Immersive Virtual Reality as Physical and Cognitive Therapy in Acquired Brain Injury: TEVI-DCA Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
- First stage: One week long and intended to adapt the patients with ABI to the use of the cyclo-ergometer (using either their upper or lower limbs, depending on the patient).
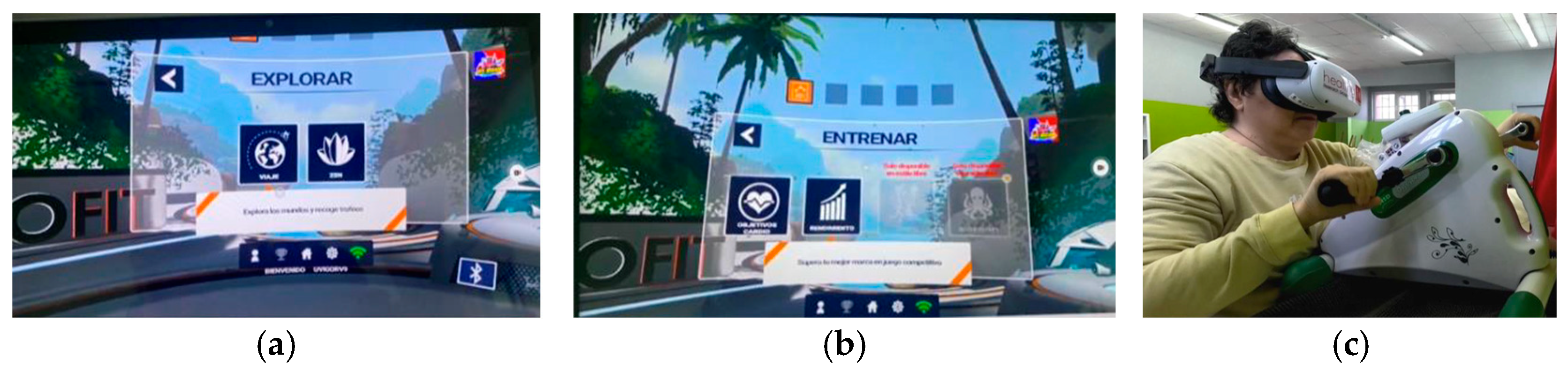
- Second stage: One week long again, with the introduction of IVR. The Explorer mode of the Holofit software was used, with each patient being able to choose between a natural or an urban environment. In this modality, the participant is able to exercise at a free pedaling pace.
- Third stage: Fourteen weeks long and subdivided into two phases of seven weeks each. The first phase is characterized by low-intensity effort (HR: 50–60%)/Borg: 3–4), where the volume of work is progressively increased (2 weeks with 5 min sessions, 3 weeks with 10 min sessions, and the last 2 weeks with 15 min sessions). If the patients’ conditions permitted, the effort was shared between the upper and lower limbs. The second phase of this stage, also lasting 7 weeks, followed the same pattern in relation to the volume of work, but in this case, it was carried out with a moderate-intensity effort (HR: 70–80%/Borg: 5–6). In this stage of the intervention, the Training modality of the Holofit software was used. In this modality, the participant must pedal at the pace set by the exergame. If the pedal rate is too slow or too fast, a message warns the participant to increase or decrease their pedaling frequency.
2.4. Evaluation Tools
- The following physical capacity tests were applied:
- Handgrip Strength Test to quantify maximum isometric hand and forearm strength [39,40,41]. A Jamar® Smart digital hand dynamometer (J.A. Preston Corporation, Clifton, NJ, USA) was used. The test was applied to both hands with the participant in a sitting position and with the elbow flexed 90 degrees.
- Five times sit-to-stand test (FTSST) to assess the functional mobility and strength of the lower extremities. The participant sat with his back against the back of the chair. The assessor counted each position out loud so the patient remained oriented. The test stopped (using a stopwatch) when the patient reached the standing position on the fifth repetition [42,43].
- Timed Up and Go Test (TUG) to assess mobility, dynamic and static balance, and fall risk. The test procedure consists of getting up from a chair, walking 3 m, turning around, returning to the chair, and sitting down. The time required to complete the predetermined route was measured with a stopwatch in seconds. Shorter times indicate better performance [44,45].
- Tinetti Test to assess gait and balance, as well as to determine the level of early stage fall risk. This consists of a gait subscale and a balance subscale. The maximum possible total score is 28: 12 for the gait subscale and 16 for the balance subscale. The Tinetti score subdivides patients into three groups according to the level of risk of falls: major risk (≤18 points), moderate risk (19–23 points), and minimal risk (≥24 points) [46,47].
- The following quality of life assessments were also applied:
- Short-Form-8 (SF-8) health survey to assess health-related quality of life. The SF-8 measures the same eight health domains as the SF-36 Health Survey with only eight questions. The scale was Likert type with five points (1–5). The higher the value, the better the quality of life in relation to health. These eight dimensions are grouped into two global components (physical and mental), which result from the sum of the defining dimensions of each component [48,49].
- The following neurocognitive tests were used:
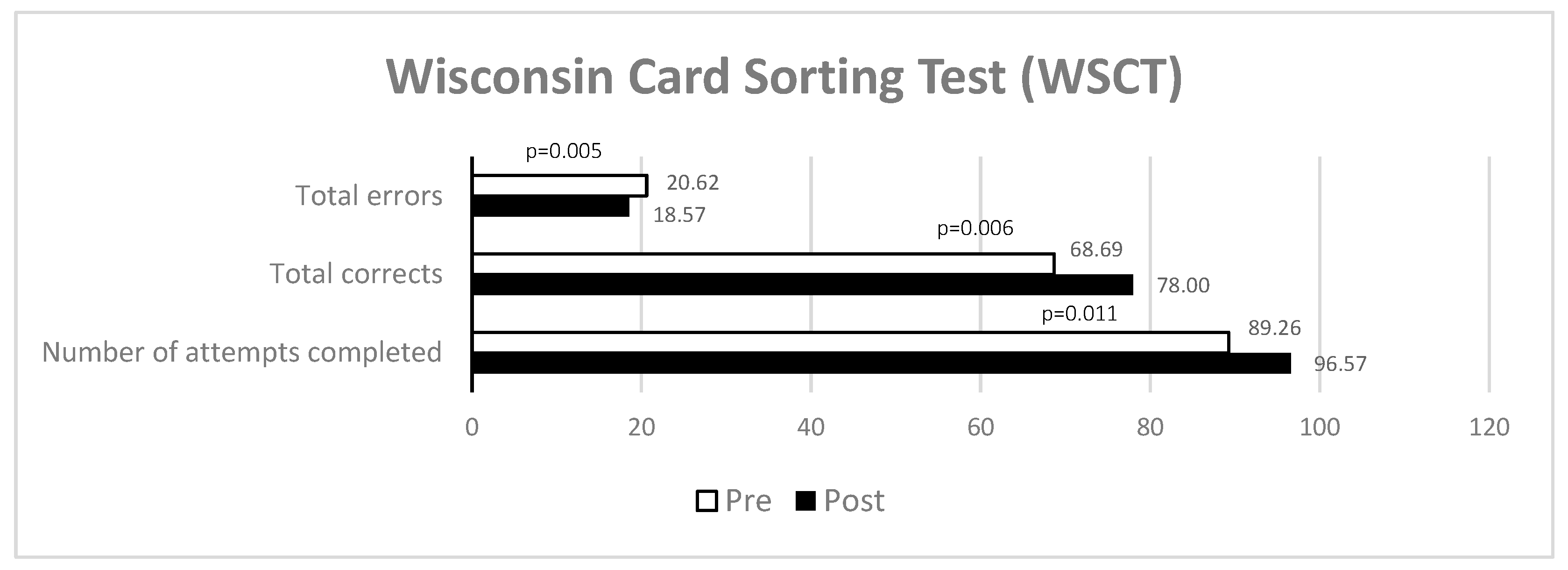
- Wisconsin Card Sorting Test (WCST) to assess executive functions and identify cognitive flexibility deficits. It consists of 4 stimulus cards and two sets of 64 cards (in the manual version) each, comprising 128 cards in total. The cards are composed of a combination of three types of attributes or characteristics: shape (triangle, star, cross, and circle), color (red, blue, green, and yellow), and number (1, 2, 3, or 4 elements). The task is to distribute the cards that match a particular criterion. When the subject makes 10 consecutive correct answers, it is considered that a category has been completed; from that moment on, the classification criterion is changed without prior notice. If you continue classifying the cards using the criterion of the previous category, you will score perseverative errors. The number of attempts is also recorded [50,51].
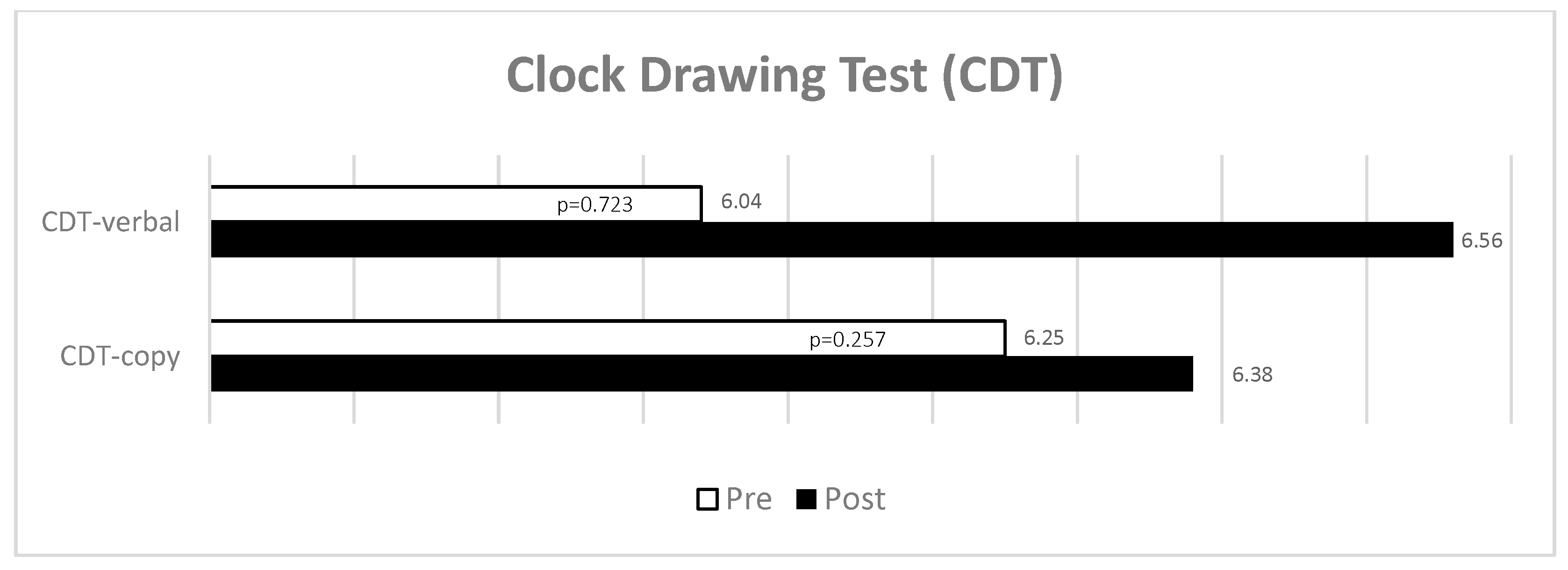
- Clock Drawing Test (CDT) to assess executive function and possible cognitive impairment linked to visuospatial abilities and constructive practice [52,53,54]. This was carried out under two conditions: asking the patients to draw a clock following a verbal command (CDT—verbal) and asking them to copy a drawing of a clock (CDT—copy). The participant was asked to draw a clock face with all the numbers and hands and to say the time shown on the clock. The number 12 must appear at the top (3 points), there must be 12 numbers present (1 point), there must be two distinguishable hands (1 point), and the time must be correctly identified (1 point) to obtain the maximum score.
- Regarding questionnaires on issues intrinsic to IVR exposure, the following were used:
- Simulator Sickness Questionnaire (SSQ) to assess the safety of IVR exposure by assessing potential associated symptomatology in three broad domains [55,56,57]: 1. oculomotor symptoms; 2. disorientation; 3. nausea. Each item is assessed on a four-point scale (0 = do not feel anything; 1 = a little; 2 = medium; and 3 = a lot) and the total score (maximum of 48 points) is the sum of the scores of the three subscales.
- System Usability Scale (SUS) to assess the usability of the device/protocol. This consists of ten questions on a Likert-type scale. Each question is scored from 1 to 5 according to the level of agreement or disagreement with each statement, with 5 meaning completely agree and 1 meaning completely disagree. The algorithm that results from these answers creates a score out of a maximum of 100 points [58,59].
- The post-game module of the Game Experience Questionnaire (GEQ-post game, to assess how players felt after they stopped playing the game. This module is also a Likert-type scale consisting of 17 items, in which responses are graded according to the intensity of the sensations experienced (0 indicates not at all and 4 indicates extremely). These items are, in turn, divided into four components (positive experiences, negative experiences, tiredness, and return to reality), which are scored individually and whose average could result in a maximum score of 4 points [38]. In the absence of a validated version of the questionnaire in Spanish, and so the current study group would have no problems with this questionnaire, a version was used that was translated by the authors and which has been used in previous research [60,61].
2.5. Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, S. Participation Based Intervention with Acquired Brain Injury: Systematic Review and Meta-Analysis. Restor. Neurol. Neurosci. 2020, 38, 419–429. [Google Scholar] [CrossRef]
- Grewal, J.; Eng, J.J.; Sakakibara, B.M.; Schmidt, J. The Use of Virtual Reality for Activities of Daily Living Rehabilitation after Brain Injury: A Scoping Review. Aust. Occup. Ther. J. 2024, 71, 868–893. [Google Scholar] [CrossRef]
- Goldman, L.; Siddiqui, E.M.; Khan, A.; Jahan, S.; Rehman, M.U.; Mehan, S.; Sharma, R.; Budkin, S.; Kumar, S.N.; Sahu, A.; et al. Understanding Acquired Brain Injury: A Review. Biomedicines 2022, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Janzen, S.; Richardson, M.; Kwok, C.; Teasell, R. An Overview of Acquired Brain Injury Rehabilitation Randomized Controlled Trials. J. Head Trauma Rehabil. 2015, 30, E47–E53. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Pinedo, F.; Cid-Gala, M.; Duque, P.; Ramirez-Moreno, J.M.; Zurdo-Hernández, J.M. Grupo de Trabajo del Plan de Atención al Daño Cerebral Sobrevenido de Extremadura [Acquired brain injury: A proposal for its definition, diagnostic criteria and classification]. Rev. Neurol. 2012, 54, 357–366. [Google Scholar]
- Dams-O’Connor, K.; Landau, A.; Hoffman, J.; St De Lore, J. Patient Perspectives on Quality and Access to Healthcare after Brain Injury. Brain Inj. 2018, 32, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, Regional, and National Burden of Neurological Disorders during 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2021, 38, 1411–1440. [Google Scholar] [CrossRef]
- Pietrzak, E.; Pullman, S.; McGuire, A. Using Virtual Reality and Videogames for Traumatic Brain Injury Rehabilitation: A Structured Literature Review. Games Health J. 2014, 3, 202–214. [Google Scholar] [CrossRef]
- Biffi, E.; Beretta, E.; Cesareo, A.; Maghini, C.; Turconi, A.C.; Reni, G.; Strazzer, S. An Immersive Virtual Reality Platform to Enhance Walking Ability of Children with Acquired Brain Injuries. Methods Inf. Med. 2017, 56, 119–126. [Google Scholar] [CrossRef]
- Calderone, A.; Carta, D.; Cardile, D.; Quartarone, A.; Rifici, C.; Calabrò, R.S.; Corallo, F. Use of Virtual Reality in Patients with Acquired Brain Injury: A Systematic Review. J. Clin. Med. 2023, 12, 7680. [Google Scholar] [CrossRef]
- Levin, M.F.; Weiss, P.L.; Keshner, E.A. Emergence of Virtual Reality as a Tool for Upper Limb Rehabilitation: Incorporation of Motor Control and Motor Learning Principles. Phys. Ther. 2015, 95, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Olk, B.; Dinu, A.; Zielinski, D.J.; Kopper, R. Measuring Visual Search and Distraction in Immersive Virtual Reality. R. Soc. Open Sci. 2018, 5, 172331. [Google Scholar] [CrossRef]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The Effectiveness of Immersive Virtual Reality in Physical Recovery of Stroke Patients: A Systematic Review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.; Godart, N.; Cavalier, M.; Berard, C.; Cieślik, B.; Federico, S.; Kiper, A.; Pellicciari, L.; Meroni, R. Effects of Immersive Virtual Reality on Upper-Extremity Stroke Rehabilitation: A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 13, 146. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Rizzo, A.; Kim, G.J. A SWOT Analysis of the Field of Virtual Reality Rehabilitation and Therapy. Presence 2005, 14, 119–146. [Google Scholar] [CrossRef]
- Vilageliu Jordà, È.; Enseñat Cantallops, A.; García Molina, A. Uso de la realidad virtual inmersiva en la rehabilitación cognitiva de pacientes con daño cerebral. Revisión sistemática. Rev. Neurol. 2022, 74, 331–339. [Google Scholar] [CrossRef]
- Tieri, G.; Morone, G.; Paolucci, S.; Iosa, M. Virtual Reality in Cognitive and Motor Rehabilitation: Facts, Fiction and Fallacies. Expert Rev. Med. Devices 2018, 15, 107–117. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela, J.M.; Rodríguez-Fuentes, G. Immersive Virtual Reality as Physical Therapy in Older Adults: Present or Future (Systematic Review). Virtual Real. 2021, 25, 801–807. [Google Scholar] [CrossRef]
- Winter, C.; Kern, F.; Gall, D.; Latoschik, M.E.; Pauli, P.; Käthner, I. Immersive Virtual Reality during Gait Rehabilitation Increases Walking Speed and Motivation: A Usability Evaluation with Healthy Participants and Patients with Multiple Sclerosis and Stroke. J. NeuroEng. Rehabil. 2021, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Elhusein, A.M.; Fadlalmola, H.A.; Awadalkareem, E.M.; Alhusain, E.Y.M.; Alnassry, S.M.; Alshammari, M.; Abdulrahman, E.E.; Fadila, D.E.S.; Ibrahim, F.M.; Saeed, A.A.M.; et al. Exercise-Based Gaming in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Belitung Nurs. J. 2024, 10, 1–14. [Google Scholar] [CrossRef]
- Campo Prieto, P.; Santos García, D.; Cancela Carral, J.M.; Rodríguez Fuentes, G. Estado actual de la realidad virtual inmersiva como herramienta de rehabilitación física y funcional en pacientes con enfermedad de Parkinson: Revisión sistemática. Rev. Neurol. 2021, 73, 358. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation That Incorporates Virtual Reality Is More Effective than Standard Rehabilitation for Improving Walking Speed, Balance and Mobility after Stroke: A Systematic Review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of Immersive and Non-Immersive Virtual Reality for Upper Extremity Functional Recovery in Patients with Stroke: A Systematic Review and Network Meta-Analysis. Neurol. Sci. 2023, 44, 2679–2697. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Liu, H.; Wang, G.; Lu, A. Effects of Immersive and Non-Immersive Virtual Reality-Based Rehabilitation Training on Cognition, Motor Function, and Daily Functioning in Patients with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2024, 38, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Brassel, S.; Power, E.; Campbell, A.; Brunner, M.; Togher, L. Recommendations for the Design and Implementation of Virtual Reality for Acquired Brain Injury Rehabilitation: Systematic Review. J. Med. Internet Res. 2021, 23, e26344. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.-G.; Sheng, J.; Ma, S.-J. Virtual Reality for Improving Balance in Patients after Stroke: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2016, 30, 432–440. [Google Scholar] [CrossRef]
- Kim, W.-S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.-J.; Paik, N.-J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Virtual Reality for Balance and Mobility Rehabilitation Following Traumatic Brain Injury: A Systematic Review of Randomized Controlled Trials. J. Clin. Neurosci. 2022, 105, 115–121. [Google Scholar] [CrossRef]
- Shen, J.; Johnson, S.; Chen, C.; Xiang, H. Virtual Reality for Pediatric Traumatic Brain Injury Rehabilitation: A Systematic Review. Am. J. Lifestyle Med. 2020, 14, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Semnani, A.V.; Mirmohammadkhani, M.; Grampurohit, N. Effects of Virtual Reality Compared to Conventional Therapy on Balance Poststroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, S.; MacKenzie, A.; Batcho, C.S.; D’Amour, J. Making Physical Activity Fun and Accessible to Adults with Intellectual Disabilities: A Pilot Study of a Gamification Intervention. J. Appl. Res. Intellect. Disabil. 2024, 37, e13213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.S.; Bodt, B.A.; Galloway, J.C. Real-World Environmental Enrichment Rehabilitation Paradigm in People with Severe Traumatic Brain Injury: A Pilot Feasibility Study. Brain Inj. 2024, 38, 742–749. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Campo-Prieto, P.; Cancela-Carral, J.M. Immersive Virtual Reality High-Intensity Aerobic Training to Slow Parkinson’s Disease: The ReViPark Program. Appl. Sci. 2024, 14, 4708. [Google Scholar] [CrossRef]
- Cancela-Carral, J.M.; Campo-Prieto, P.; Rodríguez-Fuentes, G. The IntegraPark Study: An Opportunity to Facilitate High-Intensity Exercise with Immersive Virtual Reality in Parkinson’s Disease Patients. J. Funct. Morphol. Kinesiol. 2024, 9, 156. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, L.-Y. Grip Strength in Older Adults: Test-Retest Reliability and Cutoff for Subjective Weakness of Using the Hands in Heavy Tasks. Arch. Phys. Med. Rehabil. 2010, 91, 1747–1751. [Google Scholar] [CrossRef]
- Uygur, M.; Barone, D.A.; Dankel, S.J.; DeStefano, N. Isometric Tests to Evaluate Upper and Lower Extremity Functioning in People with Multiple Sclerosis: Reliability and Validity. Mult. Scler. Relat. Disord. 2022, 63, 103817. [Google Scholar] [CrossRef]
- Matsushita, T.; Nishioka, S.; Yamanouchi, A.; Okazaki, Y.; Oishi, K.; Nakashima, R.; Tokunaga, Y.; Onizuka, S. Predictive Ability of Hand-Grip Strength and Muscle Mass on Functional Prognosis in Patients Rehabilitating from Stroke. Nutrition 2022, 102, 111724. [Google Scholar] [CrossRef]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The Five-Times-Sit-to-Stand Test: Validity, Reliability and Detectable Change in Older Females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef]
- Mong, Y.; Teo, T.W.; Ng, S.S. 5-Repetition Sit-to-Stand Test in Subjects with Chronic Stroke: Reliability and Validity. Arch. Phys. Med. Rehabil. 2010, 91, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, T.B.; Rensink, M.; Schuurmans, M. Clinimetric Properties of the Timed Up and Go Test for Patients with Stroke: A Systematic Review. Top. Stroke Rehabil. 2014, 21, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Franklin Williams, T.; Mayewski, R. Fall Risk Index for Elderly Patients Based on Number of Chronic Disabilities. Am. J. Med. 1986, 80, 429–434. [Google Scholar] [CrossRef]
- Canbek, J.; Fulk, G.; Nof, L.; Echternach, J. Test-Retest Reliability and Construct Validity of the Tinetti Performance-Oriented Mobility Assessment in People with Stroke. J. Neurol. Phys. Ther. 2013, 37, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.; Dewey, J.E.; Gandek, B. How to Score and Interpret Single-Hem Health Status Measures: A Manual for Users of the SF-8 Health Survey; Quality Metric, Incorporated: Lincoln, RI, USA, 2001; ISBN 978-1-891810-08-4. [Google Scholar]
- Wang, P.; Luo, N.; Tai, E.S.; Lee, J.; Wee, H.L.; Thumboo, J. PRM35 Relative Efficiency of the SF-8, SF-12, and SF-36 in the General Population. Value Health 2012, 15, A651. [Google Scholar] [CrossRef]
- Grant, D.A.; Berg, E. A Behavioral Analysis of Degree of Reinforcement and Ease of Shifting to New Responses in a Weigl-Type Card-Sorting Problem. J. Exp. Psychol. 1948, 38, 404–411. [Google Scholar] [CrossRef]
- Chiu, E.-C.; Wu, W.-C.; Hung, J.-W.; Tseng, Y.-H. Validity of the Wisconsin Card Sorting Test in Patients with Stroke. Disabil. Rehabil. 2018, 40, 1967–1971. [Google Scholar] [CrossRef]
- Shulman, K.I. Clock-Drawing: Is It the Ideal Cognitive Screening Test? Int. J. Geriat. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef]
- Sunderland, T.; Hill, J.L.; Mellow, A.M.; Lawlor, B.A.; Gundersheimer, J.; Newhouse, P.A.; Grafman, J.H. Clock Drawing in Alzheimer’s Disease: A Novel Measure of Dementia Severity. J. Am. Geriatr. Soc. 1989, 37, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Champod, A.S.; Gubitz, G.J.; Phillips, S.J.; Christian, C.; Reidy, Y.; Radu, L.M.; Darvesh, S.; Reid, J.M.; Kintzel, F.; Eskes, G.A. Clock Drawing Test in Acute Stroke and Its Relationship with Long-Term Functional and Cognitive Outcomes. Clin. Neuropsychol. 2019, 33, 817–830. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Kennedy, R.S.; Drexler, J.; Kennedy, R.C. Research in Visually Induced Motion Sickness. Appl. Ergon. 2010, 41, 494–503. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Rodríguez-Fuentes, G.; Cancela Carral, J.M. Traducción y Adaptación Transcultural al Español Del Simulator Sickness Questionnaire (Translation and Cross-Cultural Adaptation to Spanish of the Simulator Sickness Questionnaire). Retos 2021, 43, 503–509. [Google Scholar] [CrossRef]
- Brooke, J. SUS-A Quick and Dirty Usability Scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Hedlefs Aguilar, M.I.; Garza Villegas, A.A. Análisis Comparativo de La Escala de Usabilidad Del Sistema (EUS) En Dos Versiones. RECI 2016, 5, 44. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Feasibility and Effects of an Immersive Virtual Reality Exergame Program on Physical Functions in Institutionalized Older Adults: A Randomized Clinical Trial. Sensors 2022, 22, 6742. [Google Scholar] [CrossRef]
- Campo-Prieto, P.; Rodríguez-Fuentes, G.; Cancela-Carral, J.M. Immersive Virtual Reality Exergame Promotes the Practice of Physical Activity in Older People: An Opportunity during COVID-19. Multimodal Technol. Interact. 2021, 5, 52. [Google Scholar] [CrossRef]
- Moraes, T.M.; Zaninotto, A.L.; Neville, I.S.; Hayashi, C.Y.; Paiva, W.S. Immersive Virtual Reality in Patients with Moderate and Severe Traumatic Brain Injury: A Feasibility Study. Health Technol. 2021, 11, 1035–1044. [Google Scholar] [CrossRef]
- Pau, M.; Arippa, F.; Leban, B.; Porta, M.; Casu, G.; Frau, J.; Lorefice, L.; Coghe, G.; Cocco, E. Cybersickness in People with Multiple Sclerosis Exposed to Immersive Virtual Reality. Bioengineering 2024, 11, 115. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Ferreiro-Gómez, E.; Campo-Prieto, P.; Cancela-Carral, J.M. Exergames and Immersive Virtual Reality as a Novel Therapy Approach in Multiple Sclerosis: Randomised Feasibility Study. J. Clin. Med. 2024, 13, 5845. [Google Scholar] [CrossRef] [PubMed]
- Campo-Prieto, P.; Cancela-Carral, J.M.; Rodríguez-Fuentes, G. Wearable Immersive Virtual Reality Device for Promoting Physical Activity in Parkinson’s Disease Patients. Sensors 2022, 22, 3302. [Google Scholar] [CrossRef]
- Sevcenko, K.; Lindgren, I. The Effects of Virtual Reality Training in Stroke and Parkinson’s Disease Rehabilitation: A Systematic Review and a Perspective on Usability. Eur. Rev. Aging Phys. Act. 2022, 19, 4. [Google Scholar] [CrossRef]
- Lee, J.H.; Ku, J.; Cho, W.; Hahn, W.Y.; Kim, I.Y.; Lee, S.-M.; Kang, Y.; Kim, D.Y.; Yu, T.; Wiederhold, B.K.; et al. A Virtual Reality System for the Assessment and Rehabilitation of the Activities of Daily Living. Cyberpsychol. Behav. 2003, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Prats-Bisbe, A.; López-Carballo, J.; García-Molina, A.; Leno-Colorado, D.; García-Rudolph, A.; Opisso, E.; Jané, R. Virtual Reality–Based Neurorehabilitation Support Tool for People with Cognitive Impairments Resulting from an Acquired Brain Injury: Usability and Feasibility Study. JMIR Neurotechnol. 2024, 3, e50538. [Google Scholar] [CrossRef]
- Aulisio, M.C.; Han, D.Y.; Glueck, A.C. Virtual Reality Gaming as a Neurorehabilitation Tool for Brain Injuries in Adults: A Systematic Review. Brain Inj. 2020, 34, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Téllez, P.; Moral Muñoz, J.A.; Casado Fernández, E.; Salazar Couso, A.; Lucena Antón, D. Efectos de la realidad virtual sobre el equilibrio y la marcha en el ictus: Revisión sistemática y metaanálisis. Rev. Neurol. 2019, 69, 223–234. [Google Scholar] [CrossRef]
- Garay-Sánchez, A.; Suarez-Serrano, C.; Ferrando-Margelí, M.; Jimenez-Rejano, J.J.; Marcén-Román, Y. Effects of Immersive and Non-Immersive Virtual Reality on the Static and Dynamic Balance of Stroke Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4473. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Lee, J.; Kim, J. Stereoscopic Objects Affect Reaching Performance in Virtual Reality Environments: Influence of Age on Motor Control. Front. Virtual Real. 2024, 5, 1475482. [Google Scholar] [CrossRef]
- Brazil, C.K.; Rys, M.J. The Effect of VR on Fine Motor Performance by Older Adults: A Comparison between Real and Virtual Tasks. Virtual Real. 2024, 28, 113. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal Clinically Important Difference for Grip Strength: A Systematic Review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, V.; Weatherall, M.; McNaughton, H. Estimating the Minimal Clinically Important Difference for the Physical Component Summary of the Short Form 36 for Patients with Stroke. J. Int. Med. Res. 2021, 49, 3000605211067902. [Google Scholar] [CrossRef]
- Corti, C.; Oprandi, M.C.; Chevignard, M.; Jansari, A.; Oldrati, V.; Ferrari, E.; Martignoni, M.; Romaniello, R.; Strazzer, S.; Bardoni, A. Virtual-Reality Performance-Based Assessment of Cognitive Functions in Adult Patients with Acquired Brain Injury: A Scoping Review. Neuropsychol. Rev. 2022, 32, 352–399. [Google Scholar] [CrossRef]
- Van De Wouw, C.L.; Visser, M.; Gorter, J.W.; Huygelier, H.; Nijboer, T.C.W. Systematic Review of the Effectiveness of Innovative, Gamified Interventions for Cognitive Training in Paediatric Acquired Brain Injury. Neuropsychol. Rehabil. 2024, 34, 268–299. [Google Scholar] [CrossRef]

| Mean/% | SD | Minimum | Maximum | ||
|---|---|---|---|---|---|
| Age (years) | 52.43 | 8.64 | 35.00 | 65.00 | |
| Gender | Male | 57.1% | |||
| Female | 42.9% | ||||
| Diagnosis | Wernicke’s encephalopathy | 7.1% | |||
| Stroke | 35.7% | ||||
| Traumatic brain injury | 28.6% | ||||
| Frontotemporal dementia | 7.1% | ||||
| Cerebral palsy | 7.1% | ||||
| Encephalitis | 7.1% | ||||
| Meningitis | 7.1% | ||||
| Diagnosis (years) | 22.29 | 17.47 | 1.00 | 58.00 | |
| Barthel Index | Independent | 28.6% | |||
| Mild dependence | 64.3% | ||||
| Severe dependence | 7.1% | ||||
| Cognitive impairment | Mild | 50.0% | |||
| Moderate | 35.7% | ||||
| Severe | 14.3% | ||||
| Pre | Post | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | |
| Handgrip [dominant, Kg] | 9.71 | 2.63 | 5.00 | 12.50 | 11.21 | 2.08 | 9.00 | 13.50 |
| Handgrip [non-dominant, Kg] | 7.88 | 5.09 | 2.50 | 20.00 | 9.50 | 5.39 | 2.50 | 20.00 |
| Five sit to stand (s) | 23.25 | 10.21 | 10.00 | 50.00 | 20.50 | 7.98 | 11.00 | 37.00 |
| Timed up and go (s) | 30.27 | 25.34 | 9.00 | 96.00 | 29.38 | 22.96 | 9.00 | 89.00 |
| Tinetti test—gait | 7.06 | 2.26 | 0.00 | 9.00 | 9.08 | 2.06 | 6.00 | 12.00 |
| Tinetti test—balance | 6.81 | 2.14 | 2.00 | 10.00 | 12.14 | 3.44 | 4.00 | 16.00 |
| Tinetti test—total score | 14.47 | 2.75 | 10.00 | 19.00 | 21.46 | 4.89 | 13.00 | 28.00 |
| Pre | Post | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | |
| General Health | 2.38 | 0.96 | 0.00 | 4.00 | 3.29 | 0.91 | 1.00 | 4.00 |
| Physical Functioning | 1.69 | 1.54 | 0.00 | 4.00 | 3.00 | 1.04 | 1.00 | 4.00 |
| Physical Role Functioning | 1.69 | 1.30 | 0.00 | 4.00 | 2.43 | 1.09 | 1.00 | 4.00 |
| Bodily Pain | 3.31 | 0.95 | 2.00 | 5.00 | 4.14 | 0.66 | 3.00 | 5.00 |
| Vitality | 2.63 | 0.89 | 1.00 | 4.00 | 3.07 | 0.73 | 2.00 | 4.00 |
| Social Functioning | 2.62 | 1.15 | 1.00 | 4.00 | 2.71 | 0.73 | 2.00 | 4.00 |
| Emotional Role Functioning | 2.75 | 0.93 | 1.00 | 4.00 | 2.50 | 0.85 | 1.00 | 4.00 |
| Mental Health | 2.50 | 1.15 | 0.00 | 4.00 | 2.79 | 0.70 | 2.00 | 4.00 |
| Physical Component Summary | 9.06 | 3.73 | 4.00 | 16.00 | 12.86 | 2.63 | 9.00 | 17.00 |
| Mental Component Summary | 10.50 | 3.16 | 5.00 | 15.00 | 11.07 | 2.13 | 9.00 | 16.00 |
| Paired Differences | |||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Std. Error Mean | t | p | Cohen’s d | Confidence Interval | |
| Handgrip [dominant, Kg] | −0.70 | 0.63 | 0.15 | −4.53 | <0.001 | 0.63 | −1.69–−0.48 |
| Handgrip [non-dominant, Kg] | −0.38 | 0.61 | 0.17 | −2.24 | 0.044 | 0.62 | −1.20–−0.01 |
| Five sit to stand (s) | 1.25 | 4.15 | 1.03 | 1.20 | 0.248 | 0.15 | −0.20–0.79 |
| Timed up and go (s) | −0.60 | 5.09 | 1.31 | −0.45 | 0.655 | 0.09 | −0.62–0.39 |
| Tinetti test—gait | −1.69 | 1.60 | 0.44 | −3.81 | 0.002 | 0.60 | −1.72–−0.35 |
| Tinetti test—balance | −5.14 | 1.61 | 0.43 | −11.94 | <0.001 | 0.61 | −4.50–−1.86 |
| Tinetti test—total score | −6.92 | 2.62 | 0.72 | −9.49 | <0.001 | 0.62 | −3.79–−1.44 |
| PCS—SF-8 | −3.26 | 3.91 | 1.01 | −3.22 | 0.006 | 0.91 | −1.41–0.34 |
| MCS—SF-8 | −0.66 | 4.01 | 1.03 | −0.64 | 0.530 | 0.02 | −0.67–0.34 |
| WCST—total errors | 6.00 | 6.80 | 1.81 | 3.29 | 0.006 | 0.84 | 0.26–1.51 |
| WCST—total corrects | 7.42 | 9.34 | 2.49 | 2.97 | 0.011 | 0.34 | 0.17–1.38 |
| WCST—number of attempts completed | 13.42 | 14.84 | 3.96 | 3.38 | 0.005 | 0.80 | 0.24–1.49 |
| CDT—verbal | 0.16 | 1.58 | 0.45 | 0.36 | 0.723 | 0.58 | −0.46–0.67 |
| CDT—copy | −0.58 | 1.68 | 0.48 | −1.19 | 0.257 | 0.68 | −0.92–0.24 |
| Test | Measured Dimension | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| SSQ | Nausea | 0.01/4 | 0.02 | 0.00 | 0.07 |
| Oculomotor symptoms | 0.13/4 | 0.14 | 0.00 | 0.36 | |
| Disorientation | 0.10/4 | 0.13 | 0.00 | 0.26 | |
| Total Score | 0.24/4 | 0.26 | 0.00 | 0.57 | |
| SUS | Usability | 81.86/100 | 10.14 | 57.50 | 95.00 |
| GEQ—postgame | Positive experiences | 2.56/4 | 0.60 | 0.83 | 3.33 |
| Negative experiences | 0.09/4 | 0.24 | 0.00 | 0.83 | |
| Tiredness | 0.71/4 | 0.31 | 0.00 | 1.00 | |
| Return to reality | 0.51/4 | 0.24 | 0.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Fuentes, G.; Campo-Prieto, P.; Cancela-Carral, J.M. Immersive Virtual Reality as Physical and Cognitive Therapy in Acquired Brain Injury: TEVI-DCA Program. Electronics 2025, 14, 1204. https://doi.org/10.3390/electronics14061204
Rodríguez-Fuentes G, Campo-Prieto P, Cancela-Carral JM. Immersive Virtual Reality as Physical and Cognitive Therapy in Acquired Brain Injury: TEVI-DCA Program. Electronics. 2025; 14(6):1204. https://doi.org/10.3390/electronics14061204
Chicago/Turabian StyleRodríguez-Fuentes, Gustavo, Pablo Campo-Prieto, and José Mᵃ Cancela-Carral. 2025. "Immersive Virtual Reality as Physical and Cognitive Therapy in Acquired Brain Injury: TEVI-DCA Program" Electronics 14, no. 6: 1204. https://doi.org/10.3390/electronics14061204
APA StyleRodríguez-Fuentes, G., Campo-Prieto, P., & Cancela-Carral, J. M. (2025). Immersive Virtual Reality as Physical and Cognitive Therapy in Acquired Brain Injury: TEVI-DCA Program. Electronics, 14(6), 1204. https://doi.org/10.3390/electronics14061204









