Organic Bioelectronic Tools for Biomedical Applications
Abstract
:1. Introduction
2. Electronically Controlled Ion Transport
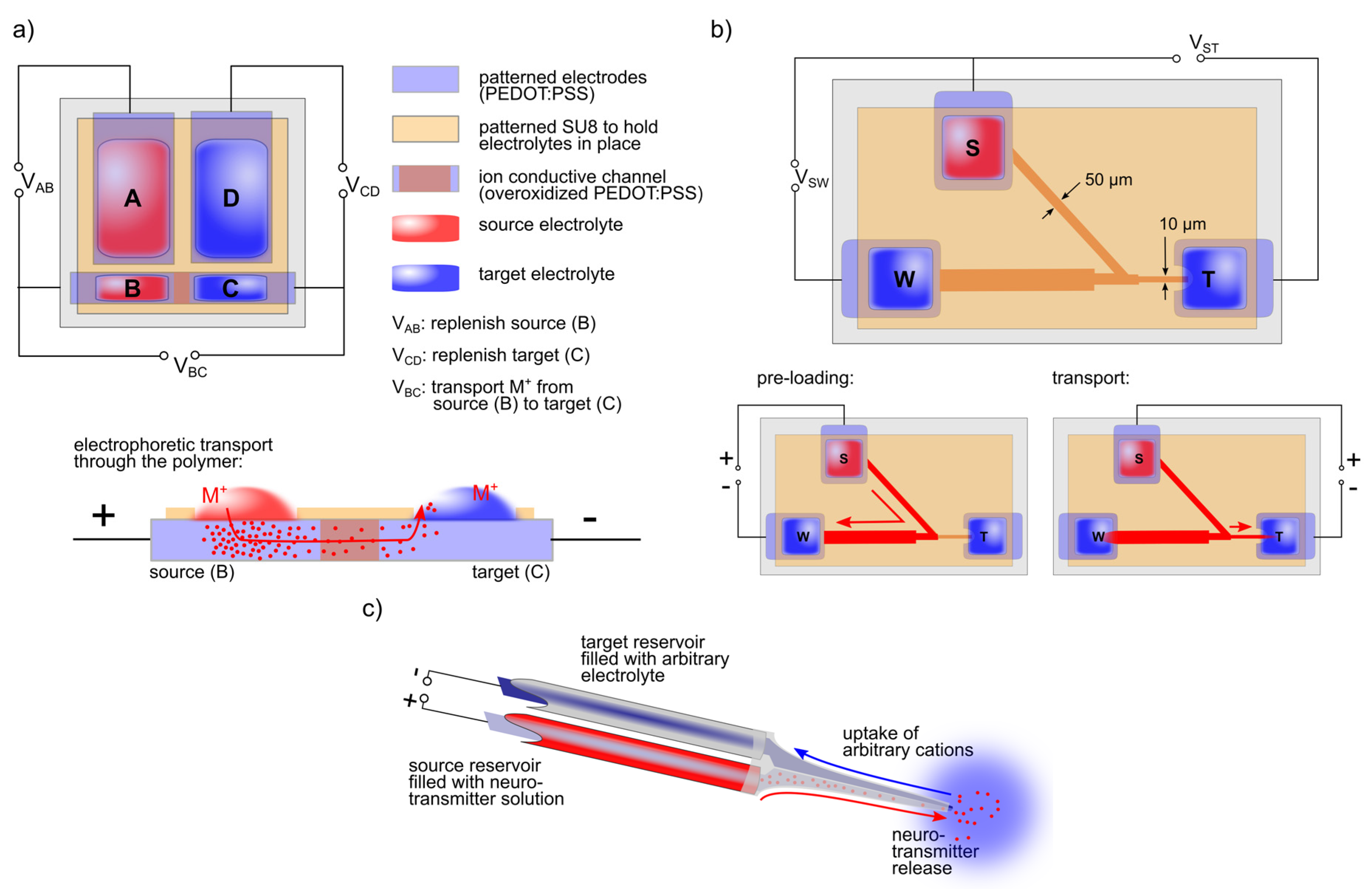
3. Self-Controlled Biomimetic Systems
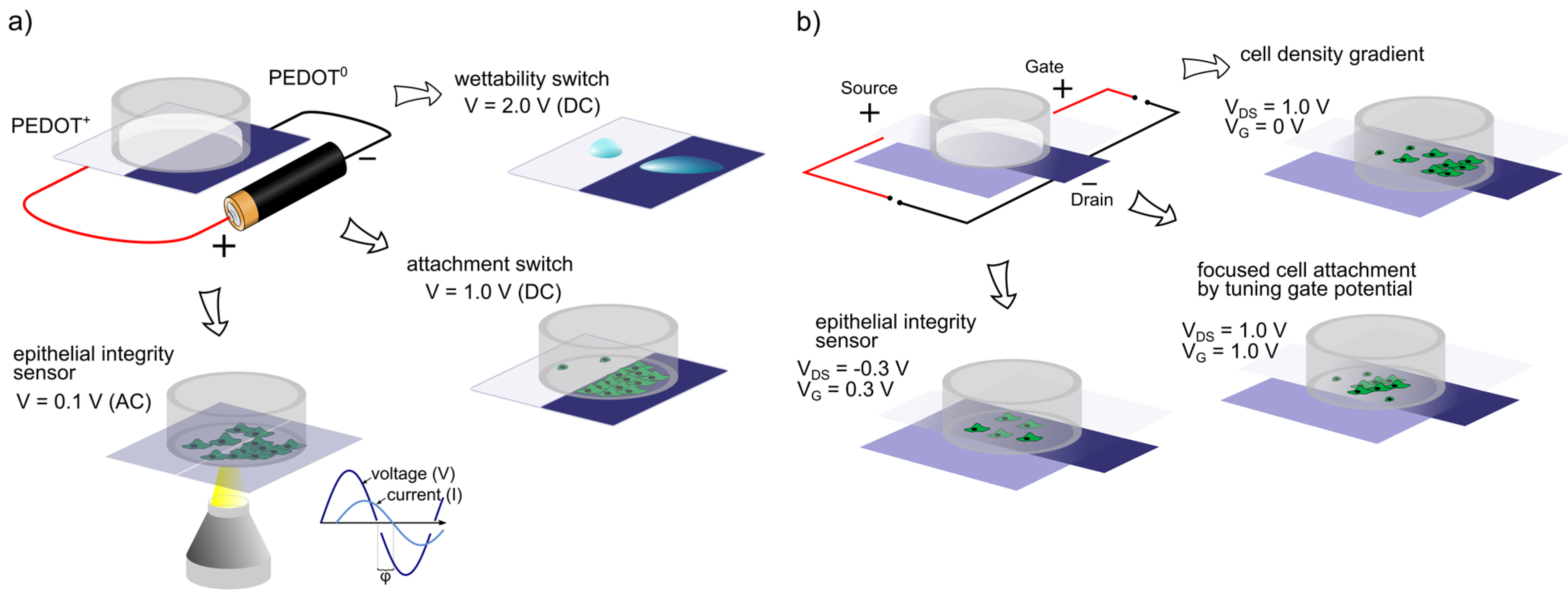
4. Organic Bioelectronic Active Surfaces
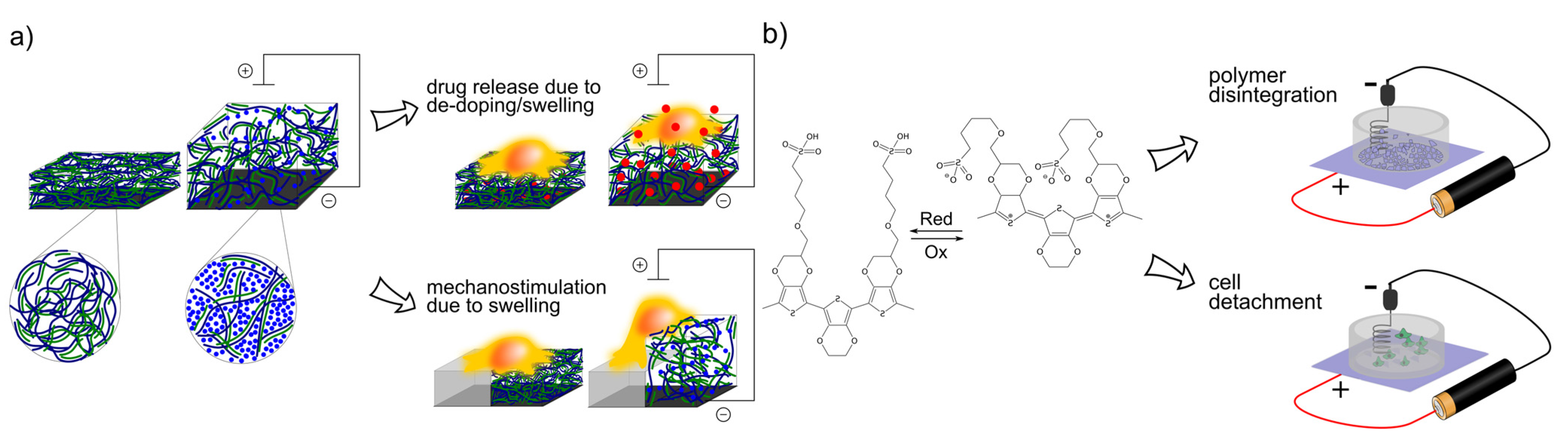
5. Sensors
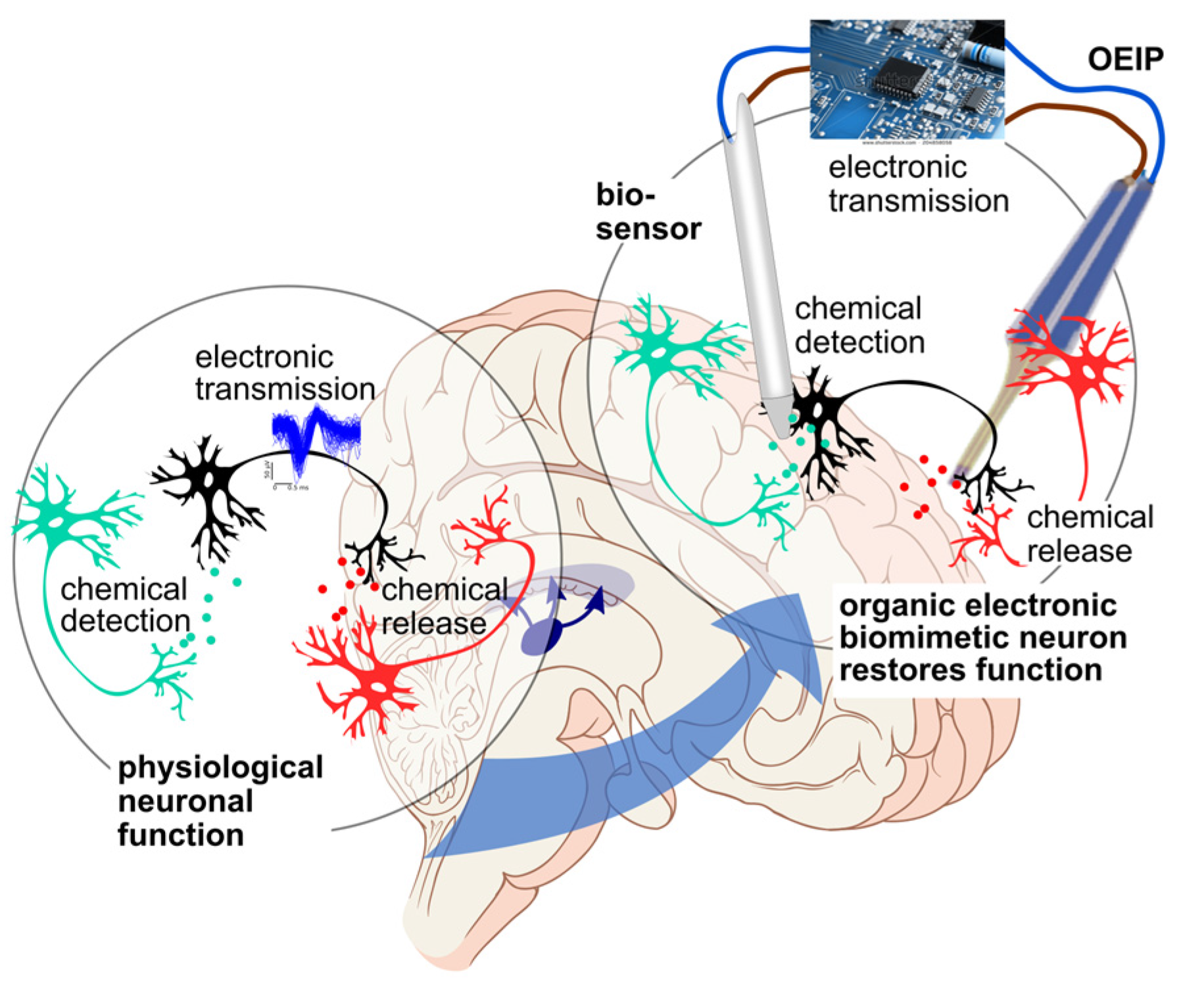
6. Biomedical Applications of Organic Bioelectronics
6.1. Neuroscience
6.2. Infection
6.3. Advanced in vitro Models
6.4. Cell Biology
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Larsson, K.C.; Kjäll, P.; Richter-Dahlfors, A. Organic bioelectronics for electronic-to-chemical translation in modulation of neuronal signaling and machine-to-brain interfacing. Biochim. Biophys. Acta 2013, 1830, 4334–4344. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.; Kjäll, P.; Richter-Dahlfors, A.; Cicoira, F. Organic bioelectronics-novel applications in biomedicine. Biochim. Biophys. Acta 2013, 1830, 4283–4285. [Google Scholar] [CrossRef] [PubMed]
- Richter-Dahlfors, A.; Kjäll, P. Nanotechnologies: Emerging applications in biomedicine. Biochim. Biophys. Acta 2011, 1810, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Svennersten, K.; Larsson, K.C.; Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics in nanomedicine. Biochim. Biophys. Acta 2011, 1810, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Muskovich, M.; Bettinger, C.J. Biomaterials-based electronics: Polymers and interfaces for biology and medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Brédas, J.L.; Heeger, A.J.; Wudl, F. Towards organic polymers with very small intrinsic band gaps. I. Electronic structure of polyisothianaphthene and derivatives. J. Chem. Phys. 1986, 85, 4673–4678. [Google Scholar] [CrossRef]
- Chiang, C.; Fincher, C.; Park, Y.; Heeger, A.; Shirakawa, H.; Louis, E.; Gau, S.; MacDiarmid, A. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting polymers: The third generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef] [PubMed]
- MacDiarmid, A.G.; Mammone, R.J.; Kaner, R.B.; Porter, S.J.; Pethig, R.; Heeger, A.J.; Rosseinsky, D.R. The concept of “doping” of conducting polymers: The role of reduction potentials (and discussion). Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1985, 314, 3–15. [Google Scholar] [CrossRef]
- Chochos, C.L.; Choulis, S.A. How the structural deviations on the backbone of conjugated polymers influence their optoelectronic properties and photovoltaic performance. Progress Polym. Sci. 2011, 36, 1326–1414. [Google Scholar] [CrossRef]
- Kroon, R.; Lenes, M.; Hummelen, J.C.; Blom, P.W.M.; de Boer, B. Small bandgap polymers for organic solar cells (polymer material development in the last 5 years). Polym. Rev. 2008, 48, 531–582. [Google Scholar] [CrossRef]
- Roncali, J. Molecular engineering of the band gap of π-conjugated systems: Facing technological applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Roncali, J. Synthetic principles for bandgap control in linear pi-conjugated systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Dai, L. Intelligent Macromolecules for Smart Devices: From Materials Synthesis to Device Applications; Springer: London, England, 2004; p. 496. [Google Scholar]
- Park, H.-S.; Ko, S.-J.; Park, J.-S.; Kim, J.Y.; Song, H.-K. Redox-active charge carriers of conducting polymers as a tuner of conductivity and its potential window. Sci. Rep. 2013, 3, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavrinidou, E.; Leleux, P.; Rajaona, H.; Khodagholy, D.; Rivnay, J.; Lindau, M.; Sanaur, S.; Malliaras, G.G. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 2013, 25, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Chung, T.D. Iontronics. Ann. Rev. Anal. Chem. 2015, 8, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A potential emergence of a transdermal drug delivery system. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Naik, A.; Garrison, J.; Guy, R.H. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 619–658. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Scudds, R.A. Iontophoresis: An overview of the mechanisms and clinical application. Arthritis Care Res. 1995, 8, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Kjäll, P.; Nilsson, D.; Robinson, N.D.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [PubMed]
- Isaksson, J.; Nilsson, D.; Kjäll, P.; Robinson, N.D.; Richter-Dahlfors, A.; Berggren, M. Electronically controlled ph gradients and proton oscillations. Org. Electron. 2008, 9, 303–309. [Google Scholar] [CrossRef]
- Tybrandt, K.; Larsson, K.C.; Kurup, S.; Simon, D.T.; Kjäll, P.; Isaksson, J.; Sandberg, M.; Jager, E.W.H.; Richter-Dahlfors, A.; Berggren, M. Translating electronic currents to precise acetylcholine induced neuronal signaling using an organic electrophoretic delivery device. Adv. Mater. 2009, 21, 4442–4446. [Google Scholar] [CrossRef]
- Tybrandt, K.; Gabrielsson, E.O.; Berggren, M. Toward complementary ionic circuits: The npn ion bipolar junction transistor. J. Am. Chem. Soc. 2011, 133, 10141–10145. [Google Scholar] [CrossRef] [PubMed]
- Tybrandt, K.; Larsson, K.C.; Richter-Dahlfors, A.; Berggren, M. Ion bipolar junction transistors. Proc. Natl. Acad. Sci. USA 2010, 107, 9929–9932. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.V.; Tybrandt, K.; Berggren, M.; Zozoulenko, I.V. Modeling of charge transport in ion bipolar junction transistors. Langmuir 2014, 30, 6999–7005. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.T.; Kurup, S.; Larsson, K.C.; Hori, R.; Tybrandt, K.; Goiny, M.; Jager, E.W.H.; Berggren, M.; Canlon, B.; Richter-Dahlfors, A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 2009, 8, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Surwade, S.P.; Chai, S.H.; Choi, J.P.; Wang, X.; Lee, J.S.; Vlassiouk, I.V.; Mahurin, S.M.; Dai, S. Electrochemical control of ion transport through a mesoporous carbon membrane. Langmuir 2014, 30, 3606–3611. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jing, W.; Li, S.; Shen, H.; Xing, W. Electric field-controlled ion transport in tio2 nanochannel. ACS Appl. Mater. Interfaces 2015, 7, 11294–11300. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Miocinovic, S.; McIntyre, C.C.; Vitek, J.L. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 2008, 5, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Osorio, I.; Frei, M.G.; Manly, B.F.; Sunderam, S.; Bhavaraju, N.C.; Wilkinson, S.B. An introduction to contingent (closed-loop) brain electrical stimulation for seizure blockage, to ultra-short-term clinical trials, and to multidimensional statistical analysis of therapeutic efficacy. J. Clin. Neurophysiol. 2001, 18, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; Schwartz, A.B.; Kettner, R.E. Neuronal population coding of movement direction. Science 1986, 233, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, A.P.; Kettner, R.E.; Schwartz, A.B. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J. Neurosci. 1988, 8, 2928–2937. [Google Scholar] [PubMed]
- Taylor, D.M.; Tillery, S.I.; Schwartz, A.B. Direct cortical control of 3d neuroprosthetic devices. Science 2002, 296, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.T.; Larsson, K.C.; Nilsson, D.; Burstrom, G.; Galter, D.; Berggren, M.; Richter-Dahlfors, A. An organic electronic biomimetic neuron enables auto-regulated neuromodulation. Biosens. Bioelectron. 2015, 71, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; McCall, J.G.; Zhang, Y.; Huang, Y.; Bruchas, M.R.; Rogers, J.A. Soft microfluidic neural probes for wireless drug delivery in freely behaving mice. In Proceedings of the International Conference on Solid-State Sensors, Actuators and Microsystems, Anchorage, AK, USA, 21–25 June 2015; pp. 2264–2267.
- Pernaut, J.-M.; Reynolds, J.R. Use of conducting electroactive polymers for drug delivery and sensing of bioactive molecules. A redox chemistry approach. J. Phys. Chem. B 2000, 104, 4080–4090. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Svennersten, K.; Berggren, M.; Richter-Dahlfors, A.; Jager, E.W.H. Mechanical stimulation of epithelial cells using polypyrrole microactuators. Lab Chip 2011, 11, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Svennersten, K.; Bolin, M.H.; Jager, E.W.H.; Berggren, M.; Richter-Dahlfors, A. Electrochemical modulation of epithelia formation using conducting polymers. Biomaterials 2009, 30, 6257–6264. [Google Scholar] [CrossRef] [PubMed]
- Löffler, S.; Libberton, B.; Richter-Dahlfors, A. Organic bioelectronics in infection. J. Mater. Chem. B 2015, 3, 4979–4992. [Google Scholar] [CrossRef]
- Isaksson, J.; Tengstedt, C.; Fahlman, M.; Robinson, N.; Berggren, M. A solid-state organic electronic wettability switch. Adv. Mater. 2004, 16, 316–320. [Google Scholar] [CrossRef]
- Liu, M.; Nie, F.Q.; Wei, Z.; Song, Y.; Jiang, L. In situ electrochemical switching of wetting state of oil droplet on conducting polymer films. Langmuir 2010, 26, 3993–3997. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Hunter, I.W. Characterization and control of the wettability of conducting polymer thin films. In Materials Research Society Symposium Proceedings; Materials Research Society: Cambridge, England, 2009. [Google Scholar]
- Bolin, M.; Svennersten, K.; Nilsson, D.; Sawatdee, A.; Jager, E.W.H.; Richter-Dahlfors, A.; Berggren, M. Active control of epithelial cell density gradients grown along the channel of an organic electrochemical transistor. Adv. Mater. 2009, 21, 4379–4382. [Google Scholar] [CrossRef] [PubMed]
- Keiichi, K.; Hisashi, F.; Masakatsu, K.; Wataru, T. Conducting polymer soft actuators based on polypyrrole films - energy conversion efficiency. Smart Mater. Struct. 2007, 16, S250. [Google Scholar]
- Kaneto, K.; Somekawa, H.; Takashima, W. Soft actuators based on conducting polymers: Recent progress. SPIE Proc. 2003, 5051. [Google Scholar] [CrossRef]
- Kaneto, K.; Nakashima, M.; Takashima, W. Improvement of electrochemical deformation of conducting polymers: Strain, force, and response. SPIE Proc. 2004, 5385. [Google Scholar] [CrossRef]
- Pattavarakorn, D.; Youngta, P.; Jaesrichai, S.; Thongbor, S.; Chaimongkol, P. Electroactive performances of conductive polythiophene/hydrogel hybrid artificial muscle. Energy Procedia 2013, 34, 673–681. [Google Scholar] [CrossRef]
- Madden, J.D. Polypyrrole actuators: Properties and initial applications. In Electroactive Polymers for Robotic Applications; Kim, K., Tadokoro, S., Eds.; Springer: London, UK, 2007; pp. 121–152. [Google Scholar]
- Wang, X.; Smela, E. Color and volume change in PPy(DBS). J. Phys. Chem. C 2009, 113, 359–368. [Google Scholar] [CrossRef]
- Hiraoka, M.; Fiorini, P.; O’Callaghan, J.; Yamashita, I.; van Hoof, C.; Op de Beeck, M. Miniature conductive polymer actuators for high pressure generation in lab on chip systems. Sens. Actuators A Phys. 2012, 177, 23–29. [Google Scholar] [CrossRef]
- Valdes-Ramirez, G.; Windmiller, J.R.; Claussen, J.C.; Martinez, A.G.; Kuralay, F.; Zhou, M.; Zhou, N.; Polsky, R.; Miller, P.R.; Narayan, R.; et al. Multiplexed and switchable release of distinct fluids from microneedle platforms via conducting polymer nanoactuators for potential drug delivery. Sens. Actuators B Chem. 2012, 161, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Conzuelo, L.V.; Arias-Pardilla, J.; Cauich-Rodriguez, J.V.; Smit, M.A.; Otero, T.F. Sensing and tactile artificial muscles from reactive materials. Sensors 2010, 10, 2638–2674. [Google Scholar] [PubMed]
- Otero, T.F.; Martinez, J.G. Structural electrochemistry: Conductivities and ionic content from rising reduced polypyrrole films. Adv. Funct. Mater. 2014, 24, 1259–1264. [Google Scholar] [CrossRef]
- Schroeder, P.; Schotter, J.; Shoshi, A.; Eggeling, M.; Bethge, O.; Hutten, A.; Bruckl, H. Artificial cilia of magnetically tagged polymer nanowires for biomimetic mechanosensing. Bioinspiration Biomimicry 2011, 6, 046007. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Y.A.; Martínez, J.G.; Al Harrasi, A.S.; Kim, S.J.; Otero, T.F. Sensing characteristics of a conducting polymer/hydrogel hybrid microfiber artificial muscle. Sens. Actuators B Chem. 2011, 160, 1180–1190. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-polymer nanotubes for controlled drug release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Esrafilzadeh, D.; Razal, J.M.; Moulton, S.E.; Stewart, E.M.; Wallace, G.G. Multifunctional conducting fibres with electrically controlled release of ciprofloxacin. J. Control. Release 2013, 169, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Poole-Warren, L.; Goding, J. Challenges of therapeutic delivery using conducting polymers. Ther. Deliv. 2012, 3, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High surface area polypyrrole scaffolds for tunable drug delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Sharma, M.; Yu, Y.; Garg, S. Electrically switchable polypyrrole film for the tunable release of progesterone. Ther. Deliv. 2013, 4, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth. Metals 2013, 163, 19–23. [Google Scholar] [CrossRef]
- Persson, K.M.; Karlsson, R.; Svennersten, K.; Löffler, S.; Jager, E.W.H.; Richter-Dahlfors, A.; Konradsson, P.; Berggren, M. Electronic control of cell detachment using a self-doped conducting polymer. Adv. Mater. 2011, 23, 4403–4408. [Google Scholar] [CrossRef] [PubMed]
- Bolin, M.; Svennersten, K.; Wang, X.; Chronakis, I.S.; Richter-Dahlfors, A.; Jager, E.; Berggren, M. Nano-fiber scaffold electrodes based on pedot for cell stimulation. Sens. Actuators 2009, 142, 451–456. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3d conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar]
- Wan, A.M.-D.; Inal, S.; Williams, T.; Wang, K.; Leleux, P.; Estevez, L.; Giannelis, E.P.; Fischbach, C.; Malliaras, G.G.; Gourdon, D. 3d conducting polymer platforms for electrical control of protein conformation and cellular functions. J. Mater. Chem. B 2015, 3, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Summerlot, D.; Kumar, A.; Das, S.; Goldstein, L.; Seal, S.; Diaz, D.; Cho, H.J. Nanoporous gold electrode for electrochemical sensors in biological environment. Procedia Eng. 2011, 25, 1457–1460. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole nanotubes conjugated with human olfactory receptors: High-performance transducers for fet-type bioelectronic noses. Angew. Chem. Int. Ed. 2009, 48, 2755–2758. [Google Scholar] [CrossRef] [PubMed]
- Dian, J.; Konečný, M.; Broncová, G.; Kronďák, M.; Matolínová, I. Electrochemical fabrication and characterization of porous silicon/polypyrrole composites and chemical sensing of organic vapors. Int. J. Electrochem. Sci. 2013, 8, 1559–1572. [Google Scholar]
- Lee, S.H.; Kwon, O.S.; Song, H.S.; Park, S.J.; Sung, J.H.; Jang, J.; Park, T.H. Mimicking the human smell sensing mechanism with an artificial nose platform. Biomaterials 2012, 33, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kwon, O.S.; Lee, S.H.; Song, H.S.; Park, T.H.; Jang, J. Ultrasensitive flexible graphene based field-effect transistor (FET)-type bioelectronic nose. Nano Lett. 2012, 12, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Kwon, O.S.; Lee, S.H.; Park, S.J.; Kim, U.K.; Jang, J.; Park, T.H. Human taste receptor-functionalized field effect transistor as a human-like nanobioelectronic tongue. Nano Lett. 2013, 13, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Chen, Y.C.; Galpern, W.R.; Brownell, A.L.; Matthews, R.T.; Bogdanov, M.; Isacson, O.; Keltner, J.R.; Beal, M.F.; Rosen, B.R.; Jenkins, B.G. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: Correlation with PET, microdialysis, and behavioral data. Magn. Reson. Med. 1997, 38, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Bong, S.; Kang, Y.-J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Sansuk, S.; Bitziou, E.; Joseph, M.B.; Covington, J.A.; Boutelle, M.G.; Unwin, P.R.; Macpherson, J.V. Ultrasensitive detection of dopamine using a carbon nanotube network microfluidic flow electrode. Anal. Chem. 2013, 85, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Han, H.-Z.; Cheng, C.-C.; Chen, L.-C.; Chang, H.-C.; Chen, J.-J.J. Modification of platinum microelectrode with molecularly imprinted over-oxidized polypyrrole for dopamine measurement in rat striatum. Sens. Actuators B Chem. 2012, 171, 93–101. [Google Scholar] [CrossRef]
- Cesarino, I.; Galesco, H.V.; Moraes, F.C.; Lanza, M.R.V.; Machado, S.A.S. Biosensor based on electrocodeposition of carbon nanotubes/polypyrrole/laccase for neurotransmitter detection. Electroanalysis 2013, 25, 394–400. [Google Scholar] [CrossRef]
- Daniel, S.; Rao, T.P.; Rao, K.S.; Rani, S.U.; Naidu, G.R.K.; Lee, H.-Y.; Kawai, T. A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sens. Actuators B Chem. 2007, 122, 672–682. [Google Scholar] [CrossRef]
- Putzbach, W.; Ronkainen, N. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [PubMed]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; You, T. Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods 2010, 2, 202–211. [Google Scholar] [CrossRef]
- Yuqing, M.; Jianrong, C.; Xiaohua, W. Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol. 2004, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.; Anderson, M.R. Electrochemical glucose sensors-developments using electrostatic assembly and carbon nanotubes for biosensor construction. Sensors 2010, 10, 8248–8274. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Chawla, S.; Pundir, C.S. An amperometric hemoglobin a1c biosensor based on immobilization of fructosyl amino acid oxidase onto zinc oxide nanoparticles-polypyrrole film. Anal. Biochem. 2012, 430, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Poturnayova, A.; Snejdarkova, M.; Hianik, T.; Korri-Youssoufi, H. Binding kinetics of human cellular prion detection by DNA aptamers immobilized on a conducting polypyrrole. Anal. Bioanal. Chem. 2013, 405, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Ramuz, M.; Hama, A.; Huerta, M.; Rivnay, J.; Leleux, P.; Owens, R.M. Combined optical and electronic sensing of epithelial cells using planar organic transistors. Adv. Mater. 2014, 26, 7083–7090. [Google Scholar] [CrossRef] [PubMed]
- Löffler, S.; Richter-Dahlfors, A. Phase angle spectroscopy on transparent conducting polymer electrodes for real-time measurement of epithelial barrier integrity. J. Mater. Chem. B 2015, 3, 4997–5000. [Google Scholar] [CrossRef]
- Quigley, A.F.; Razal, J.M.; Thompson, B.C.; Moulton, S.E.; Kita, M.; Kennedy, E.L.; Clark, G.M.; Wallace, G.G.; Kapsa, R.M. A conducting-polymer platform with biodegradable fibers for stimulation and guidance of axonal growth. Adv. Mater. 2009, 21, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, P.; Liu, M.; Song, W.; Wu, Q.; Fan, Y. Potential protective effect of biphasic electrical stimulation against growth factor-deprived apoptosis on olfactory bulb neural progenitor cells through the brain-derived neurotrophic factor-phosphatidylinositol 3′-kinase/akt pathway. Exp. Biol. Med. 2013, 238, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, F.; Barker, R.A. The glial response to intracerebrally delivered therapies for neurodegenerative disorders: Is this a critical issue? Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F.; Miocinovic, S.; Johnson, M.D.; Vitek, J.L.; McIntyre, C.C. In vivo impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng. 2009, 6, 046001. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001, 56, 261–272. [Google Scholar] [CrossRef]
- Stauffer, W.R.; Cui, X.T. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials 2006, 27, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Richardson-Burns, S.M.; Hendricks, J.L.; Sequera, C.; Martin, D.C. Effect of immobilized nerve growth factor on conductive polymers: Electrical properties and cellular response. Adv. Funct. Mater. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Thompson, B.C.; Richardson, R.T.; Moulton, S.E.; Evans, A.J.; O’Leary, S.; Clark, G.M.; Wallace, G.G. Conducting polymers, dual neurotrophins and pulsed electrical stimulation—Dramatic effects on neurite outgrowth. J. Control. Release 2010, 141, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Burns, S.M.; Hendricks, J.L.; Foster, B.; Povlich, L.K.; Kim, D.H.; Martin, D.C. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 2007, 28, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Burns, S.M.; Hendricks, J.L.; Martin, D.C. Electrochemical polymerization of conducting polymers in living neural tissue. J. Neural Eng. 2007, 4, L6–L13. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.J.; Kim, H.S.; Heo, J.; Lim, Y.G.; Park, K.S. Brain-computer interfaces using capacitive measurement of visual or auditory steady-state responses. J. Neural Eng. 2013, 10, 024001. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.Ø.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Keren, I.; Shah, D.; Spoering, A.; Kaldalu, N.; Lewis, K. Specialized persister cells and the mechanism of multidrug tolerance in E. coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Pareta, R.A.; Webster, T.J. A conductive nanostructured polymer electrodeposited on titanium as a controllable, local drug delivery platform. J. Biomed. Mater. Res. Part A 2011, 99, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N. Drug releasing polymer thin films: New era of surface-mediated drug delivery. ACS Nano 2010, 4, 2494–2509. [Google Scholar] [CrossRef] [PubMed]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses, and Medical Applications; Springer: New York, NY, USA, 2001; pp. 13–24. [Google Scholar]
- Sirivisoot, S.; Pareta, R.; Webster, T.J. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology 2011, 22, 085101. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial barriers in homeostasis and disease. Ann. Rev. Pathol. 2010, 5, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Choong, F.X.; Regberg, J.; Udekwu, K.I.; Richter-Dahlfors, A. Intravital models of infection lay the foundation for tissue microbiology. Future Microbiol. 2012, 7, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Melican, K.; Richter-Dahlfors, A. Real-time live imaging to study bacterial infections in vivo. Curr. Opin. Microbiol. 2009, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Richter-Dahlfors, A.; Rhen, M.; Udekwu, K. Tissue microbiology provides a coherent picture of infection. Curr. Opin. Microbiol. 2012, 15, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Månsson, L.E.; Melican, K.; Boekel, J.; Sandoval, R.M.; Hautefort, I.; Tanner, G.A.; Molitoris, B.A.; Richter-Dahlfors, A. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell. Microbiol. 2006, 9, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Melican, K.; Boekel, J.; Månsson, L.E.; Sandoval, R.M.; Tanner, G.A.; Källskog, O.; Palm, F.; Molitoris, B.A.; Richter-Dahlfors, A. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell. Microbiol. 2008, 10, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Jimison, L.H.; Tria, S.A.; Khodagholy, D.; Gurfinkel, M.; Lanzarini, E.; Hama, A.; Malliaras, G.G.; Owens, R.M. Measurement of barrier tissue integrity with an organic electrochemical transistor. Adv. Mater. 2012, 24, 5919–5923. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.; Jimison, L.H.; Hama, A.; Bongo, M.; Owens, R.M. Sensing of EGTA mediated barrier tissue disruption with an organic transistor. Biosensors 2013, 3, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.A.; Ramuz, M.; Huerta, M.; Leleux, P.; Rivnay, J.; Jimison, L.H.; Hama, A.; Malliaras, G.G.; Owens, R.M. Dynamic monitoring of salmonella typhimurium infection of polarized epithelia using organic transistors. Adv. Healthc. Mater. 2014, 3, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Tria, S.A.; Ramuz, M.; Jimison, L.H.; Hama, A.; Owens, R.M. Sensing of barrier tissue disruption with an organic electrochemical transistor. J. Vis. Exp. 2014, e51102. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H.V. Reductionism and complexity in molecular biology. Scientists now have the tools to unravel biological and overcome the limitations of reductionism. EMBO Rep. 2004, 5, 1016–1020. [Google Scholar] [PubMed]
- Andersson, A.-S.; Bäckhed, F.; von Euler, A.; Richter-Dahlfors, A.; Sutherland, D.; Kasemo, B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials 2003, 24, 3427–3436. [Google Scholar] [CrossRef]
- Greco, F.; Fujie, T.; Ricotti, L.; Taccola, S.; Mazzolai, B.; Mattoli, V. Microwrinkled conducting polymer interface for anisotropic multicellular alignment. ACS Appl. Mater. Interfaces 2013, 5, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Plummer, S.T.; Wang, Q.; Bohn, P.W.; Stockton, R.; Schwartz, M.A. Electrochemically derived gradients of the extracellular matrix protein fibronectin on gold. Langmuir 2003, 19, 7528–7536. [Google Scholar] [CrossRef]
- Melican, K.; Boekel, J.; Ryden-Aulin, M.; Richter-Dahlfors, A. Novel innate immune functions revealed by dynamic, real-time live imaging of bacterial infections. Crit. Rev. Immunol. 2010, 30, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Boekel, J.; Källskog, O.; Rydén-Aulin, M.; Rhen, M.; Richter-Dahlfors, A. Comparative tissue transcriptomics reveal prompt inter-organ communication in response to local bacterial kidney infection. BMC Genomics 2011, 12. [Google Scholar] [CrossRef] [PubMed]
- Inaba, R.; Khademhosseini, A.; Suzuki, H.; Fukuda, J. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 2009, 30, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.N.; Houseman, B.T.; Mrksich, M. Turning on cell migration with electroactive substrates. Angew. Chem. Int. Ed. 2001, 40, 1093–1096. [Google Scholar] [CrossRef]
- Seto, Y.; Inaba, R.; Okuyama, T.; Sassa, F.; Suzuki, H.; Fukuda, J. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials 2010, 31, 2209–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Guillaume-Gentil, O.; Akiyama, Y.; Schuler, M.; Tang, C.; Textor, M.; Yamato, M.; Okano, T.; Vörös, J. Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv. Mater. 2008, 20, 560–565. [Google Scholar] [CrossRef]
- Guillaume-Gentil, O.; Gabi, M.; Zenobi-Wong, M.; Vörös, J. Electrochemically switchable platform for the micro-patterning and release of heterotypic cell sheets. Biomed. Microdevices 2011, 13, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Kjall, P.; Ishihara, K.; Richter-Dahlfors, A.; Miyahara, Y. Biomimetic interfaces reveal activation dynamics of c-reactive protein in local microenvironments. Adv. Healthc. Mater. 2014, 3, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.S.; Luo, S.C.; Hou, S.; Zhu, B.; Sekine, J.; Kuo, C.W.; Chueh, D.Y.; Yu, H.H.; Tseng, H.R.; Chen, P. 3d bioelectronic interface: Capturing circulating tumor cells onto conducting polymer-based micro/nanorod arrays with chemical and topographical control. Small 2014, 10, 3012–3017. [Google Scholar] [CrossRef] [PubMed]
- Sekine, J.; Luo, S.C.; Wang, S.; Zhu, B.; Tseng, H.R.; Yu, H.H. Functionalized conducting polymer nanodots for enhanced cell capturing: The synergistic effect of capture agents and nanostructures. Adv. Mater. 2011, 23, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-S.; Ho, B.-C.; Yan, H.-X.; Kuo, C.-W.; Chueh, D.-Y.; Yu, H.-H.; Chen, P. Integrated 3D conducting polymer-based bioelectronics for capture and release of circulating tumor cells. J. Mater. Chem. B 2015, 3, 5103–5110. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löffler, S.; Libberton, B.; Richter-Dahlfors, A. Organic Bioelectronic Tools for Biomedical Applications. Electronics 2015, 4, 879-908. https://doi.org/10.3390/electronics4040879
Löffler S, Libberton B, Richter-Dahlfors A. Organic Bioelectronic Tools for Biomedical Applications. Electronics. 2015; 4(4):879-908. https://doi.org/10.3390/electronics4040879
Chicago/Turabian StyleLöffler, Susanne, Ben Libberton, and Agneta Richter-Dahlfors. 2015. "Organic Bioelectronic Tools for Biomedical Applications" Electronics 4, no. 4: 879-908. https://doi.org/10.3390/electronics4040879
APA StyleLöffler, S., Libberton, B., & Richter-Dahlfors, A. (2015). Organic Bioelectronic Tools for Biomedical Applications. Electronics, 4(4), 879-908. https://doi.org/10.3390/electronics4040879




