Robotic Systems for Cochlear Implant Surgeries: A Review of Robotic Design and Clinical Outcomes
Abstract
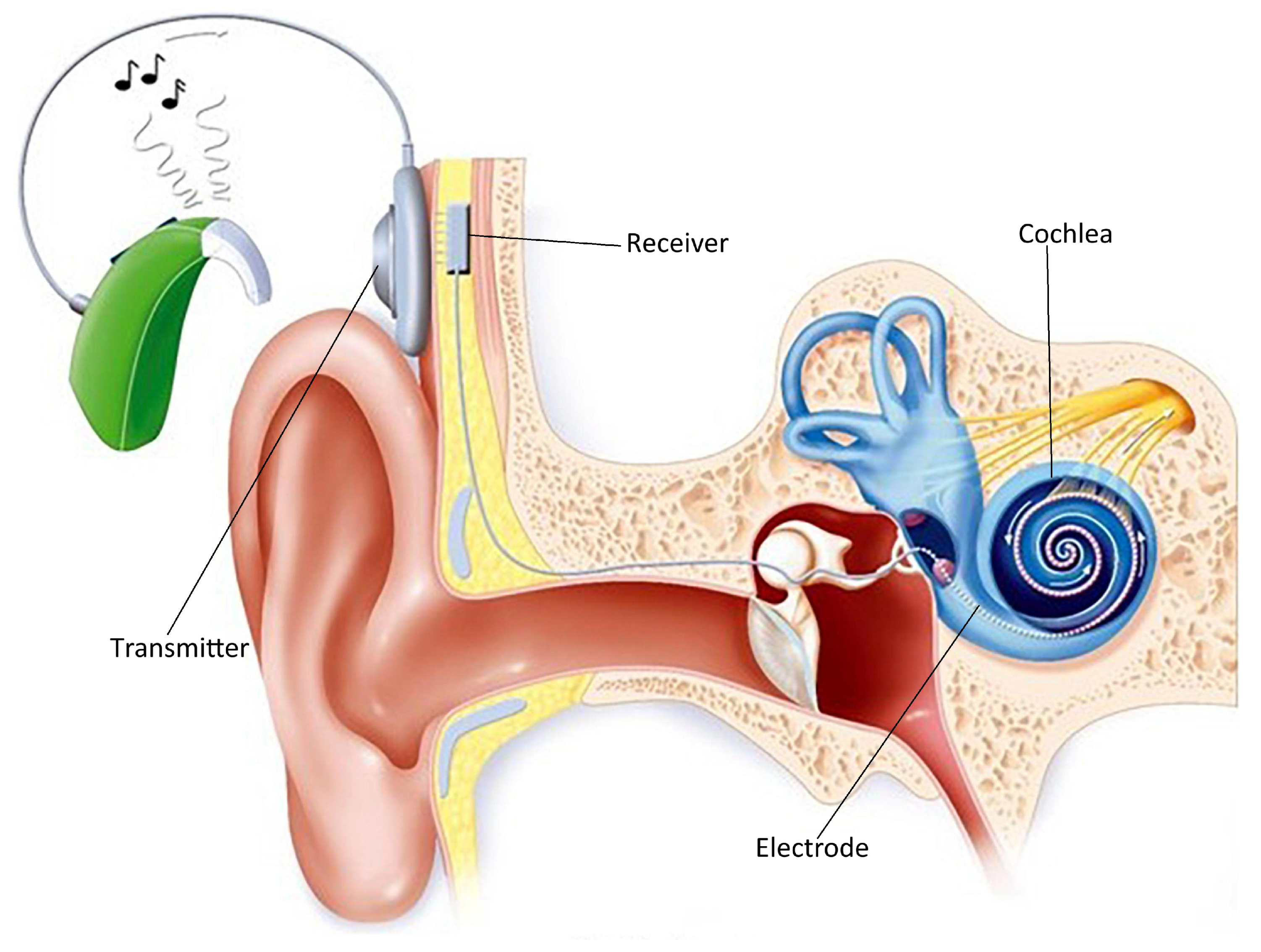
1. Introduction
- Insert the electrode deeply to capture low-frequency sounds
- Position the electrode close to the modiolus for efficient stimulation
- Preserve residual hearing
- Maximize frequency range coverage
- Minimize insertional trauma
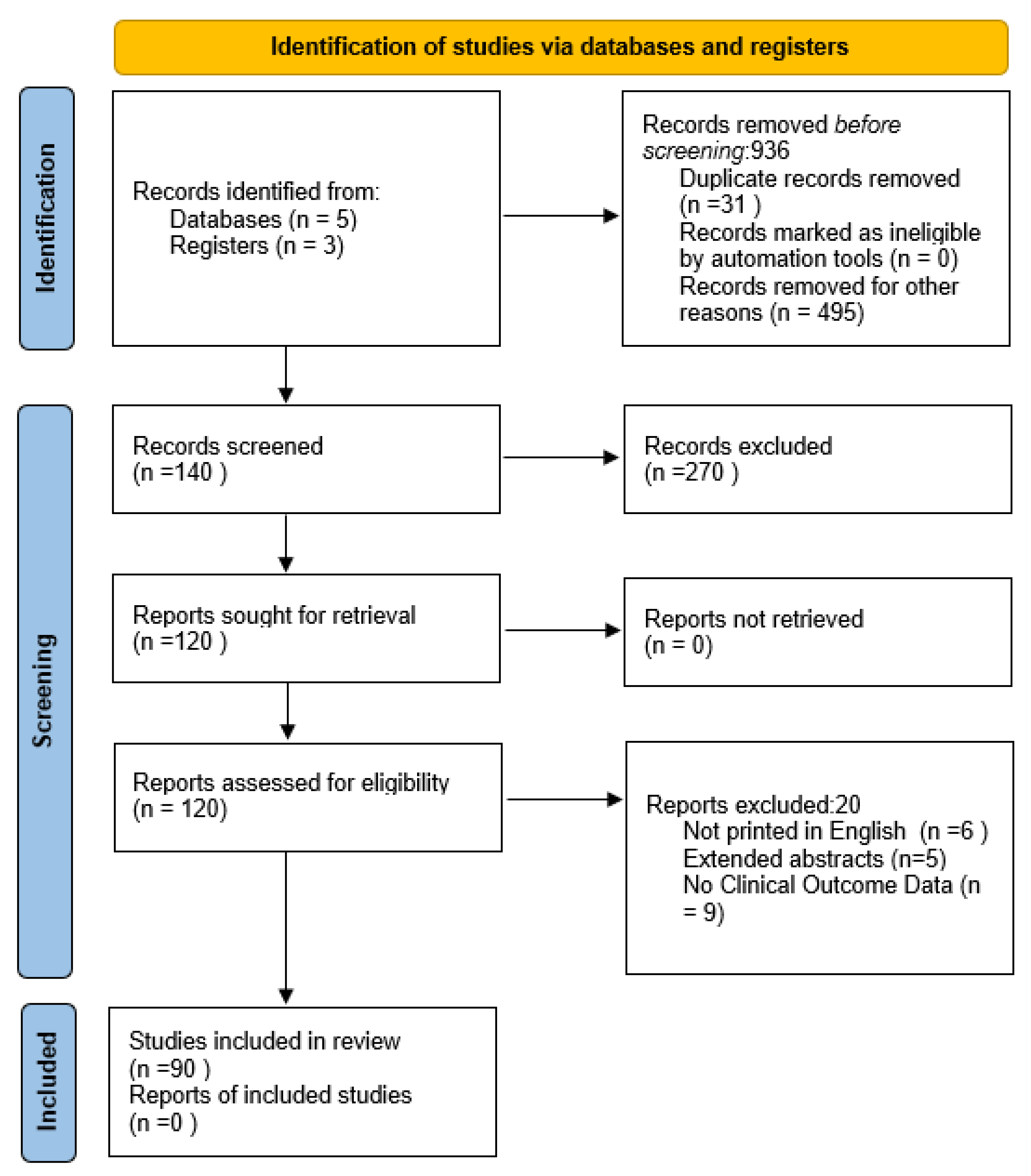
2. Methodology
- (Cochlear implant* AND robotic system)
- (Cochlear implant* AND integrated system)
- (Cochlear implant* AND insertion tool for electrodes)
- (Cochlear implant* AND HEARO)
- (Cochlear implant* AND RobOtol)
- (Cochlear implant* AND Vanderbilt)
- (Cochlear implant* AND ROSA)
- (Cochlear implant* AND review article)
- (Cochlear implant* AND clinical trials)
3. Robot-Based Approach to Inner Ear
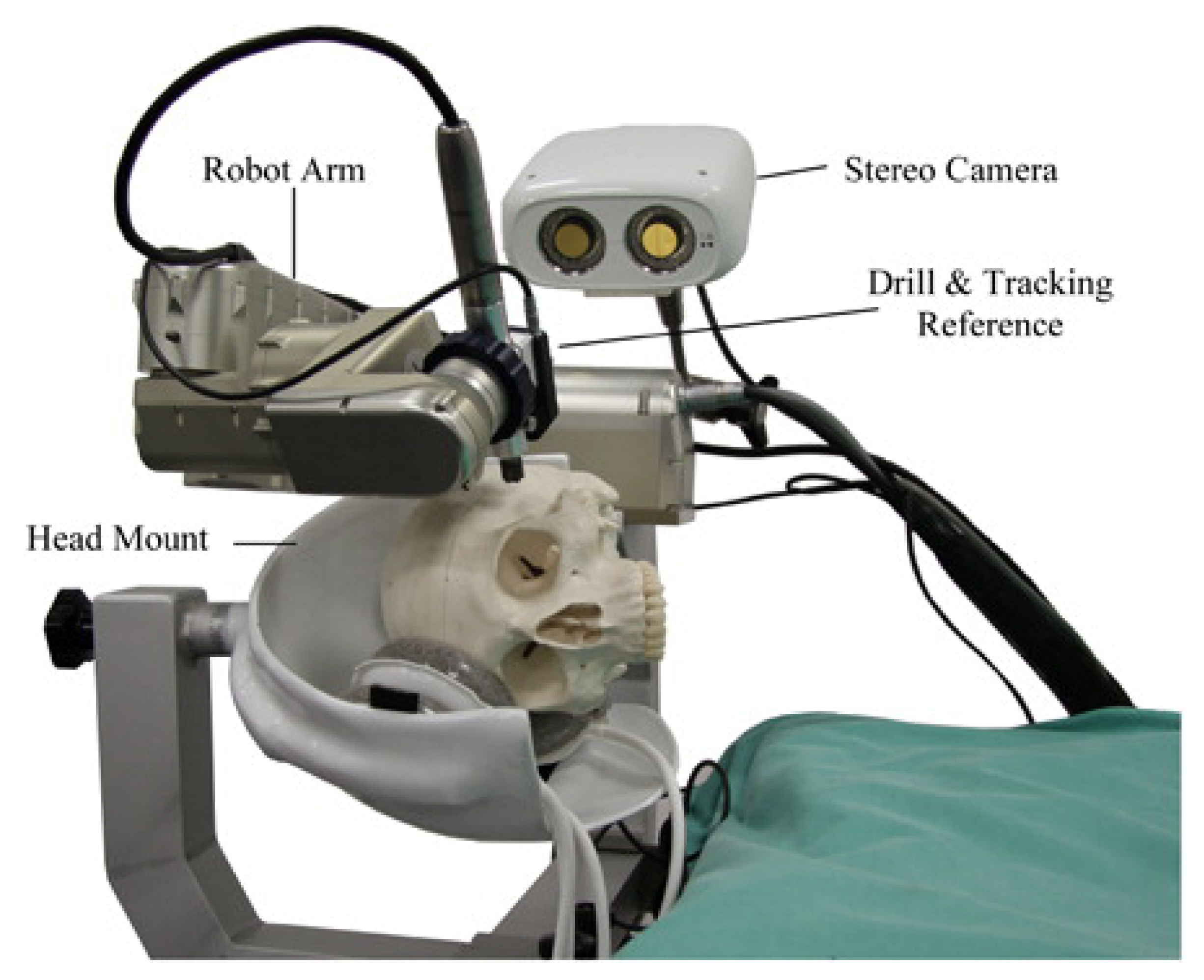
3.1. Robotics for Mastoidectomy (Vanderbilt, USA)
3.2. Robotics for Cochleostomy (London, UK)
4. Robots for Direct Cochlear Access (DCA)
4.1. ROSA® (Amiens, France)
4.2. HEARO® (Bern, Switzerland)
4.3. Vanderbilt Group (Nashville, USA)
4.4. Robots for Accessing Trans Mastoid
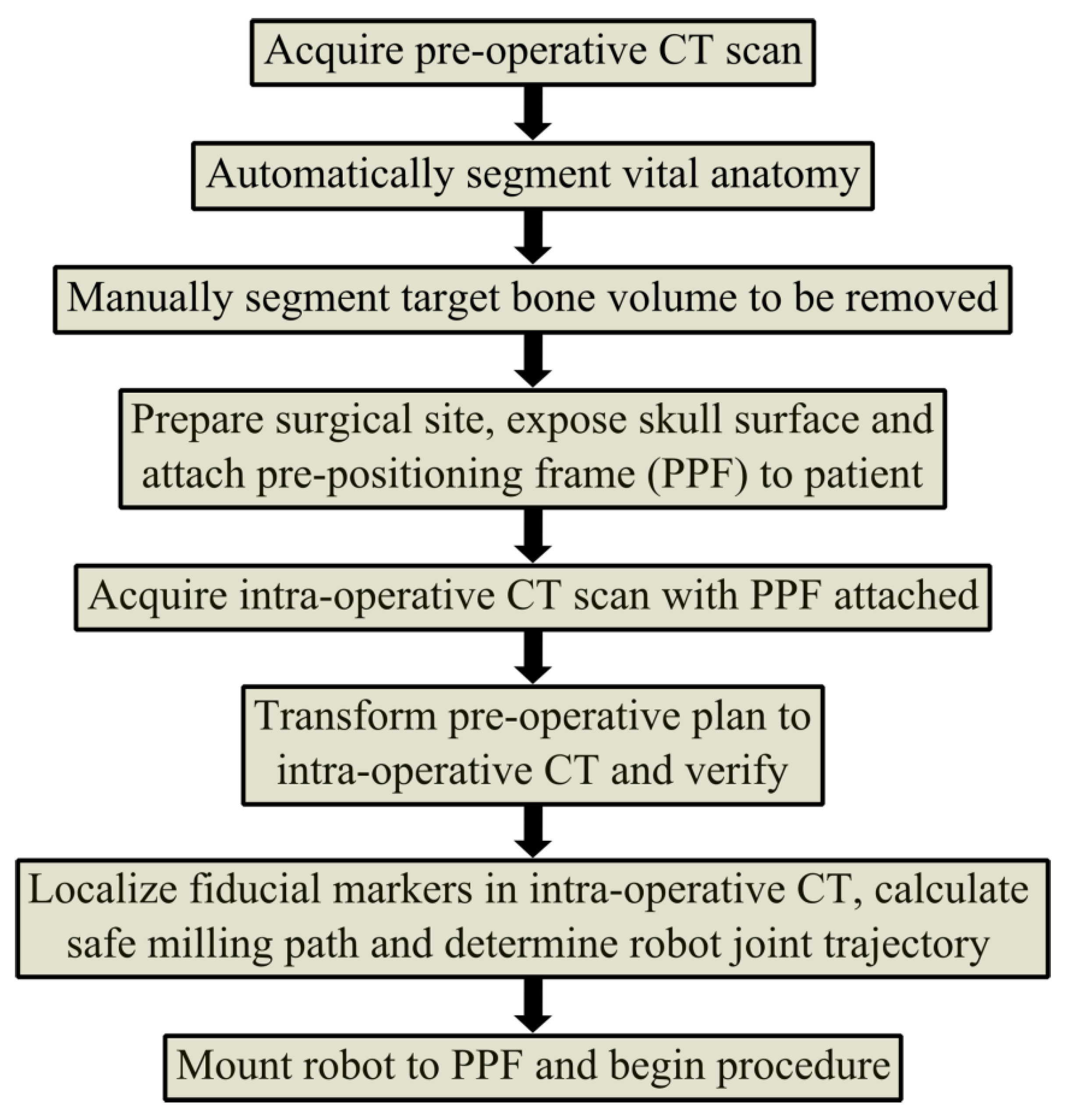
Hexapod and Micro-Stereotactic Frames (Hannover, Germany)
- Custom-Made Parallel Kinematics Motion Device (Hexapod):The Hexapod system features optimized structural elements and passive legs equipped with micrometer gauges. Surgeons mount the device directly onto the skull using magnetic bearings. The latest design includes a spherical platform with three non-rigid bone anchors, allowing for individualized placement based on patient anatomy.
- Customized Targeting Platforms:This system consists of a reusable base frame and a disposable, patient-specific surgical guide. It provides a mechanical interface for a linearly guided surgical hand drill, enabling precise positioning. The RoboJig® research project validated its preclinical feasibility. Intraoperatively, clinicians shape disposable templates using bone cement, a sterile and clinically accepted material that allows for rapid customization [18].
5. Electrode Insertion Tools
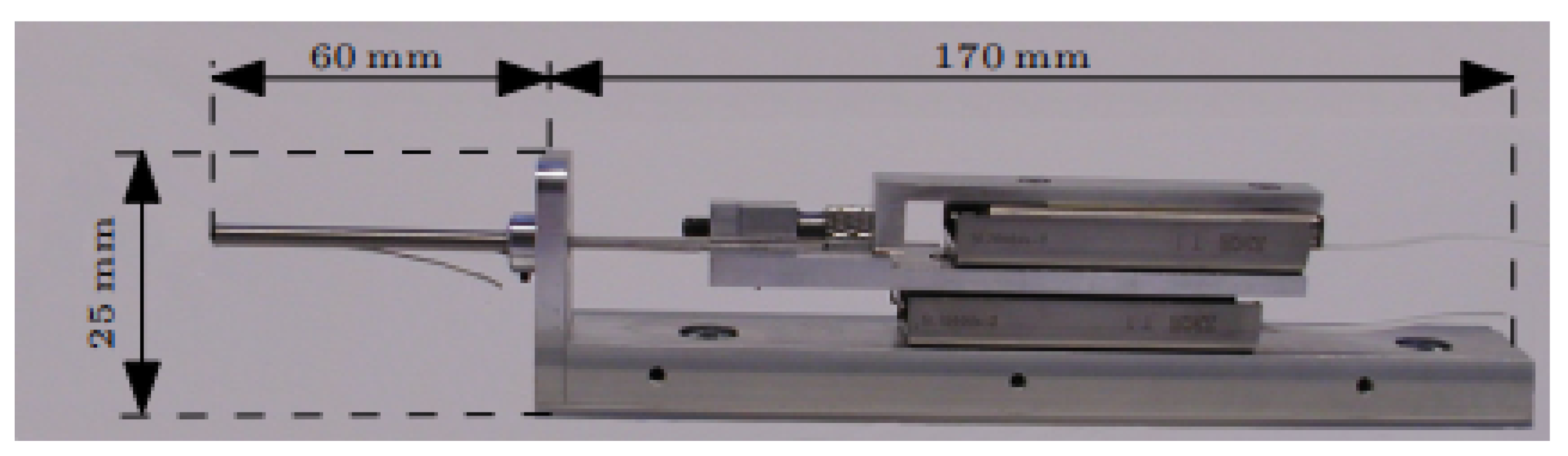
5.1. Motorized Electrode Insertion Tools (Hannover, Germany)
| Study | Lubricant | Insertion Depth | Insertion Time (s) | Speed (mm/s) | Reference |
|---|---|---|---|---|---|
| CHD | Water | 45 mm | <60 | 0.03 | [58] |
| Manual roller wheel | Soapy water | 12/12 electrodes inserted | – | [59] | |
| Manual insertion | Sodium hyaluronate | (deg) | – | [60] | |
| RobOtol | Sodium hyaluronate | (deg) | 80–100 | 0.25 | [60] |
5.2. Cochlea Hydro Drive (CHD (Hannover, Germany))
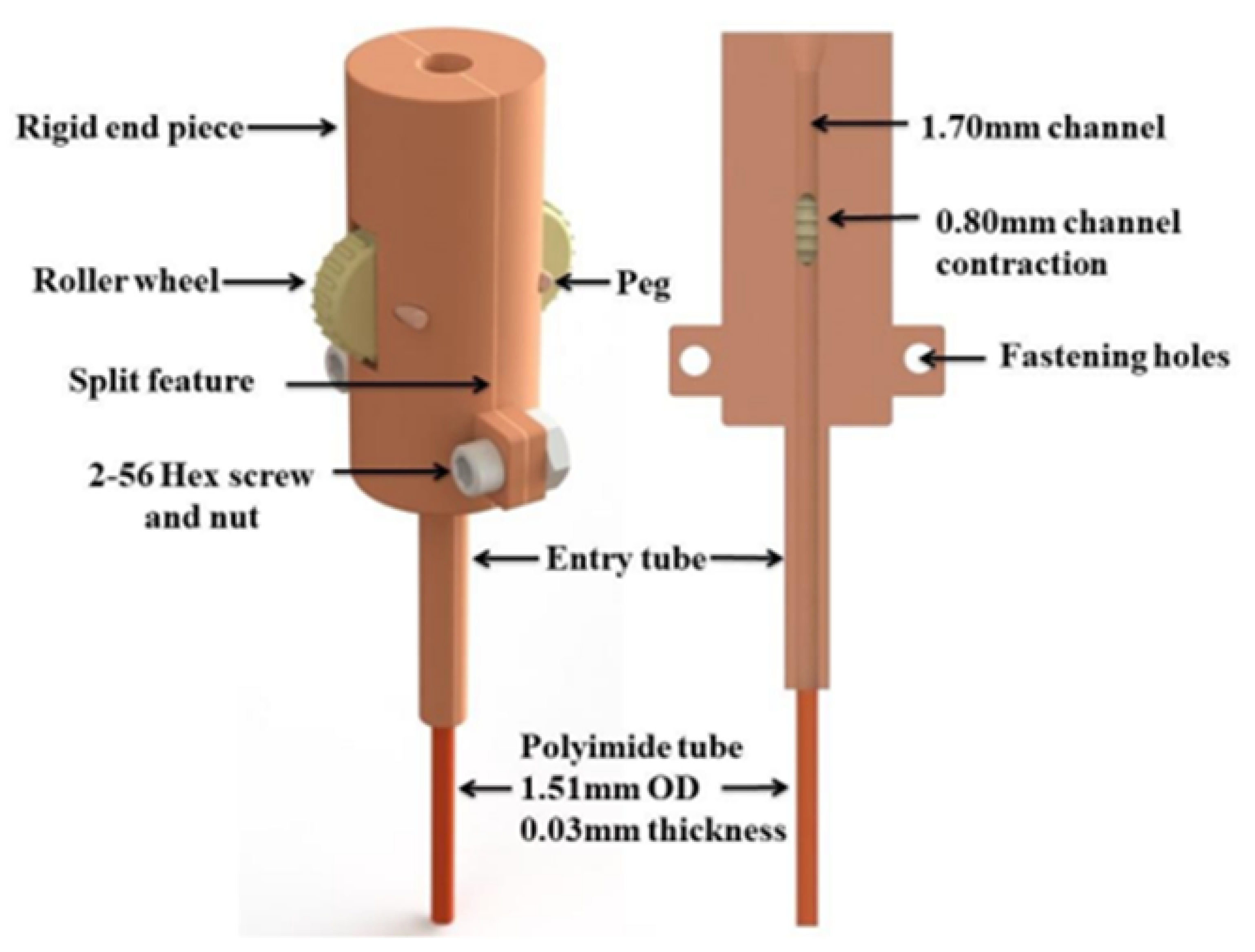
5.3. Roller Wheel Mechanism (Vanderbilt, USA)
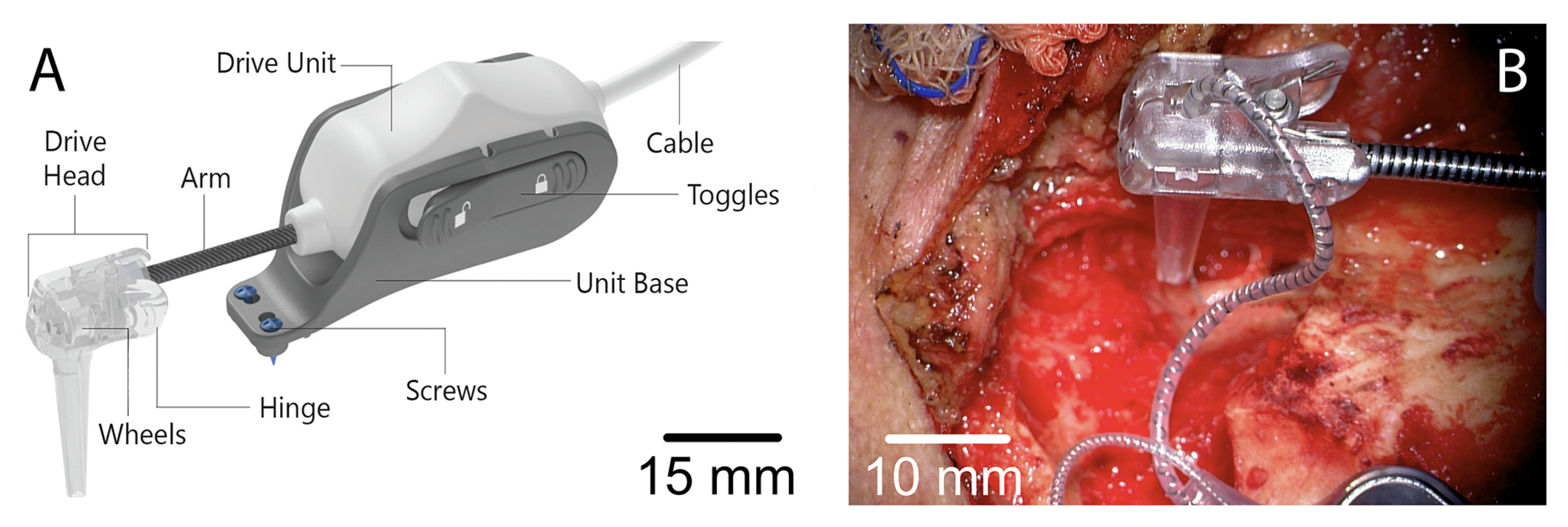
5.4. Magnetically Steered Robotic Insertion (Vanderbilt, USA)
5.5. Robot-Assisted Electrode Insertion
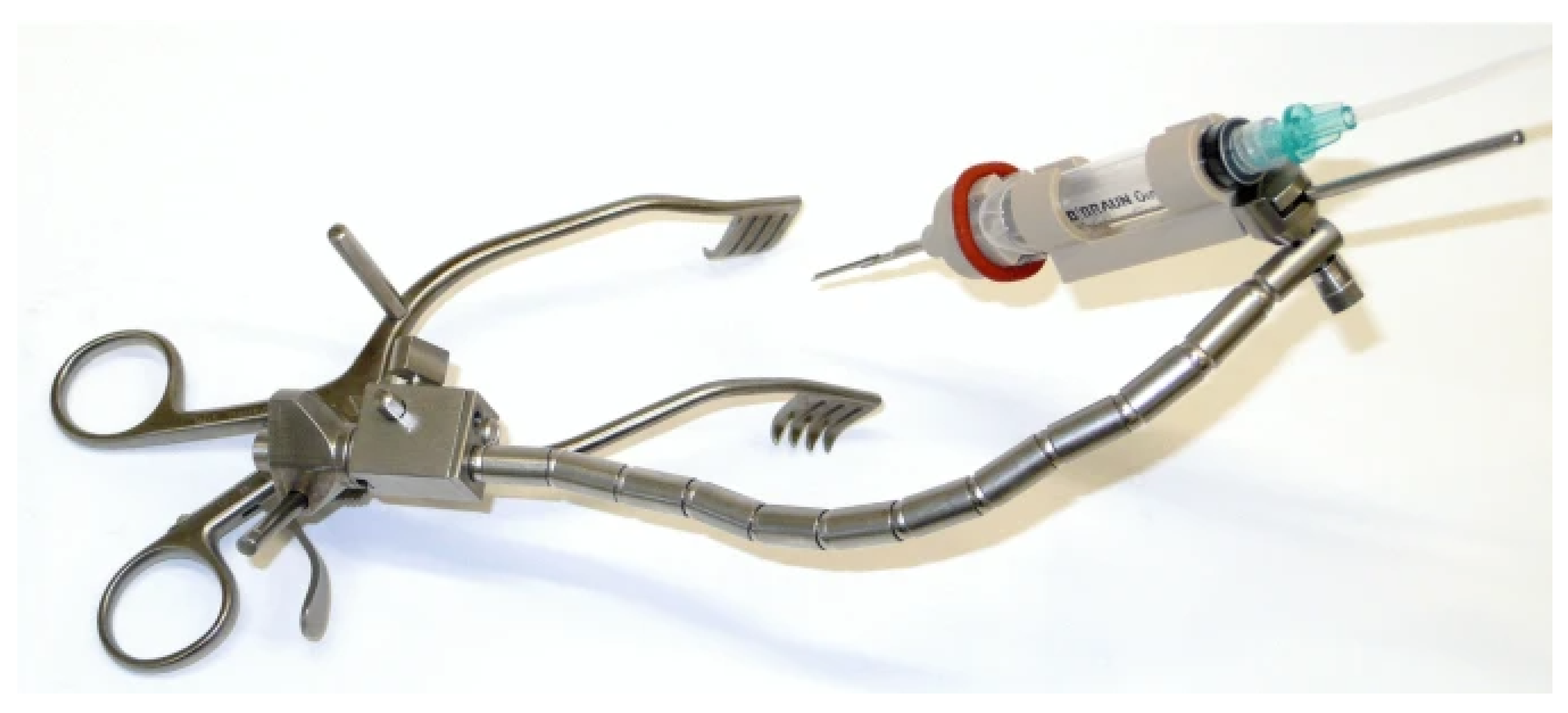
5.5.1. RobOtol® (Paris, France)
5.5.2. iotaSoft® (Iowa, USA)
5.5.3. OtoDrive® (Bern, Switzerland)
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIs | Cochlear Implants |
| DCA | Direct Cochlear Access |
| IGS | Image Guiding System |
| ST | Scala Tympani |
| EA | Electrode Array |
| AI | Artifical Intelligence |
| ML | Machine Learning |
References
- World Health Organization. Deafness and Hearing Loss. 2024. Available online: https://www.who.int/health-topics/hearing-loss#tab=tab_1 (accessed on 15 March 2024).
- World Health Organization. World Report on Hearing. 2021. Available online: https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/highlighting-priorities-for-ear-and-hearing-care (accessed on 15 March 2024).
- Raine, C. Cochlear implants in the United Kingdom: Awareness and utilization. Cochlear Implant. Int. 2013, 14, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- BIC Group. British Implant Cochlear Group. 2024. Available online: https://www.bcig.org.uk/default.aspx (accessed on 20 April 2024).
- NHS. Service Specifications. 2024. Available online: https://www.england.nhs.uk/wp-content/uploads/2013/05/D09sa-cochlear-implantation-services.pdf (accessed on 30 April 2024).
- Jia, H.; Pan, J.; Gu, W.; Tan, H.; Chen, Y.; Zhang, Z.; Jiang, M.; Li, Y.; Sterkers, O.; Wu, H. Robot-assisted electrode array insertion becomes available in pediatric cochlear implant recipients: First report and an intra-individual study. Front. Surg. 2021, 8, 695728. [Google Scholar] [CrossRef] [PubMed]
- Rebscher, S.J.; Hetherington, A.; Bonham, B.; Wardrop, P.; Whinney, D.; Leake, P.A. Considerations for Design of Future Cochlear Implant Electrode Arrays: Electrode Array Stiffness, Size, and Depth of Insertion. J. Rehabil. Res. Dev. 2008, 45, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Du, X.; Boulgouris, N.V. A Novel Sensing System for Robotic Cochlear Implants Electrode Array Placement. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 1133–1137. [Google Scholar] [CrossRef]
- Zeng, F.-G.; Popper, A.N.; Fay, R.R. (Eds.) Cochlear Implants: Auditory Prostheses and Electric Hearing, 1st ed.; Springer Handbook of Auditory Research; Springer: New York, NY, USA, 2004; p. XII, 438. [Google Scholar] [CrossRef]
- Hafeez, N. Innovative Techniques for Atraumatic and Precise Electrode Array Insertion in Cochlear Implantation. Ph.D. Thesis, Brunel University, London, UK, 2022. [Google Scholar]
- Niparko, J.K. Cochlear Implants: Principles and Practices, 2nd ed.; Lippincott Williams and Wilkins (LWW): Philadelphia, PA, USA, 2009; p. 416. [Google Scholar]
- Schendzielorz, P.; Scherzed, A.; Rak, K.; Völker, J.; Hagen, R.; Mlynski, R.; Frölich, K.; Radeloff, A. A hydrogel coating for cochlear implant arrays with encapsulated adipose-derived stem cells allows brain-derived neurotrophic factor delivery. Acta Oto-Laryngol. 2014, 134, 497–505. [Google Scholar] [CrossRef]
- Honeder, C.; Zhu, C.; Schöpper, H.; Gausterer, J.C.; Walter, M.; Landegger, L.D.; Saidov, N.; Riss, D.; Plasenzotti, R.; Gabor, F.; et al. Effects of sustained release dexamethasone hydrogels in hearing preservation cochlear implantation. Hear. Res. 2016, 341, 43–49. [Google Scholar] [CrossRef]
- Huber, M. Cochlear implant-specific risks should be considered, when assessing the quality of life of children and adolescents with hearing loss and cochlear implants—not just cochlear implant-specific benefits—Perspective. Front. Neurosci. 2022, 16, 985230. [Google Scholar] [CrossRef]
- Dillon, N.P.; Balachandran, R.; Dit Falisse, A.M.; Wanna, G.B.; Labadie, R.F.; Withrow, T.J.; Fitzpatrick, J.M.; Webster, R.J., III. Preliminary Testing of a Compact, Bone-Attached Robot for Otologic Surgery. Proc. SPIE Int. Soc. Opt. Eng. 2014, 9036, 903614. [Google Scholar] [CrossRef]
- Dillon, N.P.; Balachandran, R.; Fitzpatrick, J.M.; Siebold, M.A.; Labadie, R.F.; Wanna, G.B.; Withrow, T.J.; Webster, R.J., III. A Compact, Bone-Attached Robot for Mastoidectomy. J. Med. Devices 2015, 9, 310031–310037. [Google Scholar] [CrossRef]
- Danilchenko, A.; Balachandran, R.; Toennies, J.L.; Baron, S.; Munske, B.; Fitzpatrick, J.M.; Withrow, T.J.; Webster, R.J., III; Labadie, R.F. Robotic Mastoidectomy. Otol. Neurotol. 2011, 32, 11–16. [Google Scholar] [CrossRef]
- De Seta, D.; Daoudi, H.; Torres, R.; Ferrary, E.; Sterkers, O.; Nguyen, Y. Robotics, automation, active electrode arrays, and new devices for cochlear implantation: A contemporary review. Hear. Res. 2022, 414, 108425. [Google Scholar] [CrossRef]
- Brett, P.N.; Taylor, R.P.; Proops, D.; Coulson, C.; Reid, A.; Griffiths, M.V. A surgical robot for cochleostomy. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 1229–1232. [Google Scholar] [CrossRef]
- Du, X.; Taylor, R.; Brett, P.N.; Proops, D.; Reid, A.; Coulson, C. A smart surgical robotic for cochlear implantation. In Proceedings of the International Conference on Automation, Robotics and Control Systems 2010, ARCS 2010, Orlando, FL, USA, 12–14 July 2010; pp. 43–46. [Google Scholar]
- Du, X.; Assadi, M.Z.; Jowitt, F.; Brett, P.N.; Henshaw, S.; Dalton, J.; Proops, D.W.; Coulson, C.J.; Reid, A.P. Robustness analysis of a smart surgical drill for cochleostomy. Int. J. Med. Robot. + Comput. Assist. Surg. MRCAS 2013, 9, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Coulson, C.J.; Reid, A.P.; Proops, D.W. A cochlear implantation robot in surgical practice. In Proceedings of the 2008 15th International Conference on Mechatronics and Machine Vision in Practice, Auckland, New Zealand, 2–4 December 2008; pp. 173–176. [Google Scholar] [CrossRef]
- Coulson, C.J.; Taylor, R.P.; Reid, A.P.; Griffiths, M.V.; Proops, D.W.; Brett, P.N. An autonomous surgical robot for drilling a cochleostomy: Preliminary porcine trial. Clin. Otolaryngol. 2008, 33, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Hussong, A.; Rau, T.S.; Ortmaier, T.; Heimann, B.; Lenarz, T.M.O. An automated insertion tool for cochlear implants: Another step towards atraumatic cochlear implant surgery. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 163–171. [Google Scholar] [CrossRef]
- Taylor, R.; Du, X.; Reid, A.; Coulson, C.; Brett, P. A Sensory-Guided Surgical Micro-Drill. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2010, 224, 1531–1537. [Google Scholar] [CrossRef]
- Coulson, C.J.; Assadi, M.Z.; Taylor, R.P.; Du, X.; Brett, P.N.; Reid, A.P.; Proops, D.W. A smart micro-drill for cochleostomy formation: A comparison of cochlear disturbances with manual drilling and a human trial. Cochlear Implant. Int. 2013, 14, 98–106. [Google Scholar] [CrossRef]
- Du, X.; Brett, P.N.; Zhang, Y.; Begg, P.; Mitchell-Innes, A.; Coulson, C.; Irving, R. A hand-guided robotic drill for cochleostomy on human cadavers. Robot. Surg. Res. Rev. 2018, 5, 13–18. [Google Scholar] [CrossRef]
- Brett, P.; Du, X.; Zoka Assadi, M.; Proops, D.; Reid, A.; Coulson, C. Mechatronic hand-held surgical robots. In Proceedings of the 2012 19th International Conference on Mechatronics and Machine Vision in Practice (M2VIP), Auckland, New Zealand, 28–30 November 2012; pp. 447–449. [Google Scholar]
- Weber, S.; Bell, B.; Gerber, N.; Williamson, T.; Brett, P.; Du, X.; Caversaccio, M.; Proops, D.; Coulson, C.; Reid, A. Minimally invasive, robot assisted cochlear implantation. In Proceedings of the 3rd Joint Workshop on New Technologies for Computer/Robot Assisted Surgery (CRAS 2013), University of Southern Queensland, Toowoomba, Australia, 11–13 September 2013; pp. 11–13. [Google Scholar]
- Weber, S.; Bell, B.; Gerber, N.; Williamson, T.; Brett, P.; Du, X.; Caversaccio, M.; Proops, D.; Coulson, C.; Reid, A. Demonstration of Autonomous Atraumatic Cochleostomy by Combined Advanced Surgical Robot Systems. In The Hamlyn Symposium on Medical Robotics; Imperial College London: London, UK, 2014. [Google Scholar]
- Assadi, M.Z.; Du, X.; Dalton, J.; Henshaw, S.; Coulson, C.J.; Reid, A.P.; Proops, D.W.; Brett, P.N. Comparison on intracochlear disturbances between drilling a manual and robotic cochleostomy. Proc. Inst. Mech. Eng. Part H 2013, 227, 1002–1008. [Google Scholar] [CrossRef]
- Lefranc, M.; Capel, C.; Pruvot, A.S.; Fichten, A.; Desenclos, C.; Toussaint, P.; Gars, D.L.; Peltier, J. The Impact of the Reference Imaging Modality, Registration Method and Intraoperative Flat-Panel Computed Tomography on the Accuracy of the ROSA® Stereotactic Robot. Stereotact. Funct. Neurosurg. 2014, 92, 242–250. [Google Scholar] [CrossRef]
- Klenzner, T.; Ngan, C.C.; Knapp, F.B.; Knoop, H.; Kromeier, J.; Aschendorff, A.; Papastathopoulos, E.; Raczkowsky, J.; Wörn, H.; Schipper, J. New strategies for high precision surgery of the temporal bone using a robotic approach for cochlear implantation. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 955–960. [Google Scholar] [CrossRef]
- Klopp-Dutote, N.; Lefranc, M.; Strunski, V.; Page, C. Minimally invasive fully ROBOT-assisted cochlear implantation in humans: Preliminary results in five consecutive patients. Clin. Otolaryngol. 2021, 46, 1326–1330. [Google Scholar] [CrossRef]
- Bell, B.; Williamson, T.; Gerber, N.; Gavaghan, K.; Wimmer, W.; Kompis, M.; Weber, S.; Caversaccio, M. An Image-Guided Robot System for Direct Cochlear Access. Cochlear Implant. Int. 2014, 15 (Suppl. 1), S11–S13. [Google Scholar] [CrossRef] [PubMed]
- Caversaccio, M.; Gavaghan, K.; Wimmer, W.; Williamson, T.; Ansò, J.; Mantokoudis, G.; Weber, S. Robotic Cochlear Implantation: Surgical Procedure and First Clinical Experience. Acta Oto-Laryngol. 2017, 137, 447–454. [Google Scholar] [CrossRef]
- Bell, B.; Gerber, N.; Williamson, T.; Gavaghan, K.; Wimmer, W.; Caversaccio, M.; Weber, S. In Vitro Accuracy Evaluation of Image-Guided Robot System for Direct Cochlear Access. Otol. Neurotol. 2013, 34, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Ansó, J.; Scheidegger, O.; Wimmer, W.; Gavaghan, K.; Gerber, N.; Schneider, D.; Hermann, J.; Rathgeb, C.; Dür, C.; Rösler, K.M.; et al. Neuromonitoring During Robotic Cochlear Implantation: Initial Clinical Experience. Ann. Biomed. Eng. 2018, 46, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, V.; Heuninck, E.; Matulic, M.; Tekin, A.M.; Mertens, G.; Van Rompaey, V.; Galeazzi, P.; Zoka-Assadi, M.; van de Heyning, P. First Study in Men Evaluating a Surgical Robotic Tool Providing Autonomous Inner Ear Access for Cochlear Implantation. Front. Neurol. 2022, 13, 804507. [Google Scholar] [CrossRef]
- Müller-Graff, F.-T.; Spahn, B.; Herrmann, D.P.; Kurz, A.; Völker, J.; Hagen, R.; Rak, K. Comprehensive Literature Review on the Application of the Otological Surgical Planning Software OTOPLAN® for Cochlear Implantation. HNO 2024, 72, 89–100. [Google Scholar] [CrossRef]
- Abari, J.; Heuninck, E.; Topsakal, V. Entirely Robotic Cochlear Implant Surgery. Am. J. Otolaryngol. 2024, 45, 104360. [Google Scholar] [CrossRef]
- Labadie, R.F.; Mitchell, J.; Balachandran, R.; Fitzpatrick, J.M. Customized, Rapid-Production Microstereotactic Table for Surgical Targeting: Description of Concept and In Vitro Validation. Int. J. Comput. Assist. Radiol. Surg. 2009, 4, 273–280. [Google Scholar] [CrossRef]
- Labadie, R.F.; Balachandran, R.; Mitchell, J.E.; Noble, J.H.; Majdani, O.; Haynes, D.S.; Bennett, M.L.; Dawant, B.M.; Fitzpatrick, J.M. Clinical Validation Study of Percutaneous Cochlear Access Using Patient-Customized Microstereotactic Frames. Otol. Neurotol. 2010, 31, 94–99. [Google Scholar] [CrossRef]
- Labadie, R.F.; Noble, J.H. Preliminary Results With Image-Guided Cochlear Implant Insertion Techniques. Otol. Neurotol. 2018, 39, 922–928. [Google Scholar] [CrossRef]
- Balachandran, R.; Reda, F.; Noble, J.; Blachon, G.; Dawant, B.; Fitzpatrick, J.; Labadie, R. Minimally Invasive Image-Guided Cochlear Implantation for Pediatric Patients: Clinical Feasibility Study. Otolaryngol. Neck Surg. 2014, 150, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Labadie, R.F.; Riojas, K.; Von Wahlde, K.; Mitchell, J.; Bruns, T.; Webster, R., III; Dawant, B.; Fitzpatrick, J.M.; Noble, J. Clinical Implementation of Second-Generation Minimally Invasive Image-Guided Cochlear Implantation Surgery. Otol. Neurotol. 2021, 42, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Kobler, J.P.; Nuelle, K.; Lexow, G.J.; Rau, T.S.; Majdani, O.; Kahrs, L.A.; Kotlarski, J.; Ortmaier, T. Configuration Optimization and Experimental Accuracy Evaluation of a Bone-Attached, Parallel Robot for Skull Surgery. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.S.; John, S.; Kluge, M.; Repp, F.; Zuniga, M.G.; Stieghorst, J.; Timm, M.E.; Fröhlich, M.; Majdani, O.; Lenarz, T. Ex Vivo Evaluation of a Minimally Invasive Approach for Cochlear Implant Surgery. IEEE Trans. Biomed. Eng. 2023, 70, 390–398. [Google Scholar] [CrossRef]
- Kobler, J.-P.; Kotlarski, J.; Öltjen, J.; Baron, S.; Ortmaier, T. Design and Analysis of a Head-Mounted Parallel Kinematic Device for Skull Surgery. Int. J. Comput. Assist. Radiol. Surg. 2012, 7, 137–149. [Google Scholar] [CrossRef]
- Vollmann, B.; Müller, S.; Kundrat, D.; Ortmaier, T.; Kahrs, L. Methods for Intraoperative, Sterile Pose-Setting of Patient-Specific Microstereotactic Frames. Proc. SPIE 2015, 9415, 94150M. [Google Scholar] [CrossRef]
- Rau, T.; Witte, S.; Uhlenbusch, L.; Kahrs, L.; Lenarz, T.; Majdani, O. Concept Description and Accuracy Evaluation of a Moldable Surgical Targeting System. J. Med. Imaging 2021, 8, 015003. [Google Scholar] [CrossRef]
- Salcher, R.; John, S.; Stieghorst, J.; Kluge, M.; Repp, F.; Fröhlich, M.; Lenarz, T. Minimally Invasive Cochlear Implantation: First-in-Man of Patient-Specific Positioning Jigs. Front. Neurol. 2022, 13, 829478. [Google Scholar] [CrossRef]
- Hussong, A.; Rau, T.; Eilers, H.; Baron, S.; Heimann, B.; Leinung, M.; Lenarz, T.; Majdani, O. Conception and design of an automated insertion tool for cochlear implants. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 5593–5596. [Google Scholar] [CrossRef]
- Hrnčiřík, F.; Nagy, L.; Grimes, H.; Iftikhar, H.; Muzaffar, J.; Bance, M. Impact of Insertion Speed, Depth, and Robotic Assistance on Cochlear Implant Insertion Forces and Intracochlear Pressure: A Scoping Review. Sensors 2024, 24, 3307. [Google Scholar] [CrossRef]
- Schurzig, D.; Labadie, R.F.; Hussong, A.; Rau, T.S.; Webster, R.J., III. A Force Sensing Automated Insertion Tool for Cochlear Electrode Implantation. In Proceedings of the 2010 IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 3674–3679. [Google Scholar] [CrossRef]
- Schurzig, D.; Labadie, R.F.; Hussong, A.; Rau, T.S.; Webster, R.J., III. Design of a Tool Integrating Force Sensing With Automated Insertion in Cochlear Implantation. IEEE/ASME Trans. Mechatron. 2012, 17, 381–389. [Google Scholar] [CrossRef]
- Warren, F.; Balachandran, R.; Fitzpatrick, J.; Labadie, R. Percutaneous Cochlear Access Using Bone-Mounted, Customized Drill Guides. Otol. Neurotol. 2007, 28, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.S.; Zuniga, M.G.; Salcher, R.; Lenarz, T. A Simple Tool to Automate the Insertion Process in Cochlear Implant Surgery. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, N.; Riojas, K.E.; Bruns, T.L.; Mitchell, J.E.; Webster, R.J., III; Labadie, R.F. A Simple Manual Roller Wheel Insertion Tool for Electrode Array Insertion in Minimally Invasive Cochlear Implant Surgery. In Proceedings of the 2019 Design of Medical Devices Conference, Minneapolis, MN, USA, 15–18 April 2019. [Google Scholar] [CrossRef]
- Torres, R.; Jia, H.; Drouillard, M.; Bensimon, J.L.; Sterkers, O.; Ferrary, E.; Nguyen, Y. An Optimized Robot-Based Technique for Cochlear Implantation to Reduce Array Insertion Trauma. Otolaryngol. Neck Surg. 2018, 159, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, M.G.; Lenarz, T.; Rau, T.S. Hydraulic Insertions of Cochlear Implant Electrode Arrays into the Human Cadaver Cochlea: Preliminary Findings. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 2827–2835. [Google Scholar] [CrossRef]
- Rau, T.S.; Zuniga, M.G.; Cramer, J.; Salcher, R.; Lenarz, T. Hydraulic Actuation to Operate Ultra-Slow Insertion Velocities. Laryngo-Rhino-Otologie 2022, 101, S243–S244. [Google Scholar]
- Bottcher-Rebmann, G.; Schell, V.; Budde, L.; Zuniga, M.G.; Baier, C.; Lenarz, T.; Rau, T.S. A Tool to Enable Intraoperative Insertion Force Measurements for Cochlear Implant Surgery. IEEE Trans. Biomed. Eng. 2023, 70, 1643–1650. [Google Scholar] [CrossRef]
- Rau, T.S.; Böttcher-Rebmann, G.; Schell, V.; Cramer, J.; Artukarslan, E.; Baier, C.; Salcher, R. First clinical implementation of insertion force measurement in cochlear implantation surgery. Front. Neurol. 2024, 15, 1400455. [Google Scholar] [CrossRef]
- Morrel, W.G.; Riojas, K.E.; Webster, R.J., III; Noble, J.H.; Labadie, R.F. Custom Mastoid-Fitting Templates to Improve Cochlear Implant Electrode Insertion Trajectory. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1713–1718. [Google Scholar] [CrossRef]
- Riojas, K.E.; Tran, E.T.; Freeman, M.H.; Noble, J.H.; Webster, R.J., III; Labadie, R.F. Clinical Translation of an Insertion Tool for Minimally Invasive Cochlear Implant Surgery. ASME J. Med. Devices 2021, 15, 031001. [Google Scholar] [CrossRef]
- Zagabathuni, A.; Padi, K.K.; Kameswaran, M.; Subramani, K. Development of Automated Tool for Electrode Array Insertion and its Study on Intracochlear Pressure. Laryngoscope 2024, 134, 1388–1395. [Google Scholar] [CrossRef]
- Bruns, T.L.; Riojas, K.E.; Mitchell, J.E.; Webster, R.J., III; Labadie, R.F. Magnetically Steered Robotic Insertion of Cochlear-Implant Electrode Arrays: System Integration and First-In-Cadaver Results. IEEE Robot. Autom. Lett. 2020, 5, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, C.M.; Cavilla, M.S.; Usevitch, D.E.; Bruns, T.L.; Riojas, K.E.; Leon, L.; Webster, R.J., III; Warren, F.M.; Abbott, J.J. Magnetic Steering of Robotically Inserted Lateral-Wall Cochlear-Implant Electrode Arrays Reduces Forces on the Basilar Membrane In Vitro. Otol. Neurotol. 2021, 42, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.; Riojas, K.E.; Bruns, T.L.; Mitchell, J.E.; Webster, R.J., III; Labadie, R.F. Optimizing the Magnetic Dipole-Field Source for Magnetically Guided Cochlear-Implant Electrode-Array Insertions. J. Med. Robot. Res. 2018, 3, 1850004. [Google Scholar] [CrossRef]
- Maheo, C.; Marie, A.; Torres, R.; Archutick, J.; Leclère, J.C.; Marianowski, R. Robot-Assisted and Manual Cochlear Implantation: An Intra-Individual Study of Speech Recognition. J. Clin. Med. 2023, 12, 6580. [Google Scholar] [CrossRef]
- Gantz, J.A.; Warren, F.M.; Hansen, M.R.; Gubbels, S.P.; Cullen, R.D.; Hunter, J.B.; Wanna, G.B.; Labadie, R.F. A Steadier Hand: The First Human Clinical Trial of a Single-Use Robotic-Assisted Surgical Device for Cochlear Implant Electrode Array Insertion. Otol. Neurotol. 2023, 44, 34–39. [Google Scholar] [CrossRef]
- Kaufmann, C.R.; Wagner, F.; Wimmer, W.; Gerber, N.; Caversaccio, M.; Weber, S. Evaluation of Insertion Forces and Cochlea Trauma Following Robotics-Assisted Cochlear Implant Electrode Array Insertion. Otol. Neurotol. 2020, 41, 631–638. [Google Scholar] [CrossRef]
- Aebischer, P.; Wimmer, W.; Gavaghan, K.; Caversaccio, M.; Weber, S. Quantitative in-vitro assessment of a novel robot-assisted system for cochlear implant electrode insertion. Int. J. Comput. Assist. Radiol. Surg. 2025, 20, 323–332. [Google Scholar] [CrossRef]
- Kazmitcheff, G.; Drouillard, M.; Moreau, G.; Lavallee, S.; Ferreira, A.; Nguyen, Y. Evaluation of Command Modes of an Assistance Robot for Middle Ear Surgery. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 2532–2538. [Google Scholar] [CrossRef]
- Torres, R.; Kazmitcheff, G.; De Seta, D.; Bensimon, J.L.; Sterkers, O.; Ferrary, E.; Nguyen, Y. Improvement of the Insertion Axis for Cochlear Implantation with a Robot-Based System. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 715–721. [Google Scholar] [CrossRef]
- Daoudi, H.; Lahlou, G.; Torres, R.; Sterkers, O.; Lefeuvre, V.; Ferrary, E.; Mosnier, I.; Nguyen, Y. Robot-Assisted Cochlear Implant Electrode Array Insertion in Adults: A Comparative Study With Manual Insertion. Otol. Neurotol. 2021, 42, e438–e444. [Google Scholar] [CrossRef]
- Claussen, A.D.; Labadie, R.F.; Webster, R.J., III; Noble, J.H. Robotics-Assisted Cochlear Implant Insertion. Otol. Neurotol. 2024, 45, e459. [Google Scholar] [CrossRef]
- Kashani, R.G.; Gubbels, S.P.; Cullen, R.D.; Labadie, R.F. Combining Intraoperative Electrocochleography with Robotics-Assisted Electrode Array Insertion. Otol. Neurotol. 2024, 45, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Alhabib, S.F.; Abdullah, Z.; Alhussaini, M.; Alqarni, R.; Alalawi, M.; AlFaris, M.; Alghamdi, D. Robotic Versus Manual Electrode Insertion in Cochlear Implant Surgery: An Experimental Study. Clin. Exp. Otorhinolaryngol. 2025, 18, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Aebischer, P.; Wimmer, W.; Gavaghan, K.; Caversaccio, M.; Weber, S. Development and Evaluation of a Reusable, Force Measuring Tool for the Robot-Assisted Insertion of Cochlear Implant Electrode Arrays. IEEE Trans. Bio-Med Eng. 2025, 72, 381–387. [Google Scholar] [CrossRef]
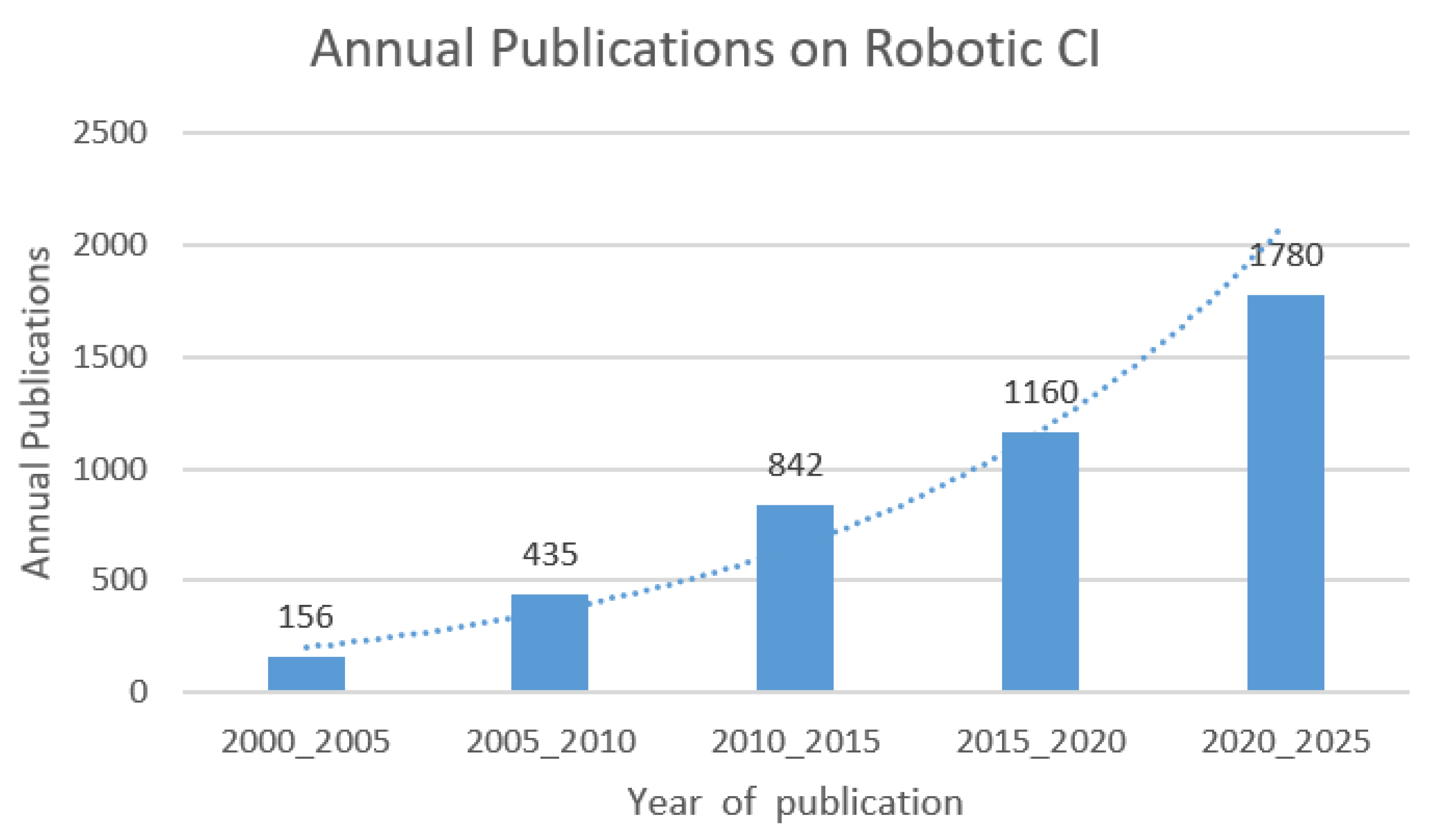
- Tessler, I.; Gecel, N.; Glicksberg, B.; Shivatzki, S.; Shapira, Y.; Zimlichman, E.; Alon, E.; Klang, E.; Wolfovitz, A. A Five-Decade Text Mining Analysis of Cochlear Implant Research: Where We Started and Where We Are Heading. Medicina 2023, 59, 1891. [Google Scholar] [CrossRef] [PubMed]
- Chakravorti, S.; Noble, J.H.; Gifford, R.H.; Dawant, B.M.; O’Connell, B.P.; Wang, J.; Labadie, R.F. Further Evidence of the Relationship Between Cochlear Implant Electrode Positioning and Hearing Outcomes. Otol. Neurotol. 2019, 40, 617–624. [Google Scholar] [CrossRef]
- Shoman, N.M. Robotics and Cochlear Implant Surgery: Goals and Developments. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 314–319. [Google Scholar] [CrossRef]
- Kashani, R.; Henslee, A.; Nelson, R.; Hansen, M. Robotic assistance during cochlear implantation: The rationale for consistent, controlled speed of electrode array insertion. Front. Neurol. 2024, 15, 1335994. [Google Scholar] [CrossRef]
- Dahroug, B.; Tamadazte, B.; Weber, S.; Tavernier, L.; Andreff, N. Review on Otological Robotic Systems: Toward Microrobot-Assisted Cholesteatoma Surgery. IEEE Rev. Biomed. Eng. 2018, 11, 125–142. [Google Scholar] [CrossRef]
- Rajan, G.P.; Kontorinis, G.; Kuthubutheen, J. The Effects of Insertion Speed on Inner Ear Function during Cochlear Implantation: A Comparison Study. Audiol. Neurotol. 2012, 18, 17–22. [Google Scholar] [CrossRef]
- Sykopetrites, V.; Sica, E.; Moalli, R.; Cocozza, D.; Razza, S.; Cristofari, E. Robot-assisted vs. manual cochlear implant electrode array insertion in four children. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 3019–3025. [Google Scholar] [CrossRef]
- Cho, H.S.; Ko, S.Y.; Isaacson, B.; Kutz, J.W., Jr. Robot-Assisted Cochlear Implant Electrode Array Insertion. Korean J. Otorhinolaryngol.-Head Neck Surg. 2024, 67, 358–364. [Google Scholar] [CrossRef]
- Gawęcki, W.; Balcerowiak, A.; Podlawska, P.; Szyfter, W.; Wierzbicka, M. Robot-assisted Cochlear Implantation via a Modified Pericanal Approach. Otolaryngol. Pol. 2023, 77, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Claussen, A.D.; Shibata, S.B.; Kaufmann, C.R.; Henslee, A.; Hansen, M.R. Comparative Analysis of Robotics-Assisted and Manual Insertions of Cochlear Implant Electrode Arrays. Otol. Neurotol. 2022, 43, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, J.; Carlson, M. Hearing preservation in pediatric cochlear implantation. Curr. Opin. Otolaryngol. Head Neck Surg. 2024, 32, 410–415. [Google Scholar] [CrossRef]
- Perkins, E.; Lee, J.; Manzoor, N.; O’Malley, M.; Bennett, M.; Labadie, R.; Rivas, A.; Haynes, D.; Gifford, R. The Reality of Hearing Preservation in Cochlear Implantation: Who Is Utilizing EAS? Otol. Neurotol. 2021, 42, 832–837. [Google Scholar] [CrossRef]
- Heuninck, E.; Van de Heyning, P.; Van Rompaey, V.; Mertens, G.; Topsakal, V. Audiological Outcomes of Robot-Assisted Cochlear Implant Surgery. Eur. Arch. Otorhinolaryngol. 2023, 280, 4433–4444. [Google Scholar] [CrossRef]
- Silva, V.A.R.; Castilho, A.M. Robotic-assisted cochlear implant surgery. Braz. J. Otorhinolaryngol. (Engl. Ed.) 2024, 90, 101530. [Google Scholar] [CrossRef]
- Thum, C.; Lenarz, T.; Fleßa, S. Direct cost of cochlear implants in Germany—A strategic simulation. Health Econ. Rev. 2022, 12, 64. [Google Scholar] [CrossRef]
- Chen, J.M.; Amoodi, H.; Shipp, D.; Nedzelski, J.M.; Lin, V.Y. Cost-utility analysis of bilateral cochlear implantation in adults: A health economic assessment from the perspective of a publicly funded program. Laryngoscope 2014, 124, 1452–1458. [Google Scholar] [CrossRef]
- Harris, M.S.; Koka, K.; Riggs, W.J.; Saleh, S.; Holder, J.T.; Dwyer, R.T.; Prentiss, S.; Lefler, S.; Kozlowski, K.; Hiss, M.M.; et al. Can Electrocochleography Help Preserve Hearing After Cochlear Implantation With Full Electrode Insertion? Otol. Neurotol. 2022, 43, 789–796. [Google Scholar] [CrossRef]
- Hafeez, N.; Du, X.; Boulgouris, N.; Begg, P.; Irving, R.; Coulson, C.; Tourrel, G. Electrical Impedance Guides Electrode Array in Cochlear Implantation Using Machine Learning and Robotic Feeder. Hear. Res. 2021, 412, 108371. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, N.; Du, X.; Boulgouris, N.; Begg, P.; Irving, R.; Coulson, C.; Tourrel, G. Real-Time Data-Driven Approach for Prediction and Correction of Electrode Array Trajectory in Cochlear Implantation. Appl. Sci. 2022, 12, 6343. [Google Scholar] [CrossRef]
- Patel, Y.D.; George, P.M. Parallel Manipulators Applications—A Survey. Mod. Mech. Eng. 2012, 2, 57–64. [Google Scholar] [CrossRef]




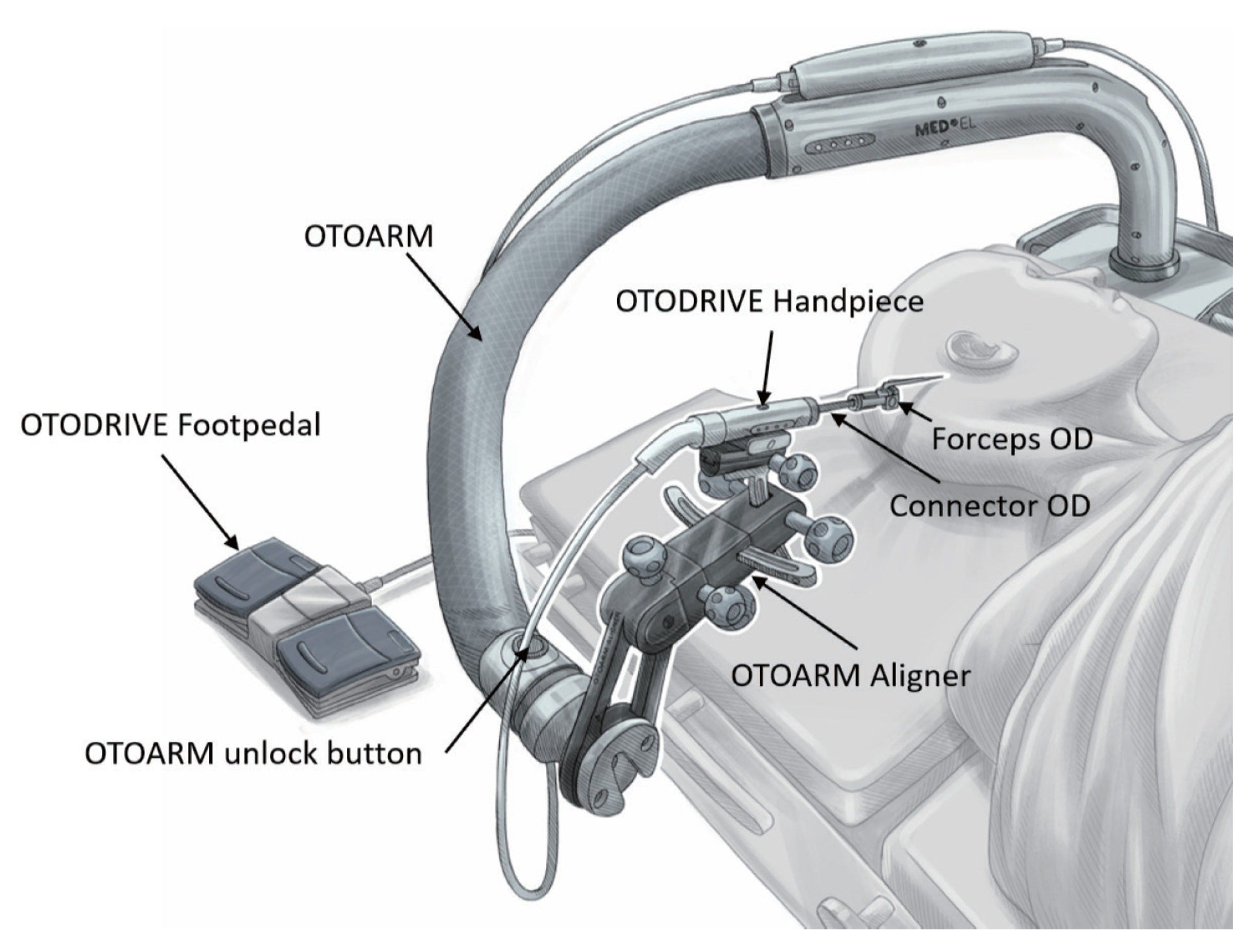
| Force (N) | Drilling Feed (mm/min) | Drill Speed (rev/s) | Drilling Time (s) | Reference |
|---|---|---|---|---|
| 1.5 | 0.1 | 25 | 30 | [24] |
| - | 0.5 | 10 | - | [19] |
| 1.5 | 0.1 | 25 | 30 | [23] |
| - | 0.5 | 10 | - | [25] |
| 2 | 0.1 | - | 10 min | [21] |
| Robot Type | Guiding System | Drill Path Trajectory Planning | Clinical Trials | Kinematic Structure | DoF | Time Needed for Surgery (min) | Accuracy of Insertion (mm) | Reference |
|---|---|---|---|---|---|---|---|---|
| Rosa® (Amiens, France) | CT scan, MRI, fpCT | Rosanna® software | Yes | Serial | 6 | 120 | 0.3, 1.59 | [32] |
| HEARO® (Bern, Switzerland) | IGS | Custom-built software | Yes | Serial | 6 | 300 | 0.15 ± 0.08 | [35] |
| Microtable® Vanderbilt (Nashville, TN, USA) | CT Scan | Custom-built software | Yes | Parallel | 6 | 60 | 0.37 ± 0.18 | [42] |
| Hexapod (Hannover, Germany) | CT Scan | Custom-built software | Yes | Parallel | 6 | 156 | 0.36 ± 0.12 | [47] |
| OtoJig® (Hannover, Germany) | CBCT | Python programming language (2.7) | Yes | - | - | - | 0.30 ± 0.11 | [48] |
| GluingJig® (Hannover, Germany) | CBCT | Python programming language (2.7) | Yes | - | - | - | 0.30 ± 0.11 | [48] |
| Robot Type | Clinical Trials | Insertion Speed (mm/s) | Insertion Time (s) | Reference |
|---|---|---|---|---|
| RobOtol® | Yes | 0.1 | 73 ± 10 | [71] |
| iotaSoft® | Yes | 0.1–1 | 195 (3 m 15 s) | [72,73] |
| OtoDrive® | Yes | 0.3 | 98 ± 35, 103 ± 19 | [74] |
| Cochlea Hydro Drive | No | 0.02–0.9 | 315 | [61] |
| Roller Wheels Tool | Yes | - | - | [66] |
| Magnetically Steered Robotic Insertion | No | 0.3–1.25 | 22.4, 103 ± 19 | [68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, O.; Wang, M.; Zhang, B.; Irving, R.; Begg, P.; Du, X. Robotic Systems for Cochlear Implant Surgeries: A Review of Robotic Design and Clinical Outcomes. Electronics 2025, 14, 2685. https://doi.org/10.3390/electronics14132685
Ahmed O, Wang M, Zhang B, Irving R, Begg P, Du X. Robotic Systems for Cochlear Implant Surgeries: A Review of Robotic Design and Clinical Outcomes. Electronics. 2025; 14(13):2685. https://doi.org/10.3390/electronics14132685
Chicago/Turabian StyleAhmed, Oneeba, Mingfeng Wang, Bin Zhang, Richard Irving, Philip Begg, and Xinli Du. 2025. "Robotic Systems for Cochlear Implant Surgeries: A Review of Robotic Design and Clinical Outcomes" Electronics 14, no. 13: 2685. https://doi.org/10.3390/electronics14132685
APA StyleAhmed, O., Wang, M., Zhang, B., Irving, R., Begg, P., & Du, X. (2025). Robotic Systems for Cochlear Implant Surgeries: A Review of Robotic Design and Clinical Outcomes. Electronics, 14(13), 2685. https://doi.org/10.3390/electronics14132685








