Conversion Electrode and Drive Capacitance for Connecting Microfluidic Devices and Triboelectric Nanogenerator
Abstract
1. Introduction
2. Microfluidics: A Rapidly Emerging Technology
2.1. The Development of Microfluidics
2.2. Microfluidics Are Used for Biochemical Analysis
2.3. Microfluidics Drive Material Delivery
2.4. Challenges Facing Microfluidics Technology
3. Triboelectric Nanogenerators and Microfluidics: Infinite Possibilities
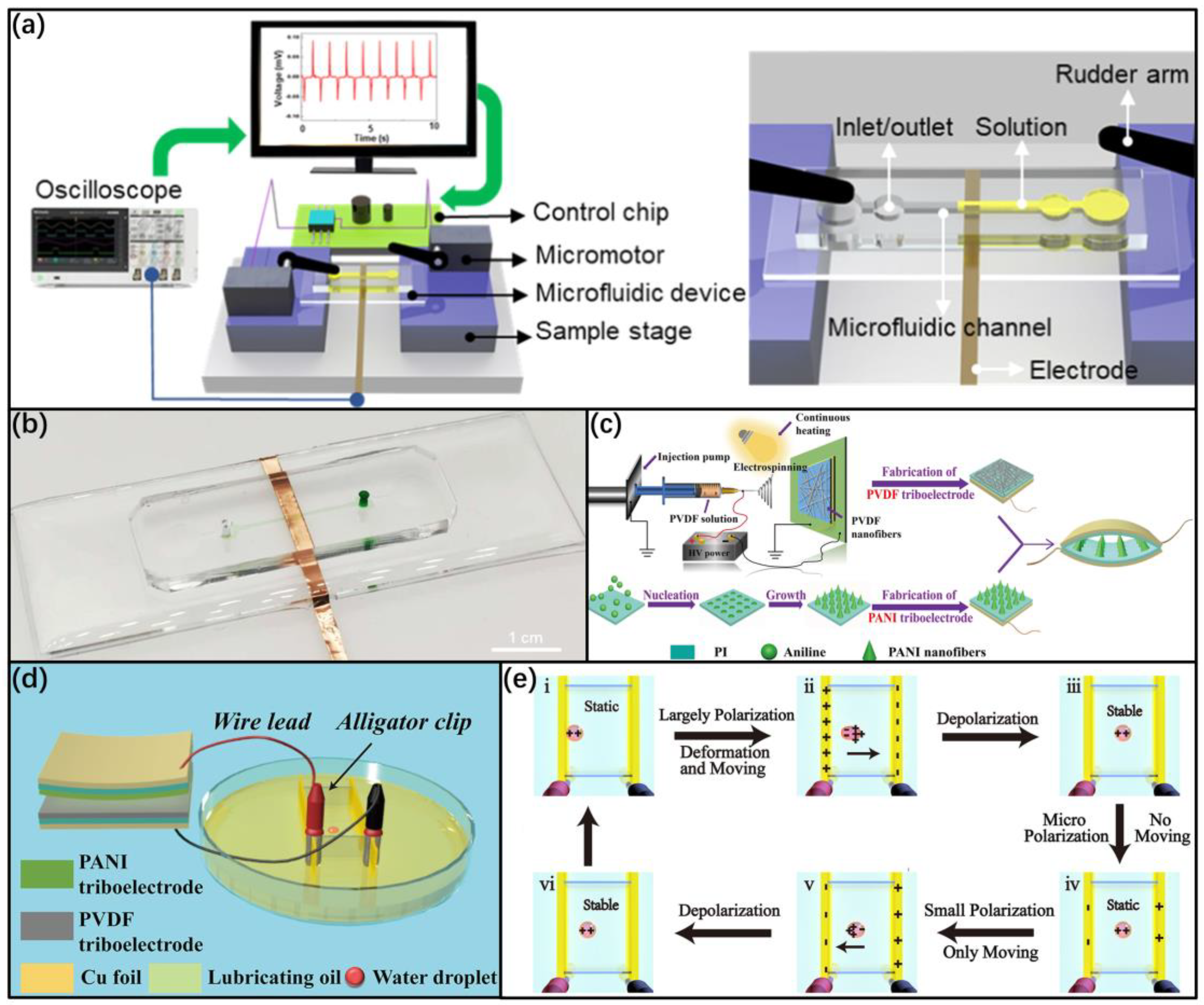
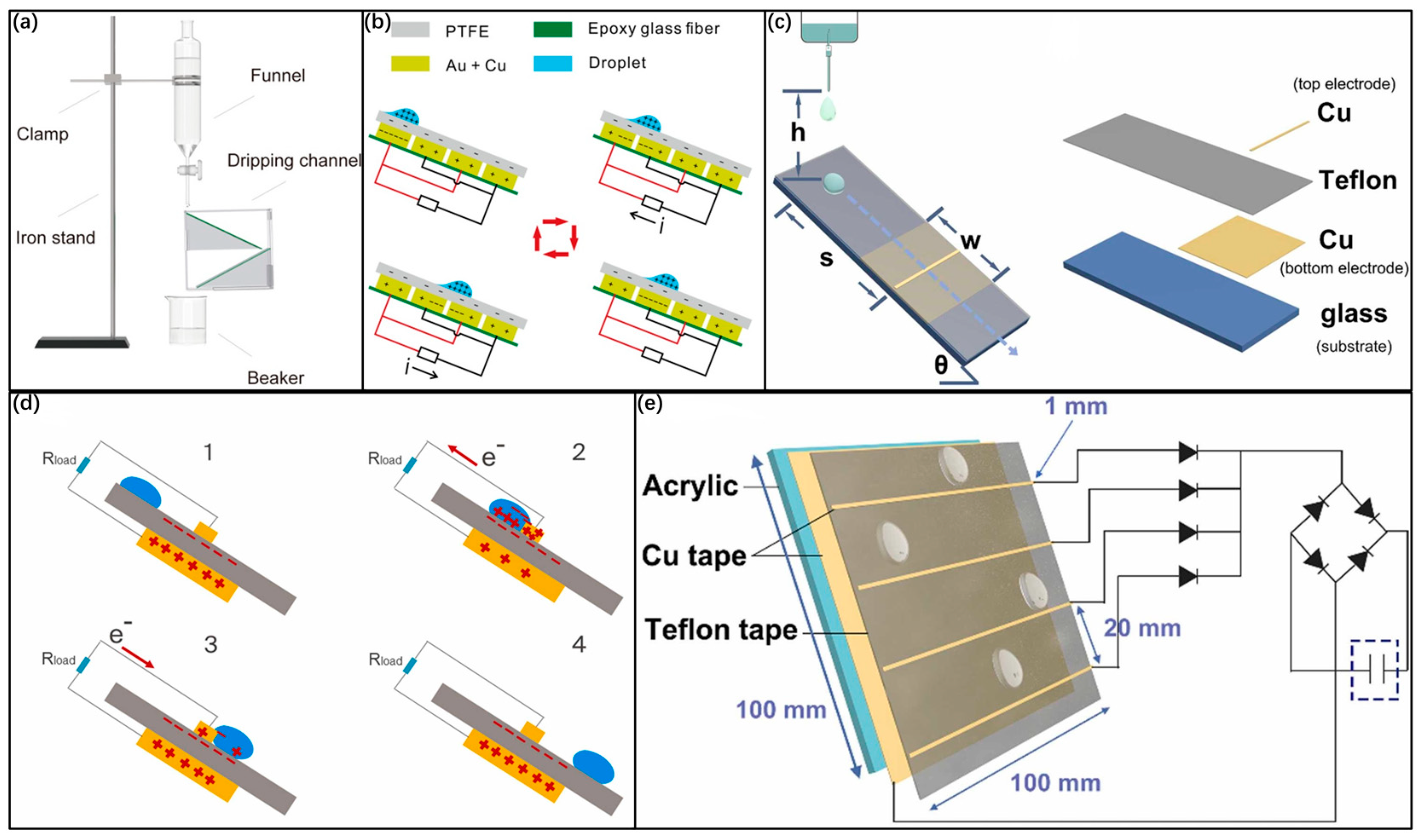
3.1. Conversion Electrode Based on Microfluidics Device
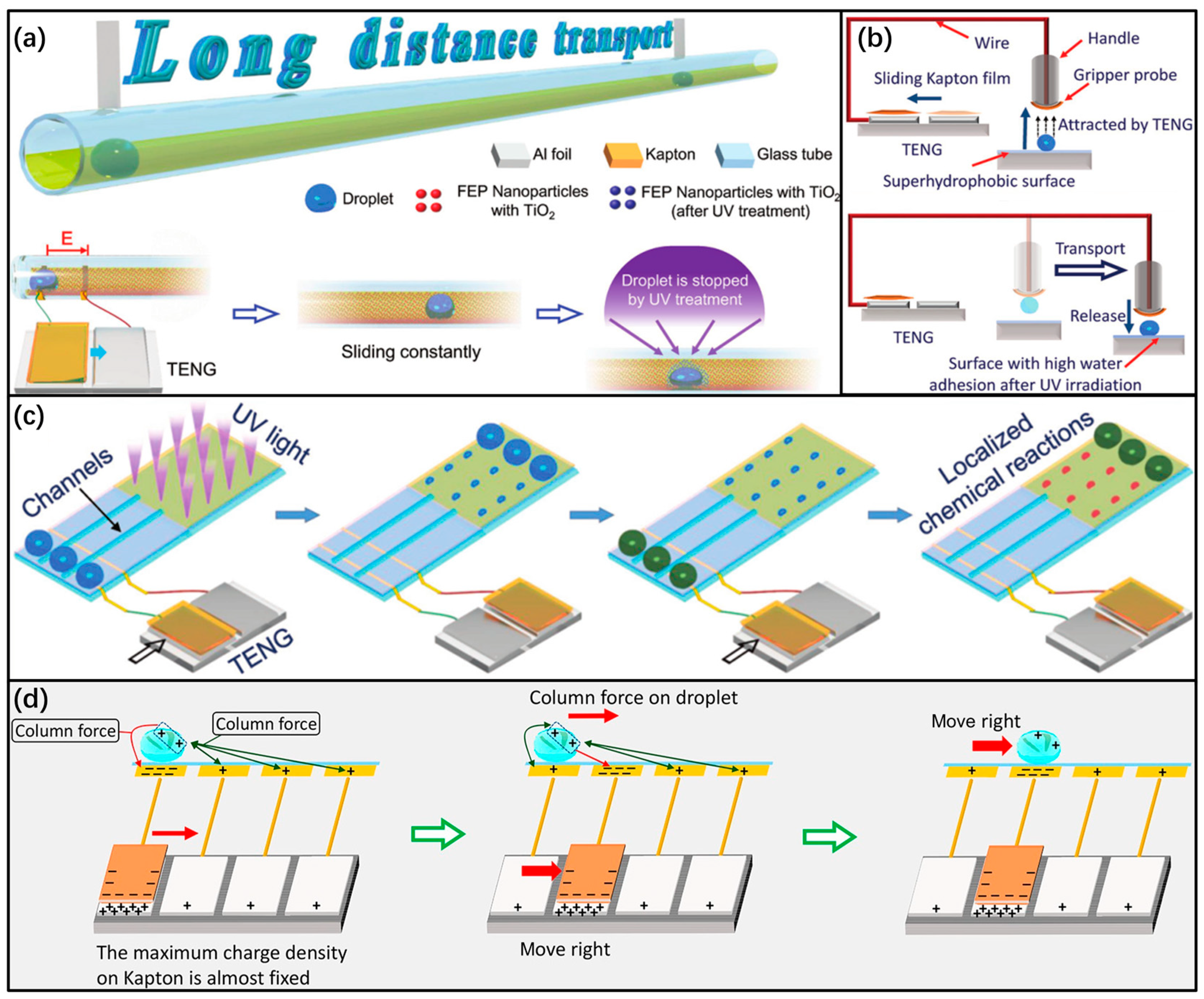
3.2. Drive Capacitance of a Dynamo-Controlled Microfluidics Device Based on Triboelectric Nanogenerators
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, H.A.; Kim, S. Microfluidics: Basic issues, applications, and challenges. AIChE J. 2001, 47, 1250. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: The promise and the challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Lin Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Campopiano, S.; Bernini, R.; Zeni, L.; Sarro, P.M. Microfluidics sensor based on integrated optical hollow waveguides. Opt. Lett. 2004, 29, 1894–1896. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Rahman, M.F.A.; Nawi, M.N.M.; Manaf, A.A.; Arshad, M.R. Characterization of Microfluidics-Based Acoustic Sensor for Immersion Application. IEEE Sens. J. 2015, 15, 1559–1566. [Google Scholar] [CrossRef]
- Waggoner, P.S.; Craighead, H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled microfluidics interfaces. Nature 2004, 437, 648–655. [Google Scholar] [CrossRef]
- Prins, M.W.J.; Welters, W.J.J.; Weekamp, J.W. Fluid Control in Multichannel Structures by Electrocapillary Pressure. Science 2001, 291, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Cho, S.K.; Garrell, R.L.; Kim, C.-J.C. Low voltage electrowetting-on-dielectric. J. Appl. Phys. 2002, 92, 4080–4087. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Y.; Zhong, Q.; Hu, Q.; Hu, B.; Wang, Z.L.; Zhou, J. Fiber-Based Generator for Wearable Electronics and Mobile Medication. ACS Nano 2014, 8, 6273–6280. [Google Scholar] [CrossRef]
- Manz, A.; Effenhauser, C.S.; Burggraf, N.; Verpoorte, E.; Raymond, D.E.; Michael, W.H.J. Capillary Electrophoresis Integrated onto a Planar Microstructure A Step towards Miniaturized Total Analysis Systems(μ-TAS). Analusis (Imprimé) 1994, 47, 1536–1541. [Google Scholar]
- Manz, A.; Verpoorte, E.; Raymond, D.E.; Effenhauser, C.S.; Burggraf, N.; Widmer, H.M. µ-TAS: Miniaturized Total Chemical Analysis Systems. In Micro Total Analysis Systems; Springer: Dordrecht, The Netherlands, 1995; pp. 5–27. [Google Scholar]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidics Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Rosenzweig, D.H.; Sawan, M.; Ajji, A. Evolution of Biochip Technology: A Review from Lab-on-a-Chip to Organ-on-a-Chip. Micromachines 2020, 11, 599. [Google Scholar] [CrossRef]
- Zhang, P.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17. [Google Scholar] [CrossRef]
- Kotz, K.; Noble, K.; Faris, G.J.A.P.L. Optical microfluidics. Appl. Phys. Lett. 2004, 85, 2658–2660. [Google Scholar] [CrossRef]
- Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef]
- Choi, J.-W.; Oh, K.W.; Thomas, J.H.; Heineman, W.R.; Halsall, H.B.; Nevin, J.H.; Helmicki, A.J.; Henderson, H.T.; Ahn, C.H. An integrated microfluidics biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab Chip 2002, 2, 27–30. [Google Scholar] [CrossRef] [PubMed]
- McClain, M.A.; Culbertson, C.T.; Jacobson, S.C.; Allbritton, N.L.; Sims, C.E.; Ramsey, J.M. Microfluidics devices for the high-throughput chemical analysis of cells. Anal. Chem. 2003, 75, 5646–5655. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.N.; Kim, S. Microfluidics platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, A.P.; Wang, J.; Ugaz, V.M. Thermoplastic elastomer gels: An advanced substrate for microfluidics chemical analysis systems. Anal. Chem. 2005, 77, 5167–5173. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, C.-S.; Kim, B.-G.; Kim, Y.-K. An integrated microfluidics chip for the analysis of biochemical reactions by MALDI mass spectrometry. Biomed. Microdevices 2008, 10, 1–9. [Google Scholar] [CrossRef]
- Lien, K.-Y.; Chuang, Y.-H.; Hung, L.-Y.; Hsu, K.-F.; Lai, W.-W.; Ho, C.-L.; Chou, C.-Y.; Lee, G.-B. Rapid isolation and detection of cancer cells by utilizing integrated microfluidics systems. Lab Chip 2010, 10, 2875–2886. [Google Scholar] [CrossRef]
- Lin, C.-S.; Tsai, Y.-C.; Hsu, K.-F.; Lee, G.-B. Optimization of aptamer selection on an automated microfluidics system with cancer tissues. Lab Chip 2021, 21, 725–734. [Google Scholar] [CrossRef]
- Ota, S.; Kitagawa, H.; Takeuchi, S. Generation of Femtoliter Reactor Arrays within a Microfluidics Channel for Biochemical Analysis. Anal. Chem. 2012, 84, 6346–6350. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Gupta, P.; Illath, K.; Kar, S.; Nagai, M.; Tseng, F.-G.; Santra, T.S. Microfluidics mechanoporation for cellular delivery and analysis. Mater. Today Bio 2021, 13, 100193. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidics and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, e2004595. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Lee, R.; Qi, Y.; Wang, K.; Hao, F.; Zhang, Y.; Lu, J.; Meng, Q.; Li, S.; et al. Synthesis of Polymer-Lipid Nanoparticles by Microfluidics Focusing for siRNA Delivery. Molecules 2016, 21, 1314. [Google Scholar] [CrossRef] [PubMed]
- Uguz, I.; Proctor, C.M.; Curto, V.F.; Pappa, A.-M.; Donahue, M.J.; Ferro, M.; Owens, R.M.; Khodagholy, D.; Inal, S.; Malliaras, G.G. A Microfluidics Ion Pump for In Vivo Drug Delivery. Adv. Mater. 2017, 29, 1701217. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D. Chapter 13—Energy harvesting and self-powered devices in droplet microfluidics. In Multidisciplinary Microfluidics and Nanofluidic Lab-on-a-Chip; Li, X., Yang, C., Li, P.C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 361–383. [Google Scholar]
- Moarefian, M.; Davalos, R.V.; Tafti, D.K.; Achenie, L.E.; Jones, C.N. Modeling iontophoretic drug delivery in a microfluidics device. Lab Chip 2020, 20, 3310–3321. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, G.; Caprini, D.; Sinibaldi, G.; Scognamiglio, C.; Silvani, G.; Peruzzi, G.; Casciola, C.M. A Microfluidics Platform for Cavitation-Enhanced Drug Delivery. Micromachines 2021, 12, 658. [Google Scholar] [CrossRef]
- Barbot, A.; Wales, D.; Yeatman, E.; Yang, G.-Z. Microfluidics at Fiber Tip for Nanoliter Delivery and Sampling. Adv. Sci. 2021, 8, 2004643. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, G.; Wang, A.; Sun, W.; Mi, S. Pressure-Triggered Microfluidics Contact Lens for Ocular Drug Delivery. ACS Appl. Polym. Mater. 2022, 4, 7290–7299. [Google Scholar] [CrossRef]
- Chen, J.; Guo, H.; Zheng, J.; Huang, Y.; Liu, G.; Hu, C.; Wang, Z.L. Self-Powered Triboelectric Micro Liquid/Gas Flow Sensor for Microfluidics. ACS Nano 2016, 10, 8104–8112. [Google Scholar] [CrossRef]
- Ahn, J.; Ko, J.; Lee, S.; Yu, J.; Kim, Y.; Jeon, N.L. Microfluidics in nanoparticle drug delivery; From synthesis to pre-clinical screening. Adv. Drug Deliv. Rev. 2018, 128, 29–53. [Google Scholar] [CrossRef]
- Chen, B.D.; Tang, W.; He, C.; Jiang, T.; Xu, L.; Zhu, L.P.; Gu, G.Q.; Chen, J.; Shao, J.J.; Luo, J.J.; et al. Ultrafine Capillary-Tube Triboelectric Nanogenerator as Active Sensor for Microliquid Biological and Chemical Sensing. Adv. Mater. Technol. 2017, 3, 1700229. [Google Scholar] [CrossRef]
- Pan, L.; Wang, J.; Wang, P.; Gao, R.; Wang, Y.-C.; Zhang, X.; Zou, J.-J.; Wang, Z.L. Liquid-FEP-based U-tube triboelectric nanogenerator for harvesting water-wave energy. Nano Res. 2018, 11, 4062–4073. [Google Scholar] [CrossRef]
- Kim, W.; Choi, D.; Kwon, J.-Y.; Choi, D. A self-powered triboelectric microfluidics system for liquid sensing. J. Mater. Chem. A 2018, 6, 14069–14076. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Pan, L.; Gao, R.; Zhang, B.; Yang, L.; Guo, H.; Liao, R.; Wang, Z.L. Direct-Current Rotary-Tubular Triboelectric Nanogenerators Based on Liquid-Dielectrics Contact for Sustainable Energy Harvesting and Chemical Composition Analysis. ACS Nano 2019, 13, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Du, X.; Niu, H.; Li, N.; Shen, G.; Li, C.; Wang, Z.L. Motion recognition by a liquid filled tubular triboelectric nanogenerator. Nanoscale 2018, 11, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, M.; Vo, C.P.; Ahn, K.K. Self-powered Flexible PDMS Channel Assisted Discrete Liquid Column Motion Based Triboelectric Nanogenerator (DLC-TENG) as Mechanical Transducer. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 907–917. [Google Scholar] [CrossRef]
- Zhong, W.; Xu, L.; Zhan, F.; Wang, H.; Wang, F.; Wang, Z.L. Dripping Channel Based Liquid Triboelectric Nanogenerators for Energy Harvesting and Sensing. ACS Nano 2020, 14, 10510–10517. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Dong-Su, K.; Arunkumar, S.; Jongsung, P.; Yun-Jin, J.; Dong-Weon, L. Flowing water-based tubular triboelectric nanogenerators for sustainable green energy harvesting. Nano Energy 2022, 102, 107675. [Google Scholar] [CrossRef]
- Zhang, Q.; He, M.; Pan, X.; Huang, D.; Long, H.; Jia, M.; Zhao, Z.; Zhang, C.; Xu, M.; Li, S. High performance liquid-solid tubular triboelectric nanogenerator for scavenging water wave energy. Nano Energy 2022, 103, 107810. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Brugger, J.; Liu, X. Study of the enhanced electricity output of a sliding droplet-based triboelectric nanogenerator for droplet sensor design. Nano Energy 2022, 98, 107166. [Google Scholar] [CrossRef]
- Wang, J.; Zi, Y.; Li, S.; Chen, X. High-voltage applications of the triboelectric nanogenerator—Opportunities brought by the unique energy technology. MRS Energy Sustain. 2019, 6, E17. [Google Scholar] [CrossRef]
- Nie, J.; Jiang, T.; Shao, J.; Ren, Z.; Bai, Y.; Iwamoto, M.; Chen, X.; Wang, Z.L. Motion behavior of water droplets driven by triboelectric nanogenerator. Appl. Phys. Lett. 2018, 112, 183701. [Google Scholar] [CrossRef]
- Nie, J.; Ren, Z.; Bai, Y.; Shao, J.; Jiang, T.; Xu, L.; Chen, X.; Wang, Z.L. Long Distance Transport of Microdroplets and Precise Microfluidics Patterning Based on Triboelectric Nanogenerator. Adv. Mater. Technol. 2019, 4, 1800300. [Google Scholar] [CrossRef]
- Nie, J.; Ren, Z.; Shao, J.; Deng, C.; Xu, L.; Chen, X.; Li, M.; Wang, Z.L. Self-Powered Microfluidics Transport System Based on Triboelectric Nanogenerator and Electrowetting Technique. ACS Nano 2018, 12, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lee, K.; Deng, S.; Seo, S.; Xia, F.; Kim, T. Portable triboelectric microfluidics system for self-powered sensors towards in-situ detection. Nano Energy 2021, 85, 105980. [Google Scholar] [CrossRef]
- Yu, J.; Wei, X.; Guo, Y.; Zhang, Z.; Rui, P.; Zhao, Y.; Zhang, W.; Shi, S.; Wang, P. Self-powered droplet manipulation system for microfluidics based on triboelectric nanogenerator harvesting rotary energy. Lab Chip 2020, 21, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Feng, Y.; Wang, B.; Liu, Y.; Wu, Z.; Yang, D.; Zheng, Y.; Peng, J.; Feng, M.; Wang, D. A new method for the electrostatic manipulation of droplet movement by triboelectric nanogenerator. Nano Energy 2021, 86, 106115. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Y.; Liu, W.; Sun, H.; Wu, W.; Song, C.; Xue, R.; Jiang, T.; Jiang, H.; Ren, Y. Self-powered AC electrokinetic microfluidics system based on triboelectric nanogenerator. Nano Energy 2021, 89, 106451. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Qiu, Y.; Wang, L.; Li, C.; Liu, G.; Liu, G.; Zheng, Q.; Chen, X.; Tian, H.; et al. Electrowetting-on-dielectric powered by triboelectric nanogenerator. Nano Energy 2022, 98, 107310. [Google Scholar] [CrossRef]
- Li, X.; Tat, T.; Chen, J. Triboelectric nanogenerators for self-powered drug delivery. Trends Chem. 2021, 3, 765–778. [Google Scholar] [CrossRef]
- Wu, C.; Wang, A.C.; Ding, W.; Guo, H.; Wang, Z.L. Triboelectric Nanogenerator: A Foundation of the Energy for the New Era. Adv. Energy Mater. 2018, 9, 1802906. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, B.; Chen, J.; Jing, Q.; Lin Wang, Z. Triboelectric nanogenerators as a new energy technology: From fundamentals, devices, to applications. Nano Energy 2015, 14, 126–138. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, Z.L. Triboelectric nanogenerators as flexible power sources. npj Flex. Electron. 2017, 1, 10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zeng, F.; Pu, Z.; Fan, J. Conversion Electrode and Drive Capacitance for Connecting Microfluidic Devices and Triboelectric Nanogenerator. Electronics 2023, 12, 522. https://doi.org/10.3390/electronics12030522
Zhu Z, Zeng F, Pu Z, Fan J. Conversion Electrode and Drive Capacitance for Connecting Microfluidic Devices and Triboelectric Nanogenerator. Electronics. 2023; 12(3):522. https://doi.org/10.3390/electronics12030522
Chicago/Turabian StyleZhu, Zhiyuan, Fan Zeng, Zhihua Pu, and Jiyu Fan. 2023. "Conversion Electrode and Drive Capacitance for Connecting Microfluidic Devices and Triboelectric Nanogenerator" Electronics 12, no. 3: 522. https://doi.org/10.3390/electronics12030522
APA StyleZhu, Z., Zeng, F., Pu, Z., & Fan, J. (2023). Conversion Electrode and Drive Capacitance for Connecting Microfluidic Devices and Triboelectric Nanogenerator. Electronics, 12(3), 522. https://doi.org/10.3390/electronics12030522