Integrated Framework for Managing Childhood Obesity Based on Biobanks, AI Tools and Methods, and Serious Games
Abstract
1. Introduction
- We propose a modular architecture that seamlessly integrates data from Biobanks, processes it through AI-driven analytics to assess obesity risk and recommend interventions, and deploys innovative applications to reinforce healthy behaviors in children. This architecture allows for a comprehensive approach, where health, behavioral, and environmental factors are all addressed within a single cohesive system.
- We implement a suite of serious games, which are designed to increase children’s nutritional knowledge and promote physical activity in an interactive and sustainable manner.
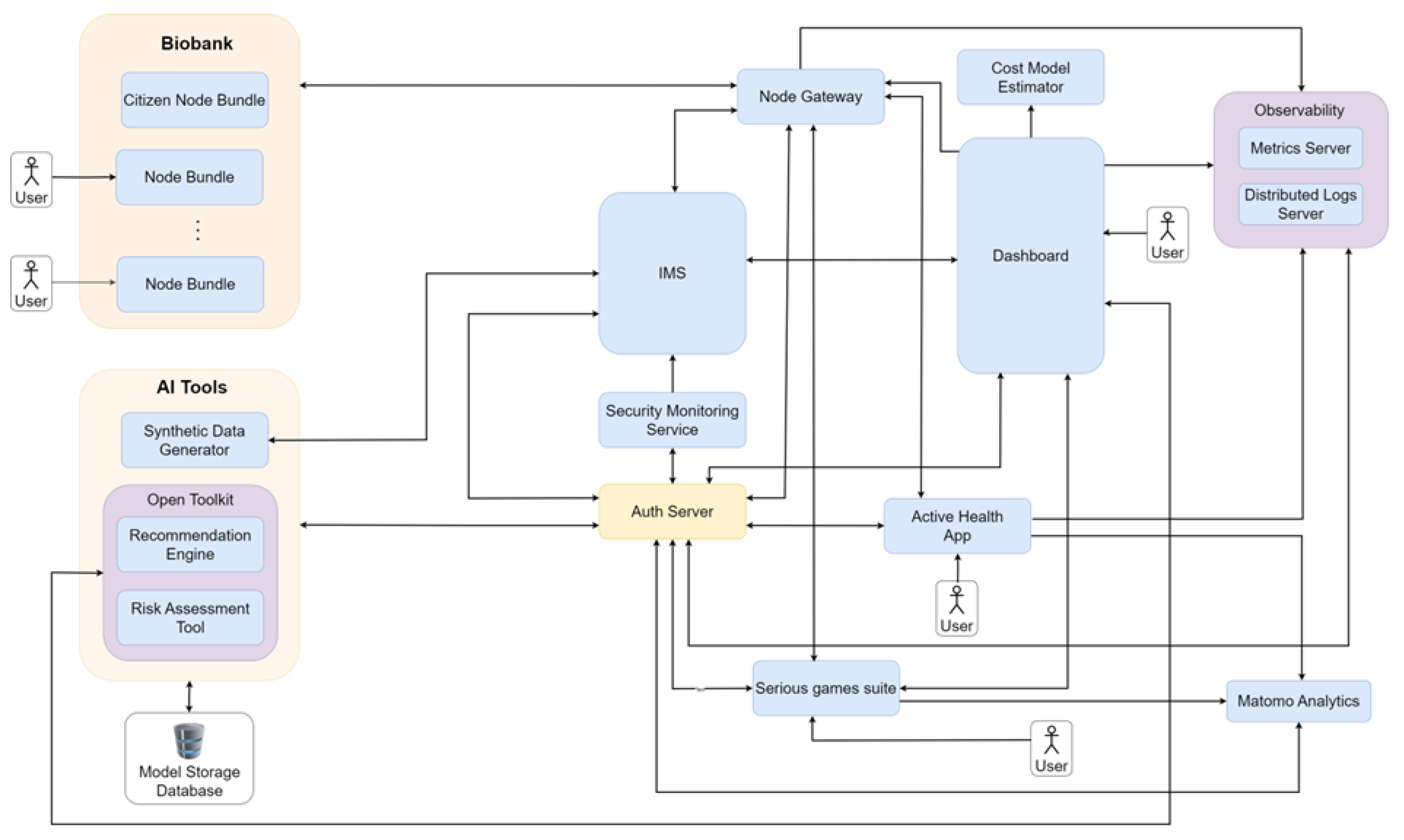
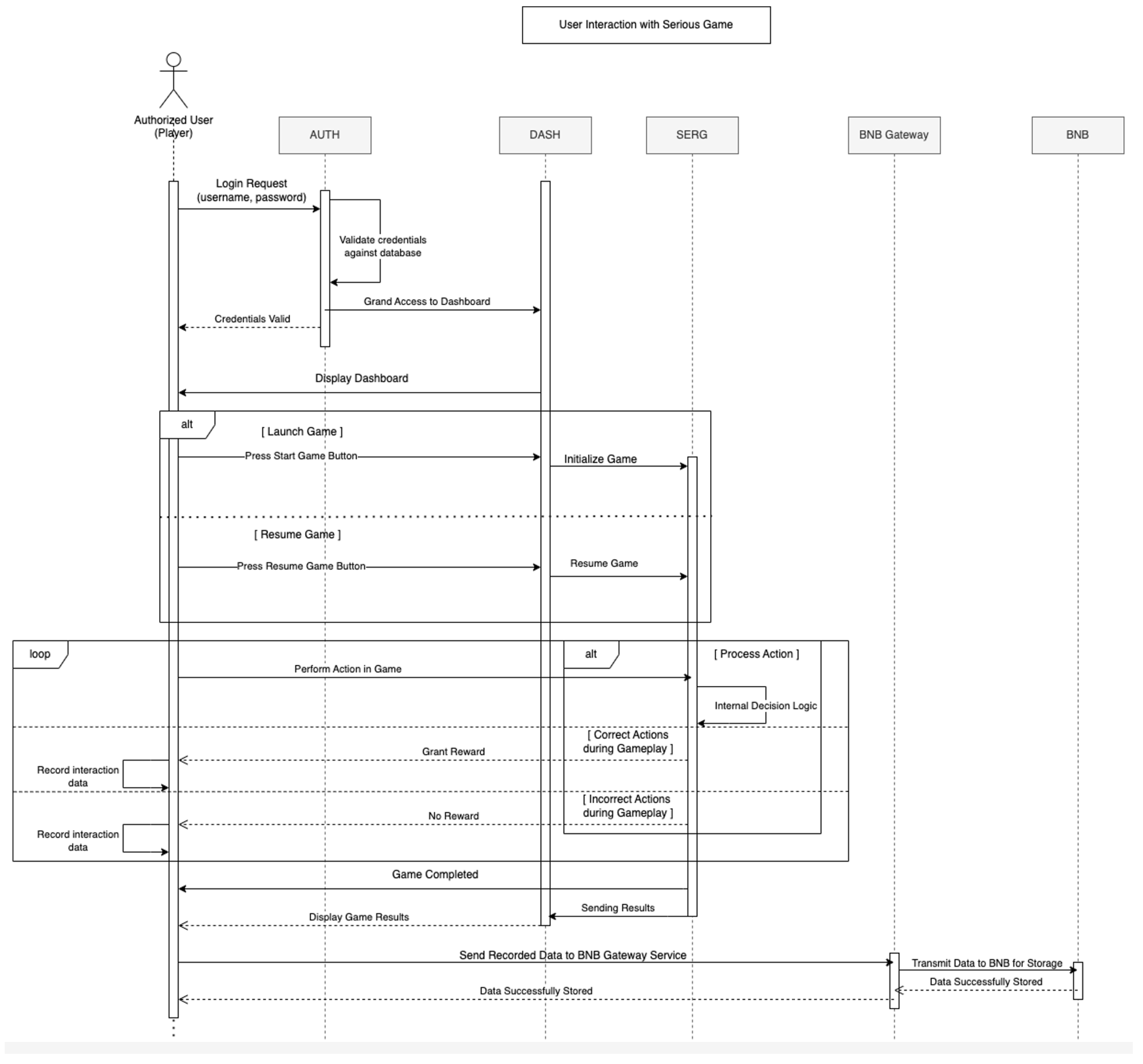
- We present architectural data-flow diagrams illustrating the information exchange between system components. These diagrams provide insight into how data and computational processes move through the framework architecture and how users interact with different elements of the architecture.
2. Evidence-Based Behavioural Modification for the Prevention of Childhood Obesity
2.1. Related Work
2.1.1. Childhood Obesity
2.1.2. AI in Healthcare
2.1.3. Biobanks
2.1.4. Serious Games
2.2. Overview of Proposed Framework
2.3. Framework Advantages
3. Description of Framework
3.1. Biobank
3.2. Data Management and Preprocessing
3.3. AI Tools
3.4. User Interface and Engagement
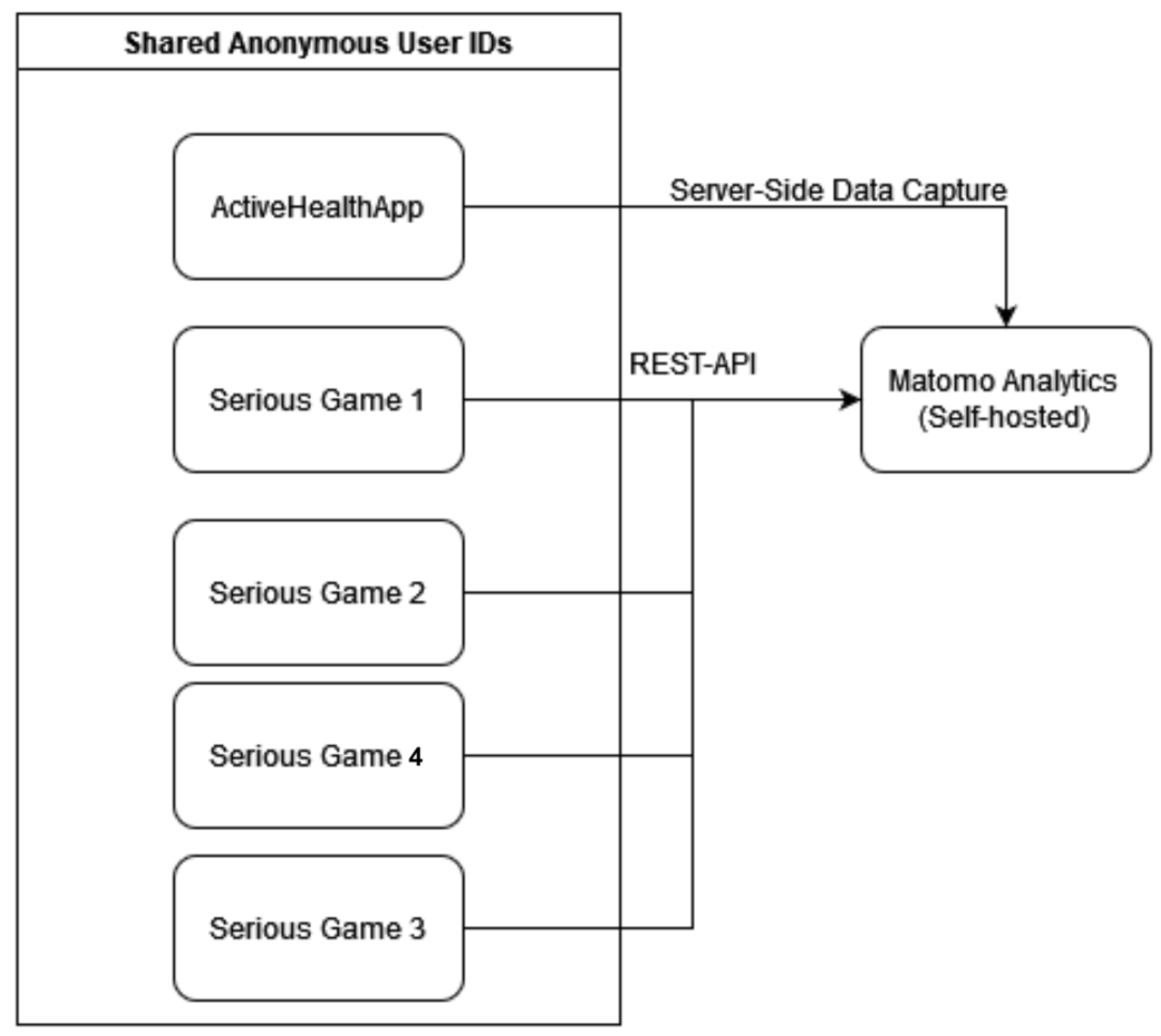
3.5. Serious Games
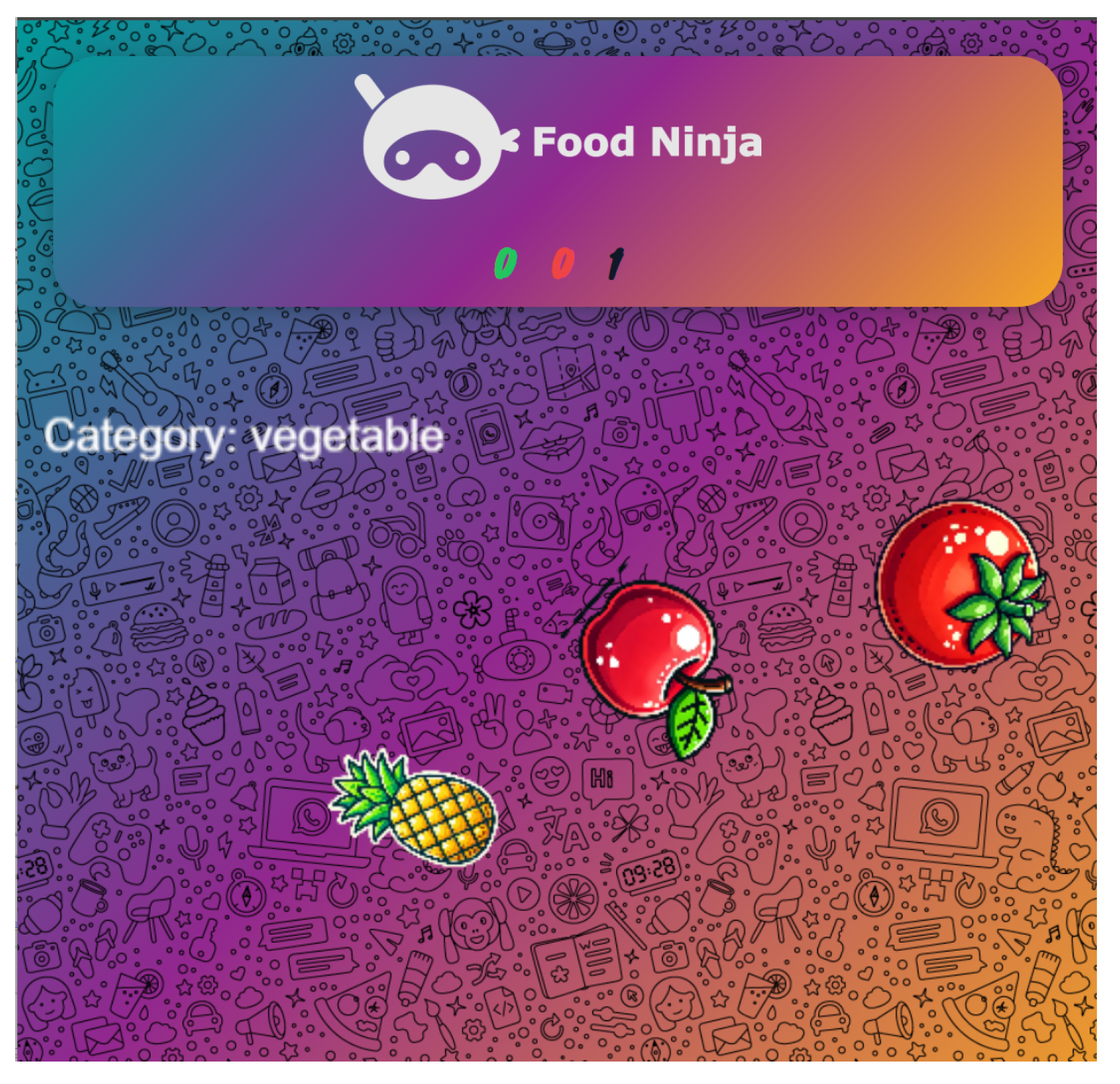
3.5.1. Food Ninja
3.5.2. Food Quiz
- In Educational mode, players engage with a predetermined set of theme-specific questions, each presenting four multiple-choice options with one correct answer. When players select incorrect answers, they receive educational messages explaining the correct choice, fostering learning without the pressure of scoring or time constraints.
- The Competitive mode introduces additional challenges through a countdown timer for each question and a sophisticated scoring system that considers both answer accuracy and response speed. Players accumulate points based on consecutive correct answers, with the game ending upon a predefined number of incorrect responses.
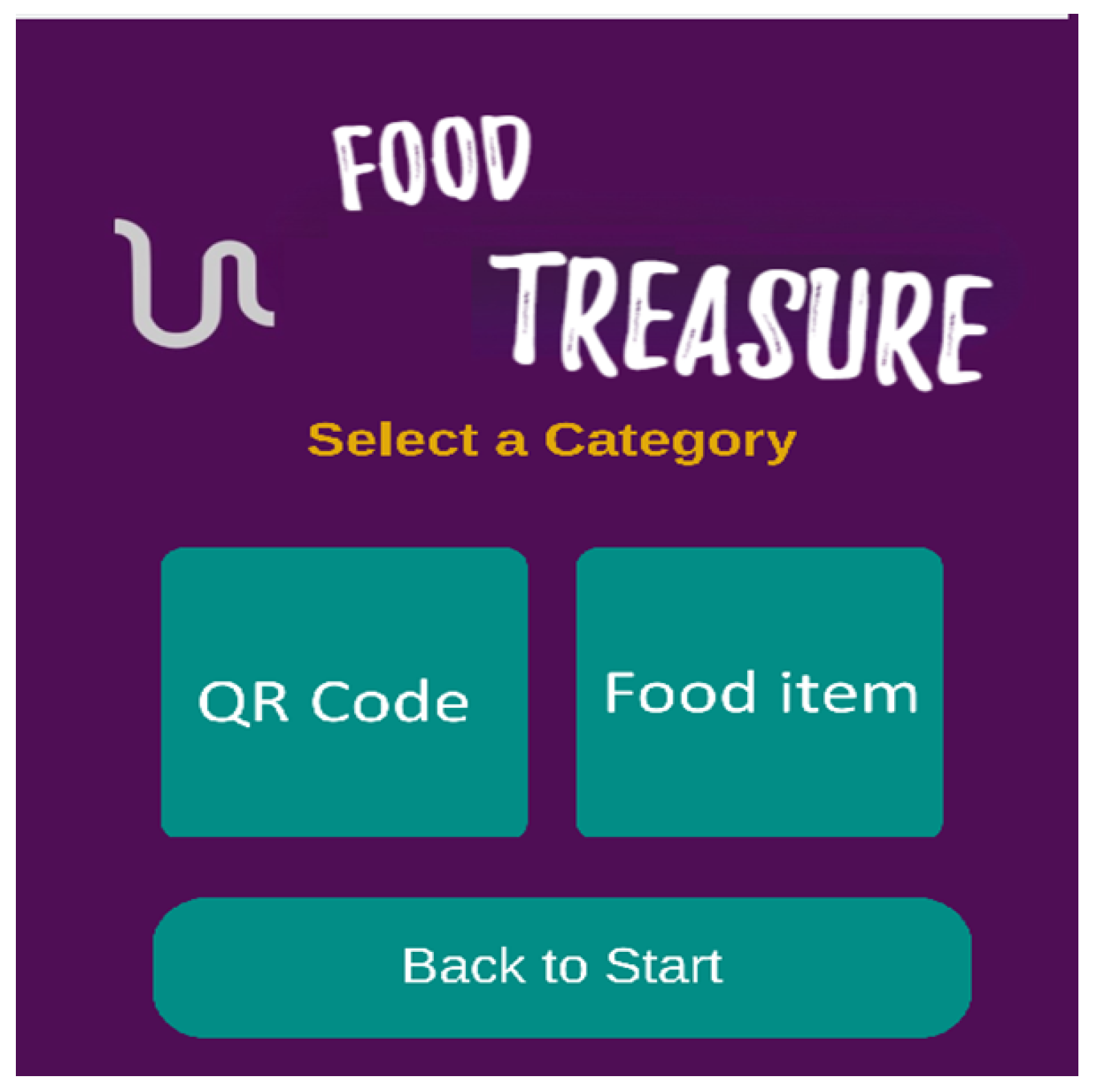
3.5.3. Food Treasure
3.5.4. Let’s Move
3.6. Framework Adaptability Across Diverse Contexts
3.7. Regulatory Compliance and Ethical Considerations
3.8. Framework Evaluation
Mapping Architectural Components to Requirements
4. System Interaction Workflows
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marcus, C.; Danielsson, P.; Hagman, E. Pediatric obesity—Long-term consequences and effect of weight loss. J. Intern. Med. 2022, 292, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Katsarova, I. Skyrocketing Obesity in Children: Why Everybody Should Be Concerned? Technical Report; European Parliamentary Research Service: Brussels, Belgium, 2024. [Google Scholar]
- Tsoi, M.F.; Li, H.L.; Feng, Q.; Cheung, C.L.; Cheung, T.T.; Cheung, B.M. Prevalence of childhood obesity in the United States in 1999–2018: A 20-year analysis. Obes. Facts 2022, 15, 560–569. [Google Scholar] [CrossRef]
- Masood, B.; Moorthy, M. Causes of obesity: A review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef] [PubMed]
- LeCroy, M.N.; Kim, R.S.; Stevens, J.; Hanna, D.B.; Isasi, C.R. Identifying key determinants of childhood obesity: A narrative review of machine learning studies. Child. Obes. 2021, 17, 153–159. [Google Scholar] [CrossRef]
- Heath, L.; Jebb, S.A.; Aveyard, P.; Piernas, C. Obesity, metabolic risk and adherence to healthy lifestyle behaviours: Prospective cohort study in the UK Biobank. BMC Med. 2022, 20, 65. [Google Scholar] [CrossRef]
- Lamas, S.; Rebelo, S.; da Costa, S.; Sousa, H.; Zagalo, N.; Pinto, E. The influence of serious games in the promotion of healthy diet and physical activity health: A systematic review. Nutrients 2023, 15, 1399. [Google Scholar] [CrossRef]
- Vourdoumpa, A.; Paltoglou, G.; Charmandari, E. The genetic basis of childhood obesity: A systematic review. Nutrients 2023, 15, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Alvarez-Pitti, J.; Kozioł-Kozakowska, A.; Brzeziński, M.; Gabbianelli, R.; Herceg-Čavrak, V.; Wühl, E.; Lucas, I.; Radovanović, D.; Melk, A.; et al. Psychosocial and environmental risk factors of obesity and hypertension in children and adolescents—A literature overview. Front. Cardiovasc. Med. 2023, 10, 1268364. [Google Scholar] [CrossRef] [PubMed]
- Albataineh, S.R.; Badran, E.F.; Tayyem, R.F. Overweight and obesity in childhood: Dietary, biochemical, inflammatory and lifestyle risk factors. Obes. Med. 2019, 15, 100112. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Jakobsen, D.D.; Brader, L.; Bruun, J.M. Association between food, beverages and overweight/obesity in children and adolescents—A systematic review and meta-analysis of observational studies. Nutrients 2023, 15, 764. [Google Scholar] [CrossRef] [PubMed]
- Liberali, R.; Kupek, E.; Assis, M.A.A.d. Dietary patterns and childhood obesity risk: A systematic review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Dallacker, M.; Hertwig, R.; Mata, J. The frequency of family meals and nutritional health in children: A meta-analysis. Obes. Rev. 2018, 19, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, H.; Wu, H. Association between diet quality scores and risk of overweight and obesity in children and adolescents. BMC Pediatr. 2023, 23, 169. [Google Scholar] [CrossRef]
- Saltaouras, G.; Kyrkili, A.; Bathrellou, E.; Georgoulis, M.; Yannakoulia, M.; Bountziouka, V.; Smrke, U.; Dimitrakopoulos, G.; Kontogianni, M.D. Associations between Meal Patterns and Risk of Overweight/Obesity in Children and Adolescents in Western Countries: A Systematic Review of Longitudinal Studies and Randomised Controlled Trials. Children 2024, 11, 1100. [Google Scholar] [CrossRef]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Executive summary: Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics 2023, 151. [Google Scholar] [CrossRef]
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 US Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197. [Google Scholar] [CrossRef]
- Bourdier, P.; Simon, C.; Bessesen, D.H.; Blanc, S.; Bergouignan, A. The role of physical activity in the regulation of body weight: The overlooked contribution of light physical activity and sedentary behaviors. Obes. Rev. 2023, 24, e13528. [Google Scholar] [CrossRef]
- Biddle, S.J.H.; García Bengoechea, E.; Wiesner, G. Sedentary behaviour and adiposity in youth: A systematic review of reviews and analysis of causality. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 43. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Saavedra, J.M.; Ramírez-Vélez, R.; Ekelund, U.; Del Pozo-Cruz, B. Reallocating sedentary time to moderate-to-vigorous physical activity but not to light-intensity physical activity is effective to reduce adiposity among youths: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 1088–1095. [Google Scholar] [CrossRef]
- Kobes, A.; Kretschmer, T.; Timmerman, G.; Schreuder, P. Interventions aimed at preventing and reducing overweight/obesity among children and adolescents: A meta-synthesis. Obes. Rev. 2018, 19, 1065–1079. [Google Scholar] [CrossRef]
- Denova-Gutierrez, E.; Gonzalez-Rocha, A.; Mendez-Sanchez, L.; Araiza-Nava, B.; Balderas, N.; Lopez, G.; Tolentino-Mayo, L.; Jauregui, A.; Hernandez, L.; Unikel, C.; et al. Overview of systematic reviews of health interventions for the prevention and treatment of overweight and obesity in children. Nutrients 2023, 15, 773. [Google Scholar] [CrossRef] [PubMed]
- Podnar, H.; Jurić, P.; Karuc, J.; Saez, M.; Barceló, M.A.; Radman, I.; Starc, G.; Jurak, G.; Đurić, S.; Potočnik, Ž.L.; et al. Comparative effectiveness of school-based interventions targeting physical activity, physical fitness or sedentary behaviour on obesity prevention in 6- to 12-year-old children: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13160. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef]
- Wyszyńska, J.; Ring-Dimitriou, S.; Thivel, D.; Weghuber, D.; Hadjipanayis, A.; Grossman, Z.; Ross-Russell, R.; Dereń, K.; Mazur, A. Physical activity in the prevention of childhood obesity: The position of the European Childhood Obesity Group and the European Academy of Pediatrics. Front. Pediatr. 2020, 8, 535705. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Global trends in insufficient physical activity among adolescents: A pooled analysis of 298 population-based surveys with 1· 6 million participants. Lancet Child Adolesc. Health 2020, 4, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K. Biological, environmental, and social influences on childhood obesity. Pediatr. Res. 2016, 79, 205–211. [Google Scholar] [CrossRef]
- Alghalyini, B. Applications of artificial intelligence in the management of childhood obesity. J. Fam. Med. Prim. Care 2023, 12, 2558–2564. [Google Scholar] [CrossRef]
- Singh, B.; Gorbenko, A.; Palczewska, A.; Tawfik, H. Application of Machine Learning Techniques to Predict Teenage Obesity Using Earlier Childhood Measurements from Millennium Cohort Study. In Proceedings of the 2023 7th International Conference on Cloud and Big Data Computing, Chongqing, China, 2–5 June 2023; pp. 55–60. [Google Scholar]
- Dugan, T.M.; Mukhopadhyay, S.; Carroll, A.; Downs, S. Machine learning techniques for prediction of early childhood obesity. Appl. Clin. Inform. 2015, 6, 506–520. [Google Scholar]
- Bays, H.E.; Fitch, A.; Cuda, S.; Gonsahn-Bollie, S.; Rickey, E.; Hablutzel, J.; Coy, R.; Censani, M. Artificial intelligence and obesity management: An obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obes. Pillars 2023, 6, 100065. [Google Scholar] [CrossRef]
- Bond, A.; Mccay, K.; Lal, S. Artificial intelligence & clinical nutrition: What the future might have in store. Clin. Nutr. ESPEN 2023, 57, 542–549. [Google Scholar]
- Guillaudeux, M.; Rousseau, O.; Petot, J.; Bennis, Z.; Dein, C.A.; Goronflot, T.; Vince, N.; Limou, S.; Karakachoff, M.; Wargny, M.; et al. Patient-centric synthetic data generation, no reason to risk re-identification in biomedical data analysis. NPJ Digit. Med. 2023, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.M.; Barbar, A.; Refaie, M. Synthetic data generation: A privacy-preserving approach to accelerate rare disease research. Front. Digit. Health 2025, 7, 1563991. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Callender, T.; Cebere, B.; Janes, S.M.; Navani, N.; van der Schaar, M. Synthetic data for privacy-preserving clinical risk prediction. Sci. Rep. 2024, 14, 25676. [Google Scholar] [CrossRef]
- Hewitt, R.; Watson, P. Defining biobank. Biopreserv. Biobank. 2013, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kinkorová, J.; Topolčan, O. Biobanks in Horizon 2020: Sustainability and attractive perspectives. Epma J. 2018, 9, 345–353. [Google Scholar] [CrossRef]
- Laugesen, K.; Mengel-From, J.; Christensen, K.; Olsen, J.; Hougaard, D.M.; Boding, L.; Olsen, A.; Erikstrup, C.; Hetland, M.L.; Høgdall, E.; et al. A review of major danish biobanks: Advantages and possibilities of health research in Denmark. Clin. Epidemiol. 2023, 15, 213–239. [Google Scholar] [CrossRef]
- Schüttler, C.; Prokosch, H.U.; Hummel, M.; Lablans, M.; Kroll, B.; Engels, C.; development team, G.B.A.I. The journey to establishing an IT-infrastructure within the German Biobank Alliance. PLoS ONE 2021, 16, e0257632. [Google Scholar] [CrossRef]
- van’t Riet, E.; Schram, M.T.; Abbink, E.J.; Admiraal, W.M.; Dijk-Schaap, M.W.; Holleman, F.; Nijpels, G.; Özcan, B.; Pijl, H.; Schaper, N.C.; et al. The diabetes pearl: Diabetes biobanking in The Netherlands. BMC Public Health 2012, 12, 949. [Google Scholar] [CrossRef]
- Ameryoun, A.; Sanaeinasab, H.; Saffari, M.; Koenig, H.G. Impact of game-based health promotion programs on body mass index in overweight/obese children and adolescents: A systematic review and meta-analysis of randomized controlled trials. Child. Obes. 2018, 14, 67–80. [Google Scholar] [CrossRef]
- Dias, J.D.; Domingues, A.N.; Tibes, C.M.; Zem-Mascarenhas, S.H.; Fonseca, L.M.M. Serious games as an educational strategy to control childhood obesity: A systematic literature review. Rev. Lat.-Am. Enferm. 2018, 26, e3036. [Google Scholar] [CrossRef] [PubMed]
- Belghali, M.; Statsenko, Y.; Al-Za’abi, A. Improving serious games to tackle childhood obesity. Front. Psychol. 2021, 12, 657289. [Google Scholar] [CrossRef]
- Mack, I.; Bayer, C.; Schaeffeler, N.; Reiband, N.; Broelz, E.; Zurstiege, G.; Fernandez-Aranda, F.; Gawrilow, C.; Zipfel, S. Chances and limitations of video games in the fight against childhood obesity—A systematic review. Eur. Eat. Disord. Rev. 2017, 25, 237–267. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, R.; Lam, S.; Leung, S.; Wang, S.; Wan, R.; Tinker, A.; McAlpine, J.N.; Woo, M.M.; Huntsman, D.G.; Talhouk, A. From biobank and data silos into a data commons: Convergence to support translational medicine. J. Transl. Med. 2021, 19, 493. [Google Scholar] [CrossRef]
- Alkhatib, R.; Gaede, K.I. Data management in biobanking: Strategies, challenges, and future directions. BioTech 2024, 13, 34. [Google Scholar] [CrossRef]
- Murphy, M.; Garrett, S.B.; Boyd, E.; Dry, S.; Dohan, D. Engaging diverse stakeholders to inform biobank governance. Biopreserv. Biobank. 2017, 15, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Geissler, J.; Parry-Jones, A.; Keulen, H.; Schmitt, D.C.; Vavassori, R.; Matharoo-Ball, B. Biobanking from the patient perspective. Res. Involv. Engagem. 2015, 1, 4. [Google Scholar] [CrossRef]
- Staunton, C.; Tindana, P.; Hendricks, M.; Moodley, K. Rules of engagement: Perspectives on stakeholder engagement for genomic biobanking research in South Africa. BMC Med. Ethics 2018, 19, 13. [Google Scholar] [CrossRef]
- Dry, S.M.; Garrett, S.B.; Koenig, B.A.; Brown, A.F.; Burgess, M.M.; Hult, J.R.; Longstaff, H.; Wilcox, E.S.; Madrigal Contreras, S.K.; Martinez, A.; et al. Community recommendations on biobank governance: Results from a deliberative community engagement in California. PLoS ONE 2017, 12, e0172582. [Google Scholar] [CrossRef]
- Bansal, P.; Ouda, A. Study on integration of FastAPI and machine learning for continuous authentication of behavioral biometrics. In Proceedings of the 2022 International Symposium on Networks, Computers and Communications (ISNCC), Shenzhen, China, 19–22 July 2022; pp. 1–6. [Google Scholar]
- Bradshaw, S.; Brazil, E.; Chodorow, K. MongoDB: The Definitive Guide: Powerful and Scalable Data Storage; O’Reilly Media: Sebastopol, CA, USA, 2019. [Google Scholar]
- Nan, Y.; Del Ser, J.; Walsh, S.; Schönlieb, C.; Roberts, M.; Selby, I.; Howard, K.; Owen, J.; Neville, J.; Guiot, J.; et al. Data harmonisation for information fusion in digital healthcare: A state-of-the-art systematic review, meta-analysis and future research directions. Inf. Fusion 2022, 82, 99–122. [Google Scholar] [CrossRef]
- Hufstedler, H.; Roell, Y.; Peña, A.; Krishnan, A.; Green, I.; Barbosa-Silva, A.; Kremer, A.; Blacketer, C.; Fortier, I.; Howard, K.; et al. Navigating data standards in public health: A brief report from a data-standards meeting. J. Glob. Health 2024, 14, 03024. [Google Scholar] [CrossRef] [PubMed]
- Inau, E.T.; Sack, J.; Waltemath, D.; Zeleke, A.A. Initiatives, concepts, and implementation practices of the findable, accessible, interoperable, and reusable data principles in health data stewardship: Scoping review. J. Med. Internet Res. 2023, 25, e45013. [Google Scholar] [CrossRef]
- Rak, R. Anonymisation, Pseudonymisation and Secure Processing Environments Relating to the Secondary Use of Electronic Health Data in the European Health Data Space (EHDS). Eur. J. Risk Regul. 2024, 15, 928–938. [Google Scholar] [CrossRef]
- Sepas, A.; Bangash, A.H.; Alraoui, O.; El Emam, K.; El-Hussuna, A. Algorithms to anonymize structured medical and healthcare data: A systematic review. Front. Bioinform. 2022, 2, 984807. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, A.; Abuhaliqa, M.A.M.; Catal, C.; Mishra, D. RESTful API testing methodologies: Rationale, challenges, and solution directions. Appl. Sci. 2022, 12, 4369. [Google Scholar] [CrossRef]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative adversarial networks: An overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef]
- Figueira, A.; Vaz, B. Survey on synthetic data generation, evaluation methods and GANs. Mathematics 2022, 10, 2733. [Google Scholar] [CrossRef]
- Gamalielsson, J.; Lundell, B.; Butler, S.; Brax, C.; Persson, T.; Mattsson, A.; Gustavsson, T.; Feist, J.; Lönroth, E. Towards open government through open source software for web analytics: The case of Matomo. JeDEM-eJournal eDemocracy Open Gov. 2021, 13, 133–153. [Google Scholar] [CrossRef]
- Johannesson, P.; Perjons, E. Evaluate Artefact. In An Introduction to Design Science; Springer International Publishing: Cham, Switerland, 2021; pp. 141–152. [Google Scholar]



| Characteristics | Food Ninja | Food Quiz | Food Treasure | Let’s Move |
|---|---|---|---|---|
| Primary Objective | Food-group identification and categorization | Health and nutrition literacy | Combine physical activity with nutrition education | Establish regular physical activity habits |
| Target Users | 6–12 years | 8–16 years | 8–14 years | 6–14 years |
| Core Mechanics | Tapping/scrolling items in categories | Multiple-choice questions with aids | AR scanning of hidden items | Guided exercises and dance routines |
| Learning Focus | Food groups and balanced diet | Meal patterns, nutrition basics, dietary patterns | Nutritional information about specific foods | Exercise techniques and movement patterns |
| Social Elements | Individual play | Multiplayer option | Parent–child interaction | Family participation |
| Physical Activity | None | None | Moderate | High |
| Technology | Basic touchscreen | Basic device | Smartphone with AR capability | Basic device with video playback |
| Environment | Indoor screen-based | Indoor screen-based | Indoor/outdoor exploration | Indoor or outdoor space for movement |
| Feedback | Immediate feedback with educational messages | Explanations for incorrect answers | AR information displays | Visual guidance and achievement tracking |
| Professional input | Nutritional guidelines | Evidence-based questions | Nutritional information | Pediatric consultation for exercises |
| Evaluation Criterion | Our Federated Architecture | Traditional Centralized Approaches | Evidence of Improvement |
|---|---|---|---|
| Data Privacy and Security | Decentralized Biobank edges with local data storage and pseudonymization techniques | Centralized data repositories with conventional anonymization | Reduced risk of large-scale breaches; GDPR compliance through data sovereignty; advanced pseudonymization; and synthetic data generation |
| Data Integration | Harmonized data flows across distributed nodes with standardized frameworks (CDISC/OMOP) | Siloed data collection with limited cross-system compatibility | Enhanced data richness while maintaining privacy; enables comprehensive analysis across multiple sources |
| Scalability | Modular components that can be implemented independently; MongoDB for scalable storage | Often requires complete system implementation; limited by central server capacity | Institutions can adopt specific components based on resources; distributes computational load across nodes |
| User Engagement | Multi-channel approach through health app and serious games suite | Single-channel tools with limited engagement strategies | Better long-term adherence through gamification and personalization; targets multiple behavioral factors simultaneously |
| Clinical Decision Support | AI-powered risk assessment and recommendation engines with healthcare professional oversight | Manual interpretation of fragmented data sources | Evidence-based decision making with integrated dashboard; personalized intervention recommendations |
| Stakeholder Collaboration | Integrated dashboard and community knowledge hub | Limited interaction between providers, researchers, and families | Facilitates knowledge transfer and collaborative decision-making; creates feedback loops between stakeholders |
| Adaptability | Flexible implementation options for varying resource settings and cultural backgrounds | Often requires standardized implementation | Components can be adopted based on local constraints; culturally adaptable content and recommendations |
| Technical Implementation | RESTful APIs with standardized documentation; modular architecture | Proprietary interfaces, monolithic systems | Easier integration with existing healthcare systems; standards-based approach reduces implementation barriers |
| Ethical Considerations | Verifiable parental consent process; age-based access controls; opt-out mechanisms | Often limited privacy controls | Enhanced protection for minors’ data; clear governance structure for sensitive information |
| Requirement | Architectural Component | Enabling Features |
|---|---|---|
| Data Privacy Protection | Federated Biobank Edges |
|
| Comprehensive Data Integration | Data Harmonization Layer |
|
| Sustainable User Engagement | Multi-channel User Interface |
|
| Evidence-based Intervention | AI Tools and Knowledge Hub |
|
| Multi-stakeholder Collaboration | Community Network and Dashboard |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vondikakis, I.; Politi, E.; Goulis, D.; Dimitrakopoulos, G.; Georgoulis, M.; Saltaouras, G.; Kontogianni, M.; Brisimi, T.; Logothetis, M.; Kakoulidis, H.; et al. Integrated Framework for Managing Childhood Obesity Based on Biobanks, AI Tools and Methods, and Serious Games. Electronics 2025, 14, 2053. https://doi.org/10.3390/electronics14102053
Vondikakis I, Politi E, Goulis D, Dimitrakopoulos G, Georgoulis M, Saltaouras G, Kontogianni M, Brisimi T, Logothetis M, Kakoulidis H, et al. Integrated Framework for Managing Childhood Obesity Based on Biobanks, AI Tools and Methods, and Serious Games. Electronics. 2025; 14(10):2053. https://doi.org/10.3390/electronics14102053
Chicago/Turabian StyleVondikakis, Ioannis, Elena Politi, Dimitrios Goulis, George Dimitrakopoulos, Michael Georgoulis, George Saltaouras, Meropi Kontogianni, Theodora Brisimi, Marios Logothetis, Harry Kakoulidis, and et al. 2025. "Integrated Framework for Managing Childhood Obesity Based on Biobanks, AI Tools and Methods, and Serious Games" Electronics 14, no. 10: 2053. https://doi.org/10.3390/electronics14102053
APA StyleVondikakis, I., Politi, E., Goulis, D., Dimitrakopoulos, G., Georgoulis, M., Saltaouras, G., Kontogianni, M., Brisimi, T., Logothetis, M., Kakoulidis, H., Prasinos, M., Anastasiou, A., Kakkos, I., Vellidou, E., Matsopoulos, G., & Koutsouris, D. (2025). Integrated Framework for Managing Childhood Obesity Based on Biobanks, AI Tools and Methods, and Serious Games. Electronics, 14(10), 2053. https://doi.org/10.3390/electronics14102053












