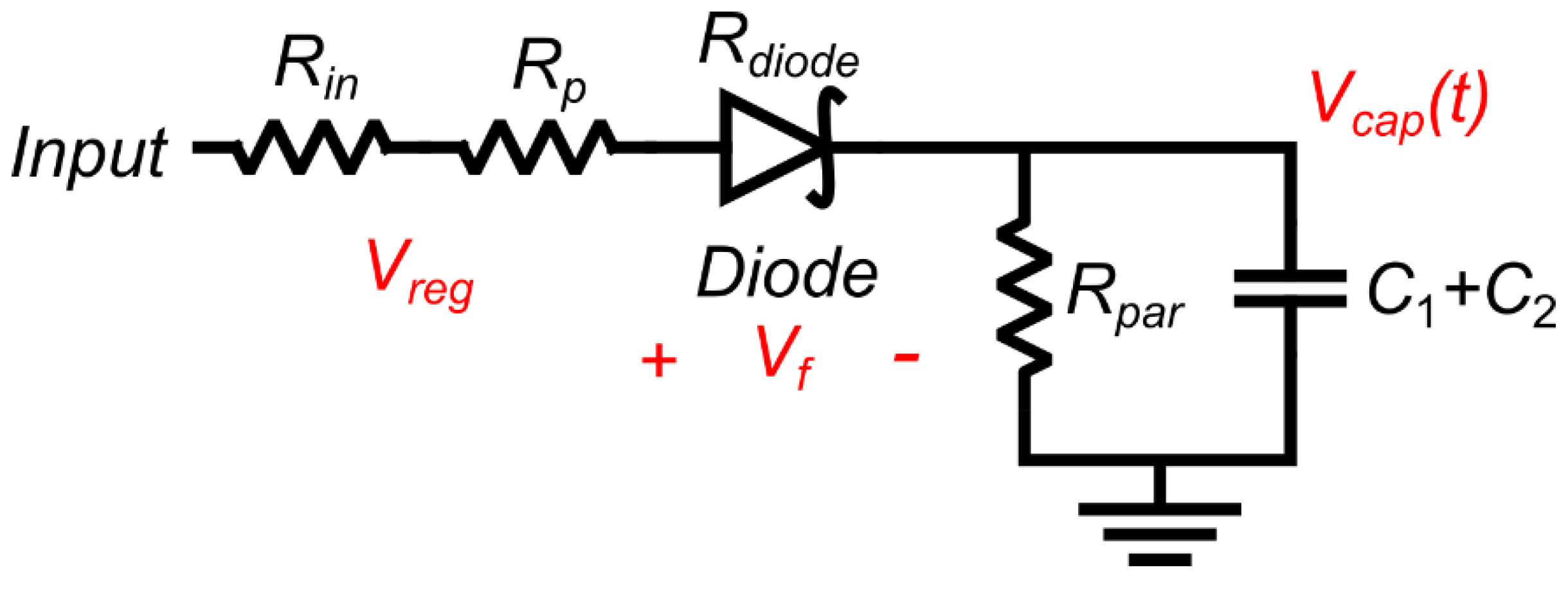
3.2. Charged Phase Capacitor Voltage
3.3. Discharging Output Waveform
4. Power Consumption Analysis
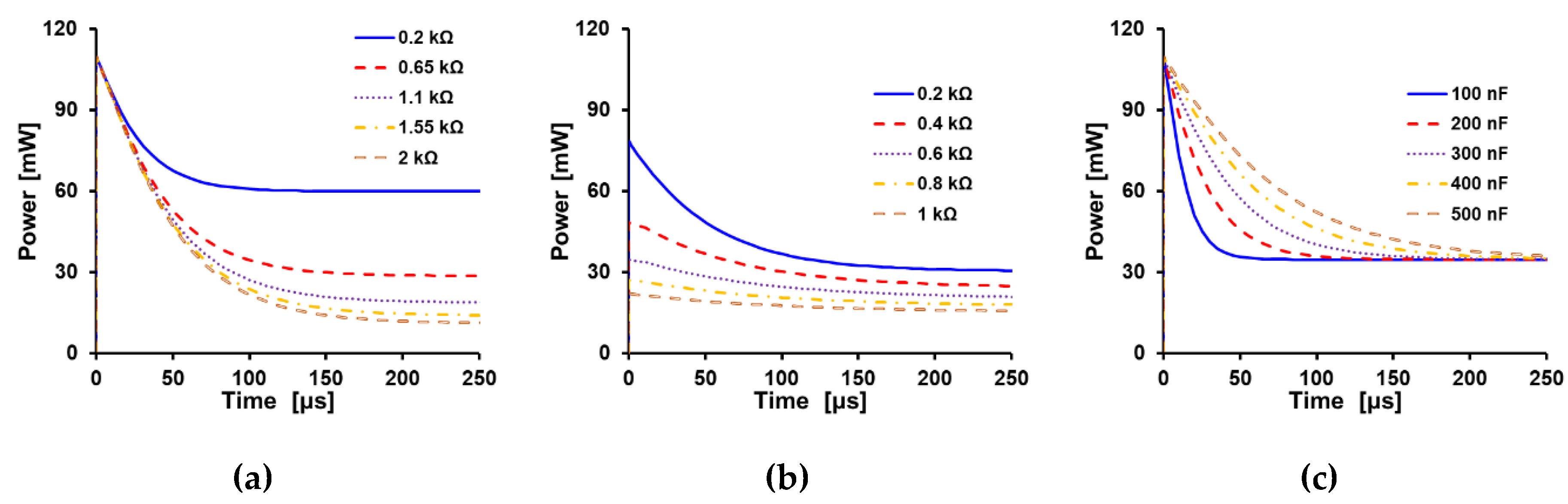
4.1. Overview of Power Analysis
4.2. Ideal Charging Case
4.3. Charged and Discharging Phase Analysis
5. Characterization Procedures of the Pulse Generator
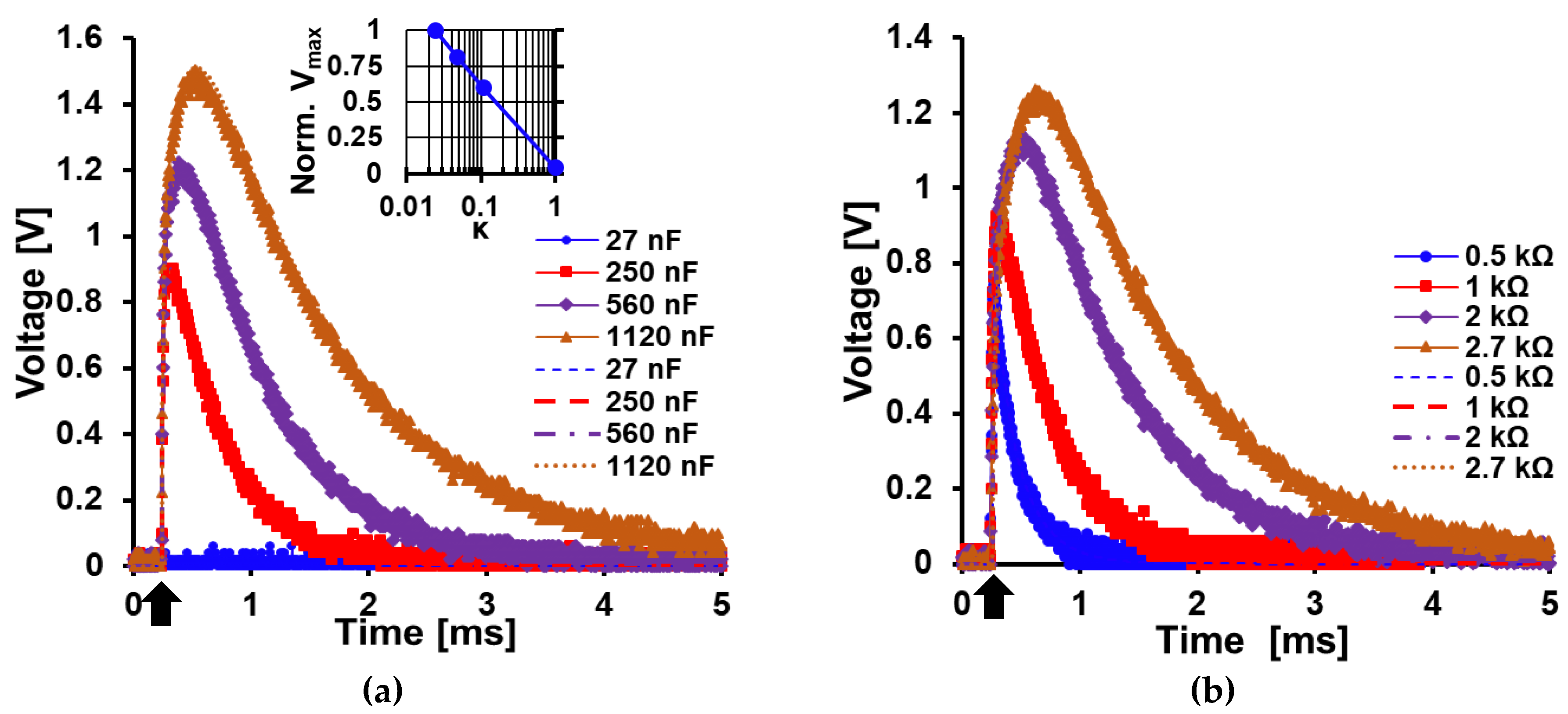
5.1. Analytical Expression Characterization and Validation
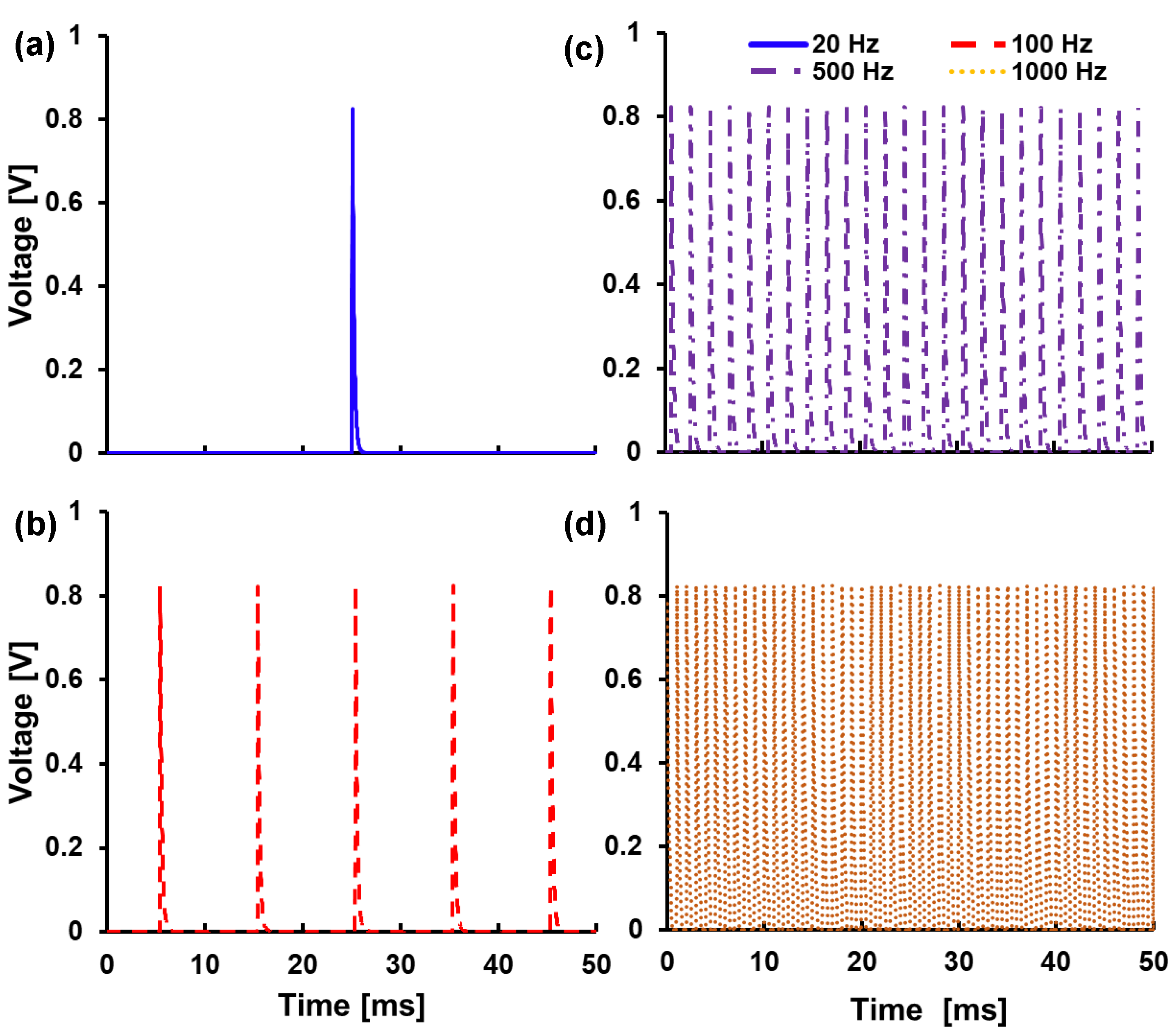
5.2. Output Frequency Characterization
5.3. Load Characterization
5.4. PBS Solution Measurements
6. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Reato, D.; Rahman, A.; Bikson, M.; Parra, L.C. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 2010, 30, 15067–15079. [Google Scholar] [CrossRef] [PubMed]
- Hoppa, M.B.; Gouzer, G.; Armbruster, M.; Ryan, T.A. Control and plasticity of the presynaptic action potential waveform at small CNS nerve terminals. Neuron 2014, 84, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Orsborn, A.L.; Moorman, H.G.; Overduin, S.A.; Shanechi, M.M.; Dimitrov, D.F.; Carmena, J.M. Closed-loop decoder adaptation shapes neural plasticity for skillful neuroprosthetic control. Neuron 2014, 82, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K.V.; Carmena, J.M. Combining decoder design and neural adaptation in brain-machine interfaces. Neuron 2014, 84, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, N.; Sandberg, A. Cognitive enhancement: methods, ethics, regulatory challenges. Sci. Eng. Ethics 2009, 15, 311–341. [Google Scholar] [CrossRef] [PubMed]
- Schambra, H.M.; Abe, M.; Luckenbaugh, D.A.; Reis, J.; Krakauer, J.W.; Cohen, L.G. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J. Neurophysiol. 2011, 106, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Matsuki, N.; Ikegaya, Y. Effects of Axonal Topology on the Somatic Modulation of Synaptic Outputs. J. Neurosci. 2012, 32, 2868–2876. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsuki, N.; Ikegaya, Y. Action-potential modulation during axonal conduction. Science 2011, 331, 599–601. [Google Scholar] [CrossRef]
- Boudkkazi, S.; Fronzaroli-Molinieres, L.; Debanne, D. Presynaptic action potential -waveform determines cortical synaptic latency. J. Physiol. 2011, 589, 1117–1131. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Grill, W.M. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 2002, 88, 1592–1604. [Google Scholar] [CrossRef]
- Grill, W.; Mortimer, J.T. The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans. Biomed. Eng. 1996, 43, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Wongsarnpigoon, A.; Woock, J.P.; Grill, W.M. Efficiency analysis of waveform shape for electrical excitation of nerve fibers. IEEE Trans. Neural. Syst. Rehabil. Eng. 2010, 18, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Harms, J.; Weyh, T.; Lemon, R.N.; Paulus, W.; Rothwell, J.C.; Siebner, H.R. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin. Neurophysiol. 2006, 117, 2292–2301. [Google Scholar] [CrossRef]
- Barth, B.B.; Henriquez, C.S.; Grill, W.M.; Shen, X. Electrical stimulation of gut motility guided by an in silico model. J. Neural. Eng. 2017, 14. [Google Scholar] [CrossRef]
- Foutz, T.J.; McIntyre, C.C. Evaluation of novel stimulus waveforms for deep brain stimulation. J. Neural. Eng. 2010, 7. [Google Scholar] [CrossRef]
- Wongsarnpigoon, A.; Grill, W.M. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J. Neural. Eng. 2010, 7. [Google Scholar] [CrossRef]
- Chipchase, L.S.; Schabrun, S.M.; Hodges, P.W. Peripheral electrical stimulation to induce cortical plasticity: a systematic review of stimulus parameters. Clin. Neurophysiol. 2011, 122, 456–463. [Google Scholar] [CrossRef]
- Kammer, T.; Beck, S.; Thielscher, A.; Laubis-Herrmann, U.; Topka, H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin. Neurophysiol. 2001, 112, 250–258. [Google Scholar] [CrossRef]
- Cassar, I.R.; Titus, N.D.; Grill, W.M. An improved genetic algorithm for designing optimal temporal patterns of neural stimulation. J. Neural. Eng. 2017, 14. [Google Scholar] [CrossRef]
- Kuncel, A.M.; Grill, W.M. Selection of stimulus parameters for deep brain stimulation. Clin. Neurophysiol. 2004, 115, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Magis, D.; Schoenen, J. Advances and challenges in neurostimulation for headaches. The Lancet Neurol. 2012, 11, 708–719. [Google Scholar] [CrossRef]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.-P.; Simpson, B.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef] [PubMed]
- Davidovics, N.S.; Fridman, G.Y.; Chiang, B.; Della Santina, C.C. Effects of biphasic current pulse frequency, amplitude, duration, and interphase gap on eye movement responses to prosthetic electrical stimulation of the vestibular nerve. IEEE Trans. Neural. Syst. Rehabil. Eng. 2011, 19, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.K.; Behrend, M.R.; Kuroda, M.; Humayun, M.S.; Weiland, J.D. An in vitro model of a retinal prosthesis. IEEE Trans. Biomed. Eng. 2008, 55, 1744–1753. [Google Scholar] [CrossRef]
- Gorman, P.H.; Mortimer, J.T. The effect of stimulus parameters on the recruitment characteristics of direct nerve stimulation. IEEE Trans. Biomed. Eng. 1983, BME-30, 407–414. [Google Scholar] [CrossRef]
- Seo, D.; Neely, R.M.; Shen, K.; Singhal, U.; Alon, E.; Rabaey, J.M.; Carmena, J.M.; Maharbiz, M.M. Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron 2016, 91, 529–539. [Google Scholar] [CrossRef]
- Qian, X.; Hao, H.-W.; Ma, B.-Z.; Wen, X.-W.; Hu, C.-H.; Li, L.-M.; Lau, R.Y.C.; Guo, X. Programmable and implantable neurostimulator with novel stimulus waveforms for rat models. Electron. Lett. 2012, 48, 1035–1036. [Google Scholar] [CrossRef]
- van Dongen, M.N.; Serdijn, W.A. A power-efficient multichannel neural stimulator using high-frequency pulsed excitation from an unfiltered dynamic supply. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 61–71. [Google Scholar] [CrossRef]
- Khalifa, A.; Karimi, Y.; Wang, Q.; Garikapati, S.; Montlouis, W.; Stanaćević, M.; Thakor, N.; Etienne-Cummings, R. The Microbead: A Highly Miniaturized Wirelessly Powered Implantable Neural Stimulating System. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 521–531. [Google Scholar] [CrossRef]
- Zaraska, W.; Thor, P.; Lipiński, M.; Cież, M.; Grzesiak, W.; Początek, J.; Zaraska, K. Design and fabrication of neurostimulator implants—selected problems. Microelectron. Reliab. 2005, 45, 1930–1934. [Google Scholar] [CrossRef]
- Lilly, J.C.; Hughes, J.R.; Alvord Jr, E.C.; Galkin, T.W. Brief, noninjurious electric waveform for stimulation of the brain. Science 1955, 121, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Malavera, A.; Doruk, D.; Morales-Quezada, L.; Carvalho, S.; Leite, J.; Fregni, F. Duration dependent effects of transcranial pulsed current stimulation (tPCS) indexed by electroencephalography. Neuromodulation 2016, 19, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Alon, G.; Roys, S.R.; Gullapalli, R.P.; Greenspan, J.D. Non-invasive electrical stimulation of the brain (ESB) modifies the resting-state network connectivity of the primary motor cortex: a proof of concept fMRI study. Brain Res. 2011, 1403, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, V.; Oliviero, A.; Mazzone, P.; Insola, A.; Pilato, F.; Saturno, E.; Accurso, A.; Tonali, P.A.; Rothwell, J.C. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp. Brain Res. 2001, 141, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Mazzone, P.; Insola, A.; Tonali, P.A.; Rothwell, J.C. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 2004, 115, 255–266. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Grill, W.M. Selective microstimulation of central nervous system neurons. Ann. Biomed. Eng. 2000, 28, 219–233. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Schmid, A. Compact, Energy-Efficient High-Frequency Switched Capacitor Neural Stimulator With Active Charge Balancing. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 878–888. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Yeh, C.-Y.; Huang, P.-Y.; Wang, Z.-Y.; Cheng, H.-H.; Li, Y.-T.; Chuang, C.-F.; Huang, P.-C.; Tang, K.-T.; Ma, H.-P.; et al. A Battery-Less, Implantable Neuro-Electronic Interface for Studying the Mechanisms of Deep Brain Stimulation in Rat Models. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 98–112. [Google Scholar] [CrossRef]
- Butson, C.R.; Maks, C.B.; McIntyre, C.C. Sources and effects of electrode impedance during deep brain stimulation. Clin. Neurophysiol. 2006, 117, 447–454. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
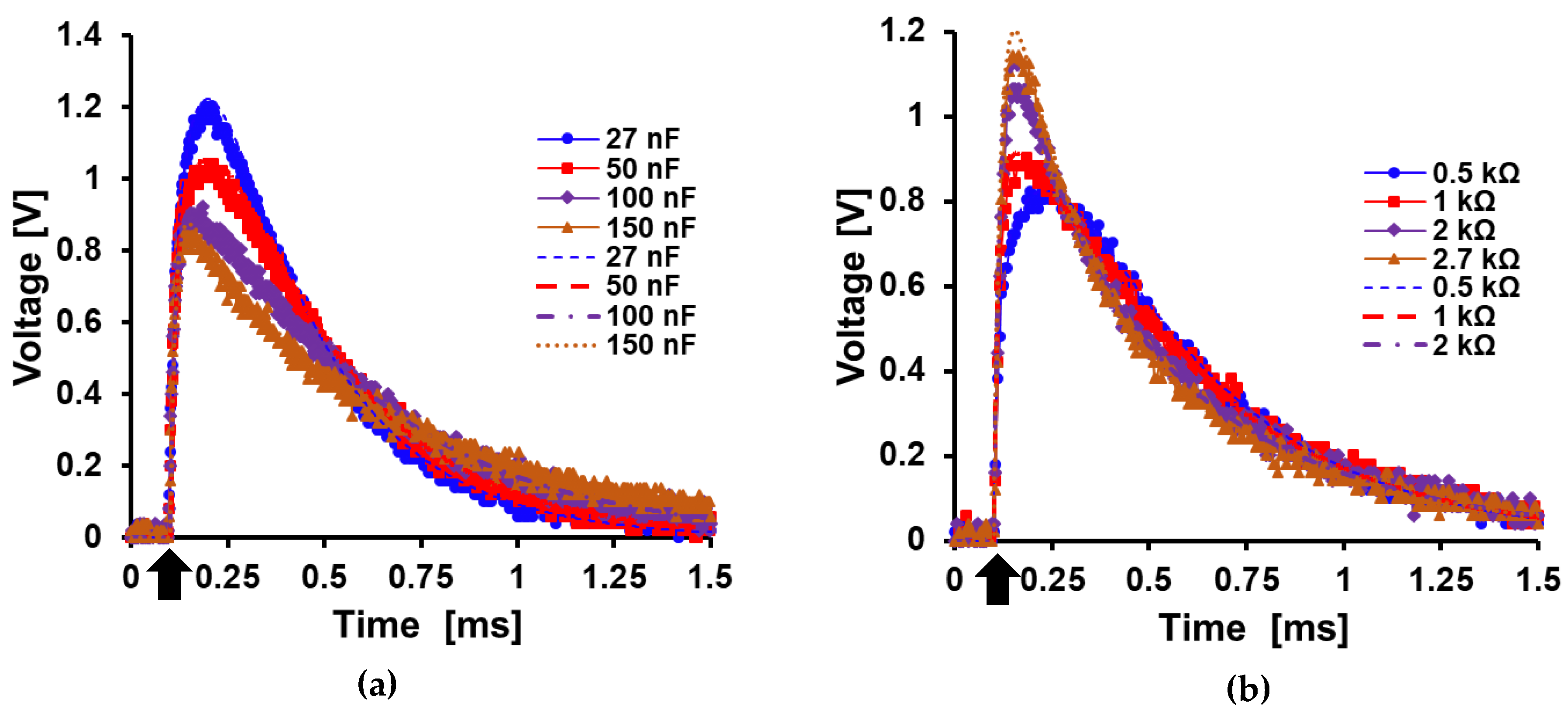
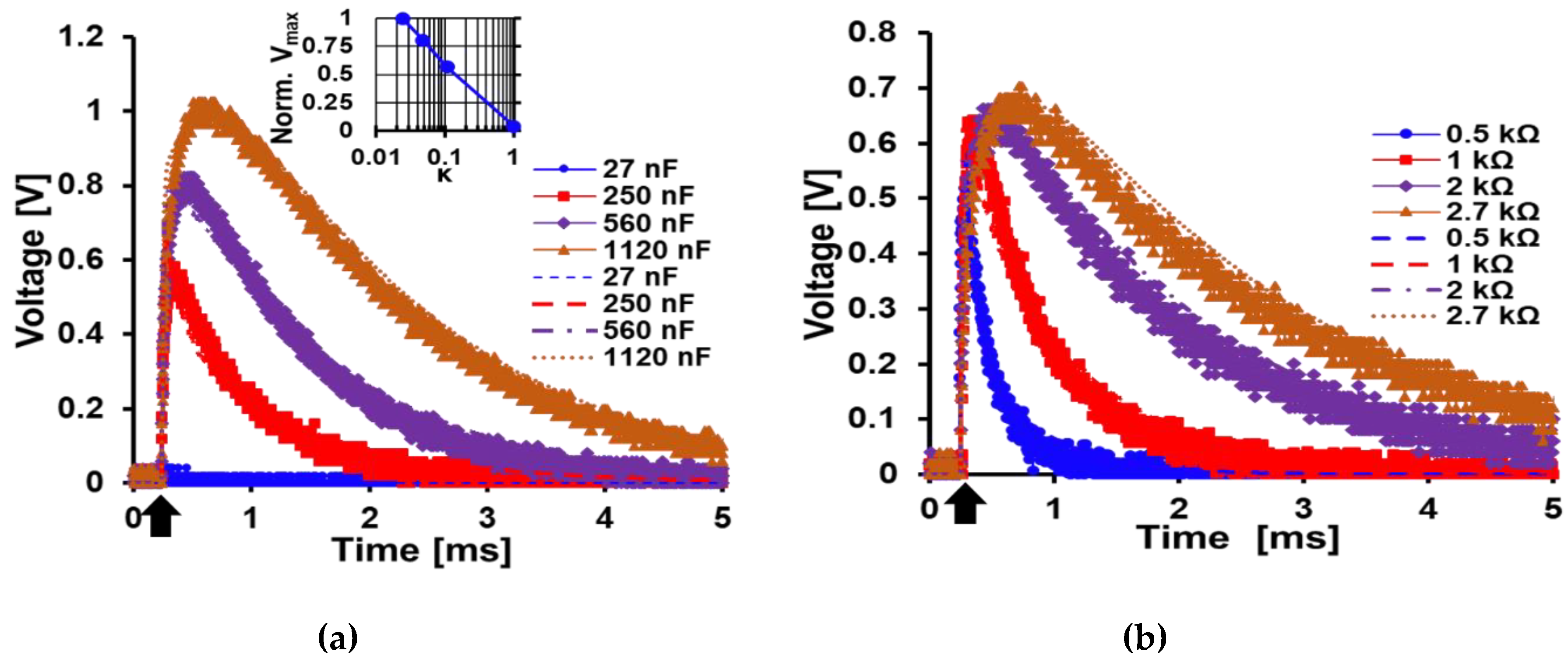
| Component | Value |
|---|---|
| RL [kΩ] | 0.5, 1, 2, 2.7 |
| CL [nF] | 27, 50, 100, 150 |
| R [kΩ] | 0.5, 1, 2, 2.7 |
| Rp [kΩ] | 0, 0.5, 1 |
| C1 [nF] | 27 |
| C2 [nF] | 27, 250, 560, 1120 |
| Input voltage [V] | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parodi, A.; Choi, J.-W. A Pulse Generation Circuit for Studying Waveform Effects on Neurostimulation. Electronics 2019, 8, 1344. https://doi.org/10.3390/electronics8111344
Parodi A, Choi J-W. A Pulse Generation Circuit for Studying Waveform Effects on Neurostimulation. Electronics. 2019; 8(11):1344. https://doi.org/10.3390/electronics8111344
Chicago/Turabian StyleParodi, Aquiles, and Jin-Woo Choi. 2019. "A Pulse Generation Circuit for Studying Waveform Effects on Neurostimulation" Electronics 8, no. 11: 1344. https://doi.org/10.3390/electronics8111344
APA StyleParodi, A., & Choi, J.-W. (2019). A Pulse Generation Circuit for Studying Waveform Effects on Neurostimulation. Electronics, 8(11), 1344. https://doi.org/10.3390/electronics8111344





