FairChain: A Trusted and Transparent Blockchain-Based Ecosystem for Drug Development for Nagoya Protocol Implementation
Abstract
1. Introduction
2. Background
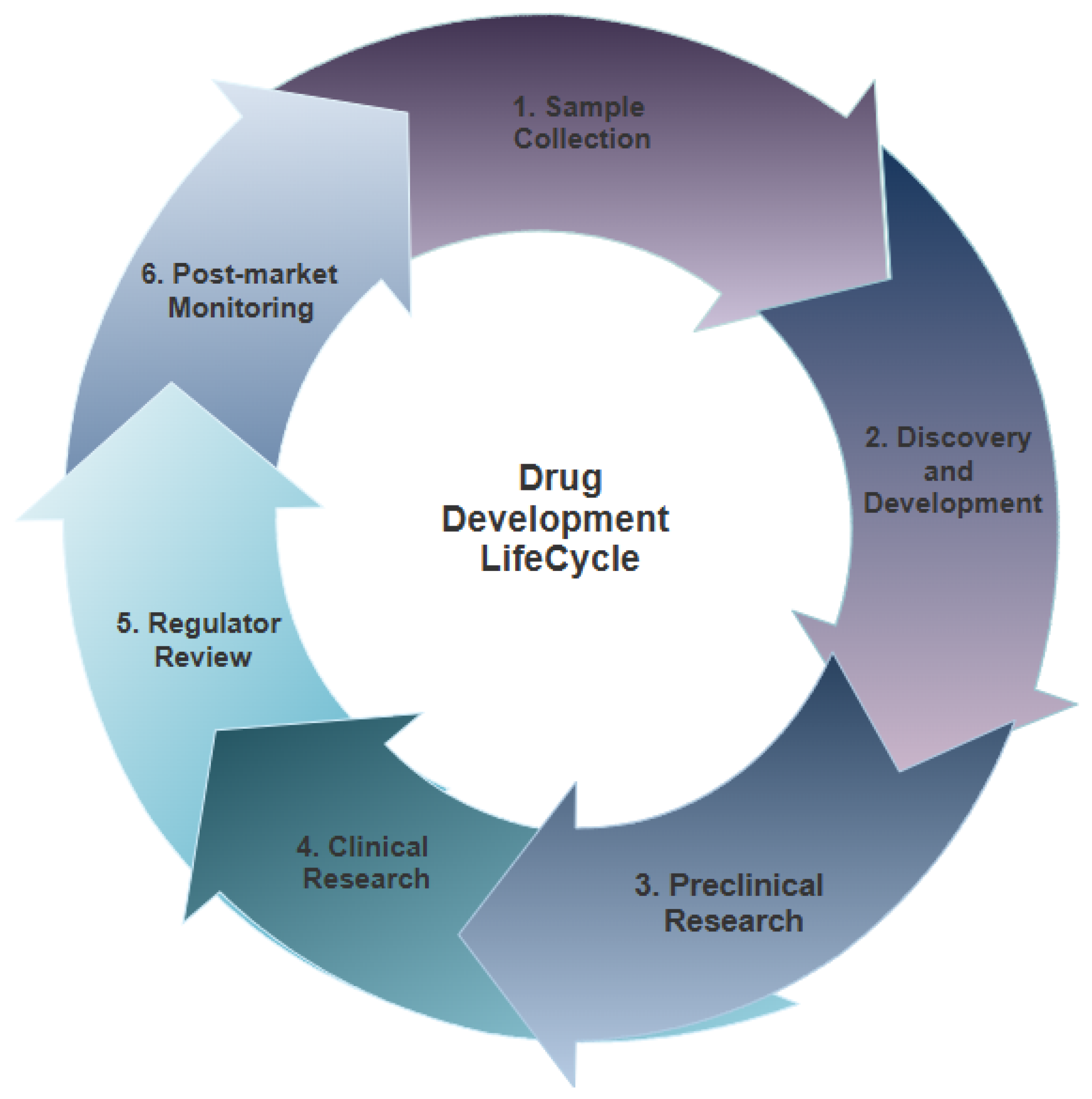
2.1. DDLC
- Sample Collection: Bio-samples are usually collected in areas far away from the laboratory; in order to make the most of these supplies and use them correctly, they must be properly preserved [4].
- Discovery and Development: This phase usually consists of sample purification and screening [4]. In sample purification, cells from macroscopic samples or proteins are isolated using different purification protocols that usually require expertise and specific facilities [4]. During sample screening, the resulting compounds from sample purification are matched to potential targets in order to identify their activity [4]. Additionally, this process might be carried out using computational approaches [4].
- Clinical Research: After the regulator (for instance, the Food and Drug Authority) reviews and approves preclinical research results, drugs are first tested on humans to ensure their safety and effectiveness [14]. Before clinical research begins, researchers must decide who qualifies to participate, how many people will participate, the duration of the research, how to limit research bias, how the drug will be provided to patients and at what dosage, what data will be collected, and how these data will be reviewed and analyzed [14]. Clinical research consists of the following four sequential phases, which are also explained briefly in Table 1:
- In phase one, 20 to 100 volunteers who are healthy or have the condition/disease under study are tested for several months in order to answer questions regarding safety and dosage and to determine how much of the drug the human body can tolerate along with any acute side effects [14].
- In phase two, several hundred people with the disease/condition are tested for several months to up to 2 years in order to find out more about the drug’s efficacy and side effects [14].
- In phase three, 300 to 3000 volunteers who have the disease or condition are tested for 1 to 4 years in order to answer questions regarding long-term or rare side effects [14].
- In phase four, several thousand volunteers who have the disease/condition are tested to find out more about the drug’s safety and efficacy [14].
- Regulator Review: A drug developer must submit a new drug application to the regulator that includes all information about the drug, including preclinical and clinical research reports, proposed labeling, safety updates, drug abuse information, patent information, and directions for use [15]. After the regulator receives the new drug application request, it has 6 to 10 months to decide whether to approve the drug or deny it [15].
- Regulator Post-Market Drug Safety Monitoring: Although clinical research results provide valuable and important information regarding drug safety and effectiveness, this information may change or be updated throughout the months and years that make up a drug’s lifetime in the marketplace. After drugs become available for public use, the regulator continues to review and monitoring drug safety reports, and might agree to apply appropriate changes to dose or usage information or any other aspects [16].
2.2. UNDP Nagoya Protocol for Access and Benefit-Sharing
2.3. Blockchain Technology
3. Related Work and Real-World Applications
Blockchain-Based Pharmaceutical Tracking Solutions
- MediLedger enables secure data exchange among pharmaceutical supply chain participants, ensuring compliance with the Drug Supply Chain Security Act (DSCSA) in the U.S. [29].
- IBM Pharma Ledger provides end-to-end supply chain tracking with enterprise-grade blockchain encryption and data security [30].
- BlockPharma offers QR code-based verification for patients to validate the authenticity of medications before purchase [33].
- Hyperledger Fabric, backed by The Linux Foundation, supports permissioned blockchain networks for enterprise pharmaceutical applications, and has been adopted by companies such as Pfizer and Merck [34].
4. Methodology
4.1. Stakeholder Engagement and Expert Interviews
- Validate the compliance bottlenecks faced by researchers and institutions.
- Identify critical trust, transparency, and auditability features expected in a blockchain ecosystem.
- Understand institutional concerns related to data governance, IP attribution, and ABS traceability.
4.2. System Requirements and Development Inputs
- Interviews with subject matter experts.
- A document review of national ABS frameworks, Nagoya Protocol requirements, and guidance from ABS Clearing-House.
- Technical architecture benchmarking of similar blockchain systems in healthcare and digital identity governance.
4.3. Bias and Scope Limitations
5. FairChain Functional Design
5.1. User Roles
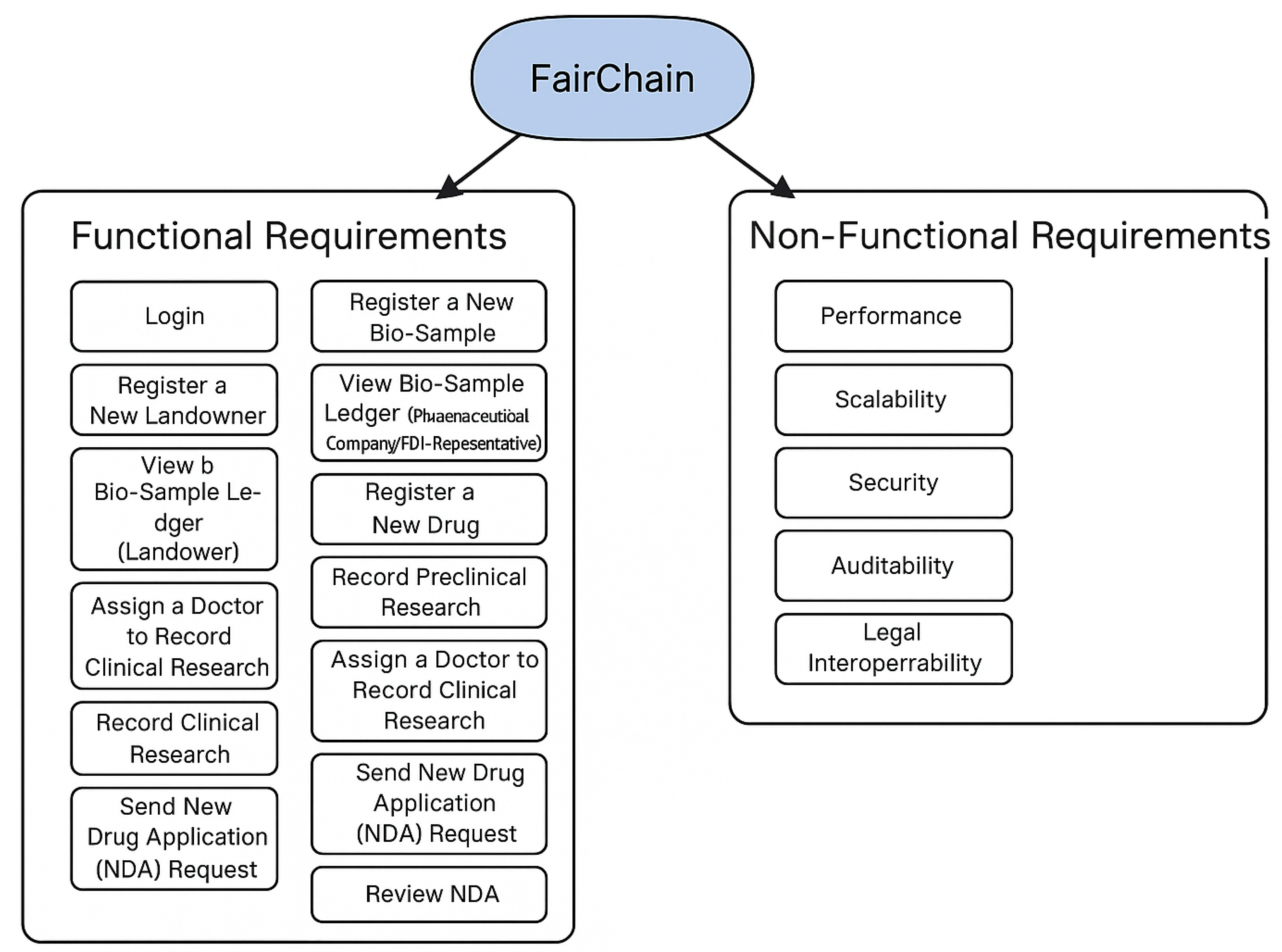
5.2. Functional Requirements
- Login: Users (including pharmaceutical company representatives, FDA representatives (reviewers), doctors, and landowners) can log into the system. The system prompts them to enter credentials, which are then validated. If correct, the user gains access; otherwise, the login is denied.
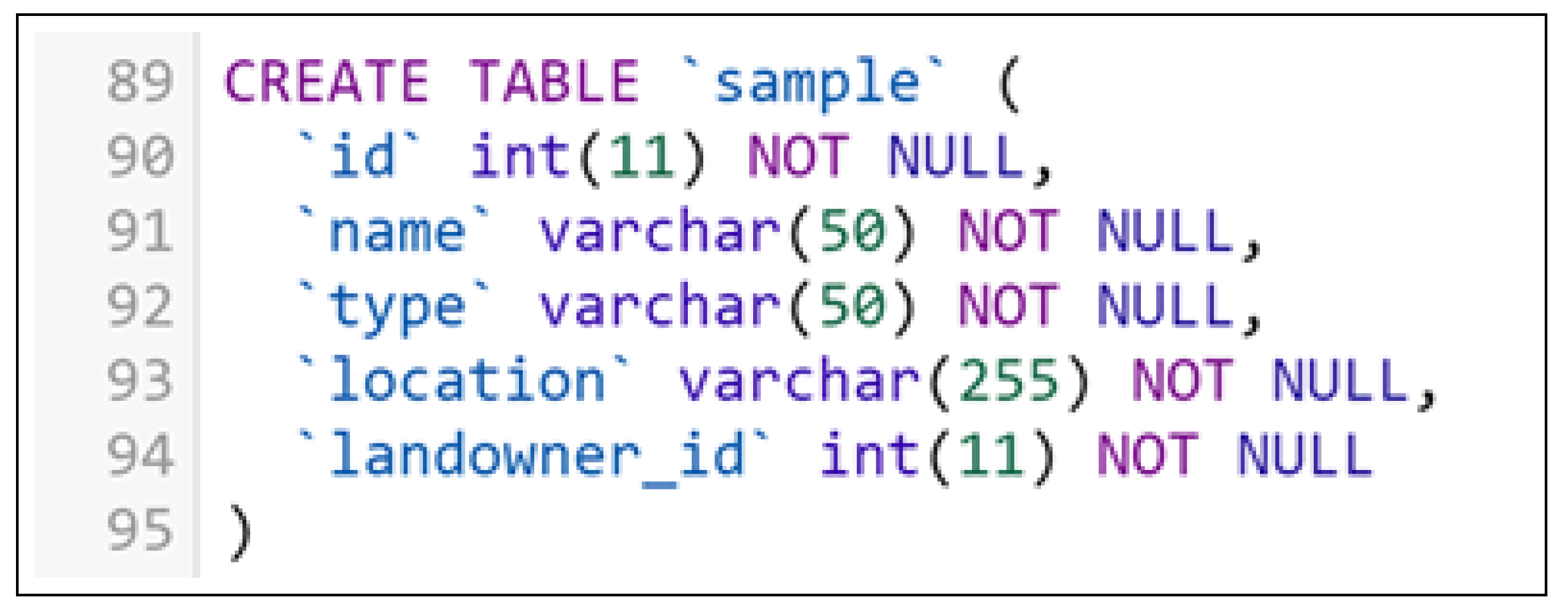
- Register a New Bio-Sample: A pharmaceutical company representative records a new bio-sample by entering details such as its name, type, and geographical location. The system verifies and stores the information, updating the bio-sample ledger on the blockchain. The sample is also assigned to a landowner.
- Register a New Landowner: A pharmaceutical company representative registers a landowner by entering identification details, contact information, and address. The system validates and stores this information, linking the landowner to a previously registered bio-sample.
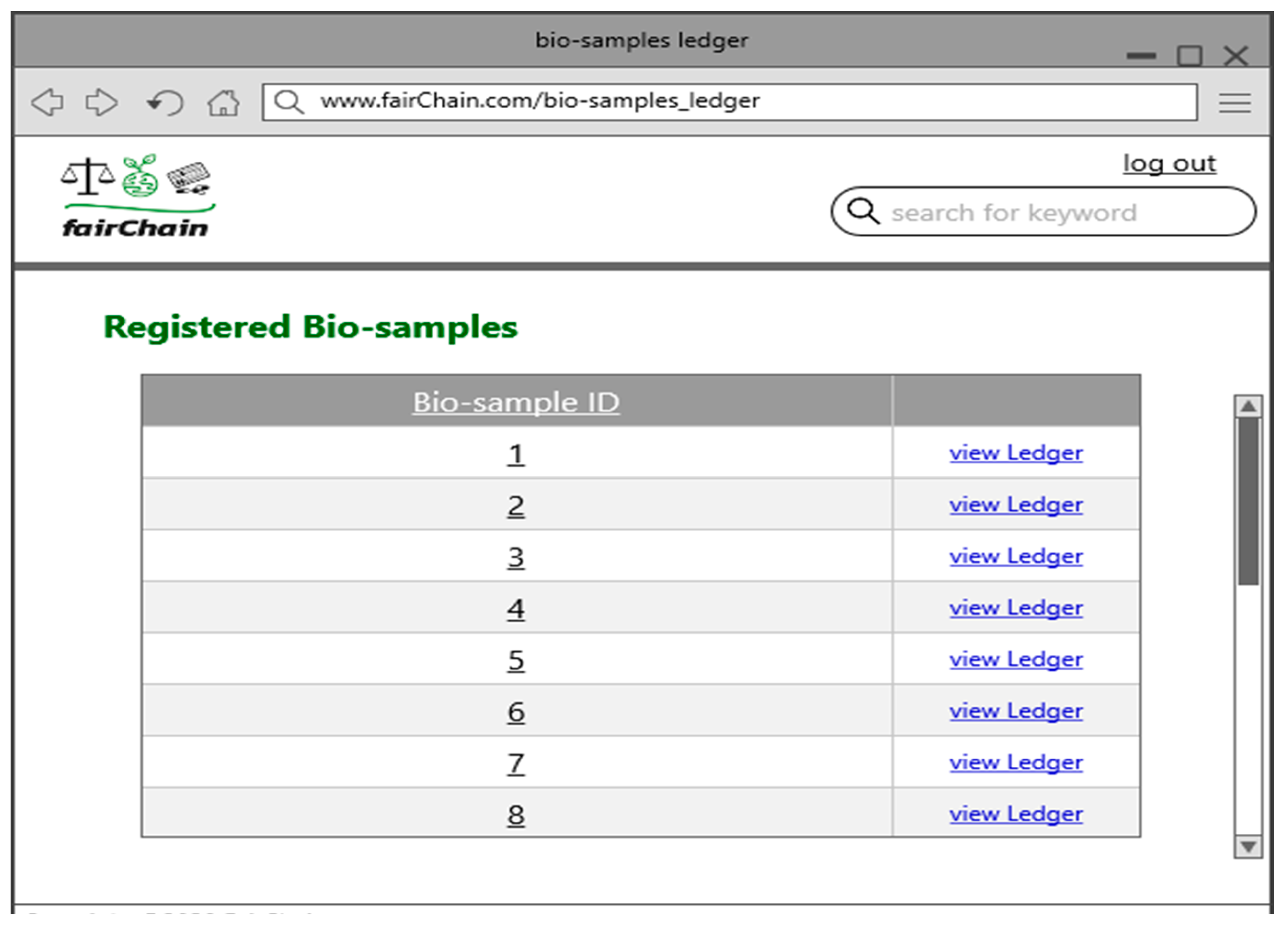
- View Bio-Sample Ledger (Pharmaceutical Company/FDA Representative): Pharmaceutical company and FDA representatives can view the bio-sample ledger, tracking all changes from registration to the latest updates. The system retrieves and displays this timeline, ensuring transparency.
- View Bio-Sample Ledger (Landowner): Landowners can log into the system and view their assigned bio-sample ledger, tracking all updates from registration onward. The system ensures that access is restricted to authorized users.
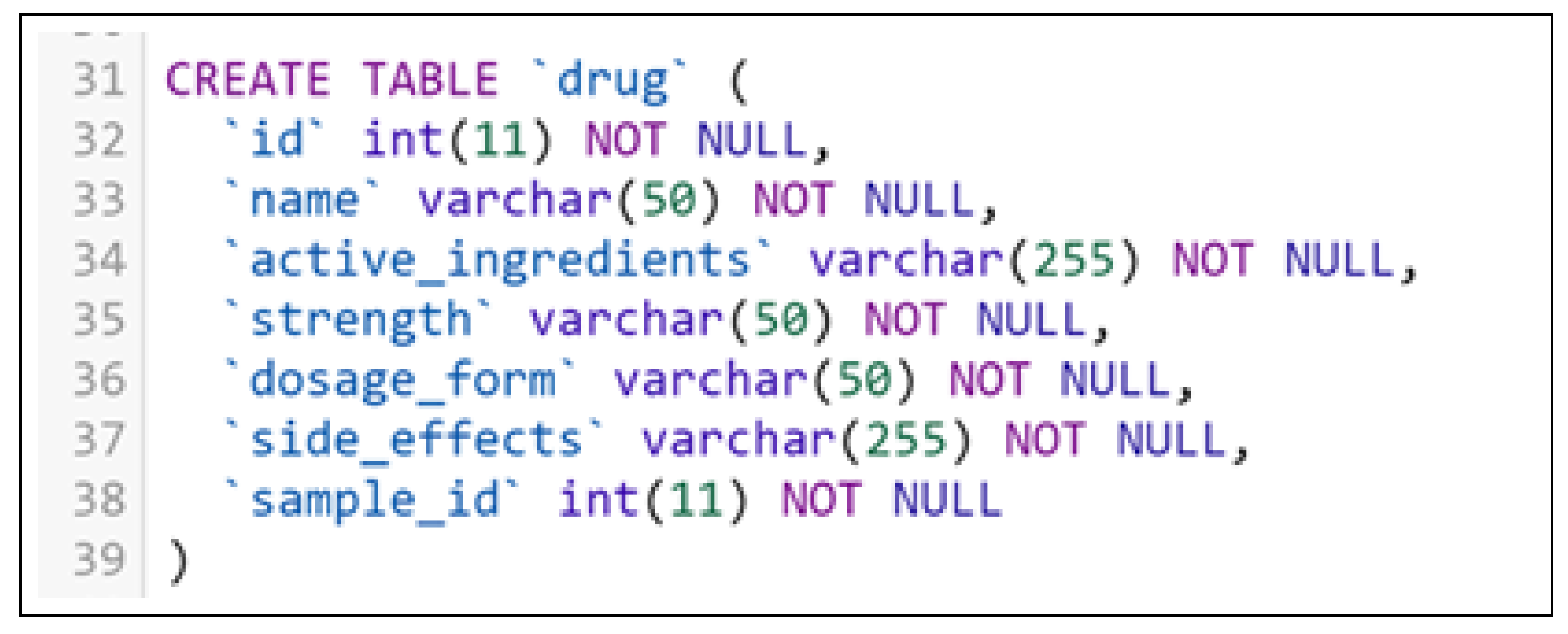
- Register a New Drug: A pharmaceutical company representative registers a new drug by providing its name and active ingredients. The system verifies and stores this information, updating the bio-sample ledger.
- Record Preclinical Research: A pharmaceutical company representative records preclinical research data, including the type and number of test subjects, toxicity levels, and human testing eligibility. The system validates and saves the data, updating the blockchain ledger.
- Assign a Doctor to Record Clinical Research: A pharmaceutical company representative assigns a doctor to clinical research by entering the doctor’s ID. After confirmation, the system grants access to record clinical trial data.
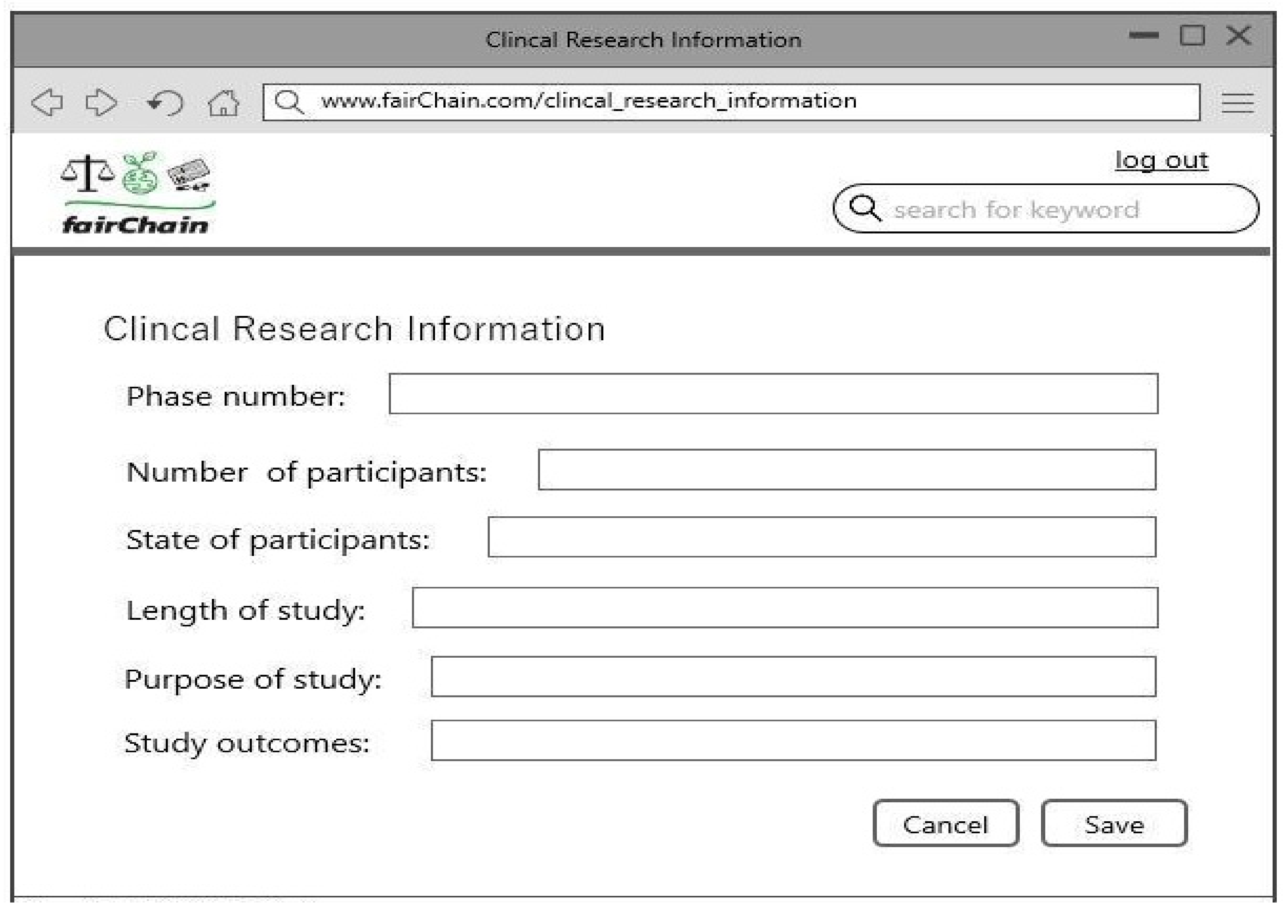
- Record Clinical Research: An authorized doctor enters clinical research data, including trial phases, participant details, study length, and outcomes. The system verifies and updates the bio-sample ledger accordingly.
- Record Labeling Information: A pharmaceutical company representative enters drug labeling details such as indications, usage, dosage, and adverse reactions. The system validates and stores the information, updating the blockchain.
- Send New Drug Application (NDA) Request: A pharmaceutical company representative submits an NDA to the FDA, including safety updates, drug abuse information, and patent details. The system imports necessary data, verifies inputs, and updates the NDA status.
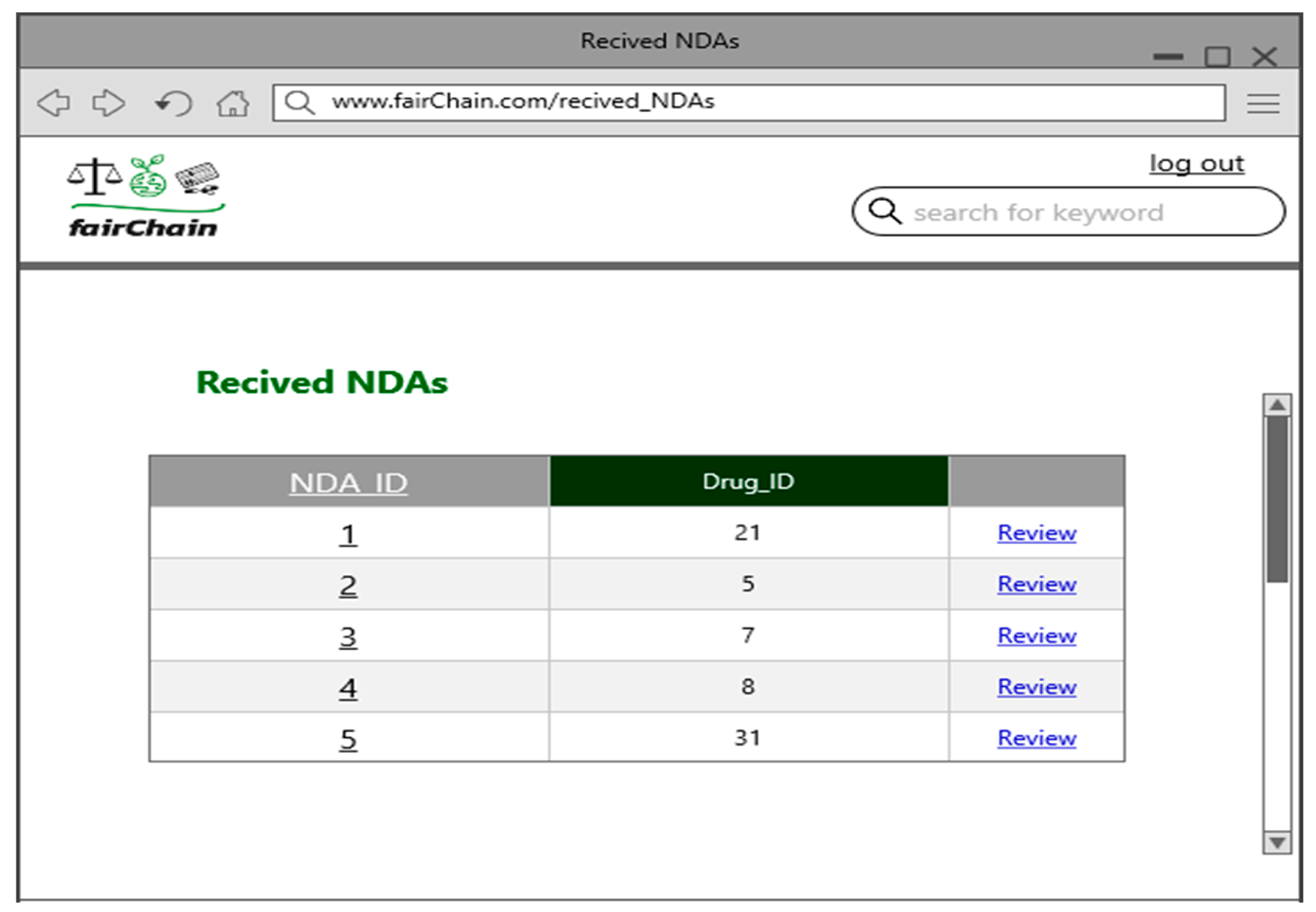
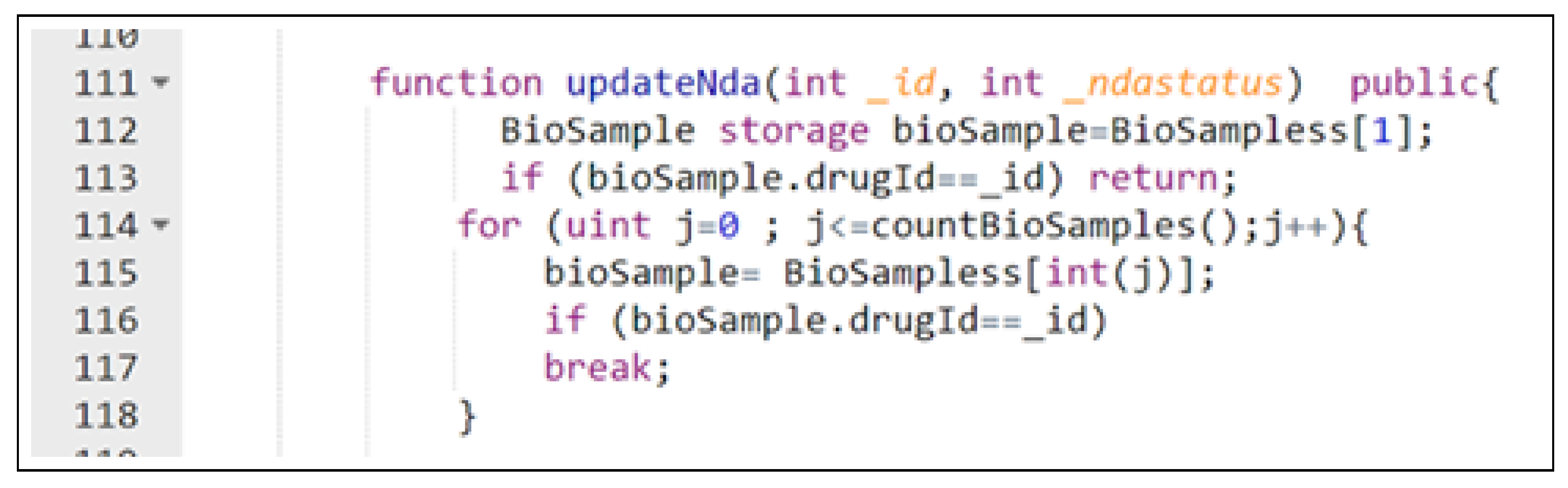
- Review NDA: An FDA representative reviews a submitted NDA request and determines whether to approve or deny it. The system updates the NDA status accordingly, ensuring transparency in the approval process.
5.3. Non-Functional Requirements (NFRs)
- Performance: The platform is designed to support efficient contract execution and sub-second response times for read operations under typical load conditions.
- Scalability: FairChain is architectured for horizontal scalability across research institutions by using modular agents and smart contracts.
- Security: Role-based access control and cryptographic signing are built into the contract deployment and token issuance mechanisms.
- Auditability: Each transaction is immutably logged to ensure transparency and verifiability during post hoc compliance reviews.
- Legal Interoperability: Metadata schemas are aligned with Nagoya Protocol terminologies to support traceable benefit-sharing reporting.
6. FairChain Design
6.1. FairChain Architecture Design
- User Interface Layer: This layer serves as the point of interaction between users and the system, providing an accessible and user-friendly interface. It encompasses elements such as dashboards, forms, and other visual components that facilitate user engagement with the underlying application functionalities.
- Application Layer: Also known as the service layer, this component contains the core business logic and rules of the system. It processes user inputs received from the User Interface Layer, executes specific operations, and manages data flows between the user interface and the data storage components.
- Authentication Layer: This security-focused layer is responsible for verifying user identities and controlling access to system resources. It ensures that only authorized users can access certain functionalities or data, typically through mechanisms such as login credentials, tokens, or multi-factor authentication.
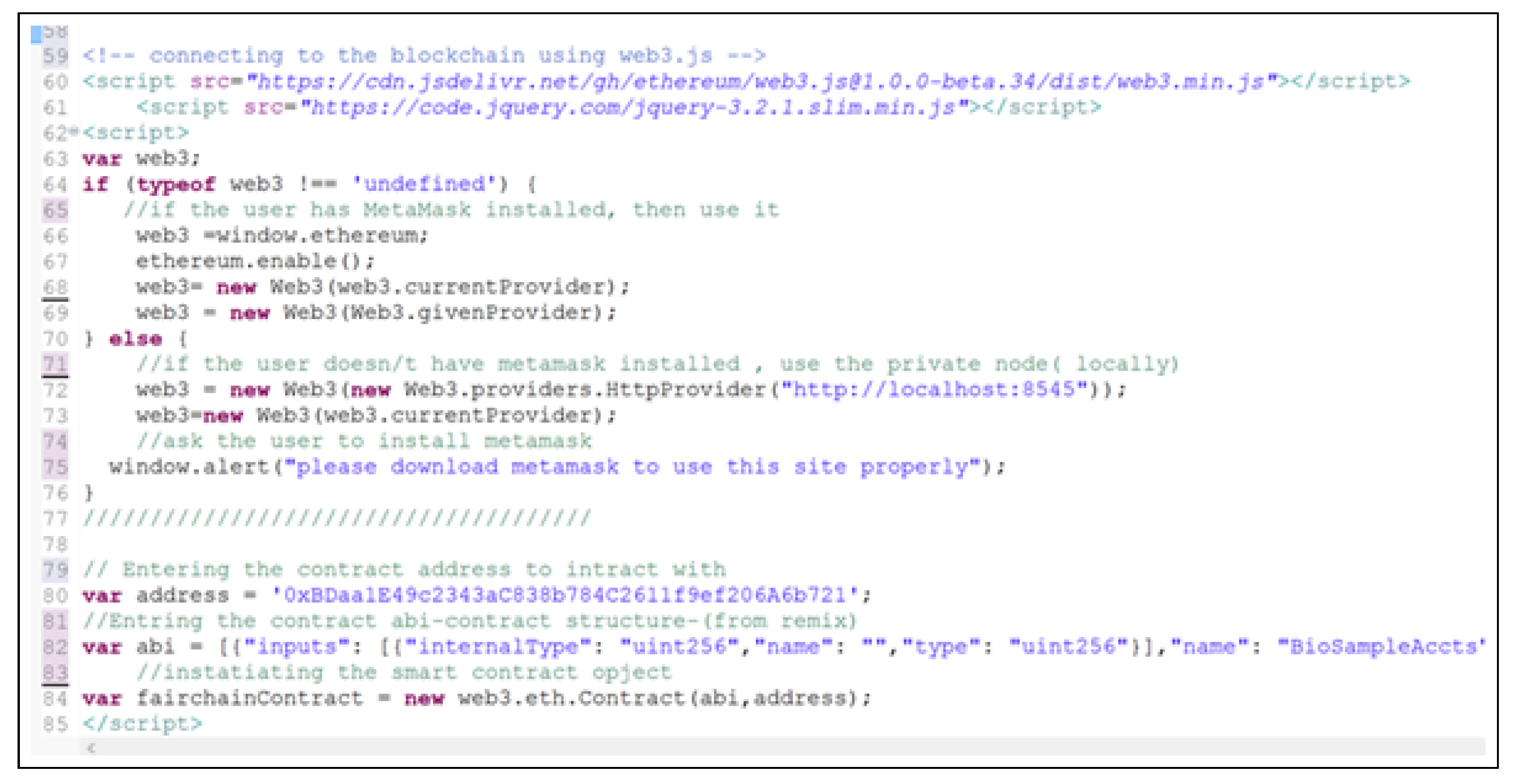
- Blockchain Layer: Serving as the decentralized ledger, this layer records all transactions and data entries in a secure and immutable manner. It operates on the Ethereum blockchain using the Proof-of-Stake (PoS) consensus mechanism, which enhances energy efficiency and network security while supporting scalability. This layer ensures transparency and trust by maintaining a tamper-proof history of activities validated by staked validators.
- Database Layer: This layer is responsible for the structured storage, retrieval, and management of data within the system. It handles operations such as querying, updating, and organizing data to support the application’s requirements, thereby ensuring efficient data access and integrity.

6.2. Database Design
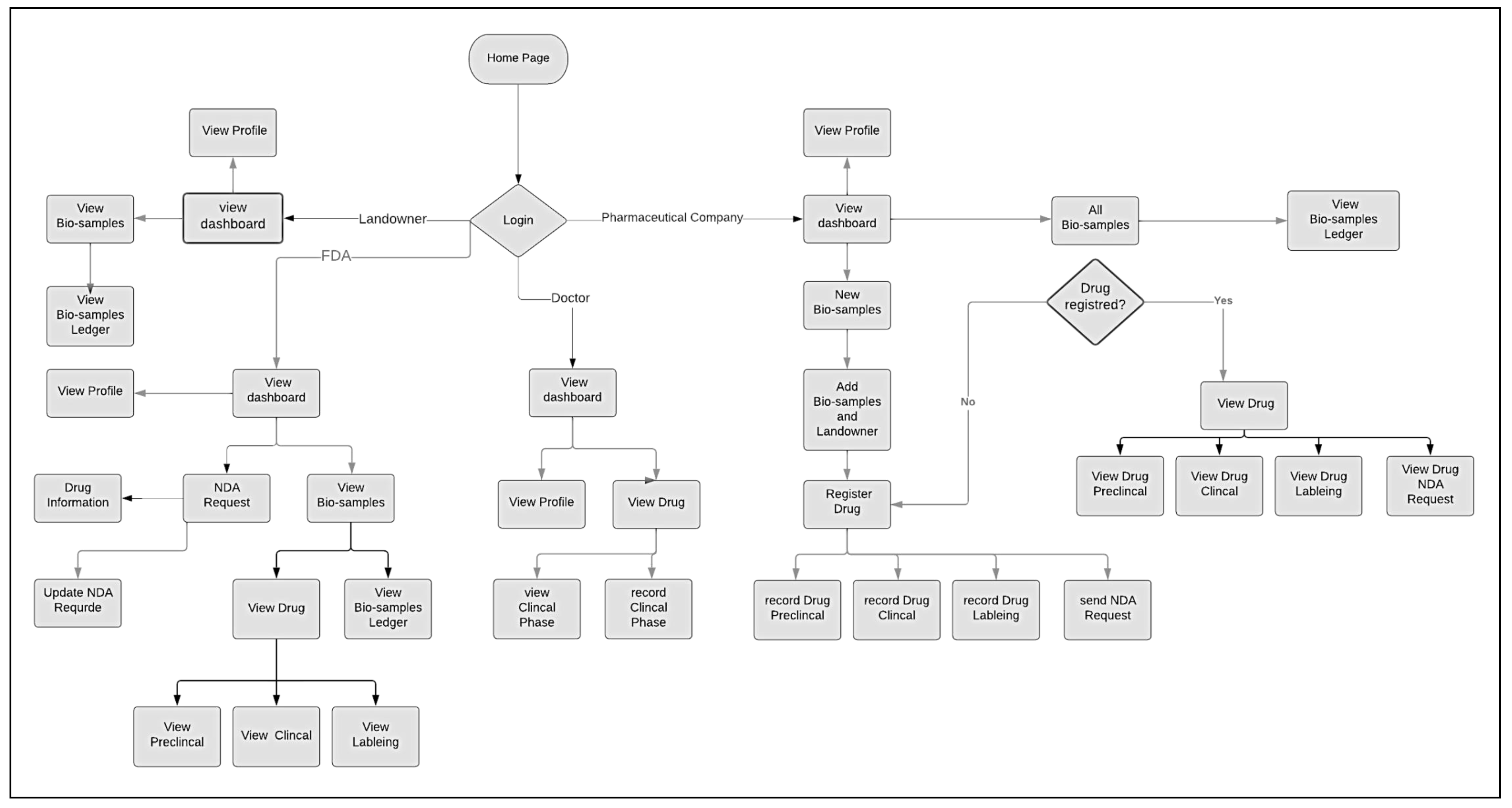
6.3. FairChain System Screen Flow
6.4. User Interface Design
7. FairChain Implementation
7.1. Database Implementation
7.2. Blockchain Ledger Implementation
7.3. Web Application Implementation
7.4. System Integration
- The off-chain MySQL database write is initiated first.
- A cryptographic hash of the stored record is computed.
- This hash is then submitted in a blockchain transaction using Web3.js and stored immutably on-chain.
- If the on-chain transaction fails, the database write is rolled back to prevent inconsistency.
7.5. Data Consistency and Security Measures
- Hash Verification: All off-chain entries include a hash submitted to the blockchain, enabling verification that stored data have not been altered.
- Role-Based Access Control (RBAC): Smart contracts and the web application enforce strict role-based permissions, limiting access to sensitive operations.
- Transactional Integrity: The system uses error-handling protocols to confirm on-chain recording before finalizing any database changes, which ensures consistent states.
- Audit Logging: All modifications are logged with timestamps and actor IDs, enabling accountability and traceability.
8. Discussion and Conclusions
8.1. Risk Analysis and Security Considerations
- Smart Contract Vulnerabilities: The use of Solidity exposes the system to risks such as re-entrance attacks and integer overflows. To mitigate this, smart contracts were tested using the Remix and Truffle frameworks. Future versions will integrate formal verification tools such as Slither or MythX.
- Oracle and Middleware Risks: The integration of off-chain data sources through Web3.js may create attack surfaces for oracle manipulation. To minimize this issue, data feeds are authenticated and logs are hash-matched with the blockchain ledger to detect tampering.
- Private Key Exposure: Private key theft could compromise system integrity; thus, FairChain recommends hardware wallets and multi-signature authorization for sensitive actions.
- Centralized Database Weaknesses: MySQL is used to store off-chain data, which could be a single point of failure. Mitigation approaches could include encryption, role-based access control, and future deployment of database replication with clustering.
- Denial of Service (DoS): Excessive or malformed transactions can disrupt smart contract functions. To prevent such abuse, FairChain introduces gas usage limits and transaction validation mechanisms.
8.2. Limitations and Jurisdictional Challenges
- Validator Selection in Proof-of-Stake (PoS): In PoS systems such as Ethereum, validators are chosen based on the quantity of staked tokens. While this promotes economic fairness and discourages malicious behavior, it may concentrate influence among large stakeholders, potentially raising concerns about decentralization. Moreover, validator availability and network liveness are subject to their economic incentives and geographical distribution, which could affect transaction confirmation reliability during high-load events.
- Security Risks beyond Smart Contracts: Although the core smart contracts have been tested and validated, broader attack vectors persist. These include endpoint vulnerabilities in Web3.js interfaces, client-side attacks such as phishing, and risks involving key management. Decentralized systems are also vulnerable to coordinated attacks (e.g., 51% attacks in smaller PoS networks), although the Ethereum mainnet has high resilience due to its size. Future work will consider integrating Hardware Security Modules (HSMs) and formal verification of contracts.
- Data Governance across Jurisdictions: Data sovereignty and privacy regulations vary across countries, affecting how off-chain data must be handled. For example, the European Union’s GDPR mandates strict controls over data portability and the right to erasure, while some jurisdictions may require data localization. FairChain supports these requirements by keeping personal data off-chain, but cross-border deployments may require compliance mapping and legal risk assessments to ensure lawful interoperability. Addressing these challenges requires regulatory harmonization or middleware solutions that adapt to local data laws.
8.3. Deployment Costs vs. Long-Term Benefits
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtenberg, F.R. Pharmaceutical Innovation, Mortality Reduction, and Economic Growth; Working Paper Series; National Bureau of Economic Research: Cambridge, MA, USA, 1998; Volume 6569. [Google Scholar]
- Buxbaum, J.D.; Chernew, M.E.; Fendrick, A.M.; Cutler, D.M. Contributions of Public Health, Pharmaceuticals, and Other Medical Care to US Life Expectancy Changes, 1990–2015. Health Aff. 2020, 39, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Strovel, J.; Sittampalam, S.; Coussens, N.P.; Hughes, M.; Inglese, J.; Kurtz, A.; Andalibi, A.; Patton, L.; Austin, C.; Baltezor, M.; et al. Early Drug Discovery and Development Guidelines: For Academic Researchers, Collaborators, and Start-up Companies. In Assay Guidance Manual; Markossian, S., Grossman, A., Baskir, H., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Balcells-Camps, M.; MIT Institute for Medical Engineering and Science (IMES), Massachusetts Institute of Technology (MIT), Cambridge, MA, USA. Personal communication, 2020.
- U.S. Food and Drug Administration. The Drug Development Process. Available online: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process (accessed on 25 February 2020).
- McCreath, S.B.; Delgoda, R. Pharmacognosy: Fundamentals, Applications and Strategies; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Renner, S.C.; Neumann, D.; Burkart, M.; Feit, U.; Groger, A.; Paulsh, A.; Paulsh, C.; Sterz, M.; Vohland, K. Import and export of biological samples from tropical countries–considerations and guidelines for research teams. Org. Divers. Evol. 2012, 12, 81–98. [Google Scholar] [CrossRef]
- Lipsky, M.S.; Sharp, L.K. From idea to market: The drug approval process. J. Am. Board Fam. Pract. 2001, 14, 362–367. [Google Scholar]
- Convention on Biological Diversity. About the Nagoya Protocol. Available online: https://www.cbd.int/abs/about/ (accessed on 14 October 2024).
- Presutti, L. Blockchain Technology: Challenges and Legal Issues. CyberLaws, 2018. Available online: https://www.cyberlaws.it/2018/blockchain-technology-challenges-legal-issues/ (accessed on 14 October 2024).
- Blockchain.com. Blockchain.com Privacy Policy. 2022. Available online: https://www.blockchain.com/legal/privacy (accessed on 14 October 2024).
- Pacific BioLabs. Stages of Drug Development-Pacific Biolabs. 2020. Available online: https://pacificbiolabs.com/stages-of-drug-development (accessed on 16 October 2024).
- U.S. Food and Drug Administration. Step 2: Preclinical Research. 2020. Available online: https://www.fda.gov/patients/drug-development-process/step-2-preclinical-research (accessed on 16 October 2024).
- U.S. Food and Drug Administration. Step 3: Clinical Research. 2020. Available online: https://www.fda.gov/patients/drug-development-process/step-3-clinical-research (accessed on 16 October 2024).
- U.S. Food and Drug Administration. Step 4: FDA Drug Review. 2020. Available online: https://www.fda.gov/patients/drug-development-process/step-4-fda-drug-review (accessed on 16 October 2024).
- U.S. Food and Drug Administration. Step 5: FDA Post-Market Drug Safety Monitoring. 2020. Available online: https://www.fda.gov/patients/drug-development-process/step-5-fda-post-market-drug-safety-monitoring (accessed on 16 October 2024).
- International Union for Conservation of Nature and Natural Resources (IUCN). The Nagoya Protocol. Available online: https://www.iucn.org/theme/global-policy/our-work/convention-biological-diversity-cbd/nagoya-protocol (accessed on 16 October 2024).
- What Is Blockchain Technology? A Step-By-Step Guide for Beginners. Available online: https://blocklr.com/guides/blockchain-technology/ (accessed on 16 October 2024).
- Itemtracker-Sample Management & Tracking Software. Available online: https://www.itemtracker.com/ (accessed on 18 October 2024).
- Laboratory Information Management Software (LIMS) LIMS Software. Available online: https://www.autoscribeinformatics.com/lims-laboratory-information-management-system (accessed on 18 October 2024).
- Brooks Life Sciences Brooks Life Sciences. Available online: https://www.brookslifesciences.com/ (accessed on 18 October 2024).
- Sustainable Sample Tracking Fluics Connect. Available online: https://fluics.com/ (accessed on 18 October 2024).
- Yazdinejad, A.; Dehghantanha, A.; Karimipour, H.; Srivastava, G.; Parizi, R.M. A robust privacy-preserving federated learning model against model poisoning attacks. IEEE Trans. Inf. Forensics Secur. 2024, 19, 6693–6708. [Google Scholar] [CrossRef]
- Mettler, M. Blockchain technology in healthcare: The revolution starts here. In Proceedings of the 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom), Munich, Germany, 14–17 September 2016; pp. 1–3. [Google Scholar]
- Yue, X.; Wang, H.; Jin, D.; Li, M.; Jiang, W. Healthcare data gateways: Found healthcare intelligence on blockchain with novel privacy risk control. J. Med. Syst. 2016, 40, 218. [Google Scholar] [CrossRef] [PubMed]
- Benchoufi, M.; Ravaud, P. Blockchain technology for improving clinical research quality. Trials 2017, 18, 335. [Google Scholar] [CrossRef] [PubMed]
- Azaria, A.; Ekblaw, A.; Vieira, T.; Lippman, A. MedRec: Using blockchain for medical data access and permission management. In Proceedings of the 2016 IEEE Open and Big Data Conference, Washington, DC, USA, 5–8 December 2016; pp. 25–30. [Google Scholar]
- Hylock, R.H.; Zeng, X. A review and evaluation of blockchain systems for healthcare applications. J. Biomed. Inform. 2022, 127, 104005. [Google Scholar]
- MediLedger. MediLedger Network: Secure Pharmaceutical Supply Chains. Available online: https://www.mediledger.com (accessed on 16 October 2024).
- IBM Blockchain. IBM Pharma Ledger. Available online: https://www.ibm.com/blockchain/solutions/pharmaceutical (accessed on 16 October 2024).
- VeChain. VeChain ToolChain™. Available online: https://www.vechain.org (accessed on 16 October 2024).
- PharmaLedger EU. PharmaLedger Blockchain Initiative. Available online: https://www.ihi.europa.eu/projects-results/project-factsheets/pharmaledger (accessed on 16 October 2024).
- BlockPharma. Blockchain Against Drug Counterfeiting. Available online: https://www.blockchainalmanac.com/supply-chain-and-logistics/blockpharma/ (accessed on 16 October 2024).
- Hyperledger Foundation. Hyperledger Fabric for Pharma. Available online: https://www.hyperledger.org (accessed on 16 October 2024).
- Phpmyadmin. Available online: https://www.phpmyadmin.net/ (accessed on 24 October 2024).
- World Health Organization (WHO). International Clinical Trials Registry Platform (ICTRP). Available online: https://www.who.int/clinical-trials-registry-platform (accessed on 24 October 2024).
- Fielding, R.T. Architectural Styles and the Design of Network-based Software Architectures. Ph.D. Thesis, University of California, Irvine, CA, USA, 2000. [Google Scholar]







| Phase No. | Number of Participants | State of Participants | Length of Study | Purpose | Outcomes |
|---|---|---|---|---|---|
| Phase 1 | 20–100 | Healthy or people with the disease/condition | Several months | Safety and dosage |
|
| Phase 2 | Several hundred | People with the disease/condition | Several months to 2 years | Efficacy and side effects | Approximately 33% of drugs move to the next phase. |
| Phase 3 | 300 to 3000 | People with the disease/condition | 1 to 4 years | Efficacy and monitoring of adverse reactions |
|
| Phase 4 | Several thousand | People with the disease/condition | Safety and efficacy | Regulator approval or denial |
| Feature | Medi-Ledger | IBM Pharma Ledger | VeChain ToolChain | Pharma-Ledger | Block-Pharma | Hyper-Ledger Fabric | FairChain |
|---|---|---|---|---|---|---|---|
| Primary Focus | Supply chain tracking | Supply chain and compliance | Supply chain and IoT | Clinical trials and patient consent | Drug authentication | Enterprise pharma blockchain | Full DDLC and Nagoya Protocol compliance. |
| Security | Permissioned blockchain | Enterprise encryption | IoT tracking (NFC and RFID) | Secure patient data sharing | QR-code verification | Permissioned enterprise blockchain | Public and permissioned blockchain, smart contracts |
| Scalability | DSCSA-compliant transactions | Enterprise scalability | IoT-enabled real-time tracking | GDPR and pharma compliance | Consumer mobile integration | High-performance ledger transactions | High-volume transactions with decentralized governance. |
| Regulatory Compliance | DSCSA (U.S.) | Global pharma regulations | Pharma supply chain laws | GDPR and EU pharma laws | Anti-counterfeit regulations | Pharma industry compliance | Nagoya Protocol for bio-sample tracking. |
| Stakeholders | Manufactur-ers, wholesalers, regulators | Pharma companies, regulators | Supply chain logistics, pharma firms | Researchers, patients, regulators | Pharmacies, patients | Pharma companies, regulators | Researchers, landowners, pharma firms, regulators. |
| Consensus Mechanism | Permissioned (custom) | PoS (IBM) | PoA | Not specified | Not specified | Raft/PBFT | PoS (Ethereum) |
| Data Privacy Model | Encrypted messaging | Private enterprise data | Encrypted IoT streams | GDPR consent tools | QR-code validation | Encrypted permissioned ledger | Off-chain storage with hashed pointers |
| Feature | FairChain Contribution |
|---|---|
| Trust Framework | Verifiable smart contracts with multi-role agents |
| Auditability | Immutable on-chain provenance using blockchain ledger |
| Legal Interoperability | Smart contract metadata aligned with Nagoya Protocol terms |
| Transparency | Executable trace logs and tokenized resource tracking |
| Modularity | Composable architecture supporting integration with research and IP workflows |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSalamah, S.; Alnehmi, S.A.; Abanumai, A.A.; Alnashri, A.H.; Alduhim, S.S.; Alnamlah, N.A.; AlGhamdi, K.; Sheerah, H.A.; Alsalamah, S.A.; Alsalamah, H.A. FairChain: A Trusted and Transparent Blockchain-Based Ecosystem for Drug Development for Nagoya Protocol Implementation. Electronics 2025, 14, 2527. https://doi.org/10.3390/electronics14132527
AlSalamah S, Alnehmi SA, Abanumai AA, Alnashri AH, Alduhim SS, Alnamlah NA, AlGhamdi K, Sheerah HA, Alsalamah SA, Alsalamah HA. FairChain: A Trusted and Transparent Blockchain-Based Ecosystem for Drug Development for Nagoya Protocol Implementation. Electronics. 2025; 14(13):2527. https://doi.org/10.3390/electronics14132527
Chicago/Turabian StyleAlSalamah, Shada, Shaima A. Alnehmi, Anfal A. Abanumai, Asmaa H. Alnashri, Sara S. Alduhim, Norah A. Alnamlah, Khulood AlGhamdi, Haytham A. Sheerah, Sara A. Alsalamah, and Hessah A. Alsalamah. 2025. "FairChain: A Trusted and Transparent Blockchain-Based Ecosystem for Drug Development for Nagoya Protocol Implementation" Electronics 14, no. 13: 2527. https://doi.org/10.3390/electronics14132527
APA StyleAlSalamah, S., Alnehmi, S. A., Abanumai, A. A., Alnashri, A. H., Alduhim, S. S., Alnamlah, N. A., AlGhamdi, K., Sheerah, H. A., Alsalamah, S. A., & Alsalamah, H. A. (2025). FairChain: A Trusted and Transparent Blockchain-Based Ecosystem for Drug Development for Nagoya Protocol Implementation. Electronics, 14(13), 2527. https://doi.org/10.3390/electronics14132527





