Abstract
The enduring interest in carbon quantum dots (CQDs) as photoluminescent material arises from their significant advantages over inorganic quantum dots (QDs), such as low toxicity and biocompatibility, which enables their application in bioimaging and drug delivery. This review is focused on the use of CQDs for light emitting devices (LED) technology and provides a guide on how to synthesize CQDs that emit blue, green, and red light, which is necessary to produce RGB LEDs. Consideration was given to the precursors, solvents, methods, and conditions of the processes, the excitation wavelength, the emission wavelength, and the photoluminescence quantum yield (QY). These unique, organic nanoparticles have the potential to revolutionize lighting and, above all, the electronics market due to their low cost and eco-friendliness, as well as the possibility of using various precursors, including waste.
1. Introduction
The term “LED” is an acronym originating from the English language, representing either “light-emitting diode” or “light-emitting device”. This ambiguity can often lead to confusion, as the same acronym is used to describe two distinct concepts. A typical light-emitting diode (LED) is constructed from semiconductor materials that, when electrically stimulated, emit light as electrons and holes recombine across a p-n junction. Different colors in such LEDs are achieved by varying the semiconductor materials, each having a unique bandgap, which determines the emitted light’s wavelength. For example, materials with wider bandgaps emit higher-energy (blue) light, while those with narrower bandgaps emit lower-energy (red) light.
Since each material requires a different threshold voltage due to its specific bandgap, LEDs of varying colors also require different driving voltages. This presents challenges in designing controllers, especially for LED panels that must simultaneously manage multiple voltage levels. The concept of LED as a light-emitting device also encompasses the use of phosphors, which can further modify the optical properties by converting light to different wavelengths. Our analysis indicates that recent advancements in materials now allow the development of LED devices that rely on a UV-emitting semiconductor diode to excite a phosphor layer, producing the desired visible light. This approach enables more flexible design possibilities for LEDs with tailored optical properties. A new star is currently rising in the phosphorescent materials market: carbon quantum dots (CQDs). These materials, when combined with UV LEDs, have the potential to form a new class of LED devices, addressing existing challenges in control circuitry while also offering expanded color palette possibilities. Carbon quantum dots could revolutionize LED technology by simplifying design requirements and enabling tunable, vibrant color options that were previously challenging to achieve.
1.1. CQDs—A New Type of Phosphorescent Material
Due to their large surface-to-volume ratio and quantum size effects, nanomaterials have unique properties, which are often different than those of the respective bulk material. Quantum dots (QDs) are excellent examples, as their optical properties depend on their size, which has affected technology since the late 20th century. However, most QDs are made of toxic elements, such as Cd, Pb, or As [1,2], which contribute to the deterioration of the environment. Despite these excellent properties, which make them an interesting material for LED (light-emitting-diode) production, their use is limited by toxicity and price [3]. The discovery of carbon quantum dots (CQDs) in 2004 [4] caused an increasing focus on this kind of nanomaterials. During the next 20 years, the number of publications focused on this subject has strongly increased (Figure 1), reaching 3000 documents in 2023 according to the Scopus database.
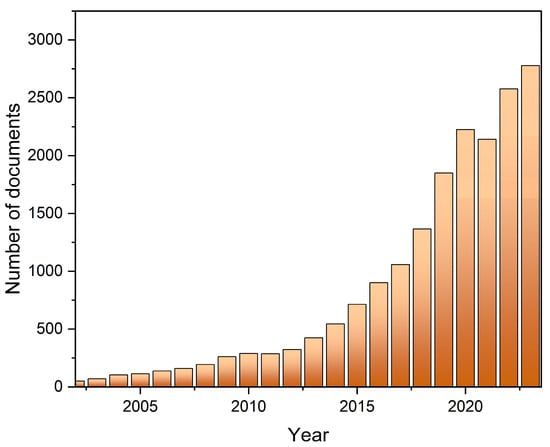
Figure 1.
Number of documents displayed in Scopus for a search query that included “carbon quantum dots” in title, abstract, or keywords, up to the end of 2023.
Carbon quantum dots (CQDs) are nanoscale carbon-based materials with sizes smaller than 10 nm [5]. The basis of their structure consists of elements such as carbon, hydrogen, and oxygen; however, they are very often doped with other elements, mostly by nitrogen. Their structure can be divided into two parts: inner-core and outer-surface functional groups, such as hydroxyl groups (–OH), carbonyl groups (–CO), carboxyl groups (–COOH), and amino groups (–NH2), which significantly affect the properties of CQDs.
CQDs have gained significant attention for their potential applications in various fields, including biosensing [6,7,8] and optoelectronics [9,10,11]. One interesting application is the use of carbon quantum dots as phosphors, which are materials that can absorb light at one wavelength and re-emit it at another, allowing the generation of different colors in LEDs [12,13]. The aim of this publication is to present CQDs as potential phosphors in LEDs. This is an overview of how synthesis conditions, methods, and precursors affect the optical properties of CQDs. In the following parts of this work, we also present a critical analysis of the collected results and highlight the gaps in the currently known literature to focus the attention of the research community on still unresolved challenges.
Some review papers about CQDs have already been published, but they are mainly focused on the structure, the light emission mechanism, and the influence of various factors on color and luminous effectivity. Although they contain lots of information, there are relatively few examples of how to synthesize CQDs that emit a particular wavelength in these papers. Even though this review considers some basic information like the other review papers, it is mainly focused on giving a recipe for the synthesis of CQDs that emit RGB colors. The greatest attention has been paid to the substrates used in the synthesis, their type, eco-friendliness, and the use of doping, as well as the type and conditions of the CQD manufacturing process. The properties of as-synthesized CQDs are also indicated in this paper: the excitation wavelength and the emitted wavelength for the highest quantum yield, as well as the quantum yield.
First, LEDs typically produce light through photoluminescence, where electrons recombine with electron holes, releasing energy in the form of photons. Photoluminescence is the most crucial feature of CQDs, as it allows their use in various technology fields, such as LEDs or sensors. The photoluminescence effect can be divided into three sub-effects: fluorescence, phosphorescence, and delayed fluorescence [3]. Fluorescence emission is created if an electron from the singlet ground state absorbs energy from UV or visible light and, through this, is excited to a singlet excited state [14]. Part of the absorbed energy can be lost through vibrational relaxation; however, the electron almost immediately emits its energy in the form of light and returns to the ground state. The phosphorescence process differs from the previous one due to the intersystem crossing transition of electrons from a singlet excited state to a triplet state. Because of the change in electron spin, the electron is not able to go back to the ground state as quickly as it was in the fluorescence effect, leading to slower decay and longer light emission. Another photoluminescence effect is called “delayed fluorescence”. It is based on a phosphorescence effect but requires a transition from the triplet state to the singlet excited state.
CQDs often exhibit size-dependent optical properties, and their emission wavelength can be tuned by controlling the size of the dots. They have a wide absorption spectrum and can emit light in various colors [15,16], making them suitable for different applications. CQDs are generally considered to be environmentally friendly and can be synthesized from readily available carbon sources. The advantages of CQDs as phosphors are as follows:
Tunability: The emission color of CQDs can be easily tuned by adjusting their size or surface functionalization, allowing for the generation of different colors in LEDs [16,17].
Cost-Effective: The synthesis of CQDs can be relatively simple and cost-effective compared with traditional phosphor materials [18].
Environmental Friendliness: Carbon-based materials are generally more environmentally friendly compared with some other phosphor materials, such as heavy-metal-based phosphors [19].
Technology based on QDs has already proven its value in the display market. QDs have many strengths, such as high quantum yield, the ability to be tuned to emit specific colors, and high environmental stability. Despite these advantages, they also have some drawbacks. Due to the use of rare earth elements, this technology is costly. Furthermore, it relies on hazardous elements, which negatively impact the environment.
CQD technology is not yet as well developed, but it has some advantages over QDs. It is worth noting that carbon is a widely available and inexpensive material, making CQD synthesis more cost-effective. Moreover, CQDs have a lower environmental impact, aligning them with future-oriented sustainable development. CQDs can also be widely used in biomedical fields because of their low toxicity and biocompatibility. They can be easily modified with different functional groups, allowing for a wide range of applications.
Despite these many advantages, there are still challenges to be addressed. The quantum yield of CQDs is not as high as that of QDs, and achieving precise wavelength emission is more difficult.
One of the most useful properties of CQDs is tunability. The maximum emitted wavelength is determined by many factors: not only the size of CQDs and surface functionalization but also the solvent, element doping, surface state, and hydrogen bonding [3].
CQDs can be synthesized by several methods. The most frequently used is the bottom-up approach, such as hydrothermal and solvothermal methods. These bottom-up approaches are based on the dissolution of the organic compound in a solvent and heating to obtain dehydration and carbonization of the precursor [20]. The advantages of these methods are low cost, ease of synthesis, and control over the degree of carbonization [3]. Using these methods, CQDs can be produced from a variety of precursors, which is key to producing carbon quantum dots that emit light in different colors. In some of the experiments, synthesis processes were performed with the use of microwaves or ultrasonication. CQDs can also be synthesized using top-down methods, such as laser ablation, chemical oxidation, electrochemical oxidation, and arc discharge [21,22]. In the top-down synthesis, large carbon compounds are fragmented into pieces to form small carbon nanoparticles. However, these types of methods are ineffective in large-scale preparation, and the photoluminescence of CQDs prepared in such a way is relatively low [3].
1.2. Synthesis of CQDs
The production process of CQDs varies depending on the utilized method. The hydrothermal and solvothermal syntheses are based on placing aqueous or non-aqueous solutions, respectively, in the autoclave and subjecting them to high temperature and high pressure for some time. This process breaks down organic matter into smaller fragments and forms carbon structures in the form of CQDs. After the process, larger and smaller fragments of molecules remain in the solution, so it needs to be purified. The microwave-assisted synthesis is a very interesting alternative for the preparation of the CQDs. This process involves using microwaves to supply heat to the carbon compounds in the solution. It causes the decomposition of organic matter and the formation of small structures. This method requires far less time and energy than the solvothermal method, so it is more eco-friendly. The electrochemical method of synthesis of the CQDs is also worth noticing. It is based on using carbon-rich substances, e.g., graphite, as one of the electrodes and passing the current through the electrolytical circuit. The small carbon particles go into the solution in the form of CQDs. Schematic illustrations of CQD syntheses by the hydrothermal, solvothermal, microwave-assisted, and electrolytical methods are shown in Figure 2.
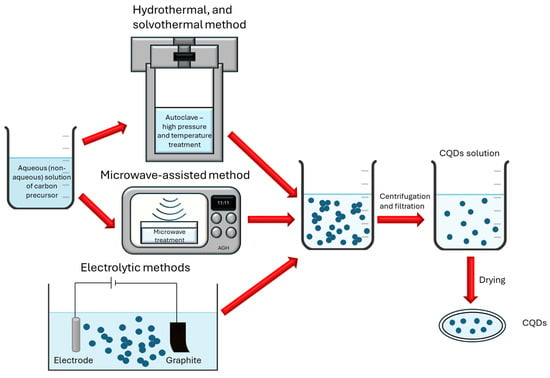
Figure 2.
Schematic of the CQD synthesis via hydrothermal, solvothermal, microwave-assisted, and electrolytic methods.
Despite the fact that LED-based technology is commonly used in display markets, there are some difficulties with producing high-quality pictures, as well as meeting the requirements of miniaturization. The direct-LED displays are made up of pixels, each of which consists of three (RGB) separately powered LEDs. LEDs of each color need to be built of different semiconductor structures, which causes difficulties in the miniaturization process. Semiconductor LEDs are frequently only used as backlighting in LCD displays. There is also a new technology being developed, micro-LEDs, which is based on the miniaturization of direct-LED displays, but it is difficult to manufacture and expensive. Another type of LED is OLED technology. OLED’s structure consists of an organic layer that emits light. OLED displays are thinner and better in color reproduction, but they have some defects, e.g., burn-in and susceptibility to environmental conditions.
Nowadays, utilizing quantum dots in LED technology is a common approach, as they have lots of advantages, for example, easy miniaturization and good color reproduction.
The long-term operational stability of CQD-based LEDs is indeed less explored compared with other nanomaterials, but existing research indicates that CQDs possess favorable stability characteristics, supporting their use in LEDs. CQDs exhibit high chemical and thermal stability, which distinguishes them from nanoparticles like AuNPs or AgNPs, which are more prone to aggregation and physicochemical structural changes over time. For example, long-term CQD performance in optoelectronic devices has demonstrated stability, maintaining approximately 95% of initial efficiency after 10 h of high-intensity light exposure [23].
Additionally, studies on CQDs suggest that any aggregation observed is often due to weak interactions and can be partially reversible through methods like ultrasonication [24]. Properly controlled surface modification of CQDs, especially through stabilizing agents, can further limit aggregation, thus supporting long-term stability [25].
Furthermore, research on CQDs indicates that their structure and photoluminescent properties are generally less susceptible to structural alterations compared with noble metal nanoparticles like AuNPs, which frequently exhibit instability due to Ostwald ripening and surface atom migration [26]. To enhance the long-term stability of CQD-LEDs, it is advisable to consider factors such as temperature and oxygen exposure, as these could accelerate CQD degradation. Managing these factors is essential for achieving stable performance in CQD-LED devices.
There are three main groups of substrates to prepare CQDs: small organic particles, aromatic chemicals, and biomaterials [3]. The most popular source of carbon dots included in the group of small organic chemicals is citric acid, which is commonly used with N-dopant [27,28]. Frequently used chemical compounds in the group of aromatic organic compounds are isomers of phenylenediamine [29,30]. CQDs can also be synthesized using green sources of carbon, such as garlic [31] or palm kernel shells [32], as well as biomass [19]. The possibility of preparing CQDs from inexpensive and widely available precursors is an important aspect for future industrial applications, as the use of inorganic QDs was strongly determined by their cost [33]. Figure 3 shows a division of precursors for CQD synthesis with examples of materials utilized in the syntheses.
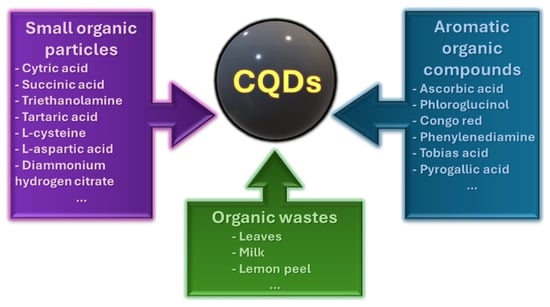
Figure 3.
Types of precursors used in CQD synthesis: small organic particles, aromatic organic compounds, and organic wastes, and their examples.
An important advantage of CQDs is that they can be synthesized according to the principles of green chemistry. According to the United States Environmental Protection Agency, “Green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances. Green chemistry applies across the life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal” [34]. Gray chemistry is an approach to chemical processes that do not fulfill the principles of green chemistry. In this paper, green synthesis is considered to be one in which natural substances and harmless solvents are used. These principles imply a significant reduction in the use and production of hazardous substances. In accordance with these principles, attention is paid to the type of chemical compounds used and their impact on the environment and human health The principles of green chemistry are a key concept in modern laboratory and industrial methods. The main idea of these principles is to strive to apply methods and processes that generate the least amount of waste, use less energy, and eliminate, reduce, or replace toxic and environmentally hazardous substances. In addition, green chemistry also promotes the efficient use of raw materials, recycling, and the production of greener chemicals, as well as seeks to develop methods and technologies that are more environmentally friendly while remaining efficient and competitive. This includes developing biodegradable materials, sustainable manufacturing processes, and minimizing chemical waste. The possibility of using precursors coming from natural sources has a significant advantage in the development of eco-friendly CQD technologies.
This work contains a literature review on the influence of synthesis conditions and the type of precursor used. While CQDs have shown great potential, improving their quantum efficiency and stability is an ongoing challenge. The collected literature was divided into three groups: CQDs emitting blue, green, and red light. Developing methods to integrate CQDs into LED devices while maintaining their optical properties is essential. Ongoing research focuses on optimizing the synthesis methods of CQDs, improving their quantum yields, and enhancing their performance as phosphors in LEDs. Surface modification techniques are explored to enhance the stability and compatibility of CQDs with LED devices.
2. Dependence of Light Emission—Factors
Among many factors that affect the emission of light by CQDs, synthesis conditions and precursor type, as well as solvents, are of crucial importance. Another significant factor is the doping of CQDs, using, e.g., nitrogen [35], sulfur [35,36], or phosphorus [36]. The vast majority of synthesized CQDs are nitrogen-doped carbon dots (N-CQDs). The addition of the source of nitrogen causes a significant increase in the quantum yield of CQDs [37].
The photoluminescence mechanism of CQDs has not been accurately explained yet [38]. Some researchers claim that the red-shift of the emission of CQDs can be explained by the quantum size effect (quantum confinement effect—QCE). It is based on the fact that decreasing the size of CQD particles causes an increase in band gap energy, so smaller CQDs require higher excitation energy, and the emitted light has a shorter wavelength [33]. However, a change in the color of the CQDs emission is observed also when the excitation wavelength is changed. This may be related to the polydistribution of size of CQDs or the participation of functional groups in photoemission.
The quantum yield and emitted wavelength of CQDs can be improved by changing the pH of their solution. The structure of CQDs contains surface functional groups, for example, -OH, -COOH, and -NH2, so in a not-neutral environment, they will be affected by H+ or OH- ions [3]. That is why an alkaline or acidic environment is able to cause shifting of the wavelength, as well as impact the intensity of CQD emission. The effect of the properties of particular CQDs is dependent on the chemicals that were used in their synthesis. In some research, an increase in the alkalinity of the CQD solution up to a certain level caused an increase in QY [37,39].
The influence of excitation wavelength dependence on the emission color of the CQDs should be mentioned. Pan et al. prepared full-color CQDs from citric acid and formamide by microwave-assisted heating [40]. The as-prepared CQDs exhibited emission of light in almost full visible spectrum if they were excited in the range of 330–600 nm. Lai et al. synthesized CQDs using L-tryptophan in NaOH aqueous solution using an electrochemical approach [41]. They obtained emission in a wide range by changing the excitation wavelength in the range of 320–560 nm, as well as the concentration of the facile solution. Yao et al. used luminol with the addition of L-tryptophan to prepare full-color CQDs by the same method and confirmed the previous results [42].
One factor that could facilitate the preparation of CQD-based LEDs is the use of one precursor to synthesize CQDs with different colors of emitted light. Generally, it seems difficult to shift the emitted wavelength without changing the precursor in the synthesis of CQDs, but there are groups that achieved that. Such prepared carbon dots are called multicolor. Yuan et al. synthesized B-CQDs and G-CQDs by solvothermal reaction of citric acid and 2,3-diaminonaphthalene in ethanol [12]. The shift in wavelength from blue to green was obtained by changing the time of the solvothermal process. Longer wavelengths were obtained by changing the isomer of diaminonaphthalene and also the time of the process. Red-emissive CQDs were synthesized using concentrated sulfuric acid as a solvent instead of ethanol. Solvent change is a common strategy for preparing multicolor CQDs. Liu et al. used Tobias acid and o-phenylenediamine in formamide, ethanol, or sulfuric acid for blue, yellow, and red emission, respectively [43]. Shen et al. applied citric acid and urea with water, dimethylacetamide, or dimethylformamide for the synthesis of B-, G-, and R-CQDs, respectively [44]. Changing the isomer of the precursor is also a common way of dealing with shifting the wavelength. Zhao et al. performed synthesis using 3,5-diaminobenzoic acid for the synthesis of B- and G-CQDs, but for R-CQD synthesis, they used 3,4-diaminobenzoic acid [45]. Additionally, obtaining G-CQDs required the use of phosphoric acid.
After synthesis, the CQDs usually require further purification as well as isolation from the rest of the solution. Early-stage purification can be performed by several different methods, such as dialysis, filtration, centrifugation, or column chromatography [20]. Many research works contain several purification approaches. Filtration and centrifugation of the solution should be utilized as they separate the nanoparticles from the larger fractions of the solution. Very often, this step is followed by dialysis of the solution, which separates small or unreacted fractions of nanoparticles. The dialysis process requires the choice of a membrane with appropriate pore size to remove only particles smaller than CQDs. This process can also be used to separate CQDs with different sizes. The column chromatography method is also utilized as a separation method by particle size or polarity. After these processes, it is also important to remove excess solvent by vacuum filtration or rotary evaporation.
The use of CQDs in many different fields requires controlling the wavelengths they emit. Particularly in LED technology, it is important to be able to obtain LEDs that shine blue, green, and red. This is essential for the production of screens, displays, and other devices that use the entire range of visible light. Information was collected on methods of producing CQDs to enable researchers to synthesize these nanoparticles, which have suitable emissive wavelengths. A division of CQDs into those emitting blue (410–495 nm), green (500–543 nm), and red light (595–715 nm) was made. In the tables, there is an overview of the methods for obtaining CQDs that emit a selected type of light. The precursor type, solvent, and doping were taken into consideration. The method for synthesizing carbon quantum dots is given, as well as the most important process parameters, mostly temperature and time. The excitation wavelength (λex) of the CQD at which the maximum quantum yield was obtained is given, as well as their emission wavelength (λem). Syntheses were divided into two groups, green and gray, due to the type of chemicals used in these processes.
Table 1 shows green and grey synthesis protocols for blue emissive CQDs. Additionally, the table contains information such as dopant, precursor, solvent, synthesis method, excitation wavelength used, observed photoemission maximum, and quantum yield.

Table 1.
Blue-emissive CQD synthesis: doped element, precursors, solvent, and method with parameters of the synthesis and optical properties: excitation wavelength (λex), emission wavelength (λem), and photoluminescence QY.
As can be observed, blue-light-emitting quantum dots can be obtained from many different precursors and solvents. The most commonly used method is the solvothermal method. However, all these works are based on bottom-up synthesis. Quantum efficiencies vary over a wide range, from 4.8 to 85%. Such high quantum efficiencies confirm the great potential of industrial application of such phosphors. Combined with their lack of toxicity, CQDs seem to be an almost perfect candidate for phosphors in blue LEDs.
The next table (Table 2) presents an identical summary as Table 1, taking into account the photoemission range corresponding to green light.

Table 2.
Green-emissive CQDs: doped element, precursors, solvent, and method with parameters of the synthesis and optical properties: excitation wavelength (λex), emission wavelength (λem), and photoluminescent quantum yield (QY).
It is worth highlighting some interesting facts. The same methods are used, as well as the same precursors, e.g., citric acid and urea, but the glow color is different. The main difference lies in the choice of solvent. The blue ones were excited at 365 nm and emitted 476 nm, while the green ones were at 365 nm and emitted 543 nm [44]. This clearly highlights the importance of the solvent used during the synthesis on their optical properties.
The next table (Table 3) presents an identical summary as Table 1 and Table 2, taking into account the photoemission range corresponding to red light.

Table 3.
Red-emissive CQDs: doped element, precursors, solvent, and method with parameters of the synthesis and optical properties: excitation wavelength (λex), emission wavelength (λem), and photoluminescent QY.
In the case of red-emission CQDs, much longer excitation wavelengths were used more often than in the case of blue-glowing CQDs. Only in a few cases was it emphasized that the length of the excitation wave did not influence the length of the emitted wave. Here, we clearly see gaps in understanding the mechanism of CQD illumination. Further research on theory and practice is required.
Table 1, Table 2 and Table 3 show that the most commonly utilized carbon source is citric acid with N-dopant, which can be, for example, urea, diaminonaphthalene, ethylenediamine. The addition of nitrogen to the carbon quantum dots structure usually significantly improves their photoluminescence quantum yield. This explains why dopants are so widely used in the synthesis of CQDs.
The synthesis of CQDs that emit different colors can be obtained by using different solvents. It is therefore very likely that changing the solvent results in modification of the CQDs structure. This phenomenon can be observed for citric acid and urea, which are the most commonly used precursors for CQD synthesis. B-CQDs are mostly synthesized in water; however, longer wavelengths require different solvents, such as DMF or formic acid. Changing the solvent in the synthesis can significantly affect the photoluminescence quantum yield. CQDs synthesized from citric acid and urea with formamide have almost four times greater quantum yield than carbon dots prepared with the use of DMF. Both types of nanoparticles were prepared using the solvothermal method. It is also important to focus on the time and temperature of the processes. The change in the time of the process can affect the quantum yield. It can be seen by comparing the syntheses with citric acid and urea as precursors and DMF as a solvent. Both syntheses were conducted using the solvothermal method.
The most popular CQD synthesis methods are the hydrothermal and solvothermal methods. Frequently used process temperatures are in the range of 160–220 °C, and the most commonly used are 180 and 200 °C. The processes typically last from 4 to 12 h, but most often, these processes take 8 or 12 h. The time and temperature of hydrothermal and solvothermal processes are important parameters, because they affect the carbonization process of the CQDs.
It is worth considering the impact of particle size on the color of the emitted light. The fact that semiconductor QDs show a close relationship between their size and the emission wavelength is known as the quantum size effect. In contrast to semiconductor quantum dots, CQDs do not always manifest this effect, or this correlation is not so prevalent. Zhan et al., Shen et al., and Yuan et al. demonstrated that the blue-, green-, and red-emitted CQDs that they prepared vary in their average size, demonstrating accordance with the quantum size effect [12,44,91]. In contrast, Kim et al. and Su et al. showed that CQDs do not exhibit a correlation between emitted radiation and their size [81,92]. This is due to the fact that not only the size of carbon dots matter for emitted light but also surface functional groups. Functional groups are attached to the carbon core and can absorb UV or visible radiation and cause the emission of light of a specific wavelength.
The information contained in the tables allows one to draw the conclusion that the emitted wavelength is strongly connected with excitation energy. Blue-, green-, and even red-emissive CQDs can be excited by UV light, but it is more likely to obtain CQDs with longer emitted wavelength by exciting them with a light of slightly shorter wavelength. This is because by exciting an electron with high energy, much of it has to be lost to thermal vibrations to emit light of a much longer wavelength than the excitation light. This situation is not very probable. The highest possibility of the electron transition from the excited state to the ground state is for the energy equal to the excited energy minus the Stokes shift. Stokes shift is the difference between the maximum absorption wavelength and the maximum emission wavelength. The electron is most often excited from the ground state to the first singlet excited state and excited vibrational energy level. Some energy is lost by vibrational relaxation and when the electron is in the lowest vibrational state. Radiation emission is therefore reduced by the value of the difference between these levels. Moreover, the transition of the electron to the ground state most often does not lead to reaching the vibrational ground state. This causes another difference between excitation energy and emitted radiation. The Stokes shift can vary from a few nanometers to several hundreds of nanometers, and it depends on the type of material. CQDs have relatively small Stokes shifts, up to tens of nanometers [93]. This causes the fact that the emission of light of slightly higher wavelength than excitation light is the most probable. Figure 4 shows the close correlation between the emitted and excitation light. It is important to note that CQD-based LED devices should emit light of different wavelengths when excited with the same wavelength, namely UV light. Such a solution greatly simplifies their construction. Researchers who focus on developing CQD-based LEDs should be mainly concerned with obtaining green and red light when excited with UV light. This would make it possible to put them into mass production and revolutionize the light-emitting diode market.
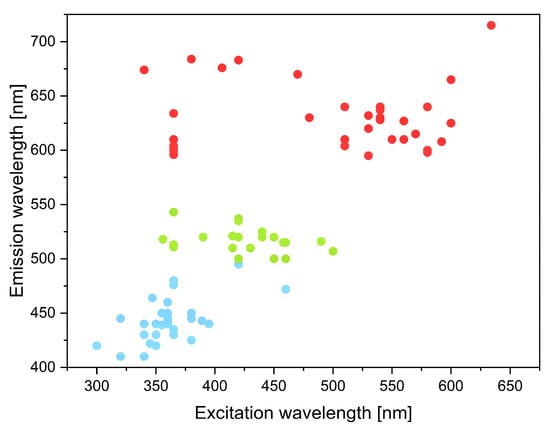
Figure 4.
The dependence of the emitted wavelength of CQDs on the excitation wavelength for the blue-, green-, and red-emissive CQDs.
The relationship between the type of solvent used in the synthesis of CQDs and the emitted wavelength of CQDs must be noticed. Figure 5 shows only those processes that used only inorganic solvents (blue) and only organic solvents (green). It can be noted that inorganic solvents are used more frequently in blue-emissive CQD synthesis than in the synthesis of these nanoparticles with longer wavelengths. This difference occurs because blue-emissive CQDs are mostly synthesized from water, sometimes with the addition of inorganic chemicals, such as phosphoric acid or ammonia. Green-emissive CQDs can also be synthesized using water, but more often, an organic solvent is used, and the most common is ethanol. Red-emissive CQDs are mostly produced with the use of organic solvents, especially ethanol, but also, e.g., DMF or formamide. R-CQDs can also be synthesized using water with some acids, such as HCl or H2SO4.
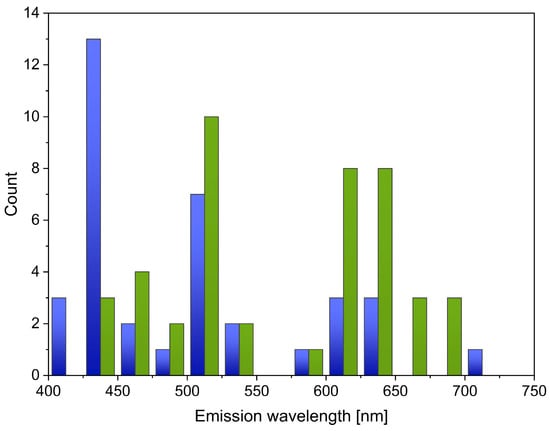
Figure 5.
The number of publications in which only inorganic (blue) and organic (green) solvents were used for the synthesis of CQDs in relation to the wavelengths they emit.
It is important to highlight that some of the CQD syntheses comply with the principles of green chemistry. Here, the main attention is paid to the wide variety of aspects of the synthesis, including both the origin of the raw materials and the energy demand, as well as the time consumed. For example, the possibility of the use of precursors of natural origin, such as garlic, has a significant advantage in the development of eco-friendly CQD technologies. Importantly, it was also demonstrated in many investigations that it is possible to synthesize CQDs using natural waste like lemon peel waste or olive solid wastes. This, in turn, fits into the rational waste management strategy. Next, concerning the green chemistry approach, many performed studies involved the use of inorganic solvents such as water, double distilled water, or deionized water. This approach allows one to avoid using organic solvents, which may be toxic. Moreover, it was also demonstrated in many experiments that the application of such methods as ultrasonication or short-term microwave heating (3 min) resulted in the fabrication of CQDs. These procedures require less energy and are less time-consuming compared with such methods as solvothermal or hydrothermal. Nonetheless, most of the above-mentioned approaches result in the fabrication of CQDs with relatively low QY. Thus, it is worth emphasizing that it is worth developing these strategies so as to achieve higher QY while simultaneously paying attention to conducting syntheses according to the principles of green chemistry. The solvothermal and hydrothermal methods have many advantages because these process conditions are well controllable, so CQDs can be synthesized from various precursors and can achieve high QY. Unfortunately, they require a lot of energy, as the processes last at least a few hours and are conducted in high temperatures, reaching up to nearly 300 °C. This energy consumption may be the reason for reducing the production of CQDs through these methods in the future, as it is possible to synthesize these nanoparticles with less energy input. The problem is also that some of these processes require the use of solvents that are not neutral for the environment. Therefore, when choosing the method of CQD synthesis, it is worth focusing on the processes that use water or other non-toxic solvents, such as ethanol. The time of the process and its temperature are also worth considering, as most of the syntheses are conducted at high temperatures and pressure. Figure 6 shows the effect of the temperature (a) and time (b) of solvothermal and hydrothermal processes on the photoluminescence quantum yield. It is clearly seen that there is no simple correlation between the process parameters, such as temperature and time, and increasing the quantum yield. One can come to the conclusion that there is no point in performing processes at temperatures above a certain value, e.g., 180 °C, or for more than a few hours.
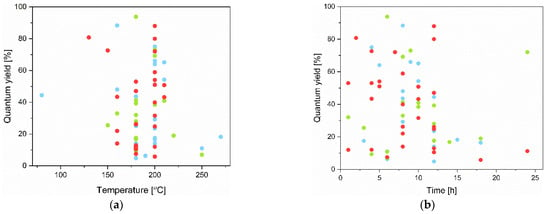
Figure 6.
Temperature (a) and time (b) dependence of solvothermal and hydrothermal syntheses on the quantum yield of CQDs. Colors on the plots are an indication of the light that CQDs emit: blue—emission of blue light, green—green light, and red—red light.
3. Discussion and Conclusions
The study of carbon quantum dots (CQDs) as phosphors in LED technology underscores their remarkable versatility and potential for innovation in lighting applications. The ability to tune the emission wavelength of CQDs through various synthesis parameters, such as dopant type, particle size, and solvent choice, is particularly compelling. This tunability enables the design of LEDs capable of emitting a wide spectrum of colors, from deep blues to vibrant reds, which is critical for applications ranging from high-definition displays to advanced lighting systems.
One of the key findings from the research is the significant impact of solvent type on the emitted wavelength of CQDs, an insight that opens a pathway for customizing the color output of CQD-based LEDs. Notably, studies such as those published in [94,95] have provided additional perspectives on the complex relationship between solvent chemistry and CQD photoluminescence. The observation that inorganic solvents are predominantly used for synthesizing blue-emissive CQDs, while organic solvents are more common for red-emissive CQDs, suggests that solvent choice profoundly influences the resulting CQD properties, a finding of critical value for the design of targeted LED applications.
Incorporating green chemistry principles in the synthesis of CQDs is another critical aspect of this research. By utilizing renewable resources, such as natural waste materials, for CQD synthesis, the environmental impact can be mitigated, aligning with global sustainability goals. Additionally, employing energy-efficient synthesis techniques, such as ultrasonication or microwave-assisted heating, significantly reduces the energy footprint of CQD production, an essential consideration in the scale-up of sustainable nanomaterial manufacturing. However, achieving eco-friendly synthesis methods must be balanced with the need for high-performance CQDs, particularly regarding quantum yield (QY). The challenge lies in optimizing synthesis conditions to achieve CQDs with high QY while adhering to sustainable practices. This balance is crucial for the widespread adoption and commercial viability of CQD-based LEDs. The challenge of quantum yield optimization, given the constraints of green chemistry, underscores the need for innovative research to advance eco-friendly methods that do not compromise efficiency.
Future research should focus on addressing the long-term stability of CQDs, a key factor for their real-world application in LED systems. Although stability studies are inherently time-intensive, previous studies on other carbon-based materials, such as carbon nanotubes and graphene oxides, suggest that CQDs could potentially exhibit high stability over extended periods. Longitudinal studies on CQD stability will provide valuable insights that could solidify their role in lighting technologies.
It is worth noting that work is already underway to create the first prototype LED devices based on CQDs as luminophores. For this purpose, CBRTP is using ALD technology to produce thin-film UV-LEDs. The optimization of luminophore application methods is ongoing to enhance light emission intensity. In these prototype diodes, a UV filter is applied as the final layer to eliminate residual UV radiation from the light source, demonstrating a practical approach to integrating CQDs into LED systems. While this integration has proven more complex than initially anticipated, a significant advantage is the potential to produce LED-CQD matrices that are easier and cheaper to mass produce and feature a wider color palette.
In conclusion, carbon quantum dots offer significant promise for revolutionizing LED technology with their tunable and potentially eco-friendly properties. Realizing their full potential, however, requires a concerted effort to overcome current challenges related to quantum yield, emission stability, and synthesis sustainability. Continued exploration and innovation in this field are essential for harnessing the full spectrum of possibilities that CQDs present for future lighting technologies.
Author Contributions
Conceptualization, M.W. and R.P.S.; methodology, K.B.; validation, R.P.S. and M.W.; formal analysis, K.B.; investigation, K.B.; resources, R.P.S.; data curation, K.B.; writing—original draft preparation, K.B.; writing—review and editing, M.W.; visualization, K.B.; supervision, M.W. and R.P.S.; project administration, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
K.B. and M.W. thank project no. DWD/7/0294/2023, financed by the Polish Ministry of Education and Science as part of the project “implementation doctorate 2023 edition”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shabbir, H.; Wojnicki, M. Recent Progress of Non-Cadmium and Organic Quantum Dots for Optoelectronic Applications with a Focus on Photodetector Devices. Electronics 2023, 12, 1327. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, X. The Emerging Development of Multicolor Carbon Dots. Small 2022, 18, 2205099. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Shabbir, H.; Csapó, E.; Wojnicki, M. Carbon Quantum Dots: The Role of Surface Functional Groups and Proposed Mechanisms for Metal Ion Sensing. Inorganics 2023, 11, 262. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, B.; Li, Z.; Wang, Y.; Zhou, J.; Li, Y. Carbon Quantum Dots as Fluorescence Sensors for Label-Free Detection of Folic Acid in Biological Samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117931. [Google Scholar] [CrossRef]
- Chellasamy, G.; Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Green Synthesized Carbon Quantum Dots from Maple Tree Leaves for Biosensing of Cesium and Electrocatalytic Oxidation of Glycerol. Chemosphere 2022, 287, 131915. [Google Scholar] [CrossRef]
- Yuan, T.; Yuan, F.; Sui, L.; Zhang, Y.; Li, Y.; Li, X.; Tan, Z.; Fan, L. Carbon Quantum Dots with Near-Unity Quantum Yield Bandgap Emission for Electroluminescent Light-Emitting Diodes. Angew. Chem. Int. Ed. 2023, 62, e202218568. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Meng, T.; Yuan, T.; Ni, R.; Li, Y.; Li, X.; Zhang, Y.; Tan, Z.; Lei, S.; et al. Red Phosphorescent Carbon Quantum Dot Organic Framework-Based Electroluminescent Light-Emitting Diodes Exceeding 5% External Quantum Efficiency. J. Am. Chem. Soc. 2021, 143, 18941–18951. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C.; Song, W.; Wei, L.; Wu, P. Preparation of Ethanediamine-Doped Carbon Quantum Dots and Their Applications in White LEDs and Fluorescent TLC Plate. Carbon Lett. 2022, 32, 581–589. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright Multicolor Bandgap Fluorescent Carbon Quantum Dots for Electroluminescent Light-Emitting Diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, F.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Adv. Mater. 2017, 29, 1702910. [Google Scholar] [CrossRef] [PubMed]
- Alhalaby, H.; Zaraket, H.; Principe, M. Enhanced Photoluminescence with Dielectric Nanostructures: A Review. Results Opt. 2021, 3, 100073. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-Color Fluorescent Carbon Quantum Dots. Sci. Adv. 2020, 6, eabb6772. [Google Scholar] [CrossRef]
- Da, X.; Han, Z.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation of Multicolor Carbon Dots with High Fluorescence Quantum Yield and Application in White LED. Chem. Phys. Lett. 2022, 794, 139497. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green Synthesis of Carbon Dots from Ocimum Sanctum for Effective Fluorescent Sensing of Pb2+ Ions and Live Cell Imaging. Sens. Actuators B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-Derived Nitrogen-Doped Carbon Quantum Dots: Highly Selective Fluorescent Probe for Detecting Fe 3+ Ions and Tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Jorns, M.; Pappas, D. A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials 2021, 11, 1448. [Google Scholar] [CrossRef]
- Shabbir, H.; Tokarski, T.; Ungor, D.; Wojnicki, M. Eco Friendly Synthesis of Carbon Dot by Hydrothermal Method for Metal Ions Salt Identification. Materials 2021, 14, 7604. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, M.; Hessel, V. Quantum Materials Made in Microfluidics—Critical Review and Perspective. Chem. Eng. J. 2022, 438, 135616. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Liu, G.; Zhang, Y.; Han, G.; Vomiero, A.; Zhao, H. Colloidal Carbon Quantum Dots as Light Absorber for Efficient and Stable Ecofriendly Photoelectrochemical Hydrogen Generation. Nano Energy 2021, 86, 106122. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.-S.; Zhang, P.; Zhou, Z.-Y.; Gao, Q.-Y.; Xiong, H.-M. Solvent-Controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths. Small 2018, 14, 1800612. [Google Scholar] [CrossRef] [PubMed]
- Dua, S.; Kumar, P.; Pani, B.; Kaur, A.; Khanna, M.; Bhatt, G. Stability of Carbon Quantum Dots: A Critical Review. RSC Adv. 2023, 13, 13845–13861. [Google Scholar] [CrossRef]
- Hofmann, M.S.; Glückert, J.T.; Noé, J.; Bourjau, C.; Dehmel, R.; Högele, A. Bright, Long-Lived and Coherent Excitons in Carbon Nanotube Quantum Dots. Nat. Nanotechnol. 2013, 8, 502–505. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.-S.; Zhong, N.; Gao, Q.-Y.; Xiong, H.-M. Highly Efficient Red-Emitting Carbon Dots with Gram-Scale Yield for Bioimaging. Langmuir 2017, 33, 12635–12642. [Google Scholar] [CrossRef]
- Miao, X.; Qu, D.; Yang, D.; Nie, B.; Zhao, Y.; Fan, H.; Sun, Z. Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Adv. Mater. 2018, 30, 1704740. [Google Scholar] [CrossRef]
- McEnroe, A.; Brunt, E.; Mosleh, N.; Yu, J.; Hailstone, R.; Sun, X. Bright, Green Fluorescent Carbon Dots for Sensitive and Selective Detection of Ferrous Ions. Talanta Open 2023, 7, 100236. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-Step Hydrothermal Synthesis of Nitrogen-Doped Conjugated Carbonized Polymer Dots with 31% Efficient Red Emission for In Vivo Imaging. Small 2018, 14, 1703919. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.-W.; Meng, X.; Lee, C.-S.; Wang, P.; Zhang, W. Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, G.; Izwan Misnon, I.; Jose, R. Solvothermal Synthesis of Green Fluorescent Carbon Dots from Palm Kernel Shells. Mater. Today Proc. 2023, In Press. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, Synthesis, and Applications of Carbon Dots: A Review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Basics of Green Chemistry. United States Environmental. Available online: https://www.epa.gov/greenchemistry/basics-green-chemistry (accessed on 31 October 2024).
- Xu, K.-F.; Jia, H.-R.; Wang, Z.; Feng, H.-H.; Li, L.-Y.; Zhang, R.; Durrani, S.; Lin, F.; Wu, F.-G. See the Unseen: Red-Emissive Carbon Dots for Visualizing the Nucleolar Structures in Two Model Animals and In Vivo Drug Toxicity. Small 2023, 19, 2205890. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Liu, Y.; Li, H.; Qi, W.; Sun, K. Luminescence of Carbon Quantum Dots and Their Application in Biochemistry. Heliyon 2023, 9, e20317. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.B.; Truong, N.T.; Wanekaya, A.K. Improving the Quantum Yield of Nitrogen-Doped Carbon Dots by Varying Dopant Ratios and PH. Sens. Actuators Rep. 2023, 6, 100165. [Google Scholar] [CrossRef]
- Yang, H.L.; Bai, L.F.; Geng, Z.R.; Chen, H.; Xu, L.T.; Xie, Y.C.; Wang, D.J.; Gu, H.W.; Wang, X.M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Khoshnood, A.; Farhadian, N.; Abnous, K.; Matin, M.M.; Ziaee, N.; Yaghoobi, E. N Doped-Carbon Quantum Dots with Ultra-High Quantum Yield Photoluminescent Property Conjugated with Folic Acid for Targeted Drug Delivery and Bioimaging Applications. J. Photochem. Photobiol. A Chem. 2023, 444, 114972. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, A.; Jiang, K.; Zhang, L.; Dong, C.; Huang, Q.; Wu, A.; Lin, H. Truly Fluorescent Excitation-Dependent Carbon Dots and Their Applications in Multicolor Cellular Imaging and Multidimensional Sensing. Adv. Mater. 2015, 27, 7782–7787. [Google Scholar] [CrossRef]
- Lai, Z.; Yang, X.; Li, A.; Qiu, Y.; Cai, J.; Yang, P. Facile Preparation of Full-Color Emissive Carbon Dots and Their Applications in Imaging of the Adhesion of Erythrocytes to Endothelial Cells. J. Mater. Chem. B 2017, 5, 5259–5264. [Google Scholar] [CrossRef]
- Yao, Z.; Lai, Z.; Chen, C.; Xiao, S.; Yang, P. Full-Color Emissive Carbon-Dots Targeting Cell Walls of Onion for in Situ Imaging of Heavy Metal Pollution. Analyst 2019, 144, 3685–3690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Wu, Y.; Zhang, R.; Cao, Y.; Xu, X.; Chen, X.; Cai, L.; Xu, Q. Multicolor Tunable Highly Luminescent Carbon Dots for Remote Force Measurement and White Light Emitting Diodes. Chem. Commun. 2019, 55, 12164–12167. [Google Scholar] [CrossRef]
- Shen, C.; Lou, Q.; Lv, C.; Zang, J.; Qu, S.; Dong, L.; Shan, C. Bright and Multicolor Chemiluminescent Carbon Nanodots for Advanced Information Encryption. Adv. Sci. 2019, 6, 1802331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zheng, X.; Zhang, H.; Xu, M.; Wang, S.; Yang, Q.; Xiong, C. Multi-Color Fluorescent Carbon Dots with Single Wavelength Excitation for White Light-Emitting Diodes. J. Alloys Compd. 2019, 793, 613–619. [Google Scholar] [CrossRef]
- Kotańska, M.; Wojtaszek, K.; Kubacka, M.; Bednarski, M.; Nicosia, N.; Wojnicki, M. The Influence of Caramel Carbon Quantum Dots and Caramel on Platelet Aggregation, Protein Glycation and Lipid Peroxidation. Antioxidants 2024, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, H.; Wojtaszek, K.; Rutkowski, B.; Csapó, E.; Bednarski, M.; Adamiec, A.; Głuch-Lutwin, M.; Mordyl, B.; Druciarek, J.; Kotańska, M.; et al. Milk-Derived Carbon Quantum Dots: Study of Biological and Chemical Properties Provides Evidence of Toxicity. Molecules 2022, 27, 8728. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, S.; Silvestri, A.; Criado, A.; Bettini, S.; Prato, M.; Valli, L. Tailoring the Sensing Abilities of Carbon Nanodots Obtained from Olive Solid Wastes. Carbon 2020, 167, 696–708. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green Synthesis of Carbon Quantum Dots from Lemon Peel Waste: Applications in Sensing and Photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Baragau, I.-A.; Gromicova, R.; Nicolaev, A.; Thomson, S.A.J.; Rennie, A.; Power, N.P.; Sajjad, M.T.; Kellici, S. Investigating the Effect of N-Doping on Carbon Quantum Dots Structure, Optical Properties and Metal Ion Screening. Sci. Rep. 2022, 12, 13806. [Google Scholar] [CrossRef]
- Liu, H.; He, Z.; Jiang, L.-P.; Zhu, J.-J. Microwave-Assisted Synthesis of Wavelength-Tunable Photoluminescent Carbon Nanodots and Their Potential Applications. ACS Appl. Mater. Interfaces 2015, 7, 4913–4920. [Google Scholar] [CrossRef]
- Prathumsuwan, T.; Jamnongsong, S.; Sampattavanich, S.; Paoprasert, P. Preparation of Carbon Dots from Succinic Acid and Glycerol as Ferrous Ion and Hydrogen Peroxide Dual-Mode Sensors and for Cell Imaging. Opt. Mater. 2018, 86, 517–529. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly Fluorescent Nitrogen-Doped Carbon Dots Derived from Phyllanthus Acidus Utilized as a Fluorescent Probe for Label-Free Selective Detection of Fe3+ Ions, Live Cell Imaging and Fluorescent Ink. Biosens. Bioelectron. 2018, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, C.; Song, J.; Li, H.; Du, D.; Lin, Y. Drug-Derived Bright and Color-Tunable N-Doped Carbon Dots for Cell Imaging and Sensitive Detection of Fe3+ in Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 7399–7405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Hu, S.; Wang, Y.; Li, Z.; Lin, H. Photo-Stimulated Polychromatic Room Temperature Phosphorescence of Carbon Dots. Small 2020, 16, 2001909. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Lu, C.; Yang, X. Efficient Room Temperature Phosphorescence Carbon Dots: Information Encryption and Dual-Channel PH Sensing. Carbon 2019, 152, 609–615. [Google Scholar] [CrossRef]
- Jiao, T.; Wen, H.; Dong, W.; Wen, W.; Li, Z. Metal Ion (Fe3+ and Cu2+) Catalyzed Synthesis of High Quantum Yield Carbon Dots. Mater. Lett. 2022, 324, 132575. [Google Scholar] [CrossRef]
- Niu, F.; Xu, Y.; Liu, J.; Song, Z.; Liu, M.; Liu, J. Controllable Electrochemical/Electroanalytical Approach to Generate Nitrogen-Doped Carbon Quantum Dots from Varied Amino Acids: Pinpointing the Utmost Quantum Yield and the Versatile Photoluminescent and Electrochemiluminescent Applications. Electrochim. Acta 2017, 236, 239–251. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.; Zhang, Y. Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Saikia, M.; Hazarika, A.; Roy, K.; Khare, P.; Dihingia, A.; Konwar, R.; Saikia, B.K. Waste-Derived High-Yield Biocompatible Fluorescent Carbon Quantum Dots for Biological and Nanofertiliser Applications. J. Environ. Chem. Eng. 2023, 11, 111344. [Google Scholar] [CrossRef]
- Gupta, A.; Nandi, C.K. PC12 Live Cell Ultrasensitive Neurotransmitter Signaling Using High Quantum Yield Sulphur Doped Carbon Dots and Its Extracellular Ca2+ Ion Dependence. Sens. Actuators B Chem. 2017, 245, 137–145. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, F.; Xu, J.; Zhang, H.; Chen, L. Solvent-Controlled Synthesis Strategy of Multicolor Emission Carbon Dots and Its Applications in Sensing and Light-Emitting Devices. Nano Res. 2022, 15, 414–422. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering Triangular Carbon Quantum Dots with Unprecedented Narrow Bandwidth Emission for Multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Huang, J.; Ding, L. Preparation of Carbon Dots with High-Fluorescence Quantum Yield and Their Application in Dopamine Fluorescence Probe and Cellular Imaging. J. Nanomater. 2019, 2019, 5037243. [Google Scholar] [CrossRef]
- Liu, J.; Kong, T.; Xiong, H.-M. Mulberry-Leaves-Derived Red-Emissive Carbon Dots for Feeding Silkworms to Produce Brightly Fluorescent Silk. Adv. Mater. 2022, 34, 2200152. [Google Scholar] [CrossRef]
- Emami, E.; Mousazadeh, M.H. Green Synthesis of Carbon Dots for Ultrasensitive Detection of Cu2+ and Oxalate with Turn On-off-on Pattern in Aqueous Medium and Its Application in Cellular Imaging. J. Photochem. Photobiol. A Chem. 2021, 418, 113443. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zheng, Y.; Wang, K.; Zhang, H.; Feng, Z.; Huang, B.; Wen, H.; Wu, J.; Xue, W.; et al. Construction of Optical Dual-Mode Sensing Platform Based on Green Emissive Carbon Quantum Dots for Effective Detection of ClO- and Cellular Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 309, 123733. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.; Wang, J.; Yang, Y.; Liu, X. The Synthesis of Green Fluorescent Carbon Dots for Warm White LEDs. RSC Adv. 2018, 8, 19585–19595. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Q.; Sun, L.; Guo, X.; Zhang, H.; Zhao, X. Green Fluorescent Carbon Dots from Chitosan as Selective and Sensitive “off-on” Probes for Nitrite and “on-off-on” Probes for Enrofloxacin Detection. J. Alloys Compd. 2022, 908, 164519. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R.; Zhang, Y.; Cheng, S.; Zhang, Y. High Quantum Yield Nitrogen Doped Carbon Dots for Ag+ Sensing and Bioimaging. J. Mol. Struct. 2023, 1283, 135212. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.; Wang, Y. High Quantum Yield Green-Emitting Carbon Dots for Fe(III) Detection, Biocompatible Fluorescent Ink and Cellular Imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef]
- Yoshinaga, T.; Shinoda, M.; Iso, Y.; Isobe, T.; Ogura, A.; Takao, K. Glycothermally Synthesized Carbon Dots with Narrow-Bandwidth and Color-Tunable Solvatochromic Fluorescence for Wide-Color-Gamut Displays. ACS Omega 2021, 6, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.R.; Li, Y.; Zou, X.; Li, W.; Liang, J.; Zhang, H.; Liu, Y.; Zhang, X.; Hu, C.; et al. Architecting Ultra-Bright Silanized Carbon Dots by Alleviating the Spin-Orbit Coupling Effect: A Specific Fluorescent Nanoprobe to Label Dead Cells. Chem. Eng. J. 2022, 428, 131168. [Google Scholar] [CrossRef]
- Mai, X.D.; Nguyen, S.H.; Tran, D.L.; Nguyen, V.Q.; Nguyen, V.H. Single-Chip Horticultural LEDs Enabled by Greenly Synthesized Red-Emitting Carbon Quantum Dots. Mater. Lett. 2023, 341, 134195. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, W.; Hong, J.; Zheng, Y.; Fan, H.; Zhang, J.; Fei, J.; Zhu, W.; Hong, J. A Molecularly Imprinted Ratiometric Fluorescence Sensor Based on Blue/Red Carbon Quantum Dots for the Visual Determination of Thiamethoxam. Biosens. Bioelectron. 2023, 238, 115559. [Google Scholar] [CrossRef]
- Shi, P.; Song, Y.; Tang, J.; Nie, Z.; Chang, J.; Chen, Q.; He, Y.; Guo, T.; Zhang, J.; Wang, H. Ultra-Narrow Bandwidth Red-Emission Carbon Quantum Dots and Their Bio-Imaging. Phys. E Low-Dimens. Syst. Nanostructures 2022, 142, 115197. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Geng, X.; Yang, R.; Qu, L.; Kani, A.N.; Li, Z. Rational Design of Far-Red to Near-Infrared Emitting Carbon Dots for Ultrafast Lysosomal Polarity Imaging. ACS Appl. Mater. Interfaces 2020, 12, 31738–31744. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.-S.; Zhang, P.; Niu, X.-Q.; Zhao, W.; Zhu, Z.-Y.; Ding, H.; Xiong, H.-M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef]
- Gao, W.; Song, H.; Wang, X.; Liu, X.; Pang, X.; Zhou, Y.; Gao, B.; Peng, X. Carbon Dots with Red Emission for Sensing of Pt2+, Au3+, and Pd2+ and Their Bioapplications in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 1147–1154. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Cheng, W.-X.; Gu, Y.; Chen, Y.; Zhou, W.; Yu, X.-F.; Chen, M.; Zhu, M.; Fan, K.; et al. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Kim, B.S.; Oh, G.H.; Song, Y.; Lee, Y.S.; Kim, S. Acid Catalyzed Microwave-Assisted Production of Full-Color Light Emissive Carbon Quantum Dots for CCT-Tunable WLEDs. Appl. Surf. Sci. 2023, 640, 158301. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward High-Efficient Red Emissive Carbon Dots: Facile Preparation, Unique Properties, and Applications as Multifunctional Theranostic Agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, G.; Li, W.; Luo, X.; Hu, Z.; Wu, F. Facile Synthesis of Efficient Red-Emissive Carbon Quantum Dots as a Multifunctional Platform for Biosensing and Bioimaging. Dye. Pigment. 2023, 215, 111303. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Zhang, Z.; Lei, J.H.; Liu, T.M.; Xing, G.; Deng, C.X.; Tang, Z.; Qu, S. One Step Synthesis of Efficient Red Emissive Carbon Dots and Their Bovine Serum Albumin Composites with Enhanced Multi-Photon Fluorescence for in Vivo Bioimaging. Light Sci. Appl. 2022, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, Y.; Chen, Y.; Lei, B.; Zhuang, J.; Xiao, Y.; Liang, Y.; Zheng, M.; Zhang, H.; Liu, Y. Solid-State Carbon Dots with Red Fluorescence and Efficient Construction of Dual-Fluorescence Morphologies. Small 2017, 13, 1700075. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gu, C.; Jiao, Y.; Gao, Y.; Liu, X.; Guo, J.; Qian, T. Novel Preparation of Red Fluorescent Carbon Dots for Tetracycline Sensing and Its Application in Trace Determination. Talanta 2023, 253, 123975. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; He, P.; Xi, Z.; Li, X.; Li, Y.; Zhong, H.; Fan, L.; Yang, S. Highly Efficient and Stable White LEDs Based on Pure Red Narrow Bandwidth Emission Triangular Carbon Quantum Dots for Wide-Color Gamut Backlight Displays. Nano Res. 2019, 12, 1669–1674. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Yuan, T.; Yuan, F.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Electroluminescent Warm White Light-Emitting Diodes Based on Passivation Enabled Bright Red Bandgap Emission Carbon Quantum Dots. Adv. Sci. 2019, 6, 1900397. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, S.; Sun, X.; Li, X.; Zeng, H.; Xie, Z.; Chen, P.; Zhou, S. Highly Efficient Carbon Dots with Reversibly Switchable Green–Red Emissions for Trichromatic White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 16005–16014. [Google Scholar] [CrossRef]
- Liu, Y.; Gou, H.; Huang, X.; Zhang, G.; Xi, K.; Jia, X. Rational Synthesis of Highly Efficient Ultra-Narrow Red-Emitting Carbon Quantum Dots for NIR-II Two-Photon Bioimaging. Nanoscale 2020, 12, 1589–1601. [Google Scholar] [CrossRef]
- Zhan, J.; Geng, B.; Wu, K.; Xu, G.; Wang, L.; Guo, R.; Lei, B.; Zheng, F.; Pan, D.; Wu, M. A Solvent-Engineered Molecule Fusion Strategy for Rational Synthesis of Carbon Quantum Dots with Multicolor Bandgap Fluorescence. Carbon 2018, 130, 153–163. [Google Scholar] [CrossRef]
- Su, R.; Guan, Q.; Cai, W.; Yang, W.; Xu, Q.; Guo, Y.; Zhang, L.; Fei, L.; Xu, M. Multi-Color Carbon Dots for White Light-Emitting Diodes. RSC Adv. 2019, 9, 9700–9708. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, G.; You, S.; Camargo, F.V.A.; Zavelani-Rossi, M.; Wang, X.; Sun, C.; Liu, B.; Zhang, Y.; Han, G.; et al. Gram-Scale Synthesis of Carbon Quantum Dots with a Large Stokes Shift for the Fabrication of Eco-Friendly and High-Efficiency Luminescent Solar Concentrators. Energy Environ. Sci. 2021, 14, 396–406. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Wang, L.; Wen, Z.; Cui, Z.; Wang, L.; He, H.; Zhang, W.; Han, Z.; Mei, S.; et al. Facile Synthesis of Red-Emissive Carbon Dots with Theoretical Understanding for Cellular Imaging. Colloids Surf. B Biointerfaces 2022, 220, 112869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.; Wang, S.; Dai, Z.; Qin, S.; Mei, S.; Zhang, W.; Guo, R. Carbon Quantum Dots for Long-Term Protection against UV Degradation and Acidification in Paper-Based Relics. ACS Appl. Mater. Interfaces 2024, 16, 5009–5018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).