Abstract
The future paradigm of early cardiac diagnostics is shifting the focus towards heart attack preventive medicine based on non-invasive medical imaging with the support of artificial intelligence. It is necessary to preventively detect its increased risk early and respond with preventive drugs before moving on to more effective, but also more invasive, forms of therapy. The main motivation of our study was to improve existing and develop new AI-based solutions for cardiac preventive medicine, with particular emphasis on the prevention of heart attacks. This is due to the fact that the epidemic of lifestyle diseases (including cardiologic ones) has been stopped but not reversed; hence, automatically supervised prevention using AI seems to be a key opportunity to introduce progress in the above-mentioned areas. This can have major effects not only scientific and clinical in nature, but also economic and social. The aim of this article is to develop and test an AI-based tool designed to predict the occurrence of a heart attack for the purposes of preventive medicine. It used the combination and comparison of multiple AI methods and techniques to determine a personalized heart attack probability based on a wide range of patient characteristics and, from a computational point of view, determine the minimum set of characteristics necessary to do so. When applied to a specific patient, this represents progress in this field of research, resulting in improvements in preclinical care and diagnostics, as well as predictive accuracy in preventive medicine. After an initial selection based on the authors’ knowledge and experience, four solutions turned out to be the best: linear support vector machine (Linear SVC), logistic regression, k-nearest neighbors algorithm (KNN, k-NN), and random forest. A comparison of the models developed in the study shows that models based on logistic regression proved to be the most accurate, although their predictive value is moderate, but sufficient for the initial screening diagnosis—selecting patients who require further, more accurate testing. In addition, this can be performed based on a reduced set of parameters, particularly heart rate, age, BMI, and cholesterol. This allows the development of a prevention strategy based on modifiable factors (e.g., in the form of diet, activity modification, or a hybrid combining different factors) combined with the monitoring of heart attack risk by the proposed system. The novelty and contribution of the described system lies in the use of AI for a widely available, cheap, and quick predictive analysis of cardiovascular functions in a group of patients classified as at risk, and over time in all patients as a standard periodic examination qualifying them for further, more advanced diagnosis of heart diseases.
1. Introduction
The early prediction of diseases through preventive measures results in increased patient survival rates. This requires the computerization of the healthcare system, which, through the Internet of Things (IoT) and cloud computing, provides cyclical access to accurate patient data for artificial intelligence (AI) systems, including machine learning (ML), predicting patient health status and the risk of various conditions [1,2].
The issue of preventive medicine in heart attack has so far been addressed in 111 papers published between 1979 and 2023, with some intensification since 2015, but papers using AI have been rare. Research to date has tended to look at incidence rates and seasonal variances in specific populations [3,4] and at prevention guidelines [5,6] rather than prevention models [7,8]. More attention has been paid to technical solutions, such as a remote cardiac monitoring system with an automatic warning of acute cardiac episodes, and less to the integration of different design, measurement, and optimization logics in complex adaptive healthcare systems, including for cardiology [9,10]. A part of this interest stems from the development of telerehabilitation systems for patients undergoing home rehabilitation, sometimes at a considerable distance from the nearest hospital with an interventional cardiology unit.
Incorrect, incomplete, or uncertain medical data can result in longer waiting times for patient prognosis, failure to ensure best outcomes, or even false prognoses. Similar results can be produced by a targeted action, i.e., an attack on data transfer or processing systems due to insufficient security. In this article, we will deal with the first of the aforementioned situations, ensuring the appropriate level of processing, inference, and prediction from data using AI. Preventive medicine, i.e., the medicine of healthy people aimed at predicting the health of patients and preventing their diseases, still poses challenges for engineers and medical specialists to accurately predict diseases for a specific patient.
Cardiovascular diseases as lifestyle diseases pose a special challenge: the epidemic of lifestyle diseases has been stopped, but their number is not decreasing. Perhaps, this can be achieved with the use of AI-based preventive cardiology medicine. The future paradigm of early cardiac diagnosis is shifting the focus towards heart attack prevention medicine based on non-invasive medical imaging with the support of artificial intelligence. It is necessary to detect increased risk early in a preventive way and use preventive drugs before moving on to more effective, but also more invasive, forms of therapy.
The main motivation of our study was to improve existing and develop new AI-based solutions for cardiac preventive medicine, with particular emphasis on the prevention of heart attacks. This is due to the fact that the epidemic of lifestyle diseases (including cardiologic ones) has been stopped but not reversed; hence, automatically supervised prevention using AI seems to be a key opportunity to introduce progress in the above-mentioned areas. This can have major effects not only scientific and clinical in nature, but also economic and social.
The aim of this article is to develop and test an artificial intelligence-based tool for predicting the occurrence of a heart attack for the purposes of preventive medicine. It used the combination and comparison of multiple artificial intelligence methods and techniques to determine a personalized probability of a heart attack based on a wide range of patient characteristics and, from a computational point of view, determine the minimum set of characteristics necessary to do so. When applied to a specific patient, this represents progress in this field of research, resulting in improvements in preclinical care and diagnosis, as well as predictive accuracy in preventive medicine.
The novelty and contribution of the described system lies in the use of AI for widely available, cheap, and quick predictive analysis of circulatory system functions in a group of patients classified as at risk, and over time in all patients in the form of a standard periodic examination qualifying them for further tests and more advanced diagnosis of heart diseases.
Current studies showed deep learning (DL) is worth using for the quick, automated segmentation of coronary plaques based on angiography, allowing for the prediction of a future heart attack, or for automating the assessment of calcium concentration in coronary arteries during gateless computed tomography (CT) of the heart and the chest, and for automating the measurement of epicardial fat tissue. This requires predictive models based on artificial intelligence, integrating clinical and diagnostic imaging parameters [11,12,13,14]. So far, AI is used to improve the effectiveness of cardiovascular CT: for image acquisition, reconstruction and denoising, image segmentation, quantitative analysis, decision support, and data integration. The further development of AI systems may provide an opportunity to improve current diagnostic procedures as precision medicine tools [11,15]. Furthermore, even freely available data can provide a reading of risk at the population level. The routine assessment of biomarkers (including hematological ones) for inclusion in clinical risk models is sometimes carried out on the basis of data that are already available to most patients. This allows for a trade-off between data availability, analysis cost, and the ease of implementing such a system in the existing healthcare system. The ideal input data for risk assessment in population health initiatives will be those that are already available to most patients [15].
Recent research focuses on three areas, i.e., prediction, classification, and detection, including, e.g., atrial fibrillation. This creates a certain way of proceeding. Most of the research to date concerns analysis based on AI (40% is based on DL and 60% on conventional ML), with a strong increase in the number of publications after 2015 [16]. Additionally, lipid-based methods currently used to predict the risk of coronary heart disease (CHD) have limitations that can be eliminated by incorporating epigenetic information (including the history of diseases in the patient’s ancestors) into AI-based risk prediction algorithms. The identification of genetic and epigenetic biomarkers in people with coronary artery disease already outperforms other risk assessment methods [17].
This is even more important because current guidelines may underestimate the risk of some cardiovascular diseases, including atherosclerotic cardiovascular disease (CVD), in high-risk people. An ML-based risk calculator based on support vector machines (SVM) eliminates this error based on data from a 13-year multi-ethnic atherosclerosis study (6459 participants) that achieved a sensitivity of 0.86, a specificity of 0.95, and an AUC of 0.92, even forall CVDs, but still requires validation [18]. Researchers are encouraged to explore alternative ML-based diagnostic methods that use non-invasive clinical data to diagnose the disease and assess its severity [19]. It is also important to have the support of medical experts in assessing the predicted risk of diseases in order to validate AI-based software 2024 [20].
The opportunities created by AI-based preventive medicine systems primarily relate to the early detection of disease through monitoring, enhanced surveillance, and the early warning of disease prediction. This applies to both individual patients and population health risks, facilitating timely and targeted interventions. Personalized risk assessment allows the creation of personalized health plans, i.e., personalized risk profiles and prevention strategies based on them. This includes the integration of genomic data, allowing genetic predispositions to be understood and taken into account when formulating individualized disease prevention recommendations. Lifestyle data and health-seeking behaviors offer insights into habits (beneficial and harmful) that influence health status, and indirectly are used to design interventions and support individuals to make informed, stimulated healthier choices. An important element of preventive medicine is individually optimized vaccination schedules to ensure timely and effective vaccination against infectious diseases, the elimination of vaccination gaps, etc. As a part of preventive medicine, early interventions for chronic diseases are viable and support AI in identifying people at risk of developing chronic diseases, confirming their risks/symptoms and implementing early interventions to avoid or mitigate these conditions. This integrated approach remotely monitors individuals with chronic diseases and their groups, improving the effectiveness of therapy and reducing the risk of complications. Environmental health monitoring, which includes monitoring the quality of air, water, and soil, allows to take into account the impact of air or water quality on public health, contributing to the introduction of preventive measures against threats resulting from periodic or permanent environmental pollution. Due to the above reason, preventive medicine systems play a key role in global health surveillance, increasing preparedness for pandemics and poisonings. Through international cooperation, environmental data and health information should be shared, contributing to a more coordinated global response to health challenges. In the long run, this will result in reduced healthcare costs by avoiding some of the high expenses associated with the treatment (e.g., in hospital) of advanced diseases and by optimizing the allocation of resources, moving saved financial resources to where they are more needed. To this end, targeted health education and mental health programs in the workplace become key areas of impact. Targeted health education campaigns that build awareness and encourage healthy habits can be actively supported by AI-powered digital health platforms, making reliable knowledge more accessible to society. Government, organizational, and corporate health initiatives targeting chronic fatigue syndrome, prolonged stress, burnout, and depression can proactively support workplace well-being programs, thanks to AI providing insight into objectively measured employee health, guiding the development of targeted interventions and promoting healthier habits among employees. Their successful implementation requires addressing technological, social, and ethical challenges, such as privacy issues, data security, and ensuring equal access to preventive interventions. Collaboration between the patient community, healthcare providers, policy makers, and technology developers is essential to achieve the full potential of preventive medicine systems, especially for cardiovascular diseases.
2. Materials and Methods
2.1. Dataset
The dataset is a raw dataset (.csv file) from Kaggle [21], representing 8763 generated(having all characteristics, including statistical characteristics, of the population under study) patient records described by the following 26 characteristics (Table 1, first is patient ID):

Table 1.
Dataset sample (8763 rows × 26 columns).
- Age—age of the patient;
- Sex—gender of the patient (male/female);
- Cholesterol—cholesterol levels of the patient;
- Blood Pressure—blood pressure of the patient (systolic/diastolic);
- Heart Rate—heart rate of the patient;
- Diabetes—whether the patient has diabetes (yes/no);
- Family History—family history of heart-related problems (1: yes, 0: no);
- Smoking—smoking status of the patient (1: smoker, 0: non-smoker);
- Obesity—obesity status of the patient (1: obese, 0: not-obese);
- Alcohol consumption—level of alcohol consumption by the patient (none/light/moderate/heavy);
- Exercise hours per week—number of exercise hours per week;
- Diet—dietary habits of the patient (healthy/average/nonhealthy);
- Previous heart problems—previous heart problems of the patient (1: yes, 0: no);
- Medication use—medication usage by the patient (1: yes, 0: no);
- Stress level—stress level reported by the patient (1–10);
- Sedentary hours per day—hours of sedentary activity per day;
- Income—income level of the patient;
- BMI—body mass index (BMI)of the patient;
- Triglycerides—triglyceride levels of the patient;
- Physical activity days per week—days of physical activity per week;
- Sleep hours per day—hours of sleep per day;
- Country—country of the patient;
- Continent—continent where the patient resides;
- Hemisphere—hemisphere where the patient resides;
- Heart attack risk—presence of heart attack risk (1: yes, 0: no).
We checked missing and incomplete values and turned categorical variables to numbers. Dataset division: training 80% (7010) and testing 20% (1753).
A dataset downloaded from Kaggle was used to demonstrate the capabilities of the proposed method. Each open dataset can create a community that allows you to create your own projects, discuss the data, discover public code, various methods, and techniques, and choose the best solution among all options. This significantly increases the possibilities of data analysis by one research team and increases the chance of quickly finding the best solution among all existing ones, not only those that can be investigated by one specific research team.
2.2. Computational Analysis
The purpose of the analysis was to compare multiple artificial intelligence methods/techniques with the expected effect in mind: selecting the best one to determine the personalized probability of a heart attack based on a wide range of patient characteristics and, from a computational point of view, determining the minimum set of features necessary for this. This will bring progress in building a personalized preventive medicine system for heart attacks and the progress in this field of research will result in improved preclinical care and diagnostics as well as predictive accuracy.
After initial selection based on the authors’ knowledge and experience, four solutions turned out to be the best: linear support vector machine (Linear SVC), logistic regression, k-nearest neighbors algorithm (KNN, k-NN), and random forest.
LinearSVC is an algorithm that attempts to find the hyperplane that maximizes the distance between classified samples. It uses a linear kernel function to perform the classification and works well with a large number of samples. Compared to the SVC model, linear SVC has additional parameters: a penalty normalization that uses “L1” or “L2” and a loss function.
Logistic regression is a ML classification algorithm that is used to predict the probability of certain classes based on some dependent variables. It computes a sum of the input features.
KNN is a non-parametric supervised learning classifier that uses proximity to classify or predict the clustering of individual data points.
Random forest is a widely used machine learning algorithm that combines the output of multiple decision trees to produce a single result. It is easy to use and flexible, dealing with both classification and regression.
3. Results
More than one-third of the study population (35.82%, Figure 1) was assessed as being at risk of a heart attack (“1”), a very high proportion that indicates the need for immediate preventive action, as therapeutic measures alone may not be sufficient in a few years time.
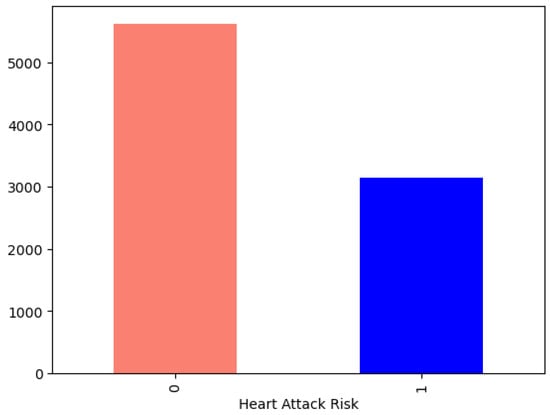
Figure 1.
Heart attack risk.
Figure 2.
Interactive correlation matrix.
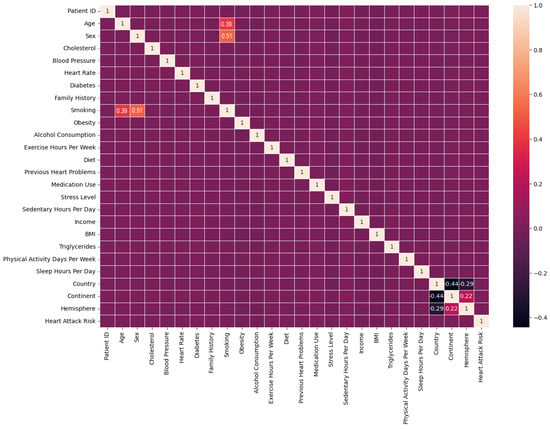
Figure 3.
Correlation matrix.
Age, sex, and smoking were the most significant risk factors for a heart attack, and smoking is a modifiable factor enabling prevention strategies.
The selection of model for classification purposes was made based on its accuracy (Figure 4):
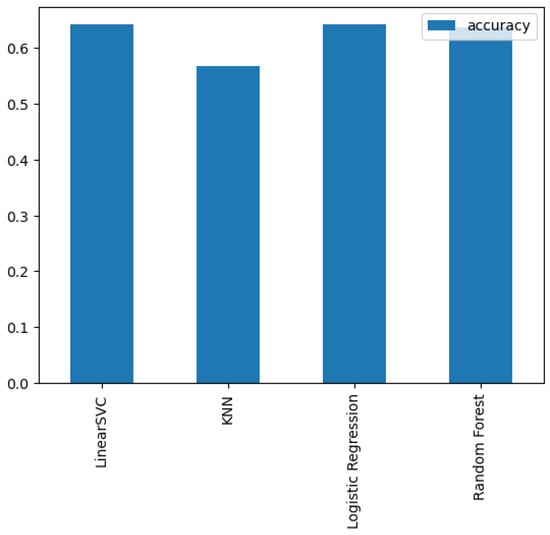
Figure 4.
Comparison of models.
- LinearSVC—LinearSupport Vector Classifier;
- Logistic Regression—LogisticRegression;
- K-Nearest Neighbors—KneighboursClassifier;
- RandomForest—RandomForestClassifier.
Hyperparameters Tuning
A study was performed to assess the impact of tuning on different models using hyperparameters specific to each model.
For the KNeighborsClassifier, model manual tuning was provided. The maximal KNN score achieved was 62.86% (Figure 5).
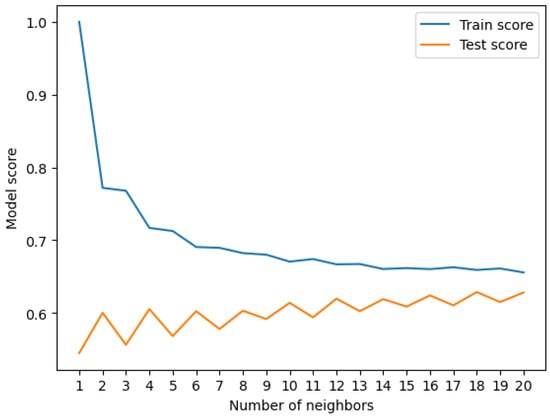
Figure 5.
Train score vs. test score.
The test score changed with an increasing number of neighbors but stabilized at the value of k = 20, and no further improvement was seen.
For the RandomForrestClassifier, manual tuning was provided. The maximal score achieved was 63.32%. Sample train scores are shown in Table 2.

Table 2.
Sample train scores.
Sample test scores are shown in Table 3.

Table 3.
Sample test scores.
For the LogisticsRegression and RandomForestClassifier, tuning using RandomizedSearchCV was provided. The maximal scores achieved were63.52% and 64.18%, respectively.
For the LogisticRegression, tuning using GridSearchCV was provided. The maximal score achieved was 64.18%.
Results achieved for 27 models’ lazy classifiers are shown in Table 4.

Table 4.
Lazy classifiers.
Receiver Operating Characteristic (ROC) is a plot of true positive rate versus false positive rate. A false positive in this case occurs when the person tests positive but does not actually have the disease. A false negative occurs when the person tests negative, suggesting they are healthy, when they actually do have the disease.
- True positive = model predicts 1 when truth is 1;
- False positive = model predicts 1 when truth is 0;
- True negative = model predicts 0 when truth is 0;
- False negative = model predicts 0 when truth is 1 (Figure 6).
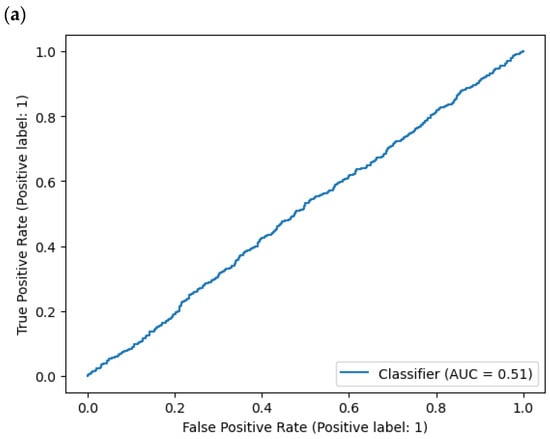
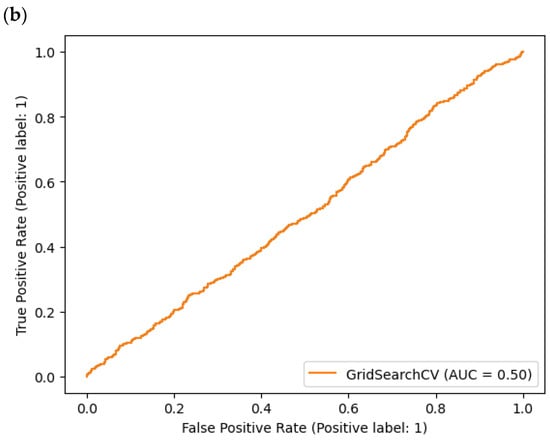 Figure 6. AUC (Area Under Curve): (a) DummyClassifier, (b) ExtraTreeClassifier.
Figure 6. AUC (Area Under Curve): (a) DummyClassifier, (b) ExtraTreeClassifier.
Area Under Curve (AUC) is the area underneath the ROC curve. A perfect model achieves a score of 1.0.
Confusion matrix shows where model made the right predictions and where it made the wrong predictions (Figure 7).
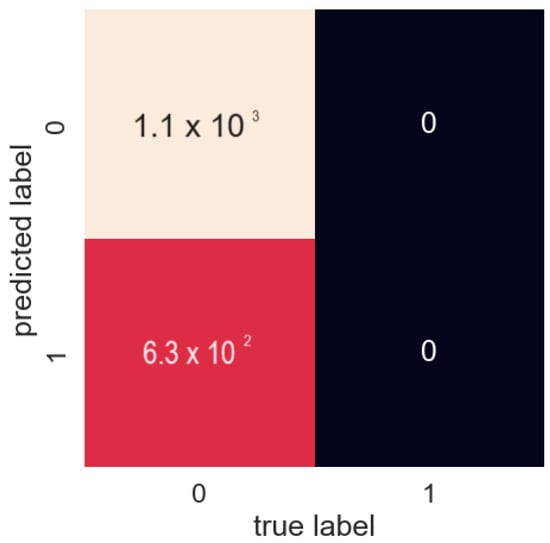
Figure 7.
Confusion matrix.
Precision indicates the proportion of positive identifications (model predicted class 1) that were actually correct. A model that produces no false positives has a precision of 1.0. Recall indicates the proportion of actual positives that were correctly classified. A model that produces no false negatives has a recall of 1.0. F1 score is a combination of precision and recall. A perfect model achieves an F1 score of 1.0. Support is the number of samples each metric was calculated on. The accuracy of the model is in the decimal form. Perfect accuracy is equal to 1.0.
Accuracy shows the accuracy of the model in the decimal form. The ideal accuracy is equal to 1.0. Precision indicates the percentage of positive identifications (predicted by the model class 1) that were actually correct. A model that does not generate false positives has a precision of 1.0. Recall indicates the percentage of actual positives that were correctly classified. A model that does not generate false negatives has a recall of 1.0. F1 score is a combination of precision and recall. An ideal model achieves an F1 score of 1.0 (Table 5).

Table 5.
Cross-validated metrics.
Additional metrics are macro average and weighted average. Macro average stands for the average of precision, recall, and F1 score between classes. Macro average does not consider class imbalances, so if there are class imbalances, pay attention to this metric. Weighted average is the weighted average of precision, recall, and F1 score between classes. Weighted means each metric is calculated with respect to the number of samples in each class.
The key features (risk factors) within the heart attack risk assessment are (from the most important) heart rate, age, BMI, and cholesterol. BMI and cholesterol are modifiable factors, including diet. Although specific risk factors allow the rapid diagnosis of heart attack, it is advisable to combine biomarkers to improve discrimination (Figure 8).
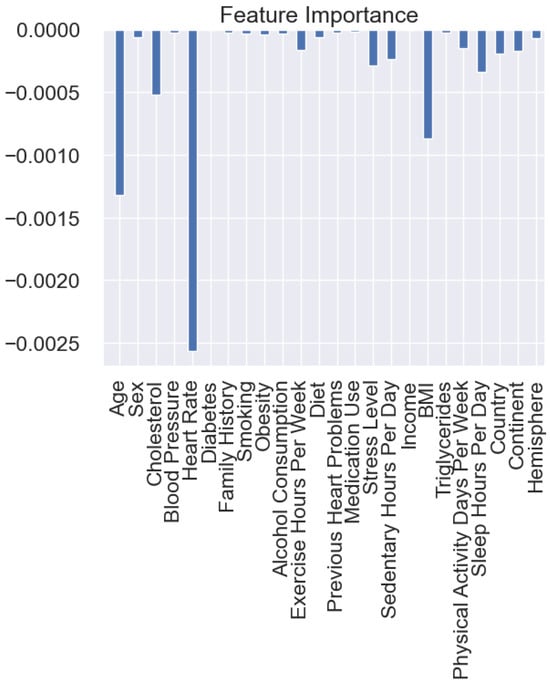
Figure 8.
Feature importance analysis.
Moreover, AI can potentially improve the prevention and self-treatment of several chronic diseases simultaneously, within one preventive medicine system. However, we are aware that the perspectives of patients, their families, and several medical specialists are combined here, which may affect both the implementation prospects and the preventive/therapeutic success of these systems. This also applies to the secondary prevention of heart attack due to polypharmacy and the coexistence of other diseases.
4. Discussion
A comparison of the models developed in the study shows that models based on logistic regression proved to be the most accurate, although their predictive value is moderate, but sufficient for the initial screening diagnosis—selecting patients who require further, more accurate testing. In addition, this can be performed based on a reduced set of parameters, particularly heart rate, age, BMI, and cholesterol. This allows the development of a prevention strategy based on modifiable factors (e.g., in the form of diet, activity modification, or a hybrid combining different factors) combined with the monitoring of heart attack risk by the proposed system.
Guidelines for the diagnosis and treatment of acute and chronic heart failure are constantly being innovated and clinically researched in the search for more effective methods of prevention—the only way, it seems, to reverse the epidemic of civilization cardiovascular conditions. This requires not only harnessing the analytical and predictive power of artificial intelligence but also developing a framework for combining health, ecological, mechanistic, and social models to design, track, and evaluate complex interventions while translating knowledge. Experienced in a variety of healthcare settings, both procedural and technological innovations can measure and optimize their impact in complex, dynamically changing interactions within the healthcare system. A review of the six largest bibliometric databases yielded 134,012 publications (1857–2024) with the keyword “preventive medicine”, including only 110 publications with the keywords “preventive medicine” + “heart attack” (1979–2023) and only 3 “preventive medicine” + “heart attack” + “artificial intelligence” or “preventive medicine” + “heart attack” + “machine learning” (2003–2023) [22,23,24]. The results from the perspective of research to date show that AI-based heart attack prediction systems offer a number of benefits that can significantly improve healthcare outcomes, but more are needed. AI algorithms analyzing large datasets (patient records, medical images, and genetic information) identify rules, mechanisms, patterns, and risk factors associated with heart attacks, and this allows for the early detection of potential problems, enabling quick intervention and taking preventive actions. AI provides a personalized risk assessment by taking into account a wide range of factors, including medical history, lifestyle, genetics, and environmental variables, allowing for more accurate predictions tailored to an individual’s specific risk profile [1,22,23,24,25]. Continuous patient monitoring provides the real-time analysis of health parameters and can detect subtle changes in health that may indicate an increased risk of heart attack. AI algorithms help medical specialists interpret complex medical data, reducing the likelihood of diagnostic errors and also providing additional information (computational biomarkers) and signaling potential problems. This can automate and accelerate the analysis of large datasets collected from patients and increase the accuracy of analyses. This relieves some of the burden of manual data analysis, allowing focus on patient care and clinical decision-making. By supporting decision-making, AI helps in developing personalized prevention, treatment, rehabilitation, and care plans [22,23,24,25,26,27,28]. AI ensures data integration and analyzes data from various sources, which enables a more comprehensive understanding of the patient’s health condition and increases the accuracy of predictions and the effectiveness of preventive/therapeutic actions taken. Conclusions from the above data may contribute to the identification of new risk factors, mechanisms, decision rules, and potential treatment options (ultimately leading to the innovative forms of prevention and therapy). Preventive medicine systems based on AI can strengthen patients’ motivation by providing them with personalized support in the area of health status and desired activities, which can encourage them to adopt a healthier lifestyle, follow treatment plans and cardiac rehabilitation (including in a hybrid form: intertwining home rehabilitation and outpatient), and actively engage with their own healthcare. Moreover, from a systems perspective, the early detection and prevention of heart attacks can lead to savings in the healthcare system, because by identifying people in high-risk groups and implementing preventive measures, the overall cost of treating heart disease can be reduced and scarce resources can be allocated more efficiently (Figure 9).
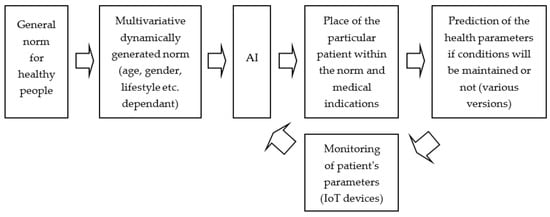
Figure 9.
AI-based preventive medicine system for predicting heart attacks.
This group of solutions offers significant benefits, but challenges include data privacy, algorithm transparency, and the need for continuous validation and improvement of artificial intelligence models for use in heart attack prevention [1,22,28,29,30,31]. It is necessary to establish a common platform of views. The study by Pelly et al. [32] identified as many as 21 concepts resulting from the views of patients and medical specialists in the area of preventive medicine. They covered five categories:
- Trust, information reliability, and security;
- Expected features, tailored feedback, and personalized advice;
- Adoption, usability, and general interest in artificial intelligence;
- Concerns and previous negative experiences with artificial intelligence;
- Perceived benefits and the usefulness of artificial intelligence in providing advice when regular contact with healthcare is not possible.
Positive public perceptions of preventive medicine systems may help increase the likelihood of successful implementation and the adoption of AI-enabled systems in the context of heart attack, as an example of broader applications in the management of chronic diseases.
4.1. Limitations
The number and severity of the aforementioned limitations will change as research progresses and further problems encountered in the daily operation of AI-based preventive medicine systems, including those dedicated to cardiovascular conditions, are solved. The limitations observed today are summarized in Table 6.

Table 6.
Current limitations of AI-based prediction of heart attack risk (own concept) [1,22,32,33,34].
Continued research, collaboration between computer scientists and medical specialists, and a commitment for validating predictive models in real-world settings will help overcome the above limitations.
In the technology area, existing prediction models based on multilayer perceptron with extreme gradient boosting provided better predictions than models based on logistic regression when analyzing diverse datasets, with different algorithm classification lists. Hence, a beneficial trend may be the hybridization of preventive medicine systems based on the use of more than one algorithm and then the aggregation of their results, e.g., based on a separate algorithm with trend extraction (based on a fuzzy system and/or multifractal analysis) [33].
The problem is much broader. Current cardiovascular mortality rates vary significantly across countries, and systems of care play a major role in determining outcomes also through shortcomings in pre-hospital and in-hospital systems and improvements in the quality of care. The time to first medical contact is critical here. It can be shortened by the preventive medicine system that we have described and by improving patients’ awareness of the importance of individual symptoms and the need to call emergency medical services [34,35]. However, this requires the coordination of preventive medicine systems with other systems used in healthcare. The algorithmic (thanks to AI) identification of patients at risk of heart attack or even with a high-risk heart attack must be synchronized with the transmission of pre-hospital electrocardiograms and appropriate referral of such patients to hospitals, e.g., for percutaneous coronary intervention [35]. Optimizing primary and secondary prevention, including patient adherence to medications, also requires data collection to facilitate the auditing and assessment of clinical improvement, but also to facilitate the detection of non-adherent patients through prediction system that produce unreliable outcomes (e.g., variability over time, jumps or gaps in data, etc.) [36,37].
4.2. Directions for Further Research
Potential areas of further interest and progress that can be explored in the future are included in Table 7.

Table 7.
Basic directions for further research on the AI-based prediction of heart attack risk (own concept) [1,22,32,33,34].
When thinking about the future of such preventive medicine systems, it is worth noting that these are long-term projects, i.e., for tens of years and centuries, because the collection and comparison of subsequent predictions with the patient’s results should be repeated no less than three times (to obtain a prediction), but ultimately cyclically throughout the life of a patient, thus obtaining increasingly accurate, personalized predictions, thereby increasing the efficiency of the system.
From the point of view of the entire system, collecting data from the patient’s entire life (not only the history of his illnesses, but also, e.g., environmental factors) will make it possible to take into account a number of new factors, the existence of which we may not be aware of today, i.e., personal (mental hygiene), local (environmental pollution), and computational (virtual indicators of quality of life or mental health) factors.
A long-term approach can create a system for counteracting lifestyle diseases, and in particular, the excessive occurrence of cardiovascular diseases in the population. However, to fully achieve this, such a system of preventive medicine must be technologically appropriately developed and, moreover, harmonize with the entire healthcare system in several areas simultaneously:
- legally and ethically (i.e.,the universal acceptance of preventive medicine);
- in terms of systematicity and the scope of periodic examinations of healthy and sick people;
- Automatic availability of data collected for analysis;
- The scope of notification and alerting about deviations from the norm.
This is already a part of periodic examination programs related to the profession or sport practiced, as well as age (children and elderly people).
It is worth noting that in this case, possible delays in the transmission and processing process are not critical parameters—even delays of the order of seconds will not significantly reduce its efficiency. The strength of the system lies in the quantity and quality of collected data and the individual approach to the user throughout the history of his research. Due to the above reason, there is a growing chance of wider use of wearable, i-wear, and smart home devices in preventive medicine systems, including those connected occasionally (such as today’s blood pressure meter or thermometer). This makes transferring the processing part of the system to the cloud more and more feasible and relieves the burden on the traditional system based on servers located throughout the country.
By reducing the time it takes for preventive medicine tools to process biomedical data (including as part of the initial feature extraction by edge computing sensors), it is possible not only to improve diagnosis, help more patients, and speed up research, but also to improve solutions hitherto only considered to be promising, such as brain–computer interfaces (BCIs) based on EEG and fNIRS [38,39,40,41,42,43].
Our study partly meets the requirements of AI-based tools recently discussed by the American Heart Association (AHA) to perform cardiac CT scans on patients with chest pain that can predict the risk of a fatal heart attack within 10 years. This technology could transform the diagnosis and treatment of heart disease in the future, saving thousands of patients’ lives. Our study aims to develop a simpler, less invasive, and less expensive system suitable for mass use, including in cardiac home care [44].
5. Conclusions
AI-based heart attack prevention systems represent a transformative approach to healthcare and offer the potential to revolutionize early detection, personalized preventive intervention, and patient care. In some cases, they will allow you to avoid treatment and rehabilitation. In order to fully use the advantages of the above-mentioned systems, further research, technological development, clinical trials, and the cooperation and involvement of scientists from many disciplines are necessary. This will mitigate the associated challenges, especially as these systems evolve, they could significantly contribute to reducing the global burden of cardiovascular disease, with tangible health, social, and economic benefits.
Author Contributions
Conceptualization, I.R. and M.K.; methodology, I.R. and M.K.; software, M.K.; validation, I.R., P.K., M.K., Z.K. and M.J.; formal analysis, I.R., P.K., M.K., Z.K. and M.J.; investigation, I.R., P.K., M.K., Z.K. and M.J.; resources, M.K.; data curation, M.K.; writing—original draft preparation, I.R., P.K., M.K., Z.K. and M.J.; writing—review and editing, I.R., P.K., M.K., Z.K. and M.J.; visualization, I.R., P.K., M.K., Z.K. and M.J.; supervision, I.R., P.K., M.K., Z.K. and M.J.; project administration, I.R.; funding acquisition, I.R. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
The study presented in this paper was financed under the grant for maintaining the research potential of Kazimierz Wielki University and Silesian University of Technology (2023).
Data Availability Statement
Kaggle dataset https://www.kaggle.com/datasets/iamsouravbanerjee/heart-attack-prediction-dataset (accessed on 10 October 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verma, A.; Agarwal, G.; Gupta, A.K.; Sain, M. Novel Hybrid Intelligent Secure Cloud Internet of Things Based Disease Prediction and Diagnosis. Electronics 2021, 10, 3013. [Google Scholar] [CrossRef]
- Rojek, I.; Kozielski, M.; Dorożyński, J.; Mikołajewski, D. AI-Based Prediction of Myocardial Infarction Risk as an Element of Preventive Medicine. Appl. Sci. 2022, 12, 9596. [Google Scholar] [CrossRef]
- Shitara, S.; Tanaka-Mizuno, S.; Takashima, N.; Fujii, T.; Arima, H.; Kita, Y.; Tsuji, A.; Kitamura, A.; Urushitani, M.; Miura, K.; et al. Population-Based Incidence Rates of Subarachnoid Hemorrhage in Japan: The Shiga Stroke and Heart Attack Registry. J. Stroke 2022, 24, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Arima, H.; Takashima, N.; Kita, Y.; Miyamatsu, N.; Tanaka-Mizuno, S.; Shitara, S.; Urushitani, M.; Miura, K.; Nozaki, K. Seasonal Variation in Incidence of Stroke in a General Population of 1.4 Million Japanese: The Shiga Stroke Registry. Cerebrovasc. Dis. 2022, 51, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M. Preventive Cardiology: The SHAPE of the future. A Synopsis from the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Herz 2007, 32, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Oeing, C.U.; Tschöpe, C.; Pieske, B. The new ESC Guidelines for acute and chronic heart failure 2016. Herz 2016, 41, 655–663. [Google Scholar] [CrossRef]
- Beleigoli, A.; Nicholls, S.J.; Brown, A.; Chew, D.P.; Beltrame, J.; Maeder, A.; Maher, C.; Versace, V.L.; Hendriks, J.M.; Tideman, P.; et al. Implementation and prospective evaluation of the Country Heart Attack Prevention model of care to improve attendance and completion of cardiac rehabilitation for patients with cardiovascular diseases living in rural Australia: A study protocol. BMJ Open 2022, 12, e054558. [Google Scholar] [CrossRef]
- Senanayake, S.; Halahakone, U.; Abell, B.; Kularatna, S.; McCreanor, V.; McPhail, S.M.; Redfern, J.; Tom, B.; Parsonage, W. Hybrid cardiac telerehabilitation for coronary artery disease in Australia: A cost-effectiveness analysis. BMC Health Serv. Res. 2023, 23, 512. [Google Scholar] [CrossRef]
- Chen, S.Q.; Xing, S.S.; Gao, H.Q. Clinical significance of automatic warning function of cardiac remote monitoring systems in preventing acute cardiac episodes. Pak. J. Med. Sci. 2014, 30, 1281–1285. [Google Scholar] [CrossRef]
- Pinero de Plaza, M.A.; Yadav, L.; Kitson, A. Co-designing, measuring, and optimizing innovations and solutions within complex adaptive health systems. Front. Health Serv. 2023, 3, 1154614. [Google Scholar] [CrossRef]
- Joshi, M.; Melo, D.P.; Ouyang, D.; Slomka, P.J.; Williams, M.C.; Dey, D. Current and Future Applications of Artificial Intelligence in Cardiac CT. Curr. Cardiol. Rep. 2023, 25, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and application of artificial intelligence in cardiac imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Liu, J.; Sun, Z.; Cui, Y.; He, Y.; Yang, Z. Deep learning analysis in coronary computed tomographic angiography imaging for the assessment of patients with coronary artery stenosis. Comput. Methods Programs Biomed. 2020, 196, 105651. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, M.A.; Selder, J.L.; Nicolas, J.; Claessen, B.E.; Mehran, R.; Bescós, J.O.; Schuuring, M.J.; Bouma, B.J.; Verouden, N.J.; Chamuleau, S.A.J. Current State and Future Perspectives of Artificial Intelligence for Automated Coronary Angiography Imaging Analysis in Patients with Ischemic Heart Disease. Curr. Cardiol. Rep. 2022, 24, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Truslow, J.G.; Goto, S.; Homilius, M.; Mow, C.; Higgins, J.M.; MacRae, C.A.; Deo, R.C. Cardiovascular Risk Assessment Using Artificial Intelligence-Enabled Event Adjudication and Hematologic Predictors. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008007. [Google Scholar] [CrossRef] [PubMed]
- Serhal, H.; Abdallah, N.; Marion, J.M.; Chauvet, P.; Oueidat, M.; Humeau-Heurtier, A. Overview on prediction, detection, and classification of atrial fibrillation using wavelets and AI on ECG. Comput. Biol. Med. 2022, 142, 105168. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.V.; Knight, S.; Dogan, T.K.; Knowlton, K.U.; Philibert, R. External validation of integrated genetic-epigenetic biomarkers for predicting incident coronary heart disease. Epigenomics 2021, 13, 1095–1112. [Google Scholar] [CrossRef]
- Kakadiaris, I.A.; Vrigkas, M.; Yen, A.A.; Kuznetsova, T.; Budoff, M.; Naghavi, M. Machine Learning Outperforms ACC/AHA CVD Risk Calculator in MESA. J. Am. Hear. Assoc. 2018, 7, e009476. [Google Scholar] [CrossRef]
- Verma, L.; Srivastava, S.; Negi, P.C. A Hybrid Data Mining Model to Predict Coronary Artery Disease Cases Using Non-Invasive Clinical Data. J. Med. Syst. 2016, 40, 178. [Google Scholar] [CrossRef]
- Zarrin-Khameh, N.; Barzi, A.; Sadeghi, S. Intelligent system and risk of different diseases in the general population. Stud. Health Technol. Inform. 2001, 81, 601–603. [Google Scholar]
- Kaggle Data Set. Available online: https://www.kaggle.com/datasets/iamsouravbanerjee/heart-attack-prediction-dataset (accessed on 10 October 2023).
- Kronish, I.M.; Lynch, A.I.; Oparil, S.; Whittle, J.; Davis, B.R.; Simpson, L.M.; Krousel-Wood, M.; Cushman, W.C.; Chang, T.I.; Muntner, P. The Association Between Antihypertensive Medication Nonadherence and Visit-to-Visit Variability of Blood Pressure: Findings from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2016, 68, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Wald, N.; Morris, J. Lowering blood pressure to prevent myocardial infarction and stroke: A new preventive strategy. Health Technol Assess. 2003, 7, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Cushman, W.; Weber, M. Roundtable discussion: The ALLHAT trial. J. Clin. Hypertens. 2003, 5, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Jasiulewicz-Kaczmarek, M.; Antosz, K.; Zhang, C.; Waszkowski, R. Assessing the barriers to Industry 4.0 implementation from a maintenance management perspective–pilot study results. IFAC-PapersOnLine 2022, 55, 223–228. [Google Scholar] [CrossRef]
- Antosz, K.; Jasiulewicz-Kaczmarek, M.; Machado, J.; Relich, M. Application of principle component analysis and logistic regression to support Six Sigma implementation in maintenance. Eksploat. I Niezawodn. Maint. Reliab. 2023, 25, 174603. [Google Scholar] [CrossRef]
- Rojek, I.; Studziński, J. Comparison of different types of neuronal nets for failures location within water-supply networks. Eksploat. I Niezawodn.-Maint. Reliab. 2014, 16, 42–47. [Google Scholar]
- Rojek, I. Hybrid neural networks as prediction models. In Artifical Intelligence and Soft Computing. ICAISC 2010; Lecture Notes in Artificial Intelligence; Rutkowski, L., Scherer, R., Tadeusiewicz, R., Zadeh, L.A., Zurada, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6114, pp. 88–95. [Google Scholar]
- Mikołajewska, E.; Prokopowicz, P.; Mikolajewski, D. Computational gait analysis using fuzzy logic for everyday clinical purposes–preliminary findings. Bio-Algorithms Med-Syst. 2017, 13, 37–42. [Google Scholar] [CrossRef]
- Rojek, I.; Mikołajewski, D.; Kotlarz, P.; Macko, M.; Kopowski, J. Intelligent system supporting technological process planning for machining and 3D printing. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, e136722. [Google Scholar]
- Mikołajewska, E.; Mikołajewski, D. Integrated IT environment for people with disabilities: A new concept. Cent. Eur. J. Med. 2014, 9, 177–182. [Google Scholar] [CrossRef]
- Pelly, M.; Fatehi, F.; Liew, D.; Verdejo-Garcia, A. Artificial intelligence for secondary prevention of myocardial infarction: A qualitative study of patient and health professional perspectives. Int. J. Med. Inform. 2023, 173, 105041. [Google Scholar] [CrossRef]
- Choi, A.; Kim, M.J.; Sung, J.M.; Kim, S.; Lee, J.; Hyun, H.; Kim, H.C.; Kim, J.H.; Chang, H.J.; Connected Network for EMS Comprehensive Technical Support Using Artificial Intelligence Investigators. Development of Prediction Models for Acute Myocardial Infarction at Prehospital Stage with Machine Learning Based on a Nationwide Database. J. Cardiovasc. Dev. Dis. 2022, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Singh, B.; Theofilatos, K.; Sörensen, N.A.; Lehmacher, J.; Hartikainen, T.; Haller, P.M.; Westermann, D.; Zeller, T.; Blankenberg, S.; et al. Serial measurements of protein and microRNA biomarkers to specify myocardial infarction subtypes. J.Mol. Cell Cardiol. Plus 2022, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Tern, P.J.W.; Vaswani, A.; Yeo, K.K. Identifying and Solving Gaps in Pre- and In-Hospital Acute Myocardial Infarction Care in Asia-Pacific Countries. Korean Circ. J. 2023, 53, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Data integration using information and communication technology for emergency medical services and systems. Clin. Exp. Emerg. Med. 2023, 10, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, G.; McGinnis, K. Integration of Tehnology Data is nice but must be turned into useful information. EMS World 2016, (Suppl. 14), 2158–7833. [Google Scholar]
- Ważny, M.; Wójcik, G.M. Shifting spatial attention–numerical model of Posner experiment. Neurocomputing 2014, 135, 139–144. [Google Scholar] [CrossRef]
- Kawala-Janik, A.; Bauer, W.; Żołubak, M.; Baranowski, J. Early-stage pilot study on using fractional-order calculus-based filtering for the purpose of analysis of electroencephalography signals. Stud. Log. Gramm. Rhetor. 2016, 47, 103–111. [Google Scholar] [CrossRef]
- Gajos-Balińska, A.; Wójcik, G.M.; Stpiczyński, P. Hybrid implementation of the fastICA algorithm for high-density EEG using the capabilities of the Intel architecture and CUDA programming. Comput. Sci. 2024, 24. [Google Scholar] [CrossRef]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Kwasniewicz, L.; Schneider, P.; Polak, N.; Gajos-Balinska, A. Mapping the Human Brain in Frequency Band Analysis ofBrain Cortex Electroencephalographic Activity for Selected Psychiatric Disorders. Front. Neuroinformatics 2018, 12, 73. [Google Scholar] [CrossRef]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Kwasniewicz, L.; Schneider, P.; Postepski, F.; Gajos-Balinska, A. Analysis of Decision-Making Process Using Methods ofQuantitative Electroencephalography and Machine Learning Tools. Front. Neuroinformatics 2019, 13, 73. [Google Scholar] [CrossRef]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Schneider, P.; Kwasniewicz, L.; Polak, N.; Gajos-Balinska, A. New Protocol for Quantitative Analysis of Brain Cortex Electroencephalographic Activity in Patients with Psychiatric Disorders. Front. Neuroinformatics 2018, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- AHA23 Conference. Available online: https://professional.heart.org/en/meetings/scientific-sessions (accessed on 25 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).