One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural Analysis
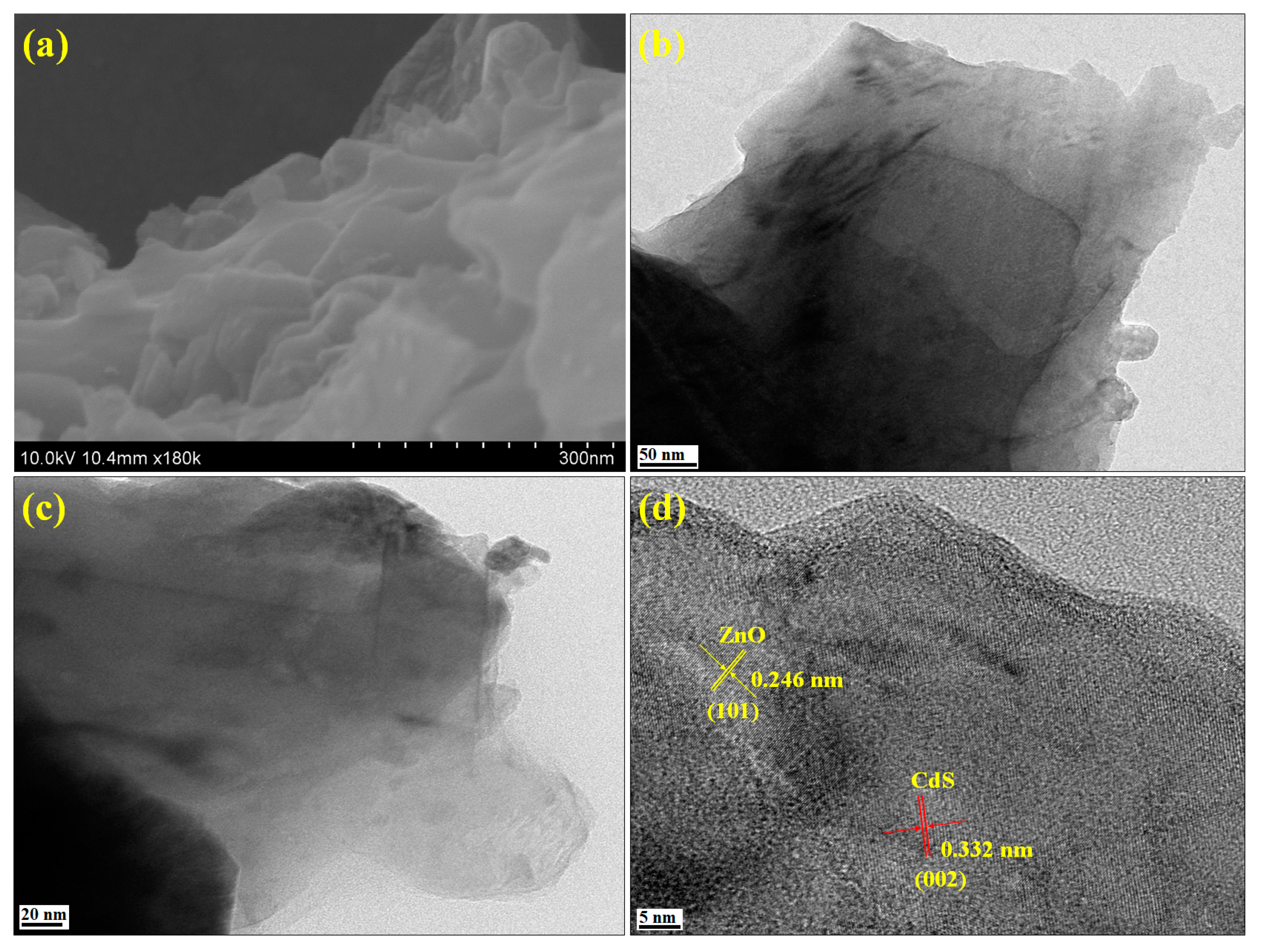
3.2. Morphological Analysis
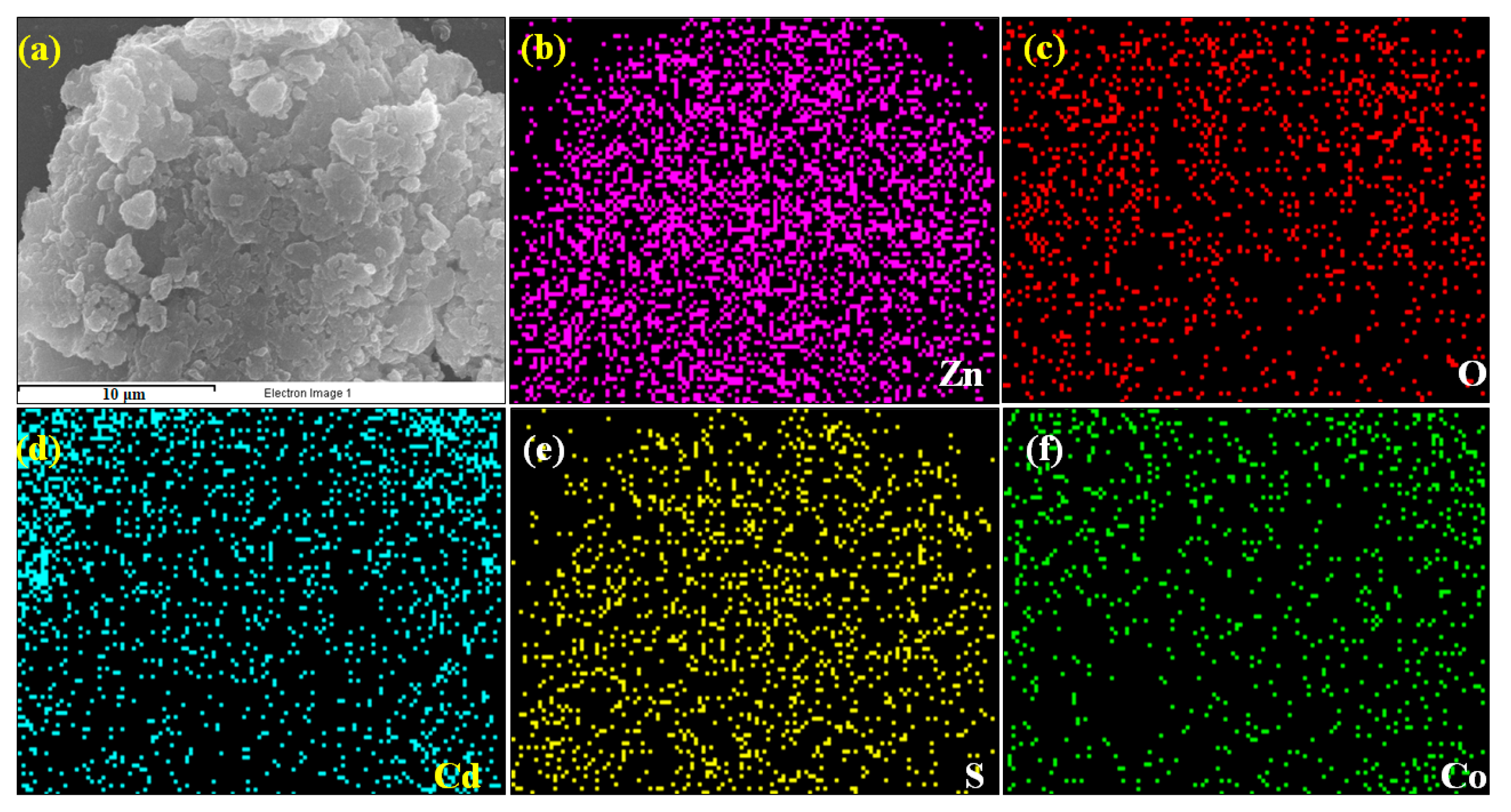
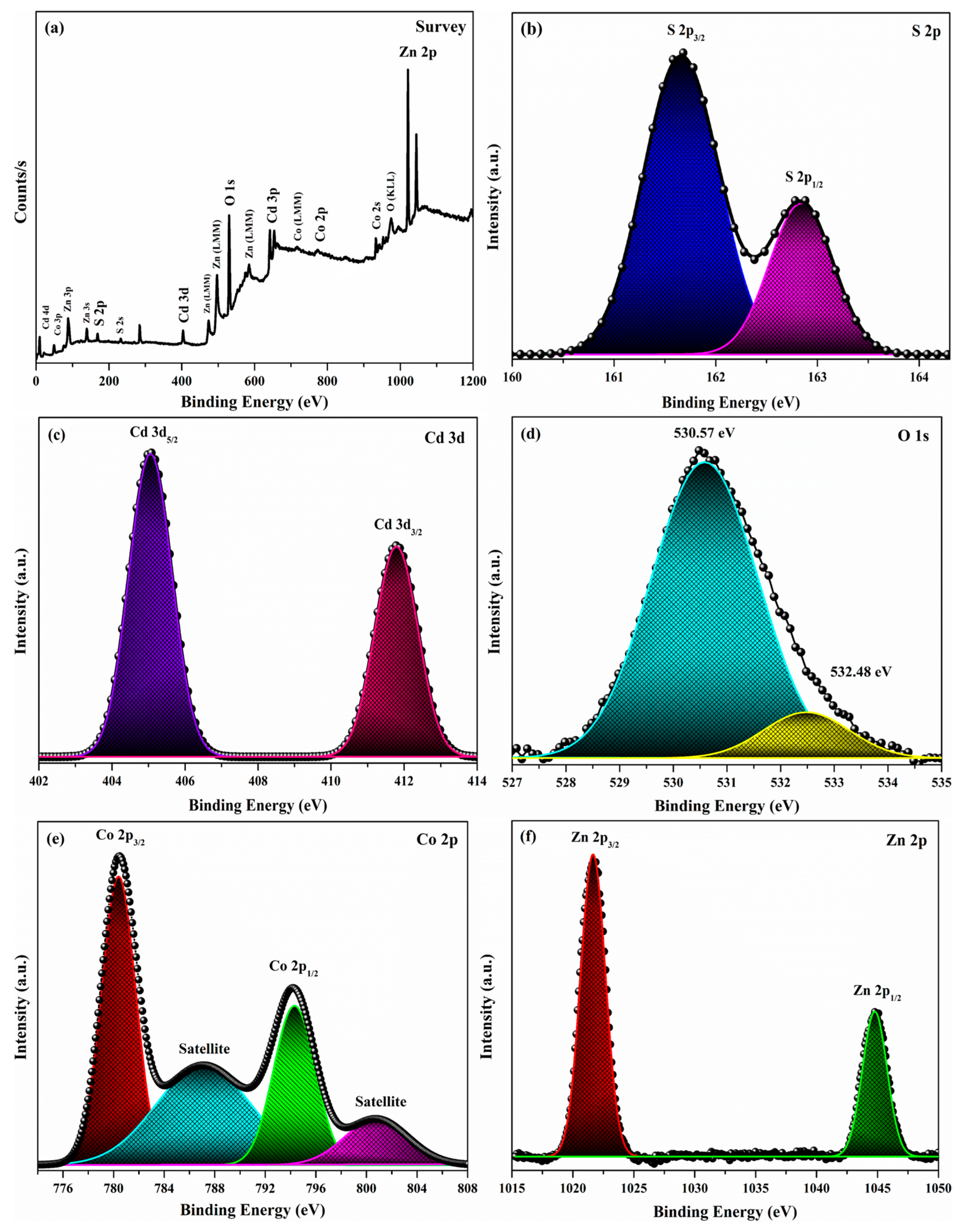
3.3. Elemental Analysis
3.4. Optical Studies
3.5. Magnetic Studies
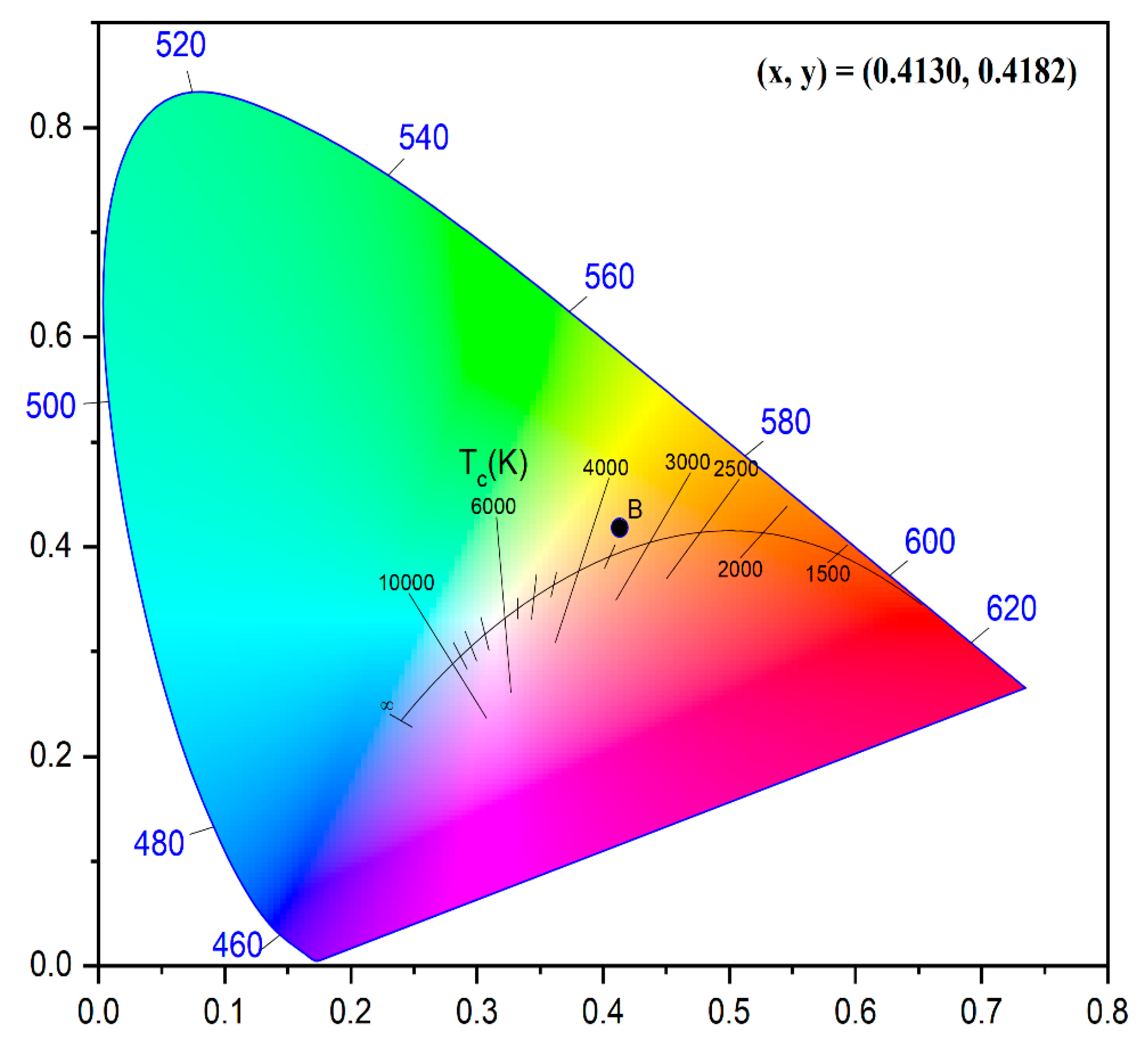
3.6. Chromaticity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radhika, P.; Achary, K.M.R.; Sreekanth, M. A study of tunable optical properties of zno/cds heterostructures with varying shell thickness structures. Mater. Today Proc. 2021, 46, 903–907. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Wang, S.; Yang, H.; Yi, Z. A simple fabrication, microstructure, optical, photoluminescence and supercapacitive performances of MgMoO4/MgWO4 heterojunction micro/nanocomposites. Solid State Sci. 2022, 129, 106909. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Fan, K.; Liu, Z.; Song, B.; Yu, J. Mof-based transparent passivation layer modified zno nanorod arrays for enhanced photo-electrochemical water splitting. Adv. Energy Mat. 2018, 8, 1800101. [Google Scholar] [CrossRef]
- Cai, L.; Du, Y.; Guan, X.; Shen, S. Cds nanocrystallites sensitized zno nanorods with plasmon enhanced photoelectrochemical performance. Chin. Chem. Lett. 2019, 30, 2363–2367. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.S.S.N.; Koutavarapu, R. Structural, optical, and luminescence properties of Ni2+-doped ZnO-CdS nanocomposite: Synthesis and investigations for green light emission. Chem. Pap. 2022, 76, 557–566. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.S.S.N.; Koutavarapu, R. Structural, optical, and photoluminescence properties of Cr3+ ion-doped ZnO-CdS nanocomposite: Synthesis and investigations for yellow emission. J. Electron. Mater. 2022, 51, 1876–1883. [Google Scholar] [CrossRef]
- Arun, V.; Manikandan, V.; AlSalhi, M.S.; Devanesan, S.; Priyadharsan, A.; Ramesh, K.K.A.; Maadeswaran, P. An efficient optical properties of sn doped ZnO/CdS based solar light driven nanocomposites for enhanced photocatalytic degradation applications. Chemosphere 2022, 300, 134460. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, X.; Wu, Y.; Gao, S.; Liu, L.; Xu, Y.; Cheng, X.; Zheng, M.; Zhou, X.; Huo, L. In-situ controllable assembly of 3d zno-zns heterojunction nanotube arrays for enhancing NO2-sensing performance at low energy consumption and sensing mechanism. Sens. Actuators B 2023, 380, 133304. [Google Scholar] [CrossRef]
- Sun, H.; Park, S.-J. Highly efficient reduction of aqueous Cr(vi) with novel zno/sns nanocomposites through the piezoelectric effect. J. Environ. Sci. 2022, 118, 57–66. [Google Scholar] [CrossRef]
- Bai, M.; Chen, M.; Li, X.; Wang, Q. One-step cvd growth of ZnO nanorod/SnO2 film heterojunction for NO2 gas sensor. Sens. Actuators B 2022, 373, 132738. [Google Scholar] [CrossRef]
- Tsai, C.-K.; Lee, Y.-C.; Nguyen, T.T.; Horng, J.-J. Levofloxacin degradation under visible-led photo-catalyzing by a novel ternary Fe–ZnO/WO3 nanocomposite. Chemosphere 2022, 298, 134285. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Meng, C.; Wang, Y.; Zhang, L.; Pan, L.; Yin, Z.; Tang, N.; Zou, J.-J. Boosting photoelectrochemical water splitting by Au@Pt modified ZnO/CdS with synergy of au-s bonds and surface plasmon resonance. J. Catal. 2022, 408, 196–205. [Google Scholar] [CrossRef]
- Khan, Z.R.; Alshammari, A.S.; Shkir, M.; AlFaify, S. Linear, third order nonlinear optical and photoluminescence properties of Cd0.99Zn0.09S/ZnO nanocomposite thin films for optoelectronics applications. Surf. Interf. 2020, 20, 100561. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Xin, Z.; Yu, Y.; Wang, L.; Zhang, W. Visible light response and heterostructure of composite CdS@ZnS–ZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Kolaei, M.; Tayebi, M.; Masoumi, Z.; Lee, B.-K. A novel approach for improving photoelectrochemical water splitting performance of ZnO-CdS photoanodes: Unveiling the effect of surface roughness of zno nanorods on distribution of cds nanoparticles. J. Alloys Compd. 2022, 906, 164314. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Yan, J.; Liu, S. Heteroepitaxial growth of core-shell zno/cds heterostructure for efficient and stable photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2022, 47, 34410–34420. [Google Scholar] [CrossRef]
- Zhang, C.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. The ultrasonic-induced-piezoelectric enhanced photocatalytic performance of ZnO/CdS nanofibers for degradation of bisphenol a. J. Alloys Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]
- Revathi, M.; Jeyakumari, A.P.; Saravanan, S. Design and fabrication of ZnO/CdS heterostructured nanocomposites for enhanced hydrogen evolution from solar water splitting. Inorg. Chem. Commun. 2021, 134, 109056. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, S.; Fang, Y.; Wang, K.; Sun, J.; Wu, W. In situ constructing ni foam supported ZnO-CdS nanorod arrays for enhanced photocatalytic and photoelectrochemical activity. J. Alloys Compd. 2021, 868, 159187. [Google Scholar] [CrossRef]
- Khan, A.; Shkir, M.; Manthrammel, M.; Ganesh, V.; Yahia, I.; Ahmed, M.; El-Toni, A.M.; Aldalbahi, A.; Ghaithan, H.; AlFaify, S. Effect of Gd doping on structural, optical properties, photoluminescence and electrical characteristics of cds nanoparticles for optoelectronics. Ceram. Int. 2019, 45, 10133–10141. [Google Scholar] [CrossRef]
- Sheng, P.; Yao, L.; Yang, P.; Yang, D.; Lu, C.; Cao, K.; Li, W. The origin of enhanced photoelectrochemical activity in metal-ion-doped ZnO/CdS quantum dots. J. Alloys Compd. 2020, 822, 153700. [Google Scholar] [CrossRef]
- Ravichandran, A.T.; Karthick, R. Enhanced photoluminescence, structural, morphological and antimicrobial efficacy of Co-doped ZnO nanoparticles prepared by co-precipitation method. Results Mater. 2020, 5, 100072. [Google Scholar] [CrossRef]
- Djerdj, I.; Jagličić, Z.; Arčon, D.; Niederberger, M. Co—doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef]
- Dietl, T. A ten-year perspective on dilute magnetic semiconductors and oxides. Nat. Mater. 2010, 9, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Krishnanunni, A.; Kompa, A.; Kekuda, D.; Murari, M.; Rao, K.M. Ferromagnetic ordering in Co-Sm co-doped ZnO prismoids grown by co-precipitation method. Inorg. Chem. Commun. 2022, 144, 109857. [Google Scholar] [CrossRef]
- Demircan, G.; Yalcin, S.; Alivi, K.; Ceyhan, G.; Acikgoz, A.; Balak, M.V.; Aktas, B.; Das, R. The effect of Co and Mn co-doping on structural and optical properties of ZnO thin films. Opt. Mater. 2022, 126, 112163. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Babu, B.; Yoo, K. Structural, optical, and magnetic properties of cobalt-doped ZnAl2O4 nanosheets prepared by hydrothermal synthesis. Energies 2021, 14, 2869. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Ravikumar, R.V.S.S.N.; Koutavarapu, R. Enhanced photocatalytic activity of ZnO/CdS composite nanostructures towards the degradation of rhodamine b under solar light. Catalysts 2022, 12, 84. [Google Scholar] [CrossRef]
- He, Y.; Hu, H.; Wang, J.; Wang, X.; Sun, M.; Tian, C.; Deng, C. Fabrication of multi-scale CdS/ZnO heteroarchitectures with boosted dual photocatalytic activities for hydrogen generation and organic dye degradation under solar light. Mater. Res. Bull. 2023, 162, 112180. [Google Scholar] [CrossRef]
- Ravindranadh, K.; Babu, B.; Rao, M.C.; Shim, J.; Reddy, C.V.; Ravikumar, R.V.S.S.N. Structural and photoluminescence studies of Co2+ doped Ca–Li hydroxyapatite nanopowders. J. Mater. Sci. Mater. Electron. 2015, 26, 6667–6675. [Google Scholar] [CrossRef]
- Rao, G.T.; Stella, R.J.; Babu, B.; Ravindranadh, K.; Reddy, C.V.; Shim, J.; Ravikumar, R. Structural, optical and magnetic properties of Mn2+ doped ZnO-CdS composite nanopowder. Mater. Sci. Eng. B 2015, 201, 72–78. [Google Scholar] [CrossRef]
- Singh, G.P.; Aman, A.K.; Singh, R.K.; Roy, M.K. Effect of low co-doping on structural, optical, and magnetic performance of ZnO nanoparticles. Optik 2020, 203, 163966. [Google Scholar] [CrossRef]
- Dharmana, G.; Rao, M.P.S.; Potukuchi, D.M. Visible light driven robust photocatalytic activity in vanadium-doped ZnO/SnS core-shell nanocomposites for decolorization of MB dye towards wastewater treatment. Inorg. Nano-Met. Chem. 2022, 52, 1059–1076. [Google Scholar] [CrossRef]




| Material | Average Crystallite Size (D) nm | Lattice Strain (ε × 10−3) | Dislocation Density (δ × 1015/m2) |
|---|---|---|---|
| ZnO/CdS [28] | 26 | 1.26 | 1.48 |
| Co doped ZnO/CdS | 23 | 1.505 | 1.89 |
| Parameter | ZnO/CdS | Co Doped ZnO/CdS |
|---|---|---|
| Coercivity | 119.32 Oe | 117.47 Oe |
| Saturation Magnetization | 13.35 × 10−3 emu/g | 17.07 × 10−3 emu/g |
| Retentivity | 1.52 × 10−3 emu/g | 1.83 × 10−3 emu/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddi, L.; Vinukonda, K.; Gurugubelli, T.R.; Koutavarapu, R. One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications. Electronics 2023, 12, 1245. https://doi.org/10.3390/electronics12051245
Maddi L, Vinukonda K, Gurugubelli TR, Koutavarapu R. One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications. Electronics. 2023; 12(5):1245. https://doi.org/10.3390/electronics12051245
Chicago/Turabian StyleMaddi, Lakshmiprasad, Khidhirbrahmendra Vinukonda, Thirumala Rao Gurugubelli, and Ravindranadh Koutavarapu. 2023. "One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications" Electronics 12, no. 5: 1245. https://doi.org/10.3390/electronics12051245
APA StyleMaddi, L., Vinukonda, K., Gurugubelli, T. R., & Koutavarapu, R. (2023). One-Step, In Situ Hydrothermal Fabrication of Cobalt-Doped ZnO/CdS Nanosheets for Optoelectronic Applications. Electronics, 12(5), 1245. https://doi.org/10.3390/electronics12051245







