Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias
Abstract
1. Introduction
2. Materials and Methods
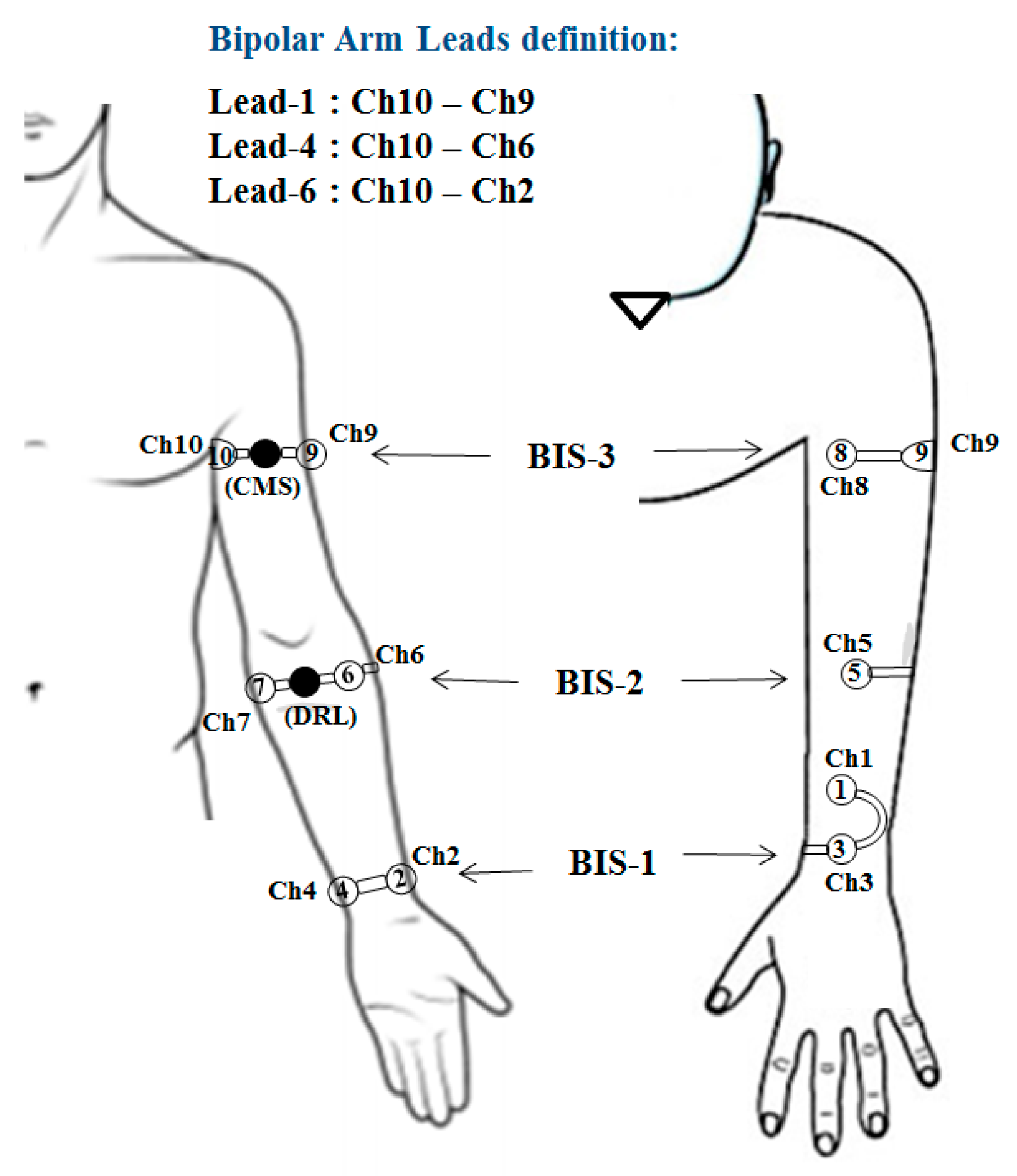
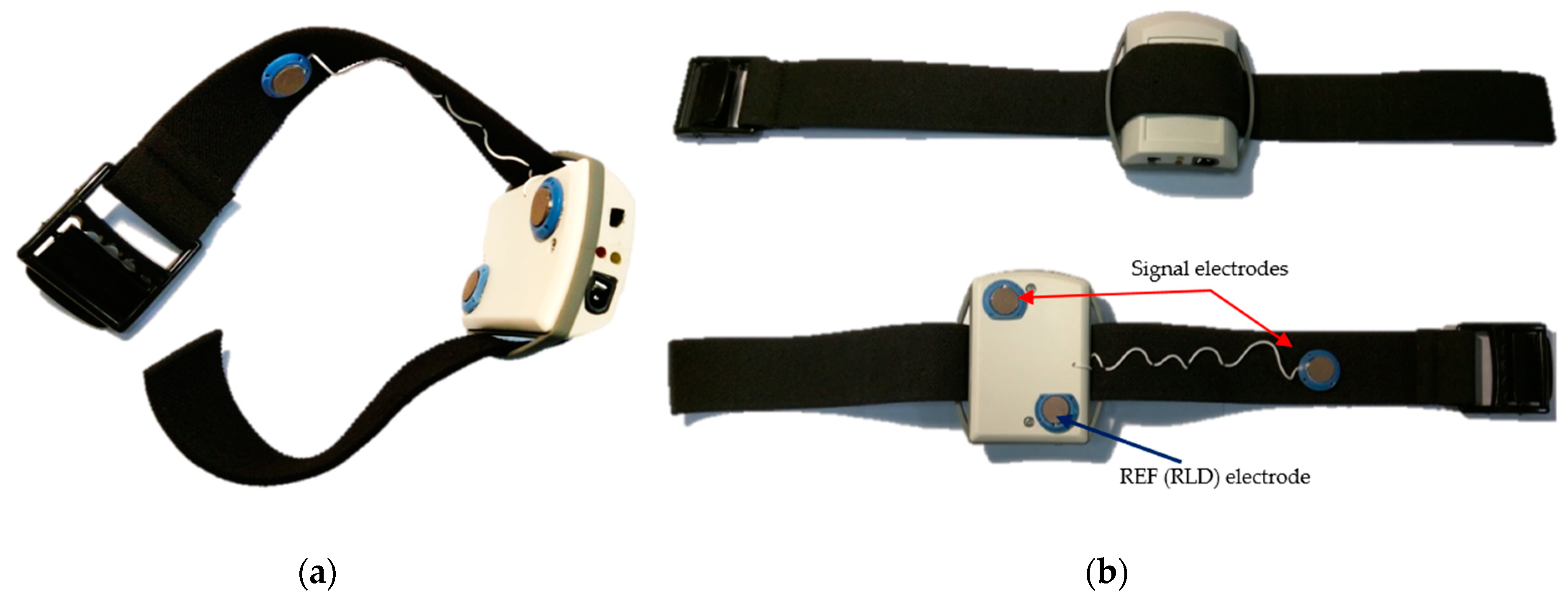
2.1. Bipolar Arm-ECG Signals
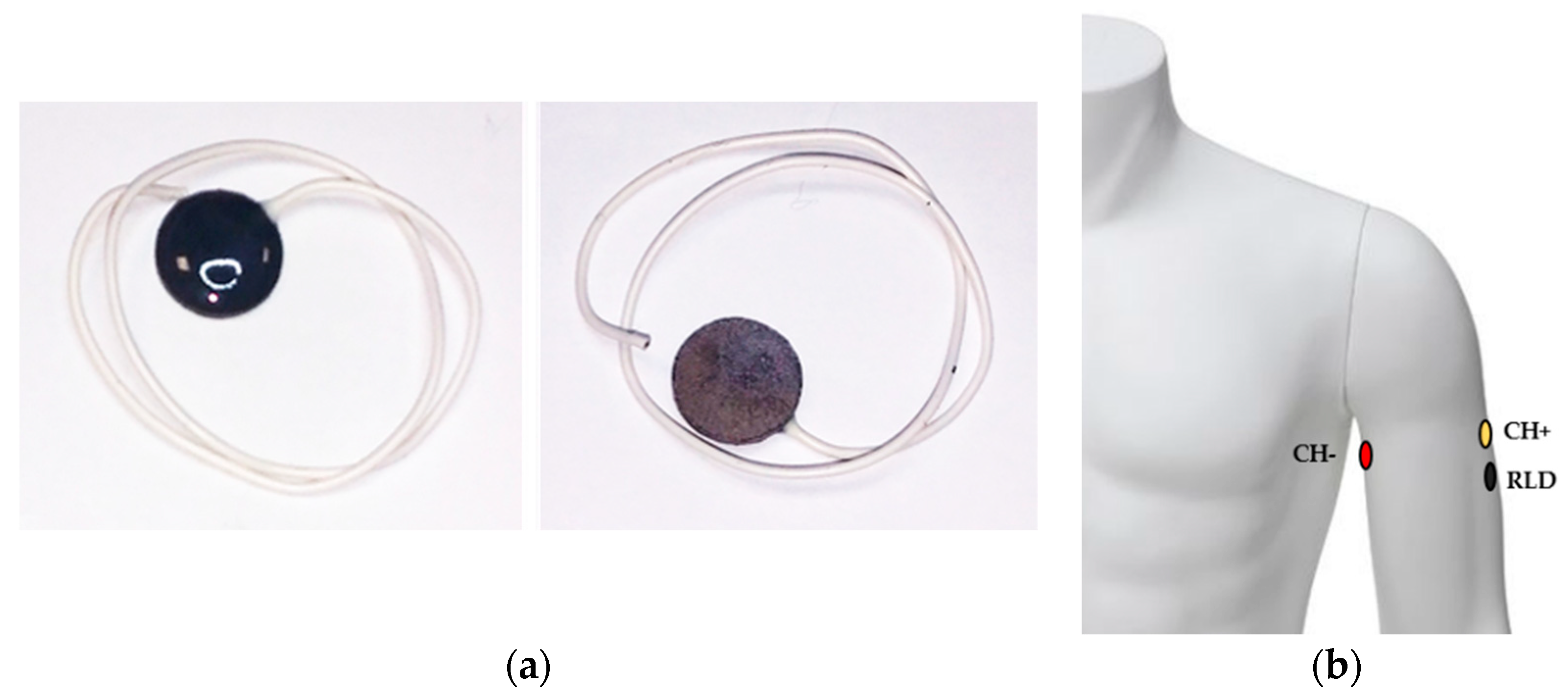
2.1.1. Dry ECG Electrode System
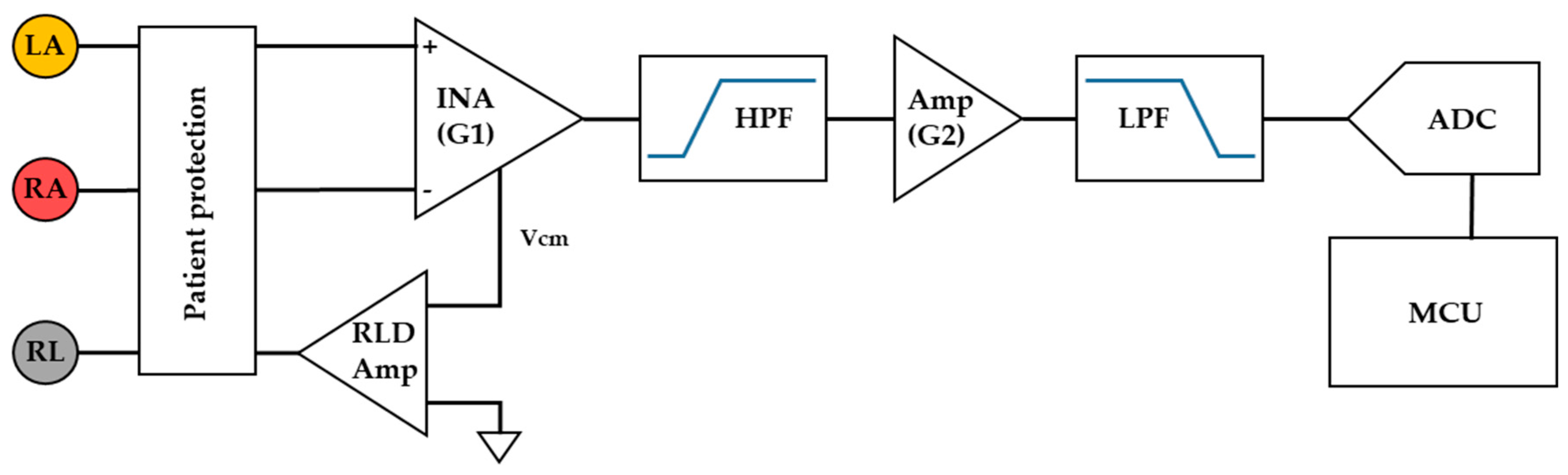
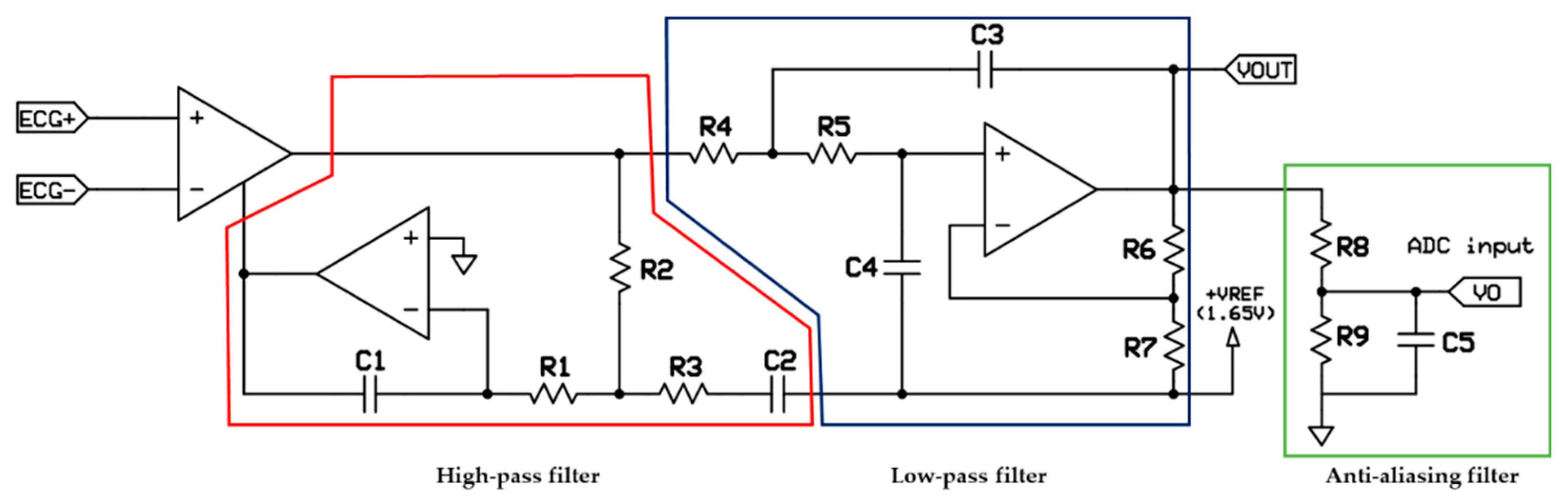
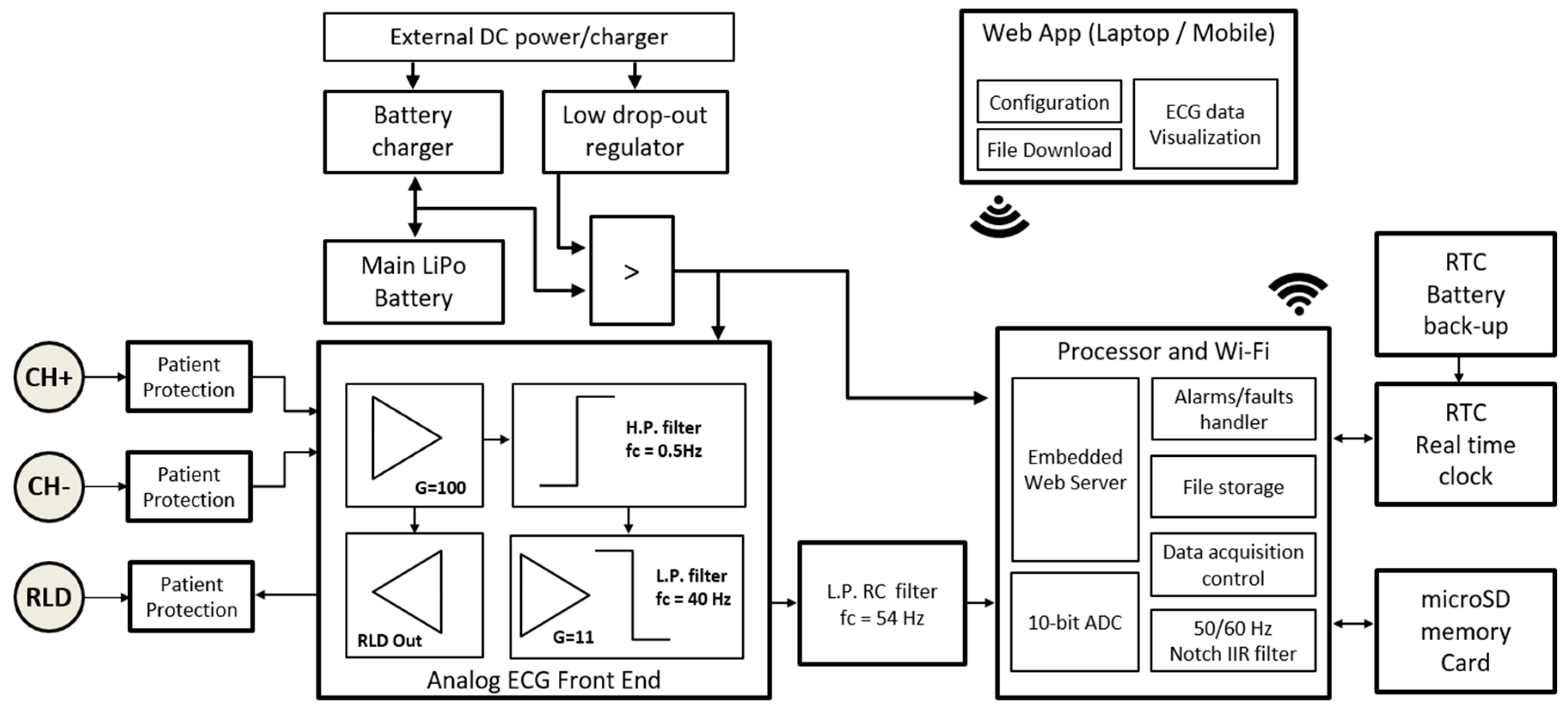
2.1.2. Front-End ECG Amplifier Configuration
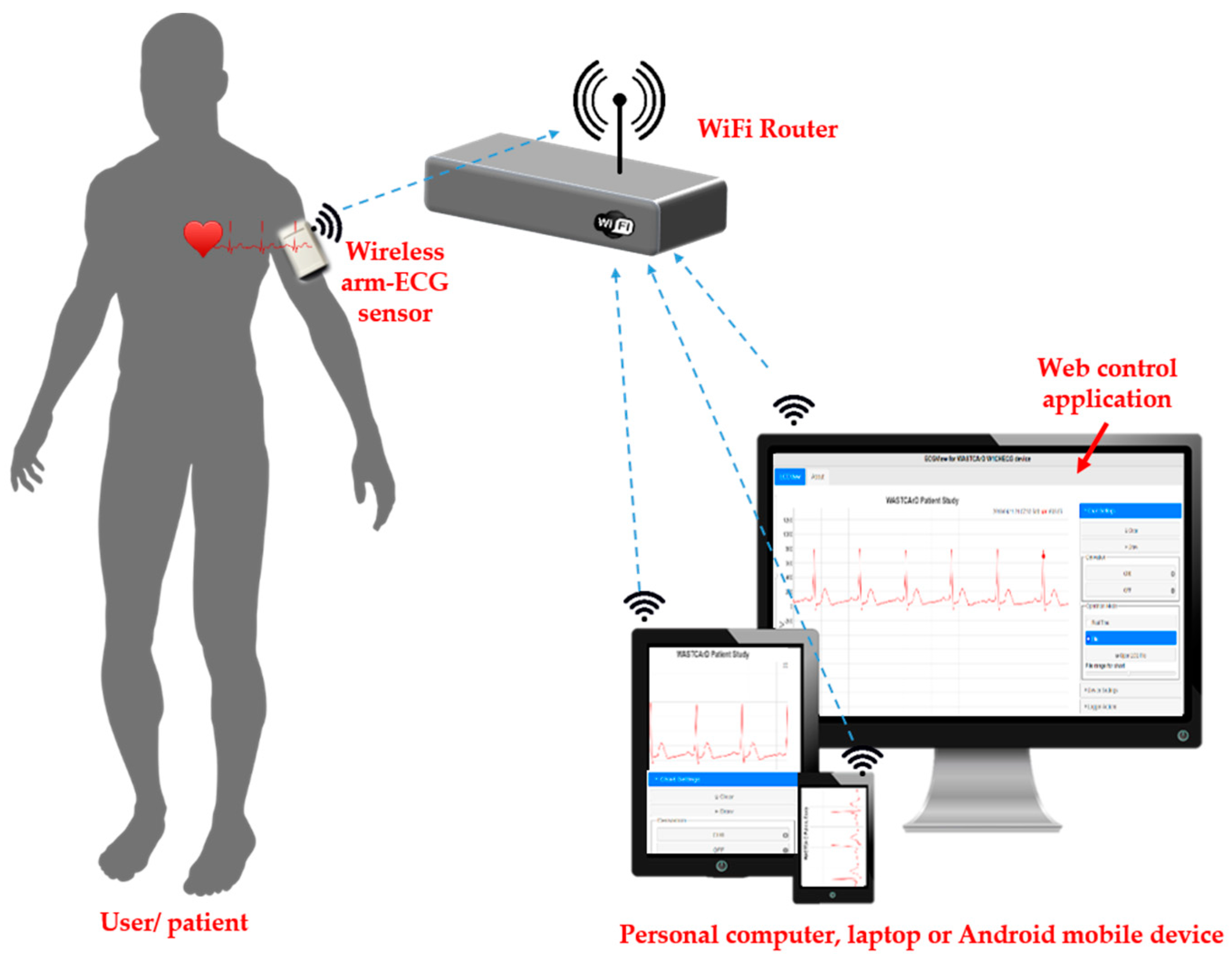
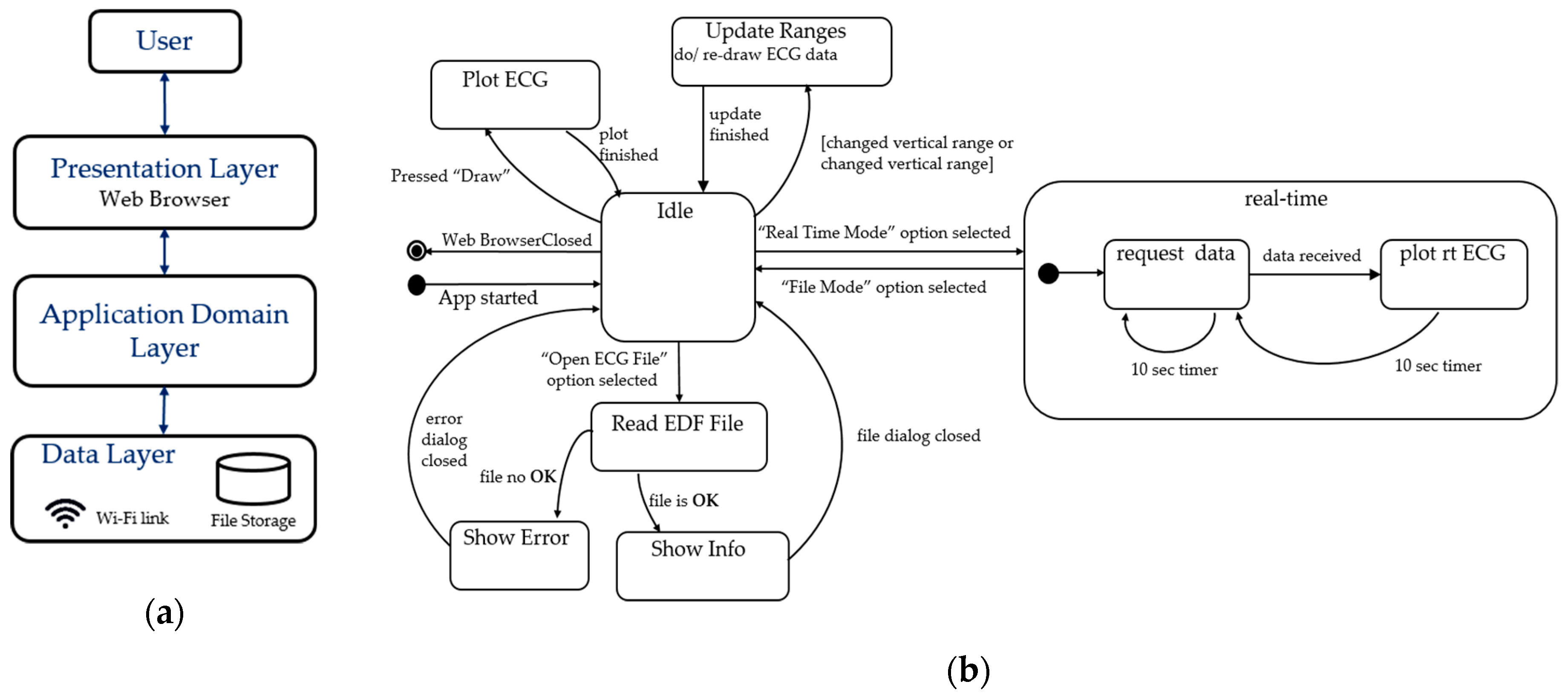
2.2. Overall System Architecture
2.2.1. Main Operational Features
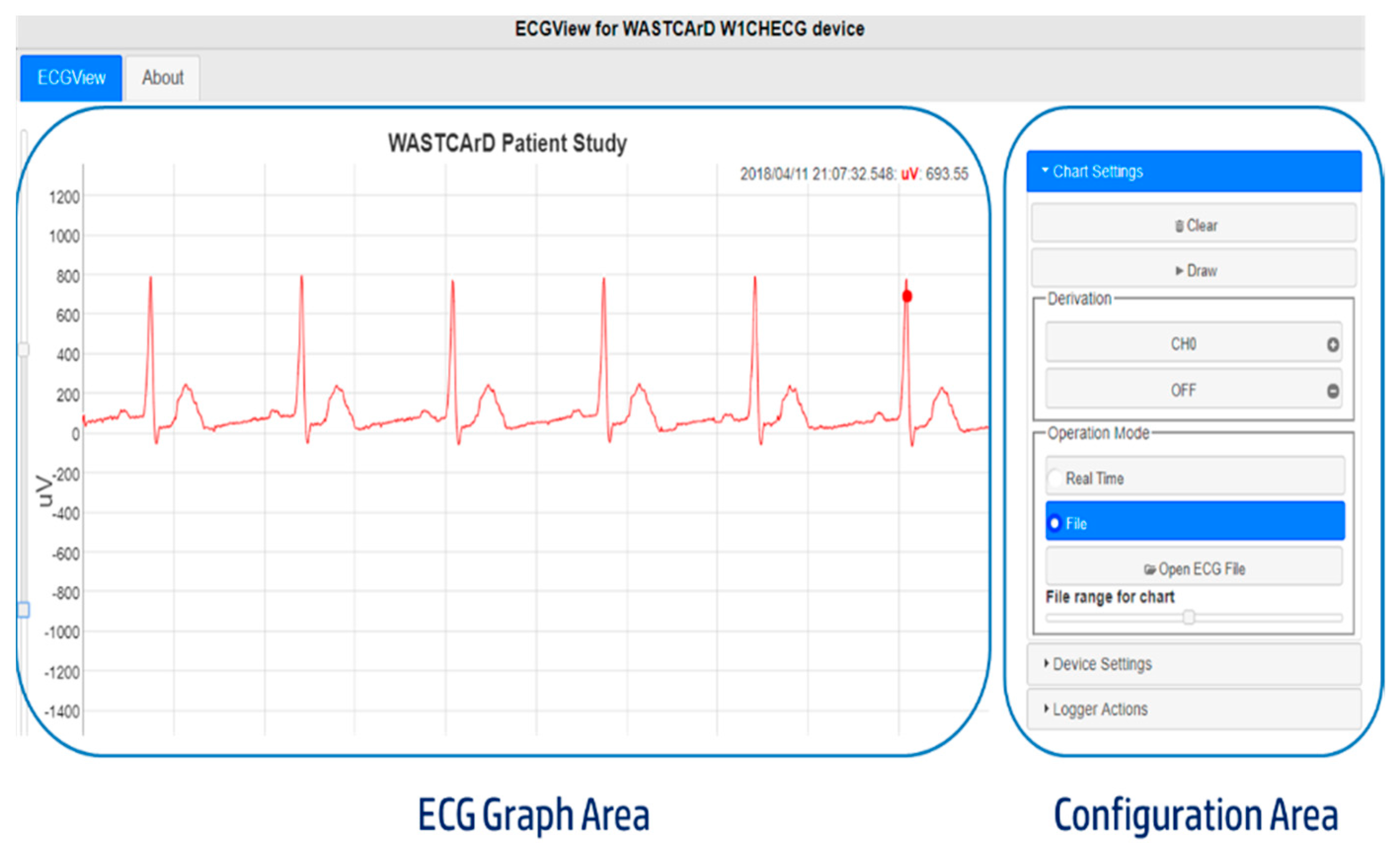
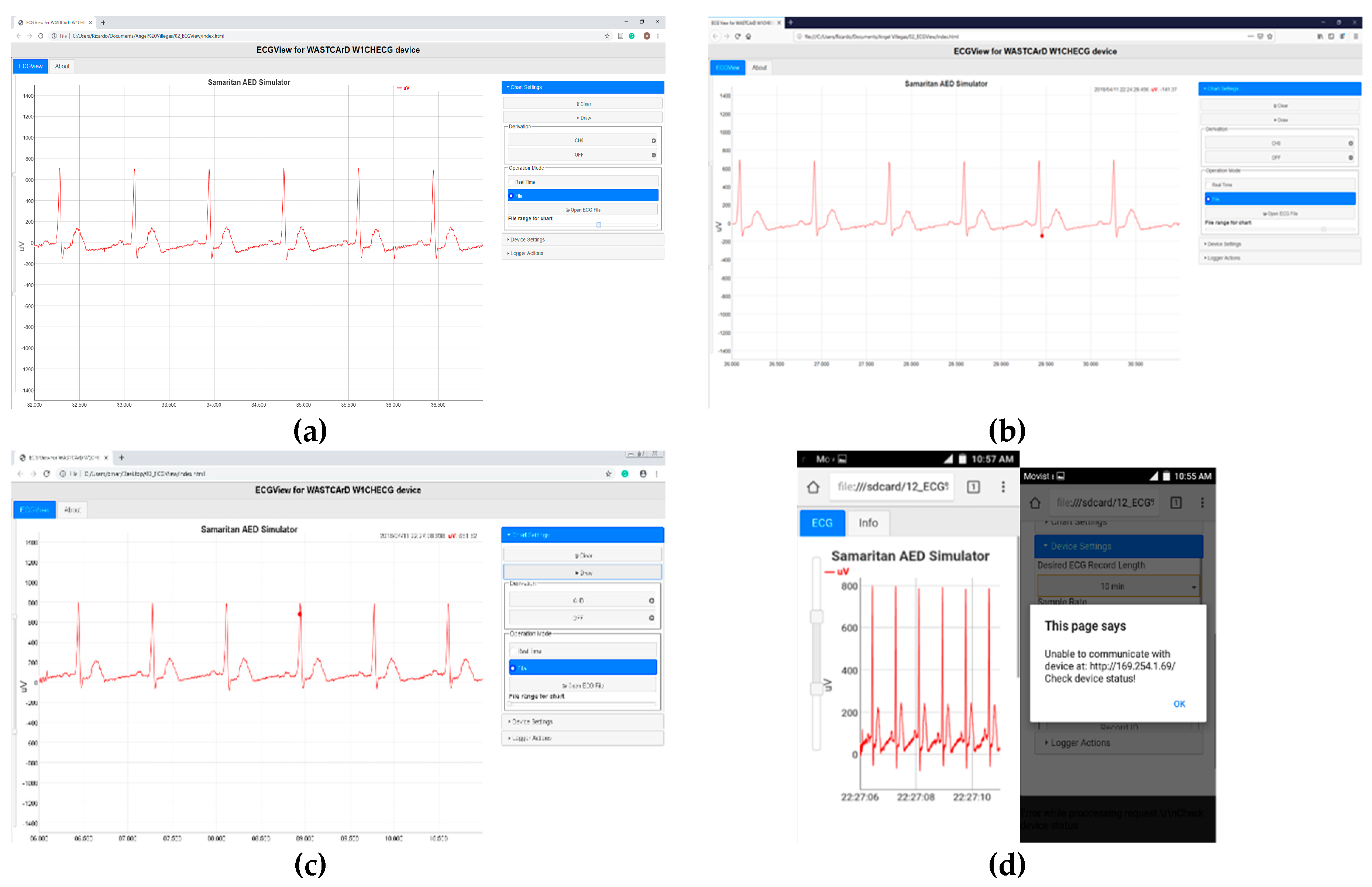
- Real-Time (monitor function): In this operation mode, the arm-ECG signal is acquired but not stored in the internal memory. Instead, it is sent to a connected client (computer or smartphone) for visualization. The main purpose of this operation mode is to initially ensure there is a good quality signal and correct skin–electrode contact before starting a longer-time recording.
- File (Holter function): The sensor acquires and stores the left-arm ECG signal in its internal memory. Later, the data can be downloaded to a personal computer or smartphone using a standard Web browser and the developed ECGView application. This is the default operation mode.
2.2.2. Data Storage Protocol
2.2.3. Wireless Communication Technology
2.3. Hardware Design
2.3.1. Power Supply and Battery Management
2.3.2. Digital Processor and Wireless Communication Management
2.3.3. Printed Circuit Board Design and Fabrication
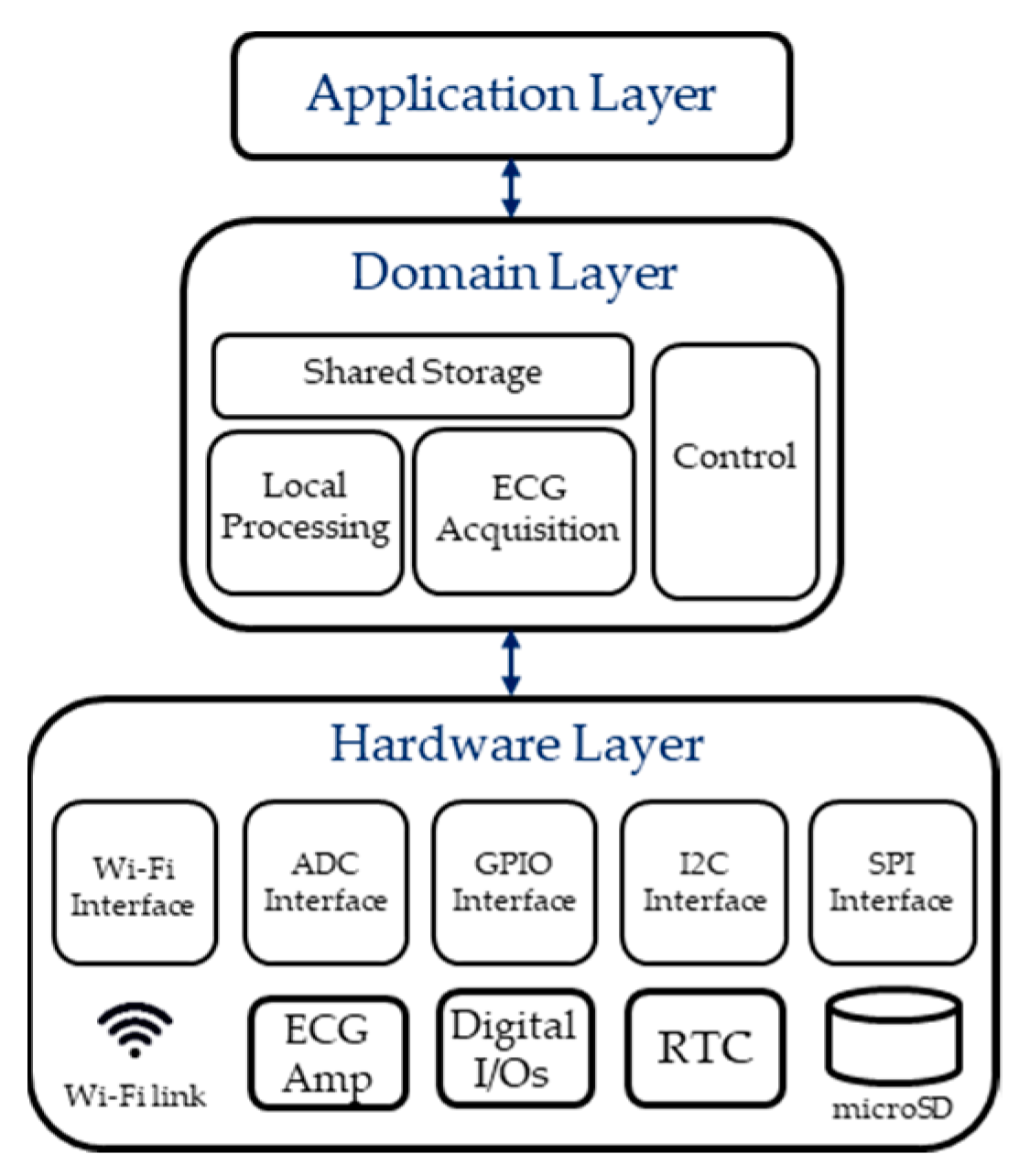
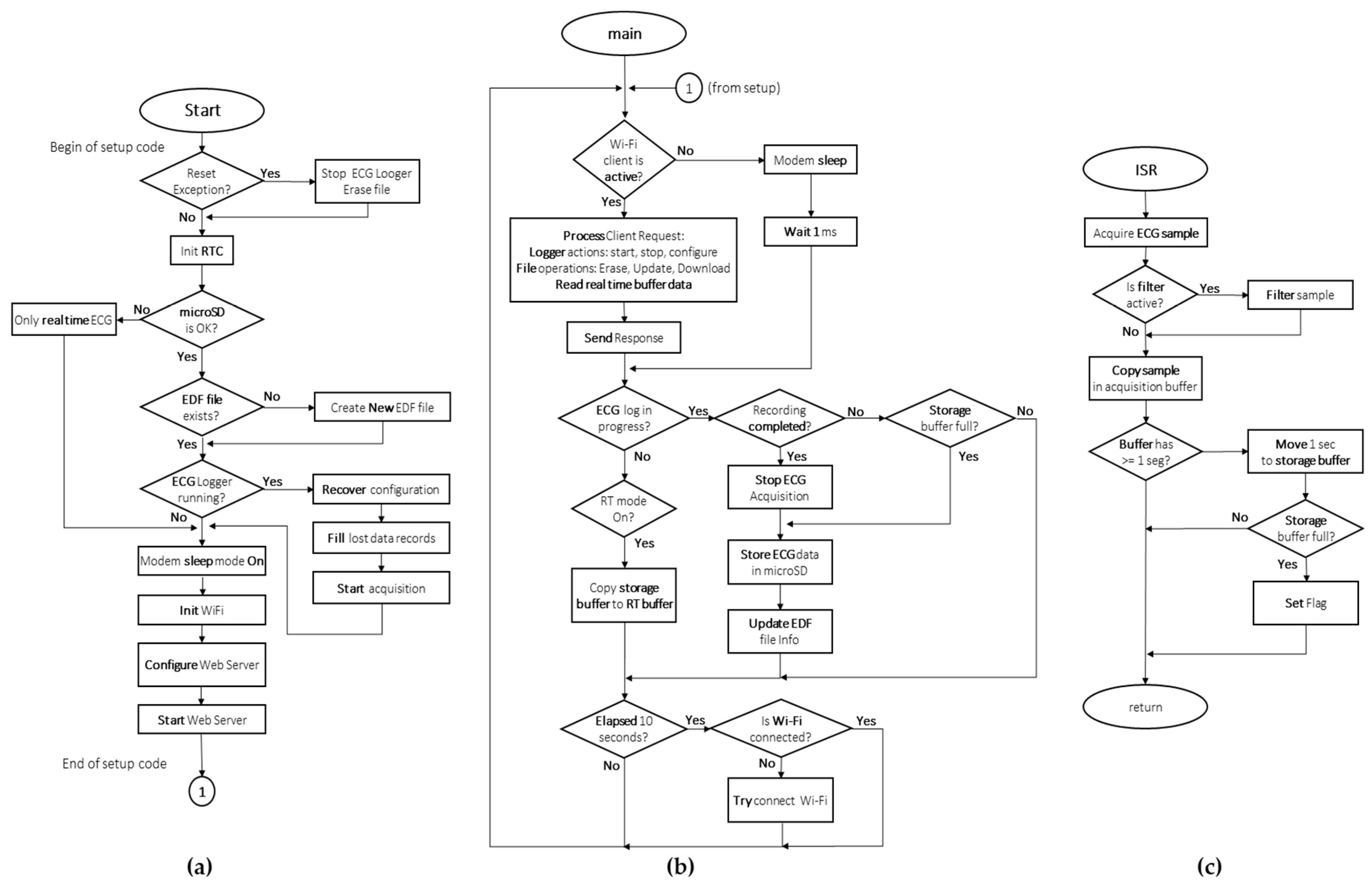
2.4. Firmware Architecture
2.4.1. Firmware Algorithm
2.4.2. Real-Time Visualization and Control Sub-System
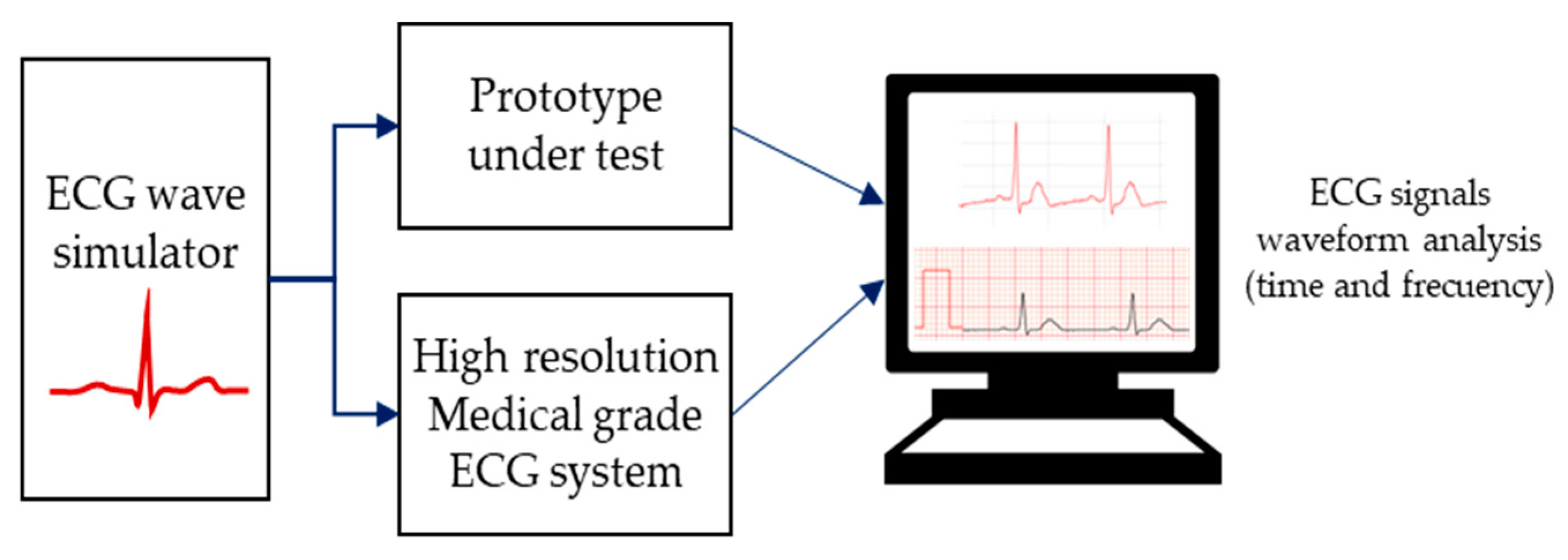
2.5. Device System Testing and Performance Assessment Methods
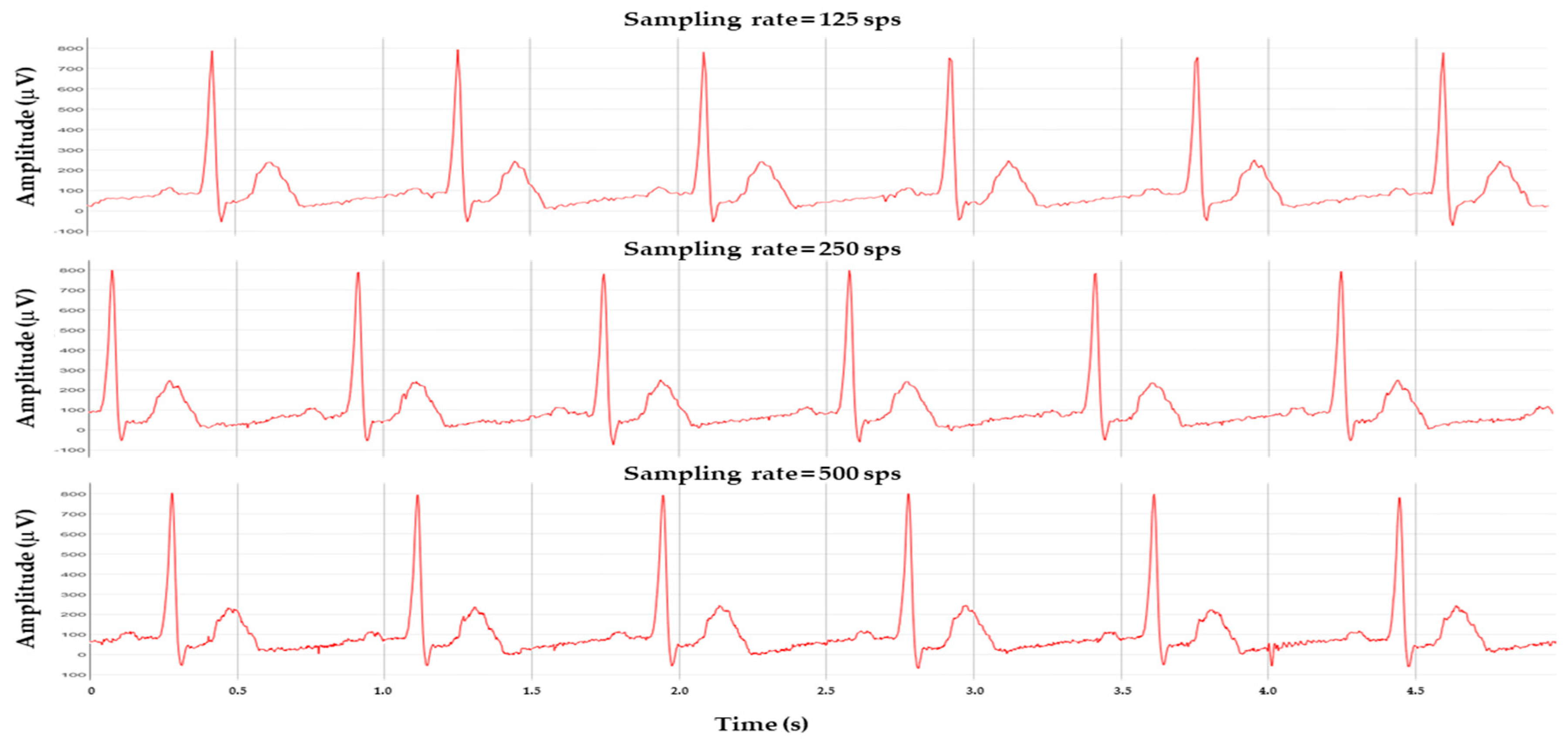
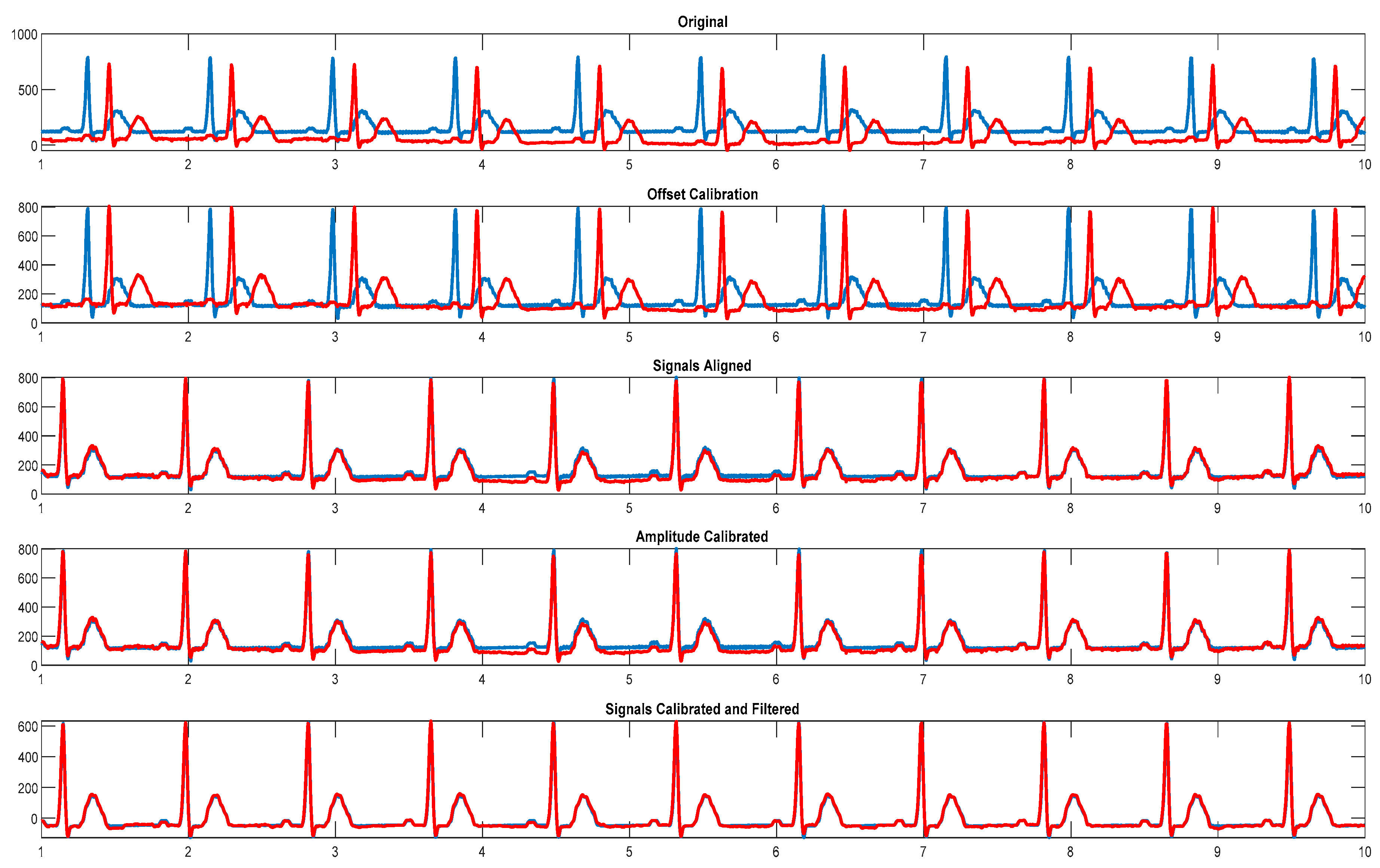
2.5.1. System Calibration and ECG Waveform Analysis
- The ECG signal is recorded on both devices for 60 seconds using a 250 Hz sampling rate.
- Resulting files are imported into Matlab software (MathWorks, Natick, MA, USA).
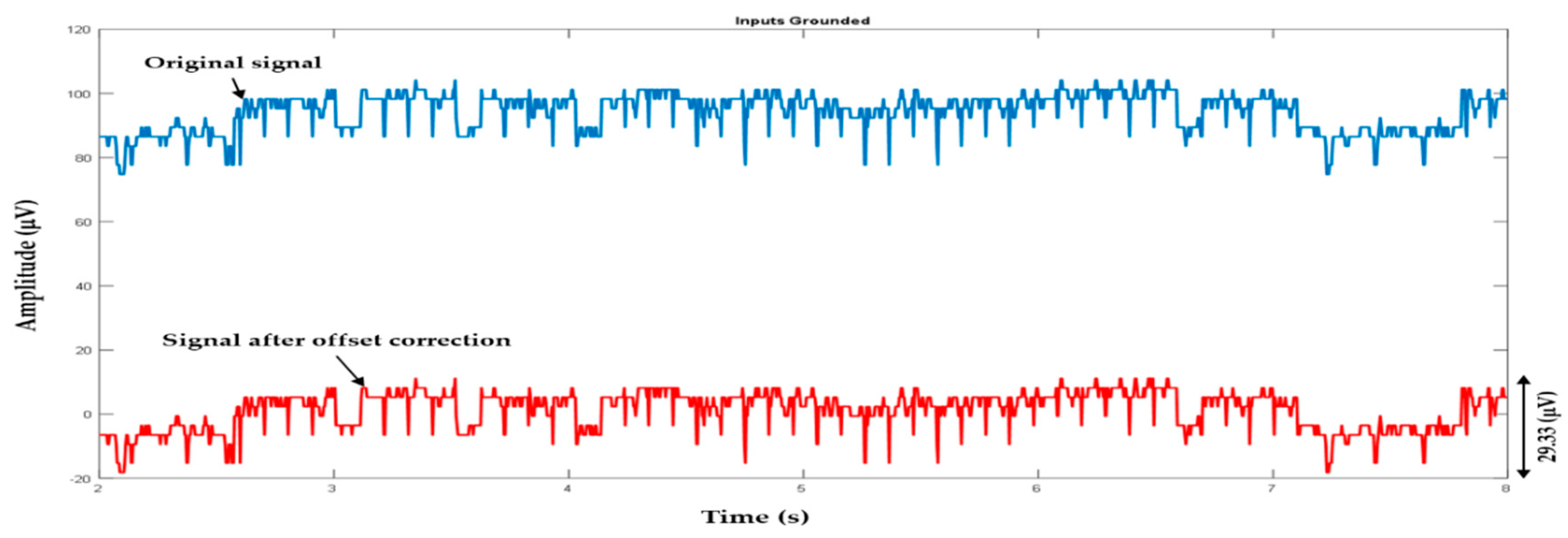
- Calculated mean value of the signals is used for offset correction.
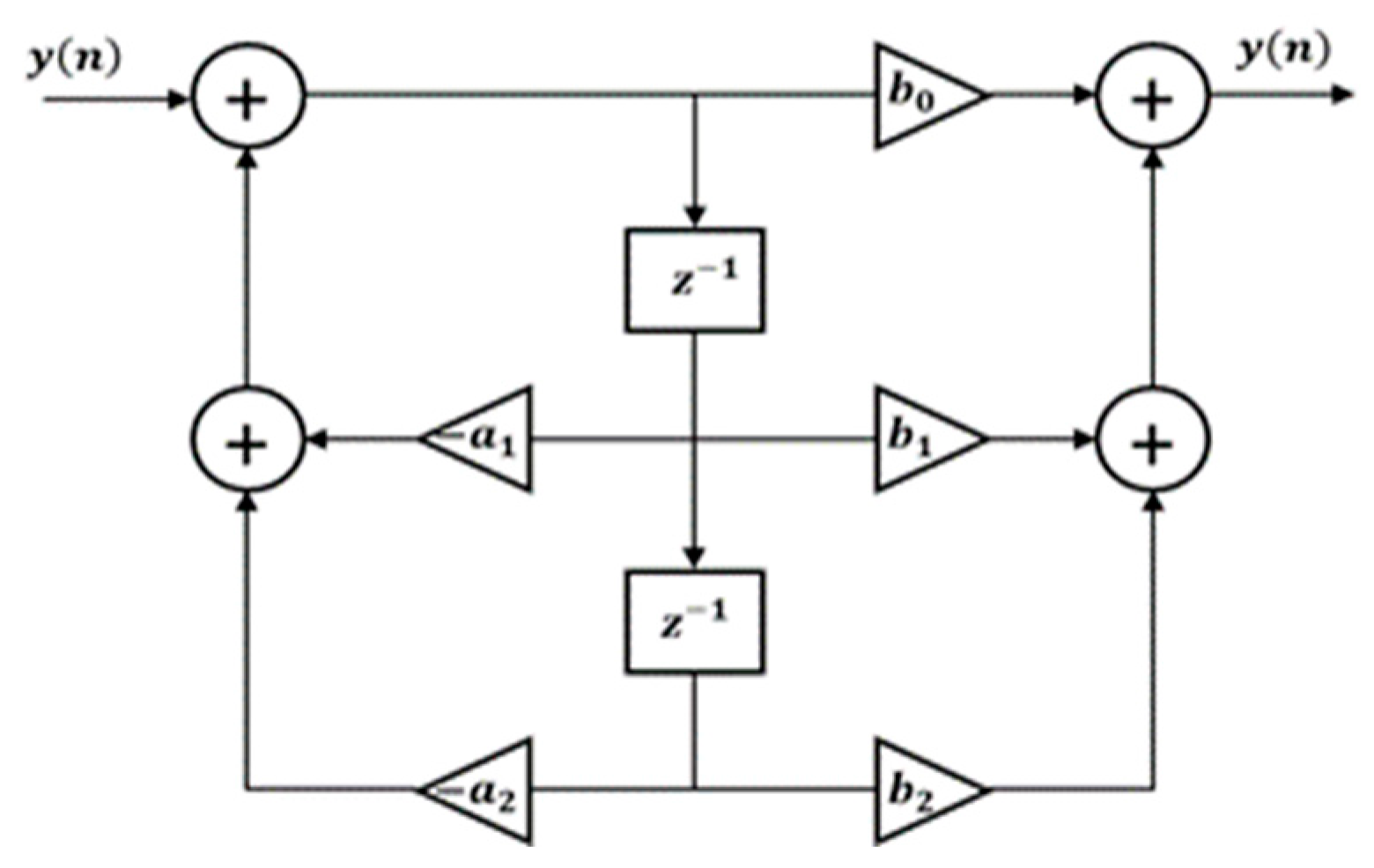
- Signals are digitally filtered using a 50 Hz notch filter with 2.5 Hz bandwidth.
- Signals are aligned using the normalized maximal cross-correlation between them.
- Difference in amplitude values of the R-peaks and S-peaks (maximal QRS complex signal amplitude span) of the aligned ECG signals is used to calculate required scaling factor value. For this calculation a 1-second signal window was used.
- The root-mean-square error (RMSE), mean absolute error (MAE) and cross-correlation coefficient (CC) are used as parameters to compare the similarity between signals after calibration.
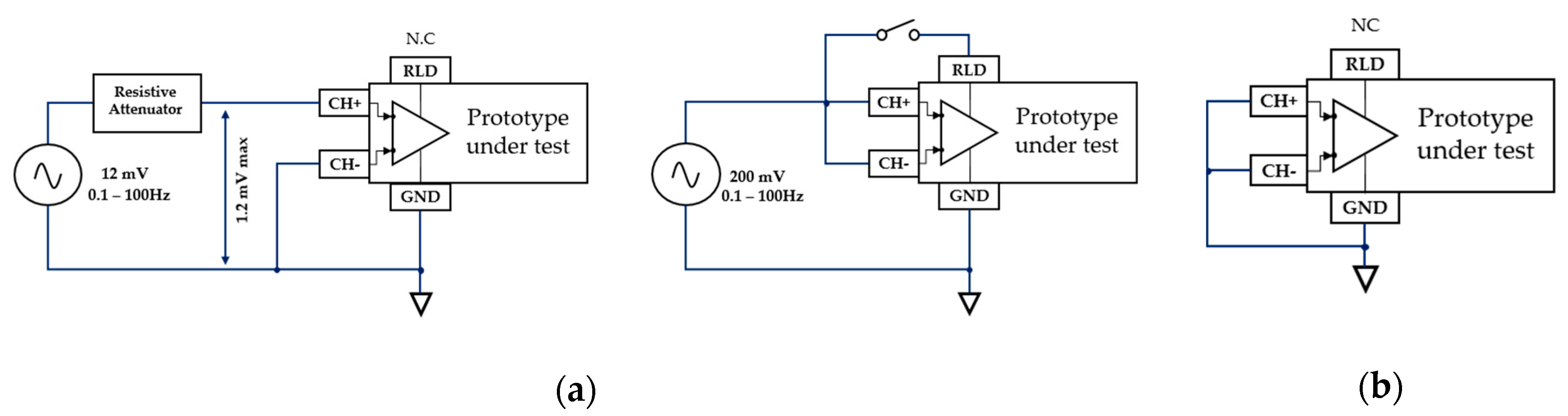
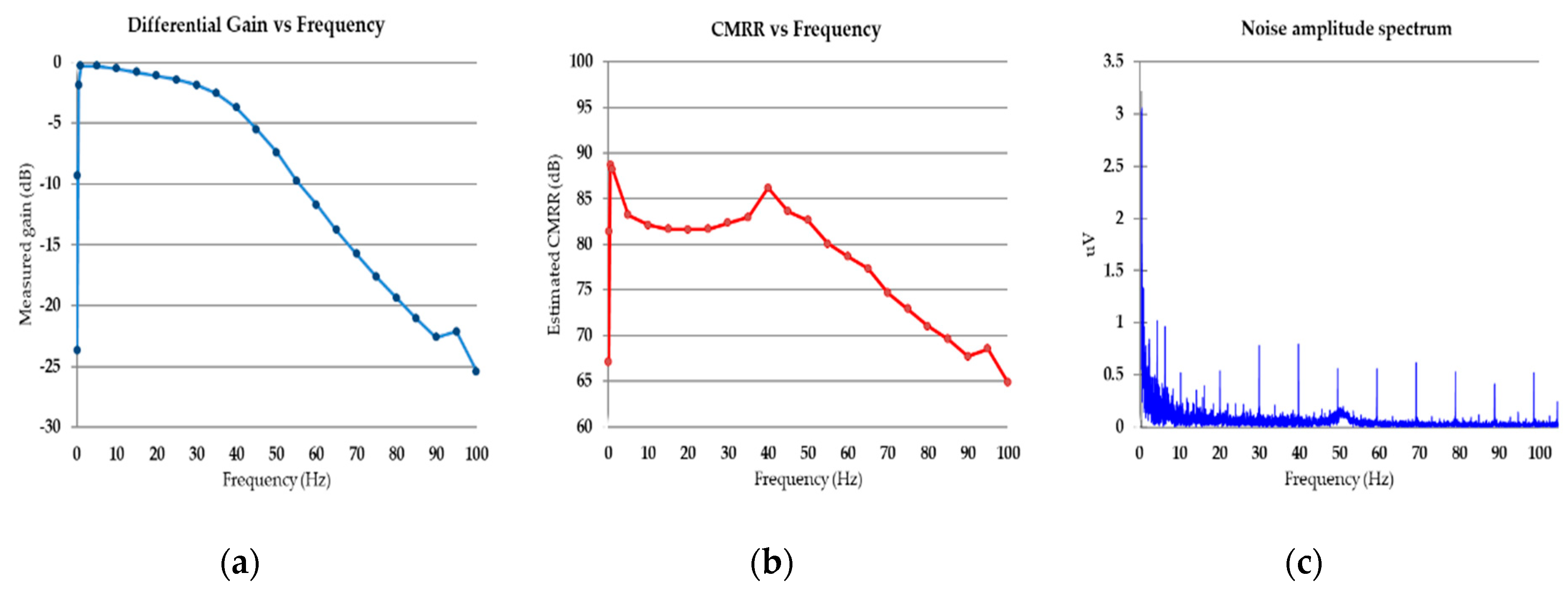
2.5.2. Amplifier Noise and CMRR Performance Assessment
2.5.3. R-peaks Detection Capacity from the Arm-ECG Signal
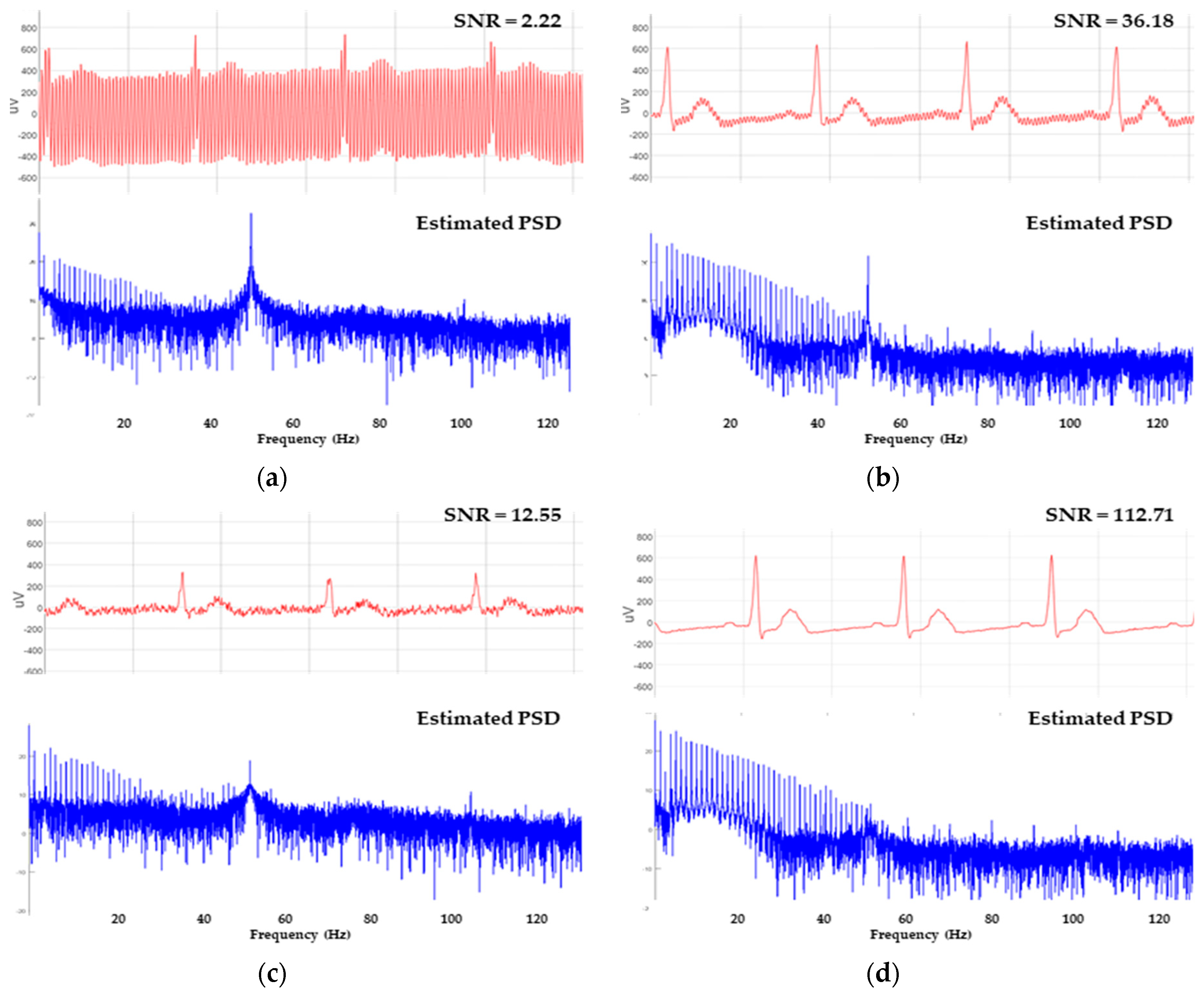
2.5.4. SNR Estimation on Recorded Signals
3. Results
3.1. Initial Offset Adjustment and System Adquisition Tests
3.2. System Calibration and Functional Validation
3.3. Front-End Amplifier Noise and CMRR Performance Assessment
3.4. Power-Line Noise Mitigation
3.5. Power Consumption, Authonomy and Other Specifications
3.6. In-Vivo Arm-ECG Recordings Assessment Results
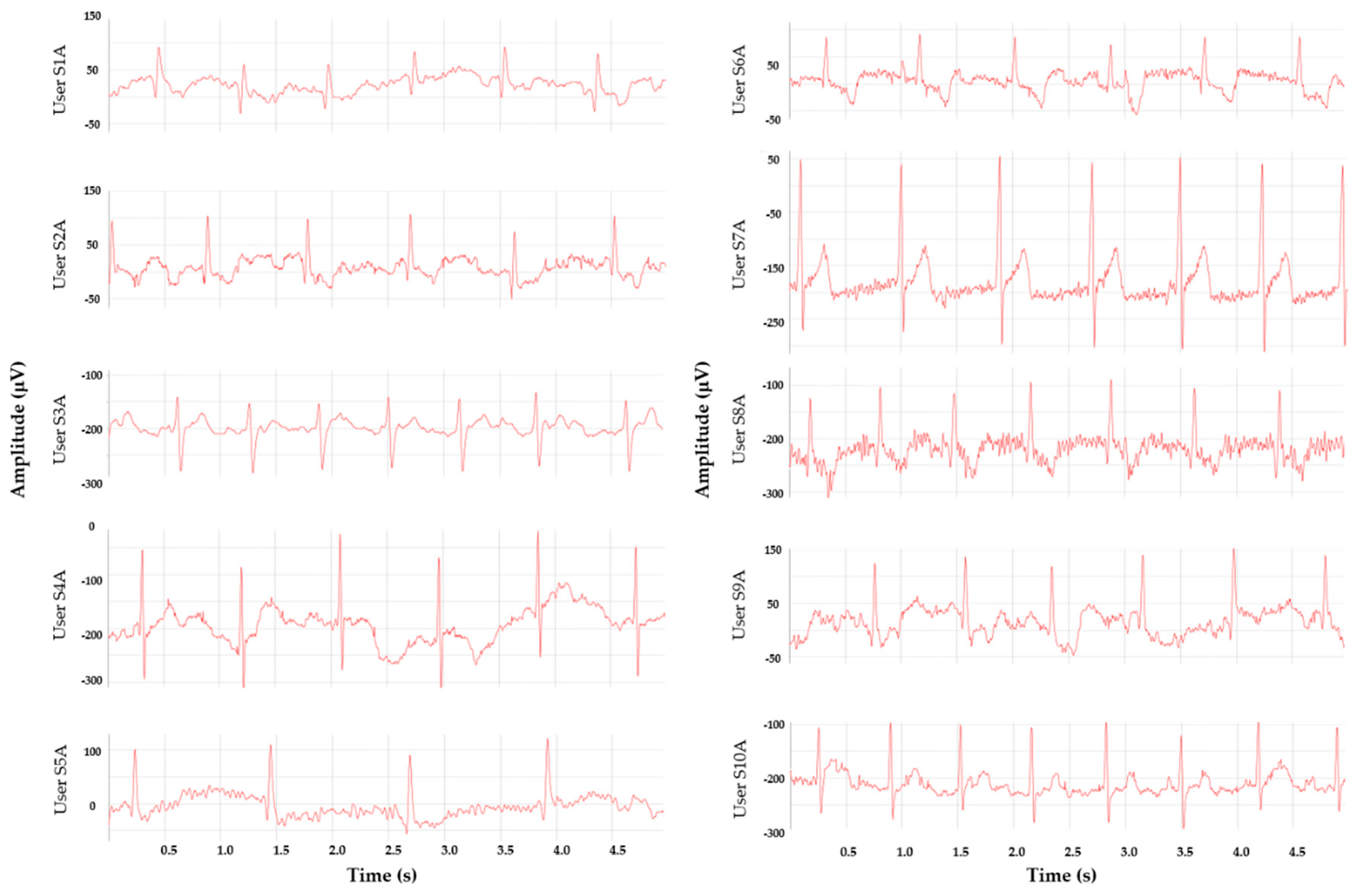
3.6.1. Arm-ECG Signals while Resting
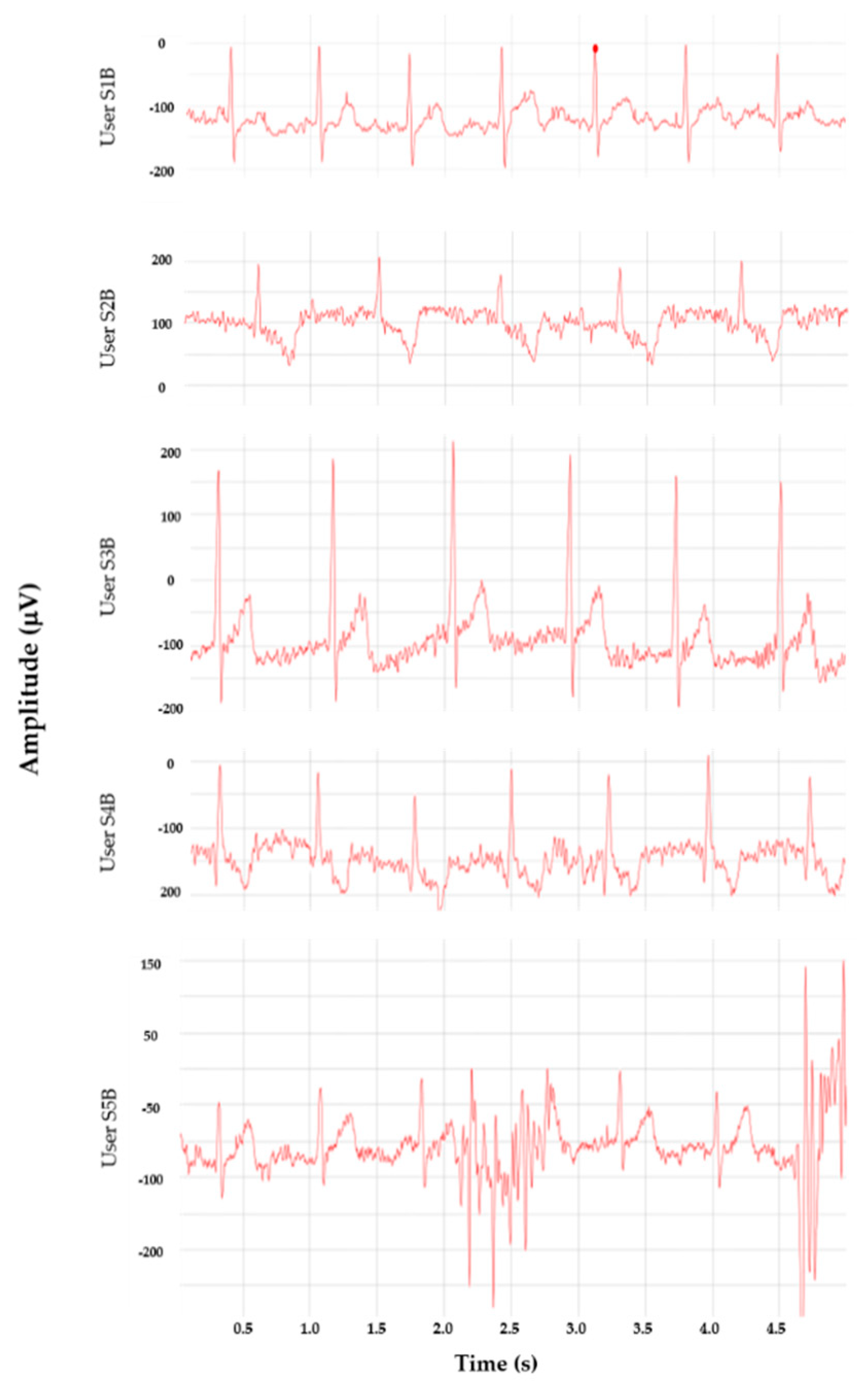
3.6.2. Arm-ECG Signals during Low-Level Physical Activity
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Katritsis, D.G.; Gersh, B.J.; Camm, A.J. A Clinical Perspective on Sudden Cardiac Death. Arrhythm. Electrophysiol. Rev. 2016, 5, 177–182. [Google Scholar] [CrossRef]
- Krahn, A.D.; Klein, G.J.; Skanes, A.C.; Yee, R. Insertable Loop Recorder Use for Detection of Intermittent Arrhythmias. Pacing Clin. Electrophysiol. 2004, 27, 657–664. [Google Scholar] [CrossRef]
- Lynn, W.D.; Escalona, O.J.; McEneaney, D.J. Arm and wrist surface potential mapping for wearable ECG rhythm recording devices: A pilot clinical study. J. Phys. Conf. Ser. 2013, 450, 3–8. [Google Scholar] [CrossRef]
- Escalona, O.J.; Lynn, W.D.; Perpiñan, G.; McFrederick, L.; McEneaney, D.J. Data-Driven ECG Denoising Techniques for Characterising Bipolar Lead Sets along the Left Arm in Wearable Long-Term Heart Rhythm Monitoring. Electronics 2017, 6, 84. [Google Scholar] [CrossRef]
- Escalona, O.; Mcfrederick, L.; Borges, M.; Linares Herrera, P.; Villegas, R.; Perpiñan, G.; McLaughlin, J.; Mceneaney, D. Wrist and Arm Body Surface Bipolar ECG Leads Signal and Sensor Study for Long-term Rhythm Monitoring. In Proceedings of the Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
- Dey, N.; Ashour, A.S.; Shi, F.; Fong, S.; Sherratt, R. Developing Residential Wireless Sensor Networks for ECG Healthcare Monitoring. IEEE Trans. Consum. Electron. 2017, 63, 442–449. [Google Scholar] [CrossRef]
- Boehm, A.; Yu, X.; Neu, W.; Leonhardt, S.; Teichmann, D. A Novel 12-Lead ECG T-Shirt with Active Electrodes. Electronics 2016, 5, 75. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef]
- Chowdhury, M.E.H.; Alzoubi, K.; Khandakar, A.; Khallifa, R.; Abouhasera, R.; Koubaa, S.; Ahmed, R.A.; Hasan, A. Wearable Real-Time Heart Attack Detection and Warning System to Reduce Road Accidents. Sensors 2019, 19, 2780. [Google Scholar] [CrossRef]
- Massot, B.; Hutu, F.; Gehin, C.; Noury, N. Design and Optimization of an Autonomous, Ambulatory Cardiac Event Monitor. In Proceedings of the IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018. [Google Scholar]
- Liu, S.; Cai, G.; Huang, Y.; Chen, Y. A wearable ECG apperatus for ubiquitous health care. In Proceedings of the IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016; pp. 4471–4476. [Google Scholar]
- Preejith, S.P.; Dhinesh, R.; Joseph, J.; Sivaprakasam, M. Wearable ECG platform for continuous cardiac monitoring. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 623–626. [Google Scholar]
- Rachim, V.P.; Chung, W. Wearable Noncontact Armband for Mobile ECG Monitoring System. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 1112–1118. [Google Scholar] [CrossRef]
- Lynn, W.; Escalona, O.; McEneaney, D. A Low Latency Electrocardiographic QRS Activity Recovery Technique for Use on the Upper Left Arm. Electronics 2014, 3, 409–418. [Google Scholar] [CrossRef]
- Vizcaya, P.R.; Perpiñan, G.I.; McEneaney, D.J.; Escalona, O.J. Standard ECG lead I prospective estimation study from far-field bipolar leads on the left upper arm: A neural network approach. Biomed. Signal Process. Control 2019, 51, 171–180. [Google Scholar] [CrossRef]
- Gemperle, F.; Kasabach, C.; Stivoric, J.; Bauer, M.; Martin, R. Design for Wearability. In Proceedings of the ISWC Second International Symposium on Wearable Computers (ISWC’98), Pittsburgh, PA, USA, 19–20 October 1998; p. 116. [Google Scholar]
- Chen, C.-Y.; Chang, C.-L.; Chang, C.-W.; Lai, S.-C.; Chien, T.-F.; Huang, H.-Y.; Chiou, J.-C.; Luo, C.-H. A Low-Power Bio-Potential Acquisition System with Flexible PDMS Dry Electrodes for Portable Ubiquitous Healthcare Applications. Sensors 2013, 13, 3077–3091. [Google Scholar] [CrossRef]
- Aleksandrowicz, A.; Leonhardt, S. Wireless and Non-contact ECG Measurement System—The “Aachen SmartChair”. Acta Polytech. 2007, 47, 68–71. [Google Scholar]
- Nemati, E.; Deen, M.J.; Mondal, M. A Wireless Wearable ECG Sensor for Long-Term Applications. IEEE Commun. Mag. 2012, 50, 36–43. [Google Scholar] [CrossRef]
- Saponara, S.; Donati, M.; Fanucci, L.; Celli, A. An Embedded Sensing and Communication Platform, and a Healthcare Model for Remote Monitoring of Chronic Diseases. Electronics 2016, 5, 47. [Google Scholar] [CrossRef]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, 47–69. [Google Scholar] [CrossRef]
- Bosnjak, A.; Kennedy, A.; Linares, P.; Borges, M.; McLaughlin, J.; Escalona, O.J. Performance assessment of dry electrodes for wearable long term cardiac rhythm monitoring: Skin-electrode impedance spectroscopy. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 1861–1864. [Google Scholar]
- Hoffmann, K.; Ruff, R.; Poppendieck, W. Long-Term Characterization of Electrode Materials for Surface Electrodes in Biopotential Recording. In Proceedings of the International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 2239–2242. [Google Scholar]
- Tallgren, P.; Vanhatalo, S.; Kaila, K.; Voipio, J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin. Neurophysiol. 2005, 16, 799–806. [Google Scholar] [CrossRef]
- Neuman, M.R. Biopotential Electrodes. In Medical Instrumentation: Application and Design; Webster, J.G., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 189–241. [Google Scholar]
- Gifari, M.W.; Zakaria, H.; Richard, M. Design of ECG Homecare: 12-lead ECG acquisition using single channel ECG device developed on AD8232 analog front end. In Proceedings of the International Conference on Electrical Engineering and Informatics (ICEEI), Denpasar, Indonesia, 10–11 August 2015; pp. 371–376. [Google Scholar]
- Bond, R.R.; Finlay, D.D.; Nugent, C.D.; Moore, G. A review of ECG storage formats. Int. J. Med Inform. 2011, 80, 681–697. [Google Scholar] [CrossRef]
- Kemp, B.; Värri, A.; Rosa, A.C.; Nielsen, K.D.; Gade, J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 391–393. [Google Scholar] [CrossRef]
- Gaxiola-Sosa, J.E.; Nasreen Mohsin, A.J.; Palliyali, R.T.; Kamran, E. A PorTable 12-Lead ECG Wireless Medical System for Continuous Cardiac-Activity Monitoring. In Proceedings of the IEEE Middle East Conference on Biomedical Engineering (MECBME), Doha, Qatar, 17–20 February 2014; pp. 123–126. [Google Scholar]
- Lee, S.H.; Jung, S.M.; Lee, C.K.; Jeong, K.S.; Cho, G.; Yoo, S.K. Wearable ECG Monitoring System Using Conductive Fabrics and Active Electrodes. In International Conference on Human-Computer Interaction; Springer: Berlin/Heidelberg, Germany, 2009; pp. 778–783. [Google Scholar]
- ElSaadany, Y.; Majumder, A.J.A.; Ucci, D.R. A Wireless Early Prediction System of Cardiac Arrest through IoT. In Proceedings of the IEEE 41st Annual Computer Software and Applications Conference, Turin, Italy, 4–8 July 2017. [Google Scholar]
- Touati, F.; Tabish, R.; Mnaouer, A.B. A Real-Time BLE enabled ECG System for Remote Monitoring. APCBEE Procedia 2013, 7, 124–131. [Google Scholar] [CrossRef]
- Pineda-López, F.; Martínez-Fernández, A.; Rojo-Álvarez, J.L.; García-Alberola, A.; Blanco-Velasco, M. A Flexible 12-Lead/Holter Device with Compression Capabilities for Low-Bandwidth Mobile-ECG Telemedicine Applications. Sensors 2018, 18, 3773. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, S.S.; Pillai, B.C. Design of Wi-Fi Based Mobile Electrocardiogram Monitoring System on Concerto Platform. Procedia Eng. 2013, 64, 65–73. [Google Scholar] [CrossRef][Green Version]
- Khalaf, A.; Abdoola, R. Wireless Body Sensor Network and ECG Android Application for eHealth. In Proceedings of the Fourth International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 19–21 October 2017. [Google Scholar]
- Luz, E.J.D.S.; Schwartz, W.R.; Cámara-Chávez, G.; Menotti, D. ECG-based heartbeat classification for arrhythmia detection: A survey. Comput. Methods Programs Biomed. 2016, 127, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Escalona, O.J.; Mitchell, R.H.; Balderson, D.E.; Harron, D.W. Fast and reliable QRS alignment technique for high-frequency analysis of signal-averaged ECG. Med. Biol. Eng. Comput. 1993, 31, 137–146. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Wang, W.J. QRS complexes detection for ECG signal: The Difference Operation Method. Comput. Methods Programs Biomed. 2008, 91, 245–254. [Google Scholar] [CrossRef]
- Jani, A.B.; Bagree, R.; Roy, A.K. Design of a low-power, low-cost ECG & EMG sensor for wearable biometric and medical application. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar] [CrossRef]
- Celik, N.B.W.; Manivannan, N.; Winter, E.; Schnalzer, B.; Burgsteiner, H. Wearable mobile ear-based ECG monitoring device using graphene-coated sensors. In Proceedings of the IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017. [Google Scholar]
- Vinciguerra, V.A.E.; Maddiona, L.; Romeo, M.; Mazzillo, M. PPG/ECG Multisite Combo System Based on SiPM Technology. In Convegno Nazionale Sensori; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Lynn, W.D.; Escalona, O.J.; Vizcaya, P.R.; McEneaney, D.J. Arm-ECG bipolar leads signal recovery methods for wearable long-term heart rate and rhythm monitoring. In Proceedings of the Computing in Cardiology (CinC), Rennes, France, 24–27 September 2017. [Google Scholar]
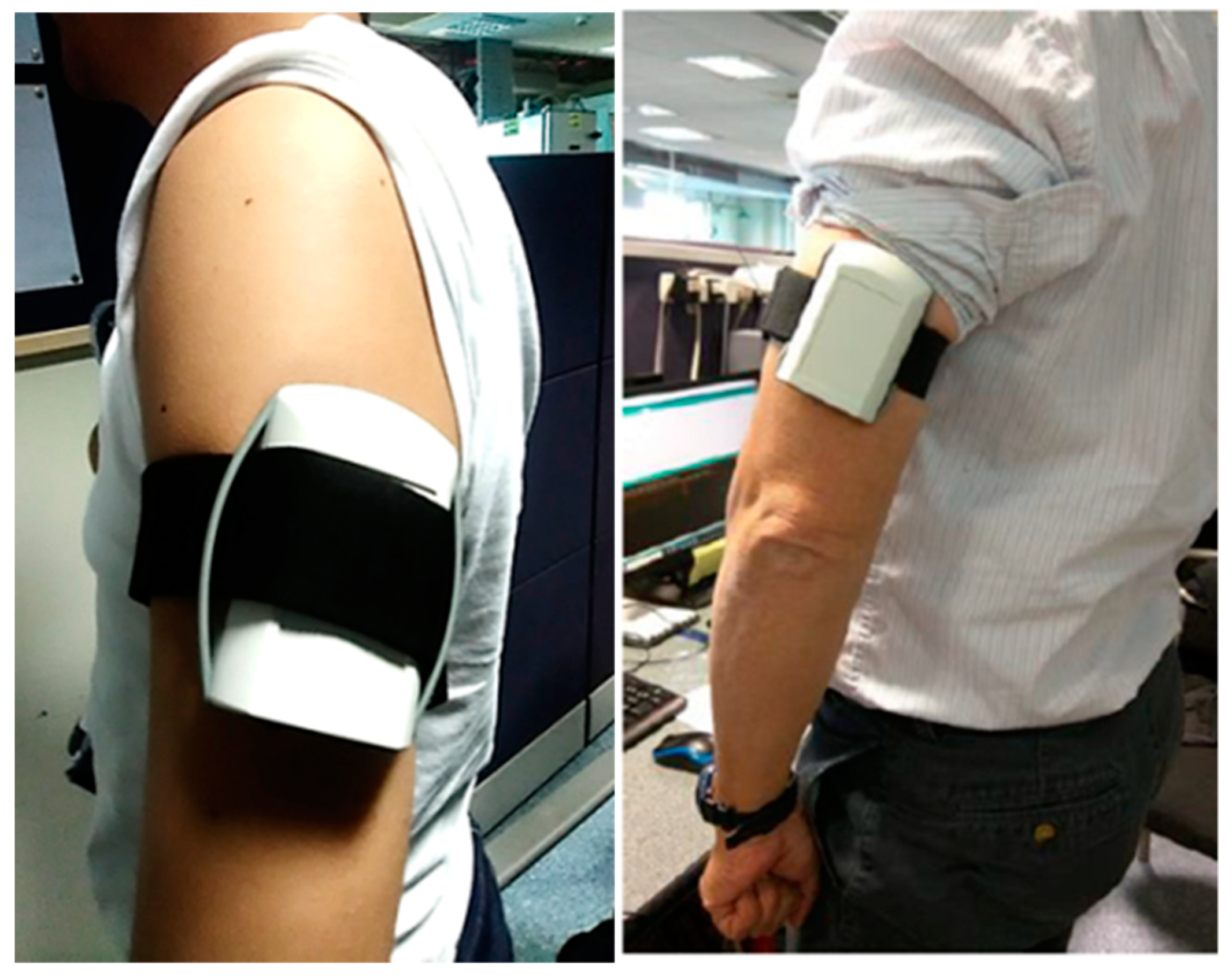
| Characteristic | ADS129X | ADS119X | ADAS1000-3 | AD8233 | AD8232 | MAX30001 |
|---|---|---|---|---|---|---|
| Input channels | 2,4,6,8 | 4,6,8 | 3 | 1 | 1 | 1 |
| Noise level (uVpp) | 4–8 | 12 | 14 | 8.5 | 12 | 6.6 |
| Single supply | Yes | Yes | No | Yes | Yes | No |
| ADC bits | 24 | 16 | 24 | N/A | N/A | 18 |
| Adjustable filters | No | No | Yes | Yes | Yes | Yes |
| Gain range | 1 to 12 | 1 to 12 | 1.4 to 4.2 | min 100 | min 100 | 20 to 160 |
| Typical package | 28/64 TQFP | 64 TQFP | 64 LQFP | 20 WLCSP | 20 LFCSP | 30 WLCSP |
| Hand soldering | Yes | Yes | Yes | No | Yes | No |
| Referential cost (£) | 10.11–31.28 | 14.24–22.85 | 17.37 | 3.68 | 3.0 | 9.13 |
| Author | Wireless Technology | Power Consumption | System Data Rate |
|---|---|---|---|
| Nemati et al. [19] | ISM 2.4 GHz (ANT) | 25 mA | 38.4 kbps |
| Gaxiola-Sosa et al. [29] | ISM 2.45 GHz | 33–40 mA | 500 kbps |
| Lee et al. [30] | ZigBee | 17.4–18.8 mA | 250 kbps |
| Aleksandrowicz and Leonhardt [18] | ZigBee | 15 mA | 250 kbps |
| Rachim and Chung [13] | Bluetooth | 19.6–24 mA | ND |
| ElSaadany et al. [31] | Bluetooth | 3–30 mA | 300 kbps |
| Touati et al. [32] | Bluetooth | 27 mA | 1 Mbps |
| Pineda-López et al. [33] | Bluetooth | 59.58–110 mA | 8.192 kbps |
| Ahammed and Pillai [34] | Wi-Fi | 260–275 mA | 24 Mbps |
| Khalaf and Abdoola [35] | Wi-Fi | 309 mA | 1 Mbps |
| Sps | SNR (Raw Data) 1 | SNR (Smoothed Data) 1 | SNR (Raw Data) 2 | SNR (Smoothed Data) 2 |
|---|---|---|---|---|
| 125 | 103.31 | 103.47 | 77.03 | 91.20 |
| 250 | 86.37 | 118.85 | 78.71 | 112.59 |
| 500 | 82.13 | 113.88 | 85.40 | 116.05 |
| Parameter | After Calibration | Calibration + Filtering |
|---|---|---|
| MAE | 18.19 uV (0.61% of fs) | 7.25 uV (0.24% of fs) |
| RMSE | 22.13uV (0.74% of fs) | 11.08 uV (0.34% of fs) |
| CC | 0.983 | 0.997 |
| Operative System | Basic Description | Web Browser | |
|---|---|---|---|
| Personal Computer (PC) | Windows 10 64 bits | Intel Core i7 8 GB RAM | Google Chrome (v76) Mozilla Firefox (v59) |
| Laptop Computer | Window 7 64 bits | Intel Pentium P6100 4 GB RAM | Google Chrome (v76) Mozilla Firefox (v) |
| Mobile | Android 5.1.1 | Qualcomm Snapdragon 210 1 GB RAM | Google Chrome (v46) |
| Operating Condition | Average Current (mA) | Estimated Autonomy (hours) |
|---|---|---|
| Holter mode (recording) | 18.0 | 127 |
| Holter mode (not recording) | 27.8 | 82 |
| Real-time mode | 35.3 | 65 |
| File transmission | 100 mA | N/A |
| ECG Recording Length (min) | File Size (KB) | Download time (s) 1 | Transfer Rate (Mbps) |
|---|---|---|---|
| 5 | 142 | 0.581 | 2.03 |
| 30 | 875 | 2.699 | 2.66 |
| 60 | 1754 | 5.85 | 2.46 |
| 360 | 10543 | 30.42 | 2.86 |
| Item | Value |
|---|---|
| Data channels | 1 |
| Sampling rate | 125, 250, 500 Hz |
| Electrode type | Sintered Ag/AgCl |
| Wireless Communication | Wi-Fi |
| Average data transfer rate | 2.5 Mbps |
| Storage media | MicroSD Card (up to 32 GB capacity) |
| Battery used | 3.7 VDC @ 2300 mAh Rechargeable LiPo |
| Battery charge current | ~425 mA |
| Operation time | Up to 72 h |
| External power input | 5 to 7.5 V DC |
| Average power consumption | 18 mA (normal operation) 36 mA (real-time mode) ~100 mA (Wi-Fi file transmission) |
| User | Manual Count | R-peaks Detected | Se (%) | PPV (%) | F1-Score (%) | SNR [5] | SNR [4] |
|---|---|---|---|---|---|---|---|
| S1A | 64 | 64 | 100 | 100 | 100 | 23.60 | 20.17 |
| S2A | 58 | 58 | 100 | 100 | 100 | 20.87 | 17.20 |
| S3A | 73 | 73 | 100 | 100 | 100 | 39.88 | 33.84 |
| S4A | 58 | 57 | 98.28 | 100 | 99.13 | 37.56 | 25.82 |
| S5A | 43 | 43 | 100 | 100 | 100 | 16.30 | 15.01 |
| S6A | 59 | 60 | 100 | 98.33 | 99.16 | 11.92 | 11.39 |
| S7A | 62 | 62 | 100 | 100 | 100 | 22.62 | 19.21 |
| S8A | 66 | 66 | 100 | 100 | 100 | 11.67 | 10.27 |
| S9A | 59 | 60 | 98.31 | 96.67 | 96.67 | 13.09 | 11.55 |
| S10A | 71 | 74 | 100 | 95.95 | 97.93 | 22.68 | 17.30 |
| User | Manual Count | R-Peaks Detected | TP | Se (%) | PPV (%) | F1-Score (%) | SNR [5] | SNR [4] | Recording Time (seconds) |
|---|---|---|---|---|---|---|---|---|---|
| S1B | 873 | 878 | 838 | 95.99 | 95.44 | 95.72 | 19.29 | 14.79 | 7190 |
| S2B | 722 | 751 | 709 | 98.20 | 94.41 | 96.27 | 18.59 | 18.86 | 3590 |
| S3B | 745 | 744 | 688 | 92.72 | 92.47 | 92.60 | 24.16 | 19.98 | 3590 |
| S4B | 764 | 790 | 693 | 91.30 | 87.83 | 89.53 | 10.95 | 10.89 | 3590 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas, A.; McEneaney, D.; Escalona, O. Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics 2019, 8, 1300. https://doi.org/10.3390/electronics8111300
Villegas A, McEneaney D, Escalona O. Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics. 2019; 8(11):1300. https://doi.org/10.3390/electronics8111300
Chicago/Turabian StyleVillegas, Angel, David McEneaney, and Omar Escalona. 2019. "Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias" Electronics 8, no. 11: 1300. https://doi.org/10.3390/electronics8111300
APA StyleVillegas, A., McEneaney, D., & Escalona, O. (2019). Arm-ECG Wireless Sensor System for Wearable Long-Term Surveillance of Heart Arrhythmias. Electronics, 8(11), 1300. https://doi.org/10.3390/electronics8111300






