Multiple Wavelength Optical Coherence Tomography Assessments for Enhanced Ex Vivo Intra-Cochlear Microstructural Visualization
Abstract
1. Introduction
2. Materials and Methods
2.1. Multiple Wavelength OCT Technical Configurations
2.2. Specimens Preparation
3. Results and Discussion
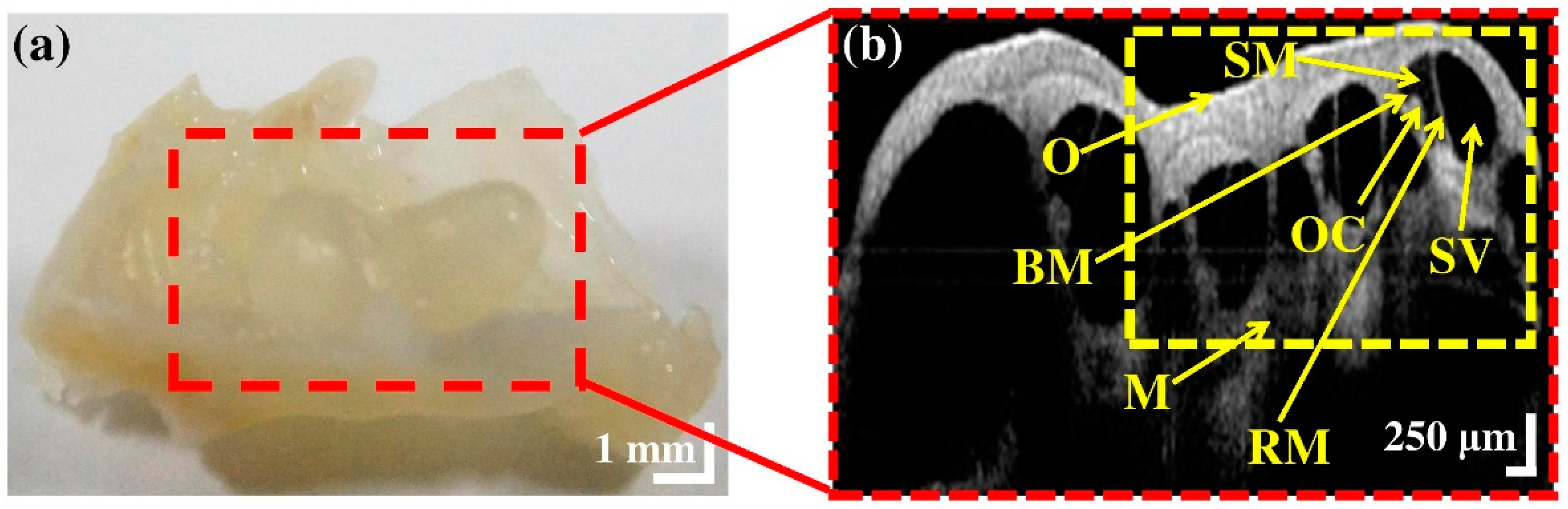
3.1. Morphological Representation of Intra-Cochlear Microstructures Ex Vivo
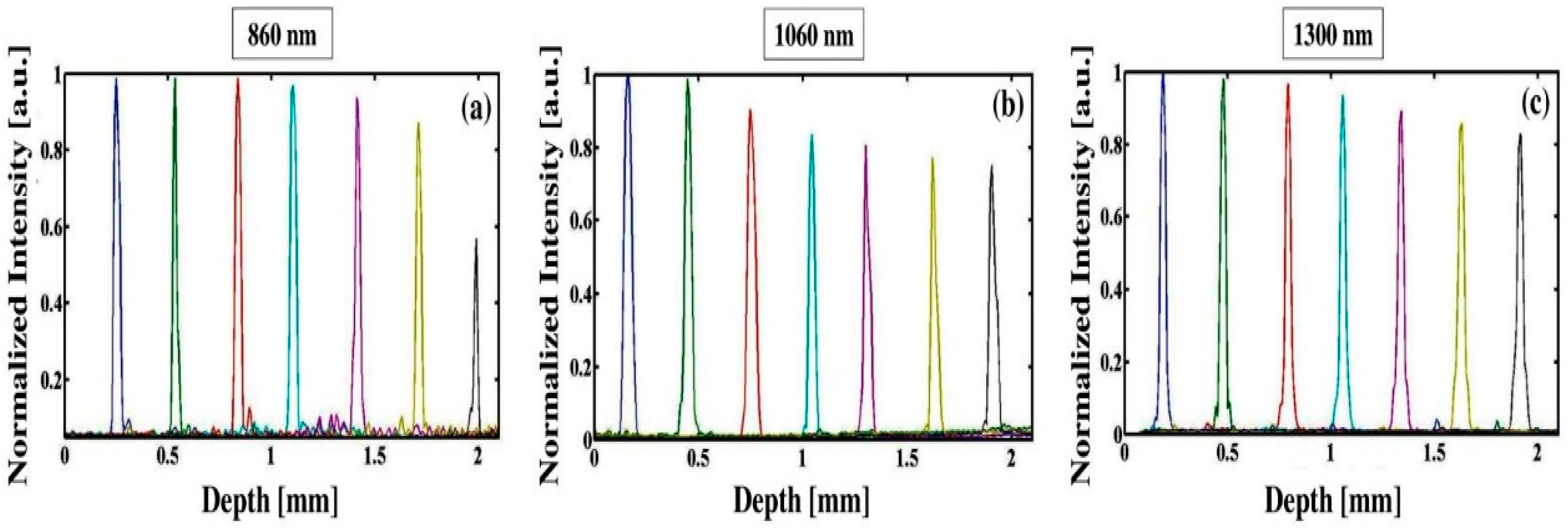
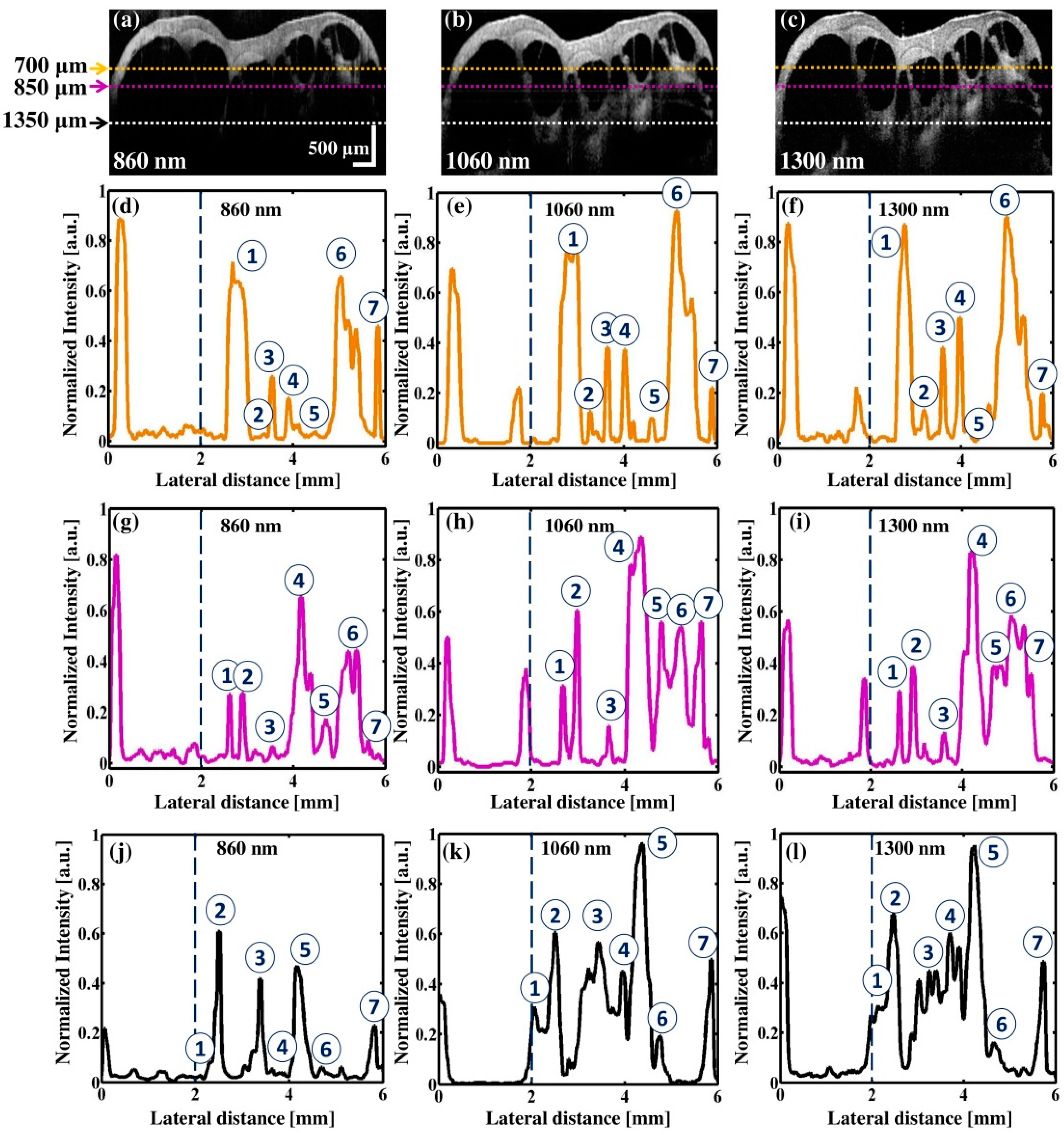
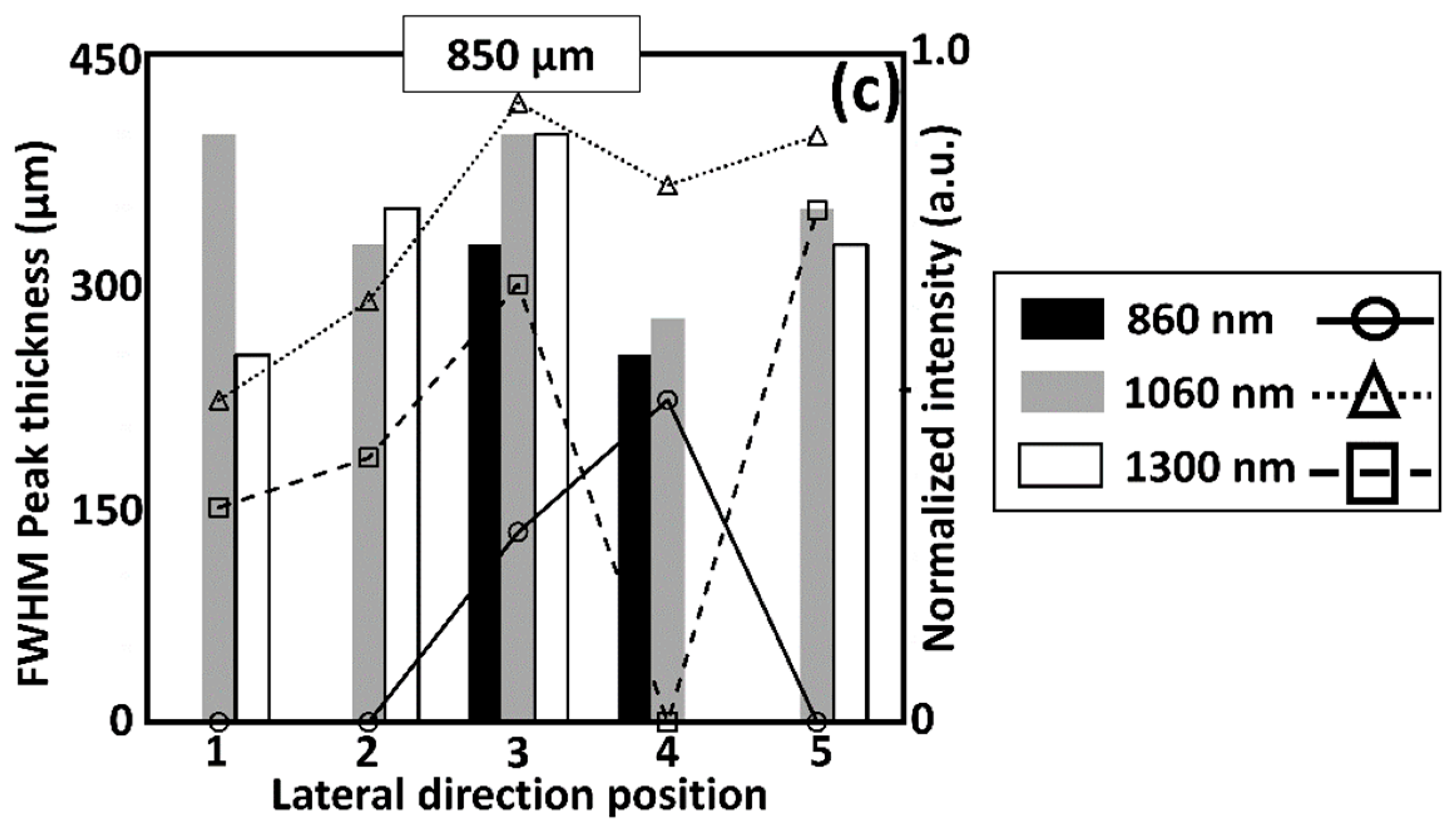
3.2. Comparison of Multiple Wavelength OCT Cross-Sectional Images and the Corresponding Normalized Intensity Fluctuations
3.3. The Comparison between Enface Representations of Multiple Wavelength OCT Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, H.; Paparella, M.M. Meniere’s disease. Lancet 2008, 372, 406–414. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M. Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Cruickshanks, K.J.; Wiley, T.L.; Tweed, T.S.; Klein, B.E.; Klein, R.; Mares-Perlman, J.A.; Nondahl, D.M. Prevalence of hearing loss in older adults in beaver dam, wisconsin the epidemiology of hearing loss study. Am. J. Epidemiol. 1998, 148, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Kesser, B.W.; Kenna, M.A. The child with hearing loss. Otolaryngol. Clin. N. Am. 2015, 48, xv–xvi. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.; Flaxman, S.; Brunskill, E.; Mascarenhas, M.; Mathers, C.D.; Finucane, M. Global and regional hearing impairment prevalence: An analysis of 42 studies in 29 countries. Eur. J. Public Health 2011, 23, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Raphael, Y.; Altschuler, R.A. Structure and innervation of the cochlea. Brain Res. Bull. 2003, 60, 397–422. [Google Scholar] [CrossRef]
- Karvonen, T.; Uranishi, Y.; Sakamoto, T.; Tona, Y.; Okamoto, K.; Tamura, H.; Kuroda, T. Estimation of the degree of endolymphatic hydrops using optical coherence tomography. Adv. Biomed. Eng. 2016, 5, 19–25. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Li, J.; Wang, Q. A contrastive analysis of laser heating between the human and guinea pig cochlea by numerical simulations. Biomed. Eng. Online 2016, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Kakigi, A.; Takubo, Y.; Egami, N.; Kashio, A.; Ushio, M.; Sakamoto, T.; Yamashita, S.; Yamasoba, T. Evaluation of the internal structure of normal and pathological guinea pig cochleae using optical coherence tomography. Audiol. Neurotol. 2013, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- De Seta, D.; Torres, R.; Russo, F.Y.; Ferrary, E.; Kazmitcheff, G.; Heymann, D.; Amiaud, J.; Sterkers, O.; Bernardeschi, D.; Nguyen, Y. Damage to inner ear structure during cochlear implantation: Correlation between insertion force and radio-histological findings in temporal bone specimens. Hear. Res. 2017, 344, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Counter, S.A.; Damberg, P.; Aski, S.N.; Nagy, K.; Berglin, C.E.; Laurell, G. Experimental fusion of contrast enhanced high-field magnetic resonance imaging and high-resolution micro-computed tomography in imaging the mouse inner ear. Open Neuroimaging J. 2015, 9, 7. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Price, D.L.; De Wilde, J.P.; Papadaki, A.M.; Curran, J.S.; Kitney, R.I. Investigation of acoustic noise on 15 mri scanners from 0.2 t to 3 t. J. Magn. Reson. Imaging 2001, 13, 288–293. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; de Boer, J.F.; Mujat, M.; Lim, H.; Yun, S.H. In vivo optical frequency domain imaging of human retina and choroid. Opt. Express 2006, 14, 4403–4411. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.E.; Lee, S.-Y.; Kim, P.; Jung, H.-Y.; Jeon, M.; Kim, J. Optical sensing method to analyze germination rate of capsicum annum seeds treated with growth-promoting chemical compounds using optical coherence tomography. J. Biomed. Opt. 2017, 22, 091502. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.E.; Lee, S.-Y.; Kim, P.; Jung, H.-Y.; Jeon, M.; Kim, J. Optical inspection and morphological analysis of diospyros kaki plant leaves for the detection of circular leaf spot disease. Sensors 2016, 16, 1282. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.F.; Park, K.; Wijesinghe, R.E.; Jeong, H.; Han, S.; Kim, P.; Jeon, M.; Kim, J. Fast industrial inspection of optical thin film using optical coherence tomography. Sensors 2016, 16, 1598. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.E.; Park, K.; Jung, Y.; Kim, P.; Jeon, M.; Kim, J. Industrial resin inspection for display production using automated fluid-inspection based on multimodal optical detection techniques. Opt. Lasers Eng. 2017, 96, 75–82. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Leitgeb, R.; Kowalczyk, A.; Bajraszewski, T.; Fercher, A.F. In vivo human retinal imaging by fourier domain optical coherence tomography. J. Biomed. Opt. 2002, 7, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.F.; Wijesinghe, R.E.; Ravichandran, N.K.; Kim, P.; Jeon, M.; Kim, J. Dual-path handheld system for cornea and retina imaging using optical coherence tomography. Opt. Rev. 2016, 2, 219–225. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Brezinski, M.E.; Tearney, G.J.; Boppart, S.A.; Bouma, B.; Hee, M.R.; Southern, J.F.; Swanson, E.A. Optical biopsy and imaging using optical coherence tomography. Nat. Med. 1995, 1, 970–972. [Google Scholar] [CrossRef] [PubMed]
- Tearney, G.J.; Brezinski, M.E.; Bouma, B.E.; Boppart, S.A.; Pitris, C.; Southern, J.F.; Fujimoto, J.G. In vivo endoscopic optical biopsy with optical coherence tomography. Science 1997, 276, 2037–2039. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.C.; Strasswimmer, J.; Park, B.H.; Cense, B.; de Boer, J.F. Advances in optical coherence tomography imaging for dermatology. J. Investig. Dermatol. 2004, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Považay, B.; Hofer, B.; Popov, S.; Glittenberg, C.; Binder, S.; Drexler, W. Multispectral in vivo three-dimensional optical coherence tomography of human skin. J. Biomed. Opt. 2010, 15, 026025. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.-C.; Lin, K.-F.; Sun, C.-W. Dental optical coherence tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Dichtl, S.; Hitzenberger, C.; Sattmann, H.; Robl, B.; Moritz, A.; Fercher, A.; Sperr, W. Polarization–sensitive optical coherence tomography of dental structures. Caries Res. 2000, 34, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.E.; Cho, N.H.; Park, K.; Jeon, M.; Kim, J. Bio-photonic detection and quantitative evaluation method for the progression of dental caries using optical frequency-domain imaging method. Sensors 2016, 16, 2076. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Lee, S.H.; Jung, W.; Jang, J.H.; Kim, J. Optical coherence tomography for the diagnosis and evaluation of human otitis media. J. Korean Med. Sci. 2015, 30, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.K.; de Boer, J.F.; Park, B.H.; Nassif, N.; Zhao, Y.; Chen, Z.; Wong, B.J. Imaging the internal structure of the guinea pig cochlea using optical coherence tomography at 1310 nm. In BIOS 2001 The International Symposium on Biomedical Optics, San Jose, CA, USA; International Society for Optics and Photonics: San Jose, CA, USA, 2001; pp. 372–378. [Google Scholar]
- Cho, N.H.; Jang, J.H.; Jung, W.; Kim, J. In vivo imaging of middle-ear and inner-ear microstructures of a mouse guided by sd-oct combined with a surgical microscope. Opt. Express 2014, 22, 8985–8995. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Cho, N.H.; Jang, J.H.; Lee, S.H.; Kim, P.; Jeon, M.; Boppart, S.A.; Kim, J.; Jung, W. In vivo 3d imaging of the human tympanic membrane using a wide-field diagonal-scanning optical coherence tomography probe. Appl. Opt. 2017, 56, D115–D119. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, A.; Djalilian, H.R.; Chang, J.E.; Chen, Z.; Wong, B.J. Optical coherence tomography of the cochlea in the porcine model. Laryngoscope 2008, 118, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shekhar, R.; Huang, D. Segmentation of 830 nm and 1310 nm Lasik Corneal Optical Coherence Tomography Images; Proc. SPIE: San Jose, CA, USA, 2002; p. 168. [Google Scholar]
- Wong, B.J.; Zhao, Y.; Yamaguchi, M.; Nassif, N.; Chen, Z.; De Boer, J.F. Imaging the internal structure of the rat cochlea using optical coherence tomography at 0.827 μm and 1.3 μm. Otolaryngol.-Head Neck Surg. 2004, 130, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Puvanathasan, P.; Forbes, P.; Ren, Z.; Malchow, D.; Boyd, S.; Bizheva, K. High-speed, high-resolution fourier-domain optical coherence tomography system for retinal imaging in the 1060 nm wavelength region. Opt. Lett. 2008, 33, 2479–2481. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelpour, M.; Považay, B.; Hermann, B.; Hofer, B.; Kajic, V.; Hale, S.L.; North, R.V.; Drexler, W.; Sheen, N.J. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5311–5316. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.S.; Batts, S.A.; Chu, K.K.; Sahin, M.I.; Leung, H.M.; Tearney, G.J.; Stankovic, K.M. Micro-optical coherence tomography of the mammalian cochlea. Sci. Rep. 2016, 6, 33288. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Raphael, P.D.; Park, J.; Ellerbee, A.K.; Applegate, B.E.; Oghalai, J.S. Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc. Natl. Acad. Sci. USA 2015, 112, 3128–3133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Wijesinghe, R.E.; Jeon, D.; Lee, S.H.; Jeon, M.; Jang, J.H. Decalcification using ethylenediaminetetraacetic acid for clear microstructure imaging of cochlea through optical coherence tomography. J. Biomed. Opt. 2016, 21, 081204. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Raphael, P.D.; Ellerbee, A.K.; Applegate, B.E.; Oghalai, J.S. Swept source optical coherence tomography for in vivo imaging and vibrometry in the apex of the mouse cochlea. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2015; p. 040010. [Google Scholar]
- Rolland, J.P.; Meemon, P.; Murali, S.; Thompson, K.P.; Lee, K.-S. Gabor-based fusion technique for optical coherence microscopy. Opt Express 2010, 18, 3632–3642. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Tuchin, V.V. Advanced Biophotonics: Tissue optical Sectioning; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Verboven, P.; Nemeth, A.; Abera, M.K.; Bongaers, E.; Daelemans, D.; Estrade, P.; Herremans, E.; Hertog, M.; Saeys, W.; Vanstreels, E. Optical coherence tomography visualizes microstructure of apple peel. Postharvest Biol. Technol. 2013, 78, 123–132. [Google Scholar] [CrossRef]
- Paragios, N.; Chen, Y.; Faugeras, O.D. Handbook of Mathematical Models in Computer Vision; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zahnd, G.; Karanasos, A.; van Soest, G.; Regar, E.; Niessen, W.; Gijsen, F.; van Walsum, T. Quantification of fibrous cap thickness in intracoronary optical coherence tomography with a contour segmentation method based on dynamic programming. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Faber, D.J.; Van Der Meer, F.J.; Aalders, M.C.; van Leeuwen, T.G. Quantitative measurement of attenuation coefficients of weakly scattering media using optical coherence tomography. Opt. Express 2004, 12, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Nishizawa, N. Quantitative comparison of contrast and imaging depth of ultrahigh-resolution optical coherence tomography images in 800–1700 nm wavelength region. Biomed. Opt. Express 2012, 3, 282–294. [Google Scholar] [CrossRef] [PubMed]





| Model | Customized | IVS-1000 | OCS1310V1 |
|---|---|---|---|
| Parameters | Specifications | ||
| Central wavelength | 860 nm | 1060 nm ± 30 nm | 1300 nm ± 15 nm |
| Spectral bandwidth | 135 nm | ≥100 nm | >97 nm (−10 dB cut off point) |
| Transverse resolution (Theoretical/Experimentally measured) | 9.7 μm/11.4 μm | 11.8 μm/14.7 μm | 14.4 μm/25 μm |
| Axial resolution air/tissue (with refractive index of 1.35) (Experimentally measured) | 10 μm/7.4 μm | 13.3 μm/9.8 μm | 16 μm/11.8 μm |
| Image acquisition speed | 140,000 line/s | 100,000 line/s | 100,000 line/s |
| Scan lens focal length | 18 mm | 18 mm | 18 mm |
| Scan lens variety wavelength range | LSM02-BB 810~1100 nm | LSM02-BB 810~1100 nm | LSM02 1250~1380 nm |
| Numerical aperture of scan lens | 0.11 | 0.11 | 0.11 |
| Optical power | 12.2 mW | 12 mW | 10 mW |
| Sensitivity | 97 dB | 101 dB | 105 dB |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Wijesinghe, R.E.; Lee, J.; Shirazi, M.F.; Kim, P.; Jang, J.H.; Jeon, M.; Kim, J. Multiple Wavelength Optical Coherence Tomography Assessments for Enhanced Ex Vivo Intra-Cochlear Microstructural Visualization. Electronics 2018, 7, 133. https://doi.org/10.3390/electronics7080133
Kim S, Wijesinghe RE, Lee J, Shirazi MF, Kim P, Jang JH, Jeon M, Kim J. Multiple Wavelength Optical Coherence Tomography Assessments for Enhanced Ex Vivo Intra-Cochlear Microstructural Visualization. Electronics. 2018; 7(8):133. https://doi.org/10.3390/electronics7080133
Chicago/Turabian StyleKim, Sungwook, Ruchire Eranga Wijesinghe, Jaeyul Lee, Muhammad Faizan Shirazi, Pilun Kim, Jeong Hun Jang, Mansik Jeon, and Jeehyun Kim. 2018. "Multiple Wavelength Optical Coherence Tomography Assessments for Enhanced Ex Vivo Intra-Cochlear Microstructural Visualization" Electronics 7, no. 8: 133. https://doi.org/10.3390/electronics7080133
APA StyleKim, S., Wijesinghe, R. E., Lee, J., Shirazi, M. F., Kim, P., Jang, J. H., Jeon, M., & Kim, J. (2018). Multiple Wavelength Optical Coherence Tomography Assessments for Enhanced Ex Vivo Intra-Cochlear Microstructural Visualization. Electronics, 7(8), 133. https://doi.org/10.3390/electronics7080133







