Abstract
Artificial intelligence (AI) advancements, especially deep learning, have significantly improved medical image processing and analysis in various tasks such as disease detection, classification, and anatomical structure segmentation. This work overviews fundamental concepts, state-of-the-art models, and publicly available datasets in the field of medical imaging. First, we introduce the types of learning problems commonly employed in medical image processing and then proceed to present an overview of commonly used deep learning methods, including convolutional neural networks (CNNs), recurrent neural networks (RNNs), and generative adversarial networks (GANs), with a focus on the image analysis task they are solving, including image classification, object detection/localization, segmentation, generation, and registration. Further, we highlight studies conducted in various application areas, encompassing neurology, brain imaging, retinal analysis, pulmonary imaging, digital pathology, breast imaging, cardiac imaging, bone analysis, abdominal imaging, and musculoskeletal imaging. The strengths and limitations of each method are carefully examined, and the paper identifies pertinent challenges that still require attention, such as the limited availability of annotated data, variability in medical images, and the interpretability issues. Finally, we discuss future research directions with a particular focus on developing explainable deep learning methods and integrating multi-modal data.
1. Introduction
The integration of artificial intelligence (AI) methods into the healthcare domain has brought transformative advancements that hold substantial potential for improving medical practices and diagnostic capabilities [1,2]. Within this paradigm, the convergence of AI with medical image analysis is a notable achievement, offering profound insights into human anatomy and physiology through the intricate interpretation of visual data [3]. This confluence of computational intelligence and medical imaging has propelled the development of sophisticated techniques with high significance for disease detection, prognosis, and treatment planning.
Medical imaging has evolved to be a foundation of modern clinical practice, enabling clinicians to gather valuable insights into the inner workings of the human body. However, the complexity of imaging modalities such as positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT) and ultrasound imaging (UI) [4,5,6,7] has presented a challenge in efficient and accurate analysis. Manual interpretation of these images is highly influenced by subjectivity and time consumption, necessitating innovative solutions to harness the full potential of these visual data.
AI methods, particularly machine learning (ML) and deep learning (DL), have emerged as transformative solutions to address the challenges posed by the complex nature of modern imaging modalities. These advanced computational techniques have revolutionized how medical professionals extract meaningful information from complex visual data. By leveraging vast amounts of annotated medical images, ML algorithms have been trained to discern intricate patterns, anomalies, and correlations that might not be easily identifiable by the human eye. DL, a subset of ML, has refined this process by employing neural networks with numerous layers, enabling the extraction of hierarchical features from raw data.
Within the domain of medical imaging, AI-powered methods have demonstrated a remarkable ability for automating tasks that were previously vulnerable to subjectivity and variability. These encompass different tasks such as image classification [8,9], object localization and detection, segmentation [10], synthetic image generation, and registration [11,12,13]. These AI-powered methods enhance the precision and accuracy of diagnostic outcomes while simultaneously expediting the analysis process.
This work aims to provide a comprehensive overview of the rapidly evolving field of AI techniques in medical image analysis. The motivation behind this paper stems from the increasing significance of AI methodologies in revolutionizing healthcare and diagnostics. As medical imaging modalities advance in complexity and volume, the need for efficient and accurate analysis becomes more pronounced. This review paper addresses this need by exploring and evaluating various ML and DL methods and commonly used datasets in medical image analysis. By synthesizing existing research, methodologies, challenges, and breakthroughs, the paper aspires to serve as a valuable resource for researchers and practitioners in the medical and computer science fields. The contributions of this paper can be summarized as follows:
- The paper synthesizes a diverse range of ML methods that have been developed and applied to medical image analysis. Through a critical analysis of various AI techniques, the paper offers insights into the strengths, limitations, and potential applications of each method.
- The paper provides a comprehensive overview of existing publicly available datasets of various anatomical structures that are suitable for use in AI-powered medical image analysis.
- The review identifies emerging trends and challenges within the field of AI-driven medical image analysis. By highlighting gaps in current research and pointing out areas that require further exploration, the paper fosters the growth of knowledge and innovation in this rapidly evolving field.
- The paper discusses the translation of AI techniques from research to clinical practice, emphasizing their potential impact on healthcare delivery.
Through comprehensive exploration and evaluation of current state-of-the-art methodologies, we aim to contribute to the advancement of both academic research and practical applications in the pursuit of improving healthcare outcomes by addressing following research questions:
- What are the prominent AI methods used in medical image analysis?
- In what medical imaging tasks have AI methods shown the most promising results?
- What are commonly observed anatomical structures and available datasets for the development of AI algorithms?
- How can AI-driven medical image analysis be effectively translated into clinical practice?
The study is organized as follows. Section 2 briefly describes the most essential learning methods in the ML field. Section 3 provides a brief theoretical background on DL architectures commonly applied for medical image processing and analysis. Section 4 overviews commonly solved medical imaging tasks, including medical image classification, object localization, detection, segmentation, synthetic image generation, and registration. Section 5 overviews commonly observed anatomical structures in medical imaging, including abdominal, brain, breast, cardiac, musculoskeletal, pulmonary, and retinal imaging. This section also includes a list of datasets for each anatomical structure. Section 6 discusses the possibility of translation of AI techniques into clinical practice. Finally, Section 7 gives a concluding remarks.
2. Learning Methods in Medical Image Analysis
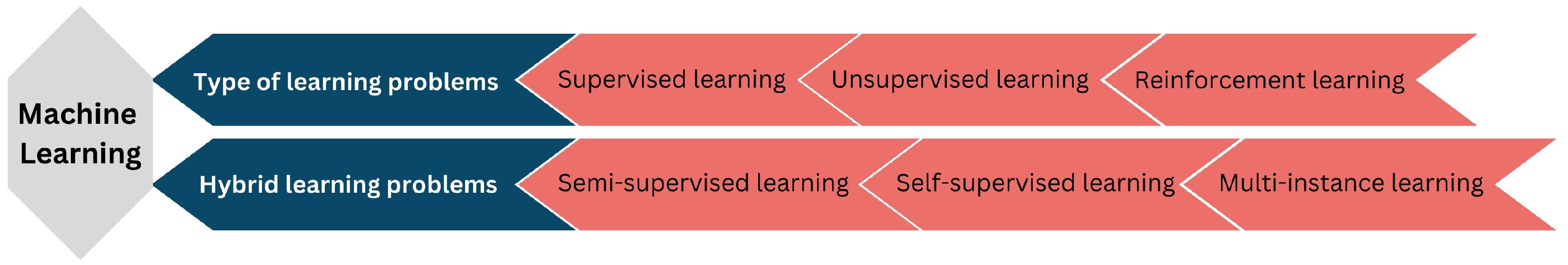
In medical image analysis, ML methods have emerged as essential tool for analyzing intricate patterns and insights encoded within medical images. These methods primarily address two distinctive problem categories, which are shown in Figure 1.

Figure 1.
An overview of ML types and techniques in medical image analysis.
The first category encompasses supervised learning, wherein algorithms learn from annotated data to predict output variables based on input features, facilitating accurate diagnoses and classifications. Unsupervised learning, in contrast, involves the exploration of unlabeled data to uncover latent structures and relationships within medical images, enabling the discovery of inherent patterns and clusters. Reinforcement learning introduces an agent–environment interaction dynamic based on cumulative reward maximization through iterative decision making. The second group of learning problems in medical image analysis encompasses hybrid methodologies. Semi-supervised learning leverages labeled and unlabeled data to enhance model performance in cases where comprehensive annotations are scarce. Self-supervised learning exploits intrinsic data relationships by predicting missing portions of the data themselves, thereby effectively harnessing unlabeled data for learning. Multi-instance learning suits scenarios where data instances are interconnected, allowing the algorithm to grasp relationships between instances within the broader context.
2.1. Supervised Learning
Supervised learning [14] is a computational approach where a model learns to map input data to corresponding output labels through exposure to a labeled training dataset. The term supervised stems from providing explicit guidance in the form of labeled examples during the model’s training phase. This guidance enables the model to generalize from the training data and subsequently predict labels for unseen instances. Supervised learning is a powerful approach in tasks with abundant labeled datasets when the goal is to make predictions or classifications based on historical patterns [15]. Generally, supervised learning is often used for tasks like classification and regression in different fields such as categorizing emails as spam or non-spam [16,17] or estimating the price of a house based on features such as square footage and location [18,19].
In medical image analysis, supervised learning is frequently utilized for image segmentation [20], and disease classification [21]. For instance, supervised learning enables the accurate delineation of anatomical structures and pathological regions within medical images in segmentation tasks, facilitating precise treatment planning and monitoring [22].
Supervised learning excels in detecting intricate patterns in medical images to accurately identify complex conditions often missed by human perception [23]. Its quantitative nature enhances analysis reliability, minimizing variability between observers and enabling disease progression prediction. The trained models process images rapidly, expediting clinical workflows, and can generalize knowledge to diverse patient cases. However, high-quality labeled data are essential but resource-intensive to acquire [24]. Moreover, overfitting often significantly affects generalization, biases in training data may lead to skewed predictions, and complex models can compromise interpretability, which is vital for informed medical decisions. To harness supervised learning’s potential in medical practice, addressing data dependencies, overfitting, bias, and interpretability is essential [25,26].
2.2. Unsupervised Learning
Unsupervised learning [27] is a computational approach where algorithms seek to uncover latent patterns and structures within unlabeled data without the guidance of explicit output labels. Unlike supervised learning, the absence of labeled examples necessitates the model’s capacity to discern inherent relationships and groupings within the data, thereby facilitating its categorization and subsequent interpretation [28]. Choosing unsupervised learning is beneficial when the goal is to explore and understand the inherent structure of the data, making it ideal for tasks where explicit output labels are either challenging to obtain or unnecessary, and in situations where labeled data are scarce or unavailable [29]. Unsupervised learning is used for tasks like clustering [30,31,32,33], dimensionality reduction [34,35], and anomaly detection [36].
In medical image analysis, unsupervised learning excels in different classification tasks like benign and malignant tumor detection [37,38,39], domain adaptation in cardiac arrhythmia classification [40,41], brain disorder classification [42,43,44], and mass detection in beast cancer [45].
The crucial benefit of unsupervised learning in medical image processing and analysis is in its ability to uncover hidden patterns and relationships, which reveals subtle variations in images. This aids in understanding complex anatomical and pathological phenomena. It facilitates novel insights and hypothesis generation by categorizing unlabeled data into clusters. Techniques like dimensionality reduction enhance interpretability by simplifying high-dimensional image analysis. Nevertheless, unsupervised models can capture noise, generating clinically irrelevant categories. Thus, applying unsupervised learning demands awareness of its challenges, reliance on clinical expertise, and rigorous validation.
2.3. Reinforcement Learning
Reinforcement learning [46] has the capacity to adaptively learn from interactions, and aligns well with dynamic medical contexts, where optimal decisions depend on evolving patient conditions and complex imaging processes. Reinforcement learning is suitable for tasks where the optimal strategy unfolds over time through trial and error. Choosing this approach is recommended when the problem involves decision making under uncertainty, and the model needs to learn from its actions and experiences. Generally, reinforcement learning is used for tasks like game playing [47,48], robotic control [49,50], and autonomous driving systems [51].
In medical image processing and analysis, reinforcement learning is used for tasks such as optimizing imaging parameters during acquisition [52], designing patient-specific treatment regimens [53,54], and automating the exploration of diverse imaging sequences [55]. Moreover, reinforcement learning is leveraged for image enhancement by tailoring post-processing algorithms to individual patients’ characteristics, which enhances diagnostic accuracy [56]. It is also significant in anatomical and biological landmark and coordinate detection [57,58] when the task is to find landmarks that can be precisely reallocated on images produced by different imaging modalities, or reducing the needed time to reach the landmark by using a continuous action space [59], or for localization of modality-invariant landmarks [60]. In object detection and extraction tasks, reinforcement learning benefits in tasks like breast lesion detection [61,62], where learning agents gradually learn the policy to choose among actions to transit, scale the bounding box, and finally localize the breast lesion. It is also used to address the lack of labeled data in brain tumor detection tasks [63,64]. Therefore, reinforcement learning models could work as robust lesion detectors with limited training data [65], reducing time consumption and providing some interpretability [66]. Moreover, in segmentation and classification tasks, learning agents are used for finding optimal local thresholds and post-processing parameters [67], finding the coarse location of region of interest, which is further used for final segmentation [68], or for hyperparameter optimization of existing approaches [69,70,71].
Reinforcement learning offers key benefits. Its adaptability supports personalized treatment strategies, aligning with precision medicine’s principles. Adaptive image acquisition optimizes protocols in real time, enhancing safety and efficiency while balancing exploration and exploitation as well as fostering innovation, and long-term planning optimizes patient outcomes [72]. A common challenge occurs in sample complexity since it requires numerous interactions, which can be limited in medical contexts. Crafting accurate reward functions demands expertise. Ethical concerns arise due to real-world consequences of learning-phase decisions [73]. Addressing these challenges ensures responsible reinforcement learning integration in clinical decision making.
2.4. Semi-Supervised Learning
Semi-supervised learning [74] has elements of both supervised and unsupervised learning. This methodology uses a limited set of labeled data alongside a more extensive pool of unlabeled data to train models that harness labeled information for guidance while extrapolating latent patterns from the unlabeled data. Choosing semi-supervised learning is appropriate when there is a need to harness the benefits of labeled data while maximizing the utility of available unlabeled examples. Generally, semi-supervised learning is specially used in automated speech recognition [75] and natural language processing [76].
In medical image processing and analysis, semi-supervised learning is leveraged to mitigate the challenges posed by limited annotated medical datasets, fostering enhanced diagnostic accuracy and improved insights. It is commonly utilized in segmentation [77], classification [78], and artificial image generation tasks [79].
Semi-supervised learning effectively uses limited labeled data by incorporating abundant unlabeled data, enhancing generalization and model robustness and overcomes supervised learning limitations by capturing latent patterns in unlabeled data. Nevertheless, designing models to balance labeled and unlabeled data requires careful consideration. Incorporating unlabeled data risks noise amplification, demanding quality control, and maintaining control over learning. To effectively employ semi-supervised learning in medical image analysis, balancing its benefits while addressing challenges is essential.
2.5. Self-Supervised Learning
Self-supervised learning [80] is an approach where the model’s training procedure leverages intrinsic information within the data to generate surrogate labels for supervision. This is advantageous when generating labeled data is impractical or costly. It has emerged as a promising strategy to overcome the challenges of limited labeled datasets and is commonly used for tasks like contrastive predictive coding [81], speech representation [82], motion and depth estimation [83], and cross-modal retrieval [84,85].
In medical image analysis, self-supervised learning is used for tasks such as finding similarities of adjacent frames in histopathological tissue images [86], or MRI scans [87], object tracking [88], and correcting frame orders in 3D medical images [89,90].
Self-supervised learning enhances feature learning and model robustness, streamlining development and alleviating annotation burdens. Designing effective pretext tasks requires domain expertise and ensuring the seamless transfer of learned features to diverse medical tasks demands validation and adaptations. Moreover, intensive computational demands for tasks like contrastive learning may extend training times. Addressing these challenges is crucial for responsible integration and harnessing self-supervised learning’s potential in medical image analysis.
2.6. Multi-Instance Learning
Multi-instance learning [91] refers to scenarios where data instances are organized into bags, with each bag containing multiple instances [92]. Unlike traditional single-instance learning, where each instance is independently labeled, in multi-instance learning, bags are labeled based on the collective behavior of their instances [93]. This introduces a level of ambiguity, as the specific instances responsible for the bag’s label are often unknown [94]. Choosing multi-instance learning is suitable when there is a common underlying structure or shared information among tasks that leads to improved generalization and efficiency across a range of related problems. Generally, it is often used for tasks like object detection [95], visual tracking in robotics [96], and human activity recognition [97], as well as in remote sensing for detecting objects in satellite imagery [98].
When using multi-instance learning in medical image analysis, images are treated as bags of sub-regions or lesions, which allows for more detailed insights and holistic analysis. For example, it is commonly utilized for identifying regions of interest in different pathologies like mass retrieval in mammograms [99]. Moreover, it is also used to incorporate patient-level data into the learning and prediction processes [100,101] and to fuse all relevant information within the examination record by including multiple potentially overlapping images or videos from different perspectives and with contextual details, which ultimately enhances performance [102].
Multi-instance learning in medical image analysis offers several advantages [103]. It enables a holistic approach by considering relationships among sub-regions within an image, enhancing understanding of intricate conditions. It reduces annotation effort by labeling entire images, streamlining the process. Enhanced interpretability highlights critical sub-regions, making diagnostic decisions more transparent. Its adaptability to image variability makes it promising for complex conditions, enhancing accuracy and depth of medical image analysis. However, integrating multi-instance learning into medical image processing brings challenges as well [104]. For example, instance ambiguity and determining contributing sub-regions complicate the identification of critical areas and diagnostic precision. Moreover, treating images as bags may result in a loss of fine-grained data, impacting accuracy if important features are missed. Modeling interactions between instances within a bag is complex, requiring careful design and validation. Addressing these issues—instance ambiguity, information loss, model complexity, and overgeneralization—is vital for the effective use of multi-instance learning in medical image analysis [105].
3. Deep Learning Architectures in Medical Image Analysis
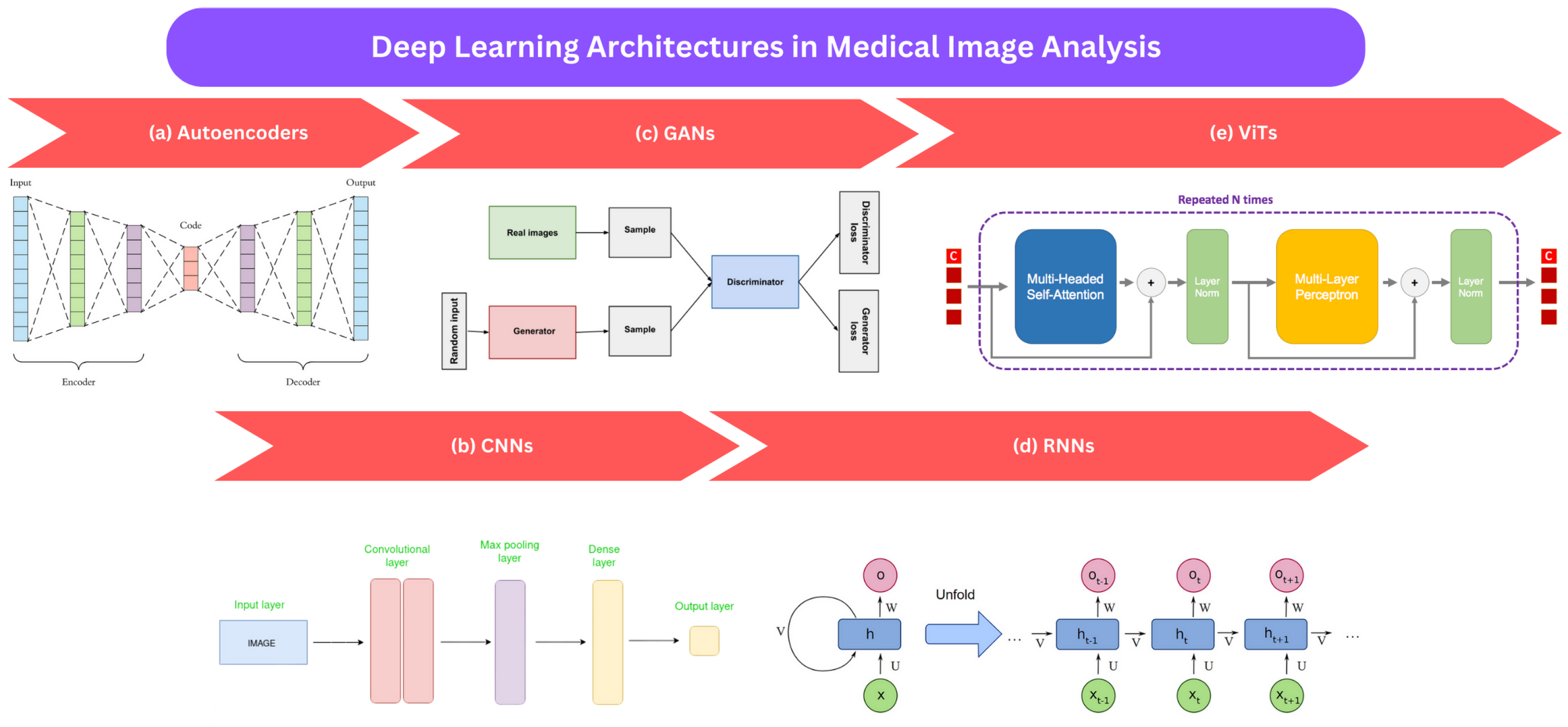
This section provides an overview of commonly employed deep learning approaches in medical image analysis. These techniques include autoencoders (AEs), convolutional neural networks (CNNs), recurrent neural networks (RNNs), generative adversarial networks (GANs), and vision transformers (ViTs), as shown in Figure 2. We explain each method’s fundamental principles and outline their advantages and limitations.
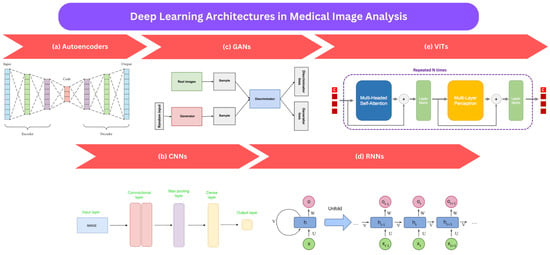
Figure 2.
An illustration of frequently employed deep learning techniques in the analysis of medical images: (a) autoencoders [106], (b) CNNs [107], (c) GANs [108], (d) RNNs [109], and (e) ViTS [110].
3.1. Autoencoders (AEs)
AEs are designed to capture the essential features and underlying patterns within high-dimensional data while simultaneously reducing their dimensionality [111]. This is achieved through a unique two-stage process: the model comprises an encoder and a decoder, with a bottleneck section in between, embodying the compact internal representation of the input. During training, AEs receive the input both as input and target output, compelling the model to reproduce the input by first encoding it into a compressed form and then decoding it back to the original state. After training, the decoder is typically removed, and the encoder is employed to produce compact input representations. In medical image processing and analysis, commonly employed AEs are denoising autoencoders [112], variational autoencoders [113], and stacked autoencoders [114].
3.2. Convolutional Neural Networks (CNNs)
CNNs consist of numerous layers of filters that acquire local features from the input image and progressively merge them to construct more abstract representations. Typically, the initial layers of a CNN learn basic features like edges and corners, while the later layers focus on higher-level features such as object shapes and textures. In contrast, fully convolutional neural networks (FCNNs), a subset of CNNs, lack fully connected layers. They employ an encoder–decoder architecture, which enables processing input of different sizes while producing outputs of the same dimensions. The encoder converts the input image into a high-level feature representation, while the decoder interprets the feature maps and restores spatial details through a series of upsampling and convolution operations, enabling pixel-wise predictions. A prominent FCNN architecture is U-Net [115], known for its capacity to locate objects within input images precisely.
3.3. Generative Adversarial Networks (GANs)
GANs represent an unsupervised learning technique for creating new data that closely mimic the provided data [79]. They consist of two key components: a generator network responsible for producing new samples and a discriminator network that distinguishes between authentic and generated samples. In essence, the generator learns to create samples resembling real data, while the discriminator learns to tell the genuine from the generated. Deep convolutional generative adversarial networks (DCGANs) [116] follow a similar GAN framework but employ deep convolutional networks for both the generator and discriminator. For example, the significant variant is the conditional GAN (cGAN), which can generate samples based on additional information like disease labels or image modalities [117].
3.4. Recurrent Neural Networks (RNNs)
RNNs [118] are especially suitable for dealing with data that unfold over time, like sequences or time-series data, where what happened before affects what happens next. Among the most popular RNN architectures is the long short-term memory (LSTM) network [119]. LSTMs are successful at capturing long-term dependencies in sequences. Unlike traditional neural networks that quickly forget previous information, LSTMs introduce memory cells capable of retaining essential information for extended periods. There are several variations of LSTMs, such as bidirectional LSTM (BLSTM) and multidimensional LSTM (MDLSTM) [120]. In medical image analysis, especially tasks involving sequential data, RNNs offer advantages over CNNs.
3.5. Vision Transformers (ViTs)
Vision Transformers (ViTs) [121] rely on a self-attention mechanism [122], enabling the network to attend to different regions of an image selectively, making them particularly well-suited for processing large, high-resolution medical images. They can efficiently capture contextual information across image patches, even in the presence of substantial anatomical variations [123]. ViTs find applications in tasks like image classification, segmentation, and feature extraction [123].
3.6. Advantages and Disadvantages of DL Architectures
As seen from previous sections, DL architectures have revolutionized various domains by offering powerful tools for automatic feature learning and complex pattern recognition. Here, we dive into the distinct advantages and challenges associated with them, since their understanding is crucial for optimizing the application of DL models, especially in the demanding field of medical image analysis.
For example, AEs have demonstrated high capability in generating synthetic medical images, which can serve as valuable data augmentation techniques for training DL models or mitigating privacy concerns by generating synthetic data while preserving original data characteristics [124]. When used in tasks like image denoising [125] or image reconstruction, their primary challenge is striking the right balance between data compression and reconstruction accuracy. Overly aggressive compression can lead to a loss of clinically relevant information, thereby impacting diagnostic accuracy [126]. Additionally, evaluating the quality of reconstructed or generated medical images can be intricate, as there may not always exist generally applicable and interpretable metrics for assessing accuracy [127]. Despite these challenges, ongoing research efforts aim to explore variations and refinements of AEs to optimize their use and mitigate their limitations in the demanding field of medical image analysis. Moreover, CNNs excel in image-related tasks by learning hierarchical features through convolutional layers. They automatically extract local features, enabling the recognition of complex patterns in images [128]. Despite their effectiveness, CNNs require large amounts of labeled data for training, which can be challenging to obtain in certain domains. Challenges like overfitting, interpretability, and computational intensity, which are shared among all DL architectures, are specifically expressed while training deep CNNs. Furthermore, GANs offer a unique approach to generative modeling by simultaneously training a generator and discriminator, leading to the creation of realistic synthetic data [79]. They find applications in data augmentation and addressing privacy concerns in medical image analysis. Nevertheless, training GANs can be challenging, because they also require careful tuning and monitoring to achieve stable convergence. RNNs excel in handling sequential data with long-term dependencies. Their memory cells enable the retention of essential information over extended periods, making them suitable for tasks such as time-series analysis in medical data [8]. Training RNNs can be computationally intensive, and may suffer from vanishing or exploding gradient problems, affecting the learning of long-term dependencies. While LSTMs address some of these issues, finding the right architecture and hyperparameter settings can be challenging. Interpreting the decisions made by RNNs, especially in complex medical tasks, remains a significant challenge. Furthermore, self-attention mechanisms in ViTs allow them to selectively attend to different regions of an image, making them well-suited for processing large, high-resolution medical images [122]. Their ability to capture contextual information across image patches, even in the presence of substantial anatomical variations, is a distinctive strength. Nevertheless, they may require substantial computational resources for training due to the self-attention mechanism’s computational complexity. The lack of spatial hierarchies might pose challenges in tasks where local features are crucial.
All mentioned DL architectures share the ability to automatically learn representations from data, adaptability to diverse data types, and proficiency in handling large-scale datasets. Common challenges include the need for substantial computational resources, interpretability concerns, and the potential for biases in training data to affect model predictions. Ethical considerations related to fairness and accountability are shared challenges, especially as these models are increasingly integrated into critical decision-making processes. Balancing performance and interpretability remains a central challenge across these diverse DL architectures.
4. Common Tasks Solved by DL in Medical Image Analysis
DL has been applied to various medical imaging tasks, such as image classification, object localization, detection, segmentation, image generation, and registration. This section will discuss some key DL applications in medical image analysis.
4.1. Image Classification
Image classification is based on assigning images to predefined classes or categories to accurately determine the class to which a given image belongs, relying on its visual characteristics. In medical image analysis, classification serves two primary purposes: object classification and exam classification. Object classification aims to classify pre-identified objects into one of several distinct classes. Conversely, exam classification focuses on categorizing a diagnostic image as either representing a normal or abnormal condition or indicating the presence or absence of a particular disease.
CNNs are widely used for image classification, with various CNN frameworks, including AlexNet [129], VGG [130], inception [131], and ResNet [132] being among the most commonly utilized DL architectures. Since they can automatically learn discriminative features from medical images and classify them into different disease categories, they have been commonly utilized for classifying lung nodules in chest CT scans [133,134], breast tumors in mammograms [135], diagnosing diabetic retinopathy in retinal fundus images [136], brain tumor classification [137,138], and cardiovascular disease classification [139]. Besides CNNs, ViTs are recently been used for medical brain and breast image classification tasks [140,141].
4.2. Object Localization and Detection
Object localization and detection are fundamental tasks within computer vision, involving identifying objects in images or videos. Object localization focuses explicitly on delineating the exact spatial boundaries of an object within an image. In contrast, object detection encompasses both localization and recognizing the object itself.
Object localization and detection are vital components of computer-aided diagnosis in medical image analysis. CNNs have demonstrated their effectiveness in identifying the presence and location of specific objects within medical images, such as lung nodules, breast tumors, and brain tumors. Different CNN architectures, including Faster R-CNN [142], You Only Look Once (YOLO) [143], and Single Shot MultiBox Detector (SSD) [144], have been employed to achieve remarkable performance in object detection and localization tasks for medical images.
For example, the Faster R-CNN framework employs a region proposal network to identify potential object locations and a subsequent network to classify these proposals as either an object of interest or background. On the other hand, the YOLO framework utilizes a single network to analyze the entire image and directly predict bounding boxes and class probabilities for objects. Meanwhile, the SSD framework incorporates multiple layers to predict bounding boxes of varying sizes and aspect ratios, enabling precise object detection and localization within medical images.
These object detection and localization techniques have found successful applications across various medical imaging tasks, encompassing tasks like lung nodule detection in chest scans [145], breast lesion identification in mammograms [146], and brain tumor segmentation [147]. Their capacity to accurately identify and locate objects of interest within medical images holds the potential to enhance diagnostic precision and support more effective clinical decision-making.
4.3. Image Segmentation
Image segmentation involves the intricate task of partitioning an image into multiple segments, each corresponding to a specific object or region of interest present within the image. These regions typically represent anatomical structures or any abnormalities that might be present. CNNs have exhibited exceptional performance in medical image segmentation, primarily due to their ability to autonomously learn features from extensive sets of annotated data.
Several architectures based on FCNNs, including U-Net [115], DeepLab [148], and Mask R-CNN [149], have been leveraged to attain state-of-the-art results in medical image segmentation tasks. For example, the U-Net framework comprises an encoder network for extracting features from input images and a decoder network for generating segmentation maps. The DeepLab architecture utilizes techniques like atrous and dilated convolutions to capture multiscale contextual information, significantly enhancing segmentation accuracy. The Mask R-CNN architecture introduces an additional segmentation branch responsible for producing pixel-level segmentation masks. Furthermore, ViTs are being applied to segmentation tasks when small training datasets are available [150], while GANs are often used as data augmentation techniques that precede segmentation tasks [151].
These advanced medical image segmentation techniques have demonstrated successful applications across various imaging modalities and tasks. Examples include the segmentation of brain tumors in MRI [152], lung nodules in chest CT scans [153], polyps [154], and vessel delineation [155]. Additionally, they find widespread use in cardiovascular image segmentation tasks, encompassing the isolation of specific structures like the aorta [156,157], heart chambers [158,159,160], epicardial tissue [161], left atrial appendage [162,163], and coronary arteries [164]. Precise segmentation is invaluable as it facilitates quantification, classification, and visualization of medical image data, ultimately supporting more informed clinical decision-making processes.
4.4. Image Generation
Image generation refers to creating novel images that closely resemble real patient data. This capability is valuable in diverse applications, including data augmentation or for creating synthetic patient data often used for training DL models. In the realm of medical imaging, GANs have emerged as a widely employed technique for image generation tasks.
For instance, GANs find common use in medical imaging tasks like generating MRI images from CT scans or producing realistic skin lesion images for dermatological purposes [165]. Moreover, GANs and AEs are essential in creating synthetic medical images for training DL models [166]. They have proven beneficial in tasks such as image denoising [167] and image super-resolution [168]. Specifically, cGANs have been harnessed in diverse medical image synthesis tasks. This includes tasks like synthesizing MRIs based on CT images and generating realistic CT images defining cardiovascular structures, particularly in cardiology applications [117].
4.5. Image Registration
Image registration is another critical task within the domain of medical image analysis. This task refers to the alignment of two or more images. These images may originate from the same patient but differ in acquisition times or stem from different patients or imaging modalities. Image registration is immensely useful in various medical imaging applications, encompassing multi-modal and deformable image registration [169]. The latter is indispensable for monitoring disease progression, formulating treatment plans, and guiding image-based medical interventions.
In deformable image registration, CNNs have found significant application [170]. The primary objective here is to estimate a dense deformation field that maps the voxels of one image to their corresponding counterparts in another image. Typically, CNN-based registration methods entail training a network to predict this deformation field based on input images. This training process employs a similarity loss function, such as cross-correlation or mutual information.
Beyond CNNs, other DL techniques, including GANs and Siamese networks [171], have been deployed for image registration tasks within medical imaging. GANs, for example, have been instrumental in multi-modal image registration tasks, aligning images acquired through distinct imaging modalities like CT and MRI [172]. Siamese networks, on the other hand, have proven effective in registration tasks centered on matching image patches. This is particularly useful in tasks such as landmark detection within brain MRI scans [173]. Despite the successes achieved by DL methods in image registration tasks, several challenges remain. These include the need for robust and efficient registration techniques and the potential for overfitting of models.
5. Commonly Observed Anatomical Structures and Publicly Available Datasets
Commonly observed anatomical structures in medical imaging, such as the brain, bones, heart, lungs, liver, and breast, have been the focus of extensive research and analysis within the field of ML. Researchers and practitioners have curated publicly available datasets encompassing various modalities and applications for these structures. These publicly available datasets play a crucial role in advancing the development and evaluation of ML algorithms for medical image analysis, fostering collaboration, and driving innovation in the field.
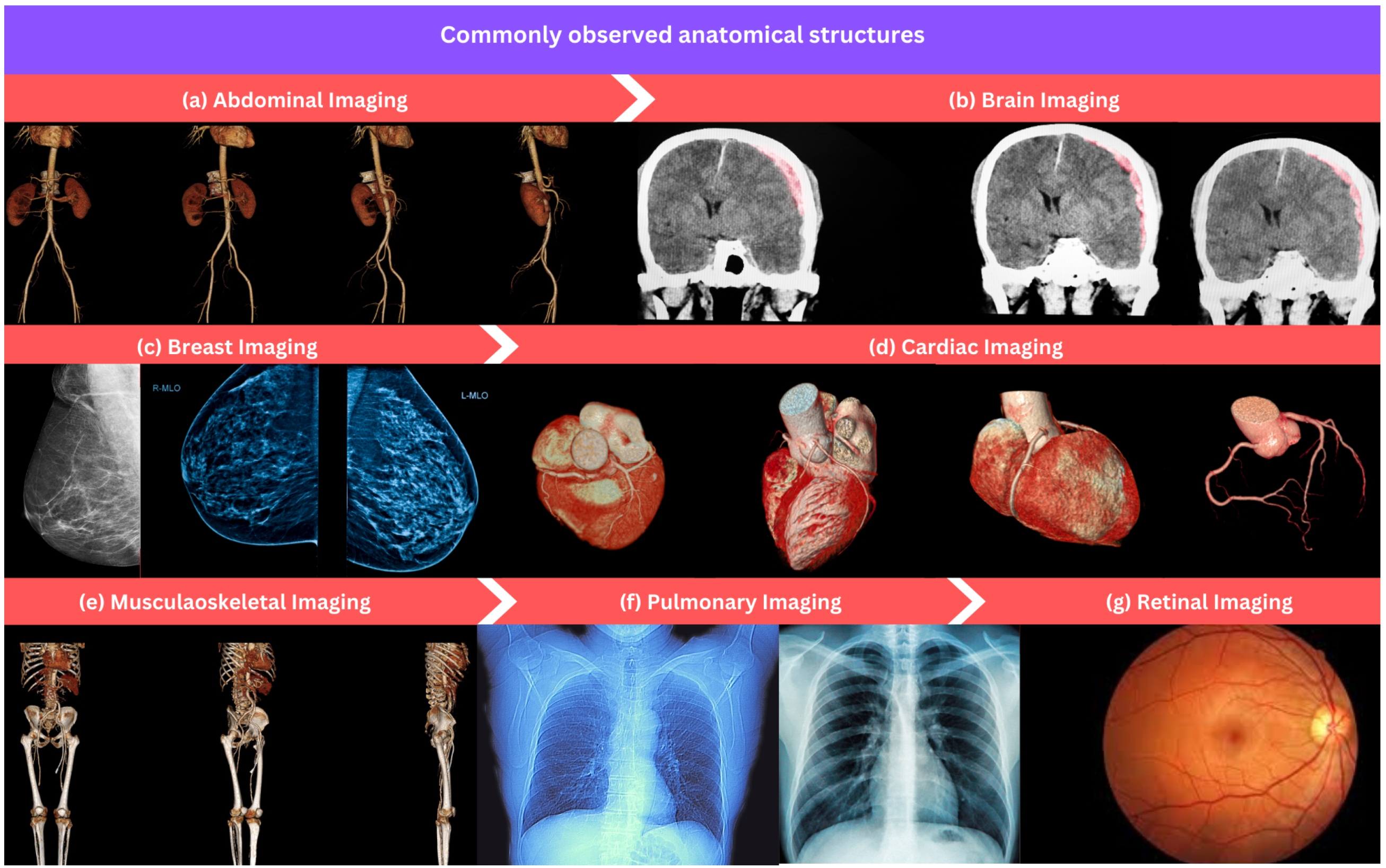
This section overviews commonly used anatomical structures for developing ML algorithms in medical image processing and analysis as shown in Figure 3. We provide a list of datasets for each anatomical structure that can serve as a valuable resource for researchers and practitioners developing ML algorithms in different medical areas.
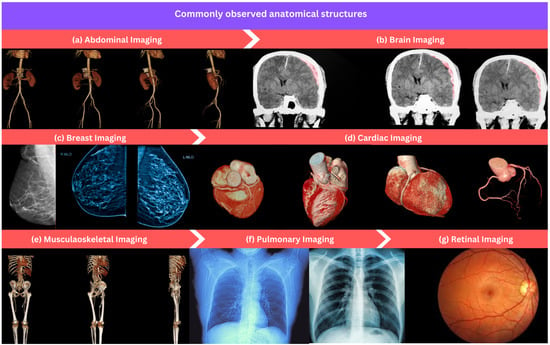
Figure 3.
An overview of commonly observed anatomical structures in the field of medical image processing and analysis: (a) abdominal imaging, (b) brain imaging, (c) breast imaging, (d) cardiac imaging, (e) musculoskeletal imaging, (f) pulmonary imaging, and (g) retinal imaging.
5.1. Abdominal Imaging
Abdominal imaging plays a vital role in the medical field, offering a comprehensive and non-invasive visualization of the internal structures and abnormalities within the abdominal region. Its primary application lies in the diagnosis and monitoring of various medical conditions, including abdominal tumors [174], gastrointestinal disorders [175], liver diseases [176], and kidney ailments. Through the detailed imaging of abdominal organs, healthcare professionals can accurately identify abnormalities, assess disease progression, and plan suitable treatment strategies [177].
Beyond its clinical applications, abdominal imaging is fundamental in medical research. It empowers scientists to explore abdominal organs’ functions, interactions, and connectivity. It contributes to a deeper understanding of physiological processes, metabolic functions, and the mechanisms underlying various abdominal-related conditions. Functional imaging techniques, such as dynamic contrast-enhanced MRI and nuclear medicine scans, can reveal how different abdominal organs respond to specific stimuli or diseases [178]. Moreover, abdominal imaging is indispensable in developing and evaluating novel medical interventions and therapies. Researchers utilize it to assess the effects of experimental treatments, study the response of abdominal organs to medications, and refine therapeutic approaches.
Table 1 summarizes commonly used abdominal organ benchmark datasets for developing and testing ML algorithms.

Table 1.
Publicly available datasets for abdominal imaging that are commonly used to develop ML learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.2. Brain Imaging
Brain imaging offers a comprehensive and non-invasive method of visualizing the brain’s internal structure, function, and abnormalities [187]. Its primary application is diagnosing and monitoring neurological disorders, including brain tumors, stroke, Alzheimer’s disease, and multiple sclerosis [188]. Healthcare professionals can identify abnormalities, assess disease progression, and plan appropriate treatment strategies by capturing detailed brain images. Brain imaging also provides precise anatomical information, aiding neurosurgeons in navigating delicate procedures during surgical planning [189]. Moreover, brain imaging helps develop and evaluate novel therapies and interventions. Researchers use it to assess the effects of experimental treatments, study the brain’s response to medications, and refine therapeutic approaches. Brain imaging has also become increasingly important in the emerging field of neuroinformatics [190], where machine learning and data-driven techniques are employed to analyze large-scale datasets and advance our understanding of brain disorders.
Beyond clinical diagnosis and treatment, brain imaging serves as a foundation of neuroscience research. It allows scientists to investigate brain functions, connectivity, and neural pathways, leading to a deeper understanding of cognitive processes, behavior, and the underlying mechanisms of various brain-related conditions. Functional brain imaging techniques such as fMRI and PET scans can reveal how different brain regions become active during specific tasks or emotional states [191].
Table 2 summarizes commonly used brain benchmark datasets for developing and testing ML algorithms.

Table 2.
Publicly available datasets for brain imaging that are commonly used to develop ML learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.3. Breast Imaging
Breast imaging is a critical medical technique that provides a non-invasive means of visualizing the internal structures of the breast. Its primary application is in detecting, diagnosing, and monitoring breast-related health concerns. Through precise imaging, healthcare professionals can identify abnormalities, assess disease stages, and formulate appropriate treatment plans [202]. Breast imaging, which includes mammography, ultrasound, and MRI, is instrumental in early breast cancer detection, significantly improving survival rates [203]. Breast imaging also contributes to the development and evaluation of novel therapies and interventions. Researchers use it to assess the effectiveness of experimental treatments, study the response of breast tissue to medications, and refine therapeutic strategies. Additionally, breast imaging is at the forefront of emerging fields like radiogenomics, where ML and data-driven approaches are applied to analyze complex imaging data and genetic information. This interdisciplinary approach holds the potential to uncover personalized treatment options and tailor healthcare strategies for individuals at risk of breast-related conditions.
Besides its clinical significance, breast imaging plays a essential role in advancing medical research. It empowers scientists to investigate breast tissue composition, hormonal influences, and genetic factors, leading to a deeper understanding of breast physiology, disease mechanisms, and risk factors. Functional imaging techniques, such as contrast-enhanced breast MRI [204], offer insights into vascularization patterns and breast tissue behavior during different phases of the menstrual cycle.
Table 3 summarizes commonly used breast benchmark datasets for developing and testing ML algorithms.

Table 3.
Publicly available datasets for breast imaging that are commonly used to develop machine learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.4. Cardiac Imaging
Cardiac imaging is a crucial medical field that provides a non-invasive means of visualizing the heart’s structure, function, and any potential abnormalities [209]. Its primary application lies in diagnosing and monitoring various cardiovascular disorders, including coronary artery disease, heart valve problems, congenital heart defects, and heart failure. By obtaining detailed images of the heart, healthcare professionals can identify issues, assess disease progression, and formulate precise treatment plans. Cardiac imaging techniques, such as echocardiography, cardiac magnetic resonance imaging (CMRI), and cardiac computed tomography (CCT), are essential tools in cardiology [139,210]. Cardiac imaging also contributes to the development and evaluation of novel cardiovascular therapies. Researchers utilize these imaging techniques to examine the effects of experimental treatments, study the heart’s response to medications, and refine therapeutic strategies. Additionally, in the era of precision medicine, cardiac imaging plays a crucial role in tailoring treatments for individuals based on their unique cardiac anatomy and function.
Beyond clinical diagnosis and treatment, cardiac imaging plays a pivotal role in advancing cardiovascular research. It enables scientists to explore cardiac functions, blood flow dynamics [211], and the interaction between different heart chambers [158,159,160]. This deeper understanding of cardiac physiology and pathology helps researchers uncover the mechanisms behind heart-related conditions and develop innovative treatment approaches. Functional imaging methods like cardiac stress tests can assess how the heart responds to physical or pharmacological stress, providing valuable insights into its performance under different conditions [212].
Table 4 summarizes commonly used cardiovascular benchmark datasets for developing and testing ML algorithms.

Table 4.
Publicly available datasets for cardiac imaging that are commonly used to develop machine learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.5. Musculaoskeletal Imaging
Musculoskeletal imaging is a pivotal field in medical diagnostics that offers a non-invasive way to visualize the bones, joints, muscles, and related structures of the body [219]. Its primary application is to diagnose and manage various musculoskeletal disorders and conditions, including fractures, arthritis, ligament injuries, and tumors [220,221]. By generating detailed images of the musculoskeletal system, healthcare professionals can accurately identify issues, assess the extent of injuries, and plan appropriate treatment strategies. This imaging domain encompasses various techniques, including X-rays, MRI, CT, and ultrasound.
In addition to clinical diagnosis and treatment planning, musculoskeletal imaging plays a vital role in advancing musculoskeletal research and orthopedic medicine [222]. It allows researchers to delve into the intricacies of bone and joint anatomy, biomechanics, and pathology. By studying musculoskeletal images, scientists can gain insights into how injuries occur, how bones and joints heal, and how the body’s mechanical systems function. This knowledge is essential in developing innovative surgical techniques, orthopedic implants, and rehabilitation strategies. Musculoskeletal imaging is also indispensable in guiding surgical interventions. Orthopedic surgeons and other specialists use pre-operative imaging to plan procedures such as joint replacements [223], fracture fixation [224], and ligament repairs. During surgery, real-time imaging, such as fluoroscopy, helps ensure the accuracy of procedures and minimizes damage to surrounding tissues. Furthermore, musculoskeletal imaging contributes to sports medicine, enabling sports physicians and trainers to assess and manage athletic injuries [225]. It aids in evaluating the severity of injuries, tracking healing progress, and determining an athlete’s readiness to return to sports activities.
Table 5 summarizes commonly used musculoskeletal benchmark datasets for developing and testing ML algorithms.

Table 5.
Publicly available datasets for musculoskeletal imaging that are commonly used to develop ML algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.6. Pulmonary Imaging
The pulmonary imaging field employs non-invasive techniques to visualize the respiratory system, including the lungs and associated structures [230]. This imaging is instrumental in diagnosing and managing a wide range of pulmonary conditions and diseases, such as pneumonia, chronic obstructive pulmonary disease (COPD), lung cancer, and pulmonary embolism. By producing detailed images of the respiratory system, healthcare professionals can accurately detect abnormalities, assess disease severity, and develop tailored treatment plans.
The primary modalities used in pulmonary imaging include chest X-rays, CT scans, MRI, and PET scans. Each of these techniques provides specific insights into lung anatomy, functionality, and pathology [231].
Beyond clinical diagnosis and treatment, pulmonary imaging plays a significant role in advancing respiratory medicine and pulmonary research. It allows scientists and clinicians to explore lung function, mechanics, and responses to various stimuli. Research involving pulmonary imaging has led to breakthroughs in understanding lung diseases’ underlying mechanisms and developing innovative therapies. For example, functional imaging techniques like pulmonary function testing (PFT) can assess lung capacity and airflow, aiding in the diagnosis and management of conditions like asthma and COPD [232].
Table 6 summarizes commonly used pulmonary benchmark datasets for developing and testing ML algorithms.

Table 6.
Publicly available datasets for pulmonary imaging that are commonly used to develop machine learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
5.7. Retinal Imaging
Retinal imaging is a specialized field of medical diagnostics that employs non-invasive imaging techniques to visualize the retina, the light-sensitive tissue at the back of the eye [239]. It plays a critical role in diagnosing and monitoring various ocular conditions, including diabetic retinopathy [240,241], macular degeneration [242], glaucoma [243], and retinal vascular diseases [244]. By providing detailed retina images, retinal imaging enables eye care professionals to detect abnormalities, assess disease progression, and tailor treatment plans for optimal outcomes.
Various modalities are used in retinal imaging, with fundus photography [245,246] and optical coherence tomography (OCT) [247] being among the most common. Fundus photography captures high-resolution color images of the retina, allowing for the identification of structural changes, such as bleeding or swelling. On the other hand, OCT provides cross-sectional images of retinal layers and helps visualize subtle changes in retinal thickness and integrity [248].
Table 7 summarizes commonly used retina benchmark datasets for developing and testing ML algorithms.

Table 7.
Publicly available datasets for retinal imaging that are commonly used to develop machine learning algorithms. “Tr/Ts” denotes training/testing set, respectively.
6. The Translation of AI Techniques from Research to Clinical Practice
The successful translation of AI-driven medical image analysis from research to clinical practice entails a multifaceted approach that encompasses technical, ethical, regulatory, and clinical considerations [256]. This section gives details about steps involved in overcoming the gap between AI innovation and its meaningful integration into the clinical domain. Addressing the question of how AI-driven medical image analysis can be effectively translated into clinical practice necessitates a thorough examination of essential factors and strategies. Here are some guiding points which must be addressed:
- Integration frameworks and workflow adaptation. A fundamental aspect of facilitating the transition of AI techniques to clinical application is in the development of integration frameworks tailored to the specific needs of healthcare environments. Collaborative efforts between computer scientists, medical professionals, and regulatory bodies are imperative to design workflows that seamlessly incorporate AI outputs into existing diagnostic and decision-making processes.
- Validation and regulatory compliance. The validation of AI algorithms to ensure their safety, accuracy, and reliability is a major step in the translation process. Rigorous validation studies that adhere to established standards, such as those outlined by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), contribute to establishing the credibility of AI methods. Comprehensively documenting algorithm behavior, training data, and validation protocols not only ensures confidence in clinical users but also facilitates regulatory compliance.
- Ethical considerations and human–AI collaboration. Ethical deliberations underpinning the integration of AI into clinical practice are of paramount significance. Transparency in AI decision-making processes, interpretability of results, and mitigation of bias are pivotal to engendering trust between clinicians and AI systems. Human–AI collaboration models, where AI outputs are presented as supportive tools rather than autonomous decision makers, foster a cooperative environment where clinical expertise harmoniously interacts with AI-generated insights.
- Education and training. The integration of AI techniques necessitates the provision of comprehensive education and training for medical professionals. Knowledge dissemination on AI’s capabilities, limitations, and clinical relevance enables clinicians to harness AI insights optimally. Robust training programs tailored to diverse healthcare settings aid in fostering the requisite proficiency to interpret, validate, and incorporate AI outputs effectively.
- Longitudinal evaluation and continuous improvement. Sustaining the translation of AI techniques into clinical practice mandates a commitment to longitudinal evaluation and continuous improvement. Regular assessment of AI-generated outcomes in real-world scenarios facilitates the identification of shortcomings, iterative refinement, and the incorporation of feedback from clinical practitioners. This iterative approach fosters the evolution of AI algorithms that align with dynamic clinical demands.
Therefore, effectively translating AI-driven medical image analysis into clinical practice involves a concerted effort encompassing integration frameworks, rigorous validation, ethical considerations, education, and continuous refinement. The confluence of technical robustness, regulatory compliance, and ethical mindfulness is essential in realizing the full potential of AI to augment and elevate clinical decision making within the medical imaging domain.
7. Discussion and Conclusions
In this work, we gave a comprehensive overview of fundamental concepts and state-of-the-art medical image processing and analysis approaches. We gave a brief overview of learning problems commonly employed in medical image processing and commonly used deep learning methods. We highlighted commonly observed anatomical areas and publicly available datasets for ML algorithm development.
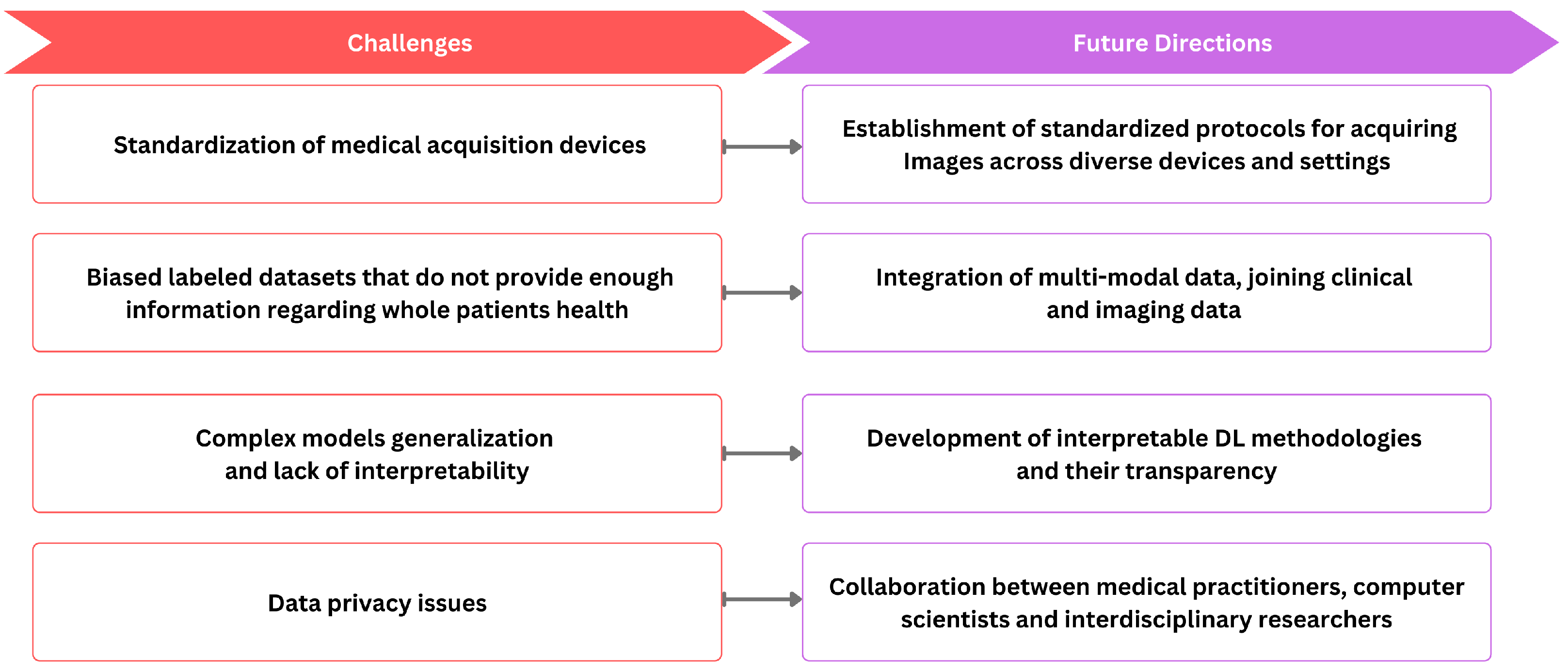
ML and DL techniques have emerged as effective tools for various medical imaging tasks, but their widespread integration faces notable challenges, as shown in Figure 4. One notable obstacle is the inherent variability of medical image data, stemming from differences in resolution, contrast, and signal-to-noise ratios due to diverse clinical protocols. The standardization of medical image acquisition to ensure uniformity remains a significant challenge. Further, precise medical image annotations are required for DL models. However, the limited availability of annotated data and the intricacies involved in annotating medical images impose constraints on their applicability. Moreover, dataset bias arises when the data used to train a model differ from the data on which the model is applied, leading to potential shortcomings in the study. For example, datasets collected as part of population studies may differ from those of patients referred to hospitals, introducing biases in the data. Biases can also stem from imaging devices or procedures, introducing specific measurement biases. For example, in chest X-rays, images may include interventions like chest drains, impacting the accuracy of automated diagnosis [257]. Labeling errors arising from systematic biases in human annotators or automatic methods extracting labels from patient reports further contribute to dataset bias. This discrepancy can result in algorithms that perform well in benchmarks but poorly in real-world scenarios. The importance of unbiased evaluation in assessing model performance emphasizes the need for independent sets of data for training and testing. It cautions against incorrect implementations that can lead to overoptimistic results. For instance, some studies on ADHD classification based on brain imaging have employed circular analysis, performing feature selection on the entire dataset before cross-validation [258]. Another issue arises when repeated measures of an individual are split between the training and test sets, causing the algorithm to recognize individual patients instead of condition markers. Additionally, data privacy concerns reduce medical data sharing for deep learning training. The inherent diversity of medical images further complicates the task of model generalization across varied datasets. The black-box nature of DL models, lacking interpretability, presents an additional limitation.
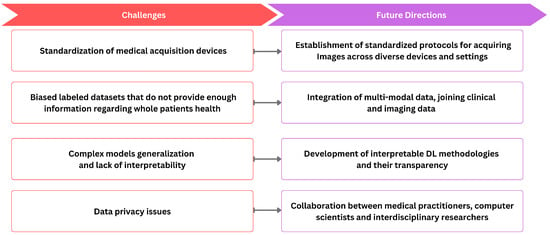
Figure 4.
The relationship between current challenges and future directions.
Addressing the challenge of standardizing medical image acquisition involves the establishment of clear protocols that define standardized procedures for acquiring images across diverse devices and settings. This includes specifying crucial imaging parameters such as resolution, contrast, and orientation. Moreover, embracing widely accepted industry standards like DICOM is essential to ensure compatibility and interoperability among different imaging systems. Simultaneously, the implementation of quality assurance programs is crucial to monitor and maintain the performance of imaging equipment, ensuring ongoing consistency in image quality. Furthermore, the future direction of research in medical image analysis should encompass the development of interpretable DL methodologies, facilitating the comprehension of DL models and augmenting transparency. Moreover, significant progression could be obtained by integrating images with additional clinical context, incorporating information from patient records, and including various clinical descriptors such as blood tests, genomics, medications, vital signs, and non-imaging data like ECG. This integration facilitates a shift from the realm of images to comprehensive patient-level information. It would allow for population-level statistical analysis, offering insights into disease manifestations, treatment responses, adverse reactions, and medication interactions. Therefore, integrating multi-modal data, joining clinical and imaging data, holds immense potential to enhance the precision of medical image analysis while fostering a holistic understanding of underlying pathologies. However, implementing this step necessitates establishing intricate infrastructure and formulating new privacy and security regulations, spanning interactions between hospitals and academic research institutes, among hospitals, and across international consortia.
Author Contributions
Conceptualization, I.G. and M.H.; writing—original draft preparation, I.G. and M.H.; writing—review and editing, I.G., M.H., H.L. and K.R.; visualization, H.L. and M.H.; supervision, I.G. and K.R.; project administration, I.G. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AE | Autoencoders |
| AI | Artificial Intelligence |
| BLSTM | Bidirectional LSTM |
| CT | Computed Tomography |
| cGAN | conditional GAN |
| CNNs | Convolutional Neural Networks |
| DCGANs | Deep Convolutional Generative Adversarial Networks |
| DL | Deep Learning |
| EMA | European Medicines Agency |
| FCNNs | Fully Convolutional Neural Networks |
| FDA | Food and Drug Administration |
| GANs | Generative Adversarial Networks |
| LSTM | Long Short-Term Memory |
| ML | Machine Learning |
| MDLSTM | Multidimensional LSTM |
| MRI | Magnetic Resonance Imaging |
| OCT | Optical Coherence Tomography |
| PET | Positron Emission Tomography |
| RNNs | Recurrent Neural Networks |
| SSD | Single Shot MultiBox Detector |
| UI | Ultrasound Imaging |
| ViTs | Vision Transformers |
| YOLO | You Only Look Once |
References
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Kuwaiti, A.A.; Nazer, K.H.A.; Al-Reedy, A.; Al-Shehri, S.Z.; Al-Muhanna, A.F.; Subbarayalu, A.V.; Muhanna, D.A.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Mohammed, M.A.; Mohammed, V.A. Impact of Artificial Intelligence on the Automation of Digital Health System. Int. J. Softw. Eng. Appl. 2022, 13. [Google Scholar] [CrossRef]
- Hurkmans, C.W.; Borger, J.H.; Pieters, B.R.; Russell, N.S.; Jansen, E.P.M.; Mijnheer, B.J. Variability in target volume delineation on CT scans of the breast. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1366–1372. [Google Scholar] [CrossRef]
- Vinod, S.K.; Jameson, M.G.; Min, M.; Holloway, L.C. Uncertainties in volume delineation in radiation oncology: A systematic review and recommendations for future studies. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 121, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cao, G.; Wang, Y.L.; Liao, S.; Wang, Q.; Shi, J.; Li, C.; Shen, D. Review and Prospect: Artificial Intelligence in Advanced Medical Imaging. Front. Radiol. 2021, 1, 781868. [Google Scholar]
- Wang, Z.; Zhang, Z.; Zheng, J.Q.; Huang, B.; Voiculescu, I.; Yang, G.Z. Deep Learning in Medical Ultrasound Image Segmentation: A Review. arXiv 2020, arXiv:2002.07703. [Google Scholar]
- Fawaz, H.I.; Forestier, G.; Weber, J.; Idoumghar, L.; Muller, P.A. Deep learning for time series classification: A review. Data Min. Knowl. Discov. 2018, 33, 917–963. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nandal, A.; Dhaka, A.; Dixit, R. Medical Image Classification Techniques and Analysis Using Deep Learning Networks: A Review. In Health Informatics: A Computational Perspective in Healthcare; Springer: Singapore, 2021. [Google Scholar]
- Liu, X.; Song, L.; Liu, S.; Zhang, Y. A Review of Deep-Learning-Based Medical Image Segmentation Methods. Sustainability 2021, 13, 1224. [Google Scholar]
- Bharati, S.; Mondal, M.R.H.; Podder, P.; Prasath, V.B.S. Deep Learning for Medical Image Registration: A Comprehensive Review. arXiv 2022, arXiv:2204.11341. [Google Scholar]
- Zou, J.; Gao, B.; Song, Y.; Qin, J. A review of deep learning-based deformable medical image registration. Front. Oncol. 2022, 12, 1047215. [Google Scholar] [PubMed]
- Fu, Y.; Lei, Y.; Wang, T.; Curran, W.J.; Liu, T.; Yang, X. Deep learning in medical image registration: A review. Phys. Med. Biol. 2019, 65, 20TR01. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.L. Foundations of Machine Learning; The MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Li, Q.; Cai, W.T.; Wang, X.; Zhou, Y.; Feng, D.D.; Chen, M. Medical image classification with convolutional neural network. In Proceedings of the 2014 13th International Conference on Control Automation Robotics and Vision (ICARCV), Singapore, 10–12 December 2014; pp. 844–848. [Google Scholar]
- Sasikala, V.; Mounika, K.; Tulasi, Y.S.; Gayathri, D.K.; Anjani, M. Performance evaluation of Spam and Non-Spam E-mail detection using Machine Learning algorithms. In Proceedings of the 2022 International Conference on Electronics and Renewable Systems (ICEARS), Tuticorin, India, 16–18 March 2022; pp. 1359–1365. [Google Scholar]
- Raj, R.; Kang, S.S. Spam and Non-Spam URL Detection using Machine Learning Approach. In Proceedings of the 2022 3rd International Conference for Emerging Technology (INCET), Belgaum, India, 27–29 May 2022; pp. 1–6. [Google Scholar]
- Matey, V.; Chauhan, N.; Mahale, A.; Bhistannavar, V.; Shitole, A. Real Estate Price Prediction using Supervised Learning. In Proceedings of the 2022 IEEE Pune Section International Conference (PuneCon), Pune, India, 15–17 December 2022; pp. 1–5. [Google Scholar]
- Using Supervised Machine Learning to Predict House Prices. J. Stud. Res. 2022, 11. [CrossRef]
- Moorthy, J.; Gandhi, U.D. A Survey on Medical Image Segmentation Based on Deep Learning Techniques. Big Data Cogn. Comput. 2022, 6, 117. [Google Scholar] [CrossRef]
- Hussain, A.; Malik, H.; Chaudhry, M.U. Supervised Learning Based Classification of Cardiovascular Diseases. Proc. Eng. Technol. Innov. 2021, 20, 24–34. [Google Scholar] [CrossRef]
- Ker, J.; Wang, L.; Rao, J.P.; Lim, T.C.C. Deep Learning Applications in Medical Image Analysis. IEEE Access 2018, 6, 9375–9389. [Google Scholar] [CrossRef]
- Aljuaid, A.; Anwar, M. Survey of Supervised Learning for Medical Image Processing. SN Comput. Sci. 2022, 3, 292. [Google Scholar] [PubMed]
- Rädsch, T.; Reinke, A.; Weru, V.; Tizabi, M.D.; Schreck, N.; Kavur, A.E.; Pekdemir, B.; Ross, T.; Kopp-Schneider, A.; Maier-Hein, L. Labelling instructions matter in biomedical image analysis. Nat. Mach. Intell. 2022, 5, 273–283. [Google Scholar] [CrossRef]
- Teng, Q.; Liu, Z.; Song, Y.; Han, K.; Lu, Y. A survey on the interpretability of deep learning in medical diagnosis. Multimed. Syst. 2022, 28, 2335–2355. [Google Scholar] [CrossRef]
- Moss, L.; Corsar, D.; Shaw, M.; Piper, I.; Hawthorne, C. Demystifying the Black Box: The Importance of Interpretability of Predictive Models in Neurocritical Care. Neurocritical Care 2022, 37, 185–191. [Google Scholar]
- Barlow, H. Unsupervised Learning. Neural Comput. 1989, 1, 295–311. [Google Scholar] [CrossRef]
- Moreno-García, C.F.; Aceves-Martins, M.; Serratosa, F. Unsupervised Machine Learning Application to Perform a Systematic Review and Meta-Analysis in Medical Research. Comput. Sist. 2016, 20, 7–17. [Google Scholar] [CrossRef]
- Mazzei, M. An Unsupervised Machine Learning Approach for Medical Image Analysis. In Advances in Information and Communication: Proceedings of the 2021 Future of Information and Communication Conference (FICC); Springer: Berlin/Heidelberg, Germany, 2021; Volume 2. [Google Scholar]
- Jain, A.K. Data clustering: 50 years beyond K-means. Pattern Recognit. Lett. 2008, 31, 651–666. [Google Scholar] [CrossRef]
- Jain, A.K.; Murty, M.N.; Flynn, P.J. Data clustering: A review. ACM Comput. Surv. 1999, 31, 264–323. [Google Scholar] [CrossRef]
- Ronan, T.; Qi, Z.; Naegle, K.M. Avoiding common pitfalls when clustering biological data. Sci. Signal. 2016, 9, re6. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Zhao, X.; Ning, S.; Lu, J.; Loh, K.P.; He, Q.; Loh, N.D.; Pennycook, S.J. Learning motifs and their hierarchies in atomic resolution microscopy. Sci. Adv. 2020, 8, eabk1005. [Google Scholar] [CrossRef] [PubMed]
- Kandel, B.M.; Wang, D.J.J.; Gee, J.C.; Avants, B.B. Eigenanatomy: Sparse dimensionality reduction for multi-modal medical image analysis. Methods 2015, 73, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Woodland, M.; Patel, N.; Taie, M.A.; Yung, J.; Netherton, T.J.; Patel, A.B.; Brock, K.K. Dimensionality Reduction for Improving Out-of-Distribution Detection in Medical Image Segmentation. In Proceedings of the International Workshop on Uncertainty for Safe Utilization of Machine Learning in Medical Imaging, Vancover, BC, Canada, 12 October 2023; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Tian, Y.; Pang, G.; Liu, F.; Chen, Y.; Shin, S.H.; Verjans, J.W.; Singh, R.; Carneiro, G. Constrained Contrastive Distribution Learning for Unsupervised Anomaly Detection and Localisation in Medical Images. arXiv 2021, arXiv:2103.03423. [Google Scholar]
- Li, Y.; Gu, H.; Wang, H.; Qin, P.; Wang, J. BUSnet: A Deep Learning Model of Breast Tumor Lesion Detection for Ultrasound Images. Front. Oncol. 2022, 12, 848271. [Google Scholar] [CrossRef]
- Venkatesh, C.; Yamini, L.S. An Efficient Method for Detection and Classification of Pulmonary Neoplasm based on Deep Learning Technique. HELIX 2021, 11, 6–12. [Google Scholar]
- Zhang, Q.; Xiao, Y.; Dai, W.; Suo, J.; Wang, C.; Shi, J.; Zheng, H. Deep learning based classification of breast tumors with shear-wave elastography. Ultrasonics 2016, 72, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, G.; Ding, Z.; Li, J.; Yang, H. Unsupervised Domain Adaptation for ECG Arrhythmia Classification. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 304–307. [Google Scholar]
- Imtiaz, M.N.; Khan, N.M. Cross-Database and Cross-Channel ECG Arrhythmia Heartbeat Classification Based on Unsupervised Domain Adaptation. arXiv 2023, arXiv:2306.04433. [Google Scholar]
- Kanchanatawan, B.; Sriswasdi, S.; Thika, S.; Stoyanov, D.S.; Sirivichayakul, S.; Carvalho, A.F.; Geffard, M.; Maes, M. Towards a new classification of stable phase schizophrenia into major and simple neuro-cognitive psychosis: Results of unsupervised machine learning analysis. J. Eval. Clin. Pract. 2018, 24, 879–891. [Google Scholar] [CrossRef]
- Cai, X.L.; Xie, D.J.; Madsen, K.H.; Wang, Y.M.; Bögemann, S.; Cheung, E.F.C.; Møller, A.; Chan, R.C.K. Generalizability of machine learning for classification of schizophrenia based on resting-state functional MRI data. Hum. Brain Mapp. 2019, 41, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Parija, S.; Sahani, M.; Bisoi, R.; Dash, P.K. Autoencoder-based improved deep learning approach for schizophrenic EEG signal classification. Pattern Anal. Appl. 2022, 26, 403–435. [Google Scholar] [CrossRef]
- Cao, P.; Liu, X.; Bao, H.; Yang, J.; Zhao, D. Restricted Boltzmann machines based oversampling and semi-supervised learning for false positive reduction in breast CAD. Bio-Med. Mater. Eng. 2015, 26 (Suppl. 1), S1541–S1547. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.S.; Barto, A.G. Reinforcement Learning: An Introduction. IEEE Trans. Neural Netw. 2005, 16, 285–286. [Google Scholar] [CrossRef]
- Silver, D.; Hubert, T.; Schrittwieser, J.; Antonoglou, I.; Lai, M.; Guez, A.; Lanctot, M.; Sifre, L.; Kumaran, D.; Graepel, T.; et al. A general reinforcement learning algorithm that masters chess, shogi, and Go through self-play. Science 2018, 362, 1140–1144. [Google Scholar] [CrossRef]
- Pérolat, J.; Vylder, B.D.; Hennes, D.; Tarassov, E.; Strub, F.; de Boer, V.; Muller, P.; Connor, J.T.; Burch, N.; Anthony, T.W.; et al. Mastering the game of Stratego with model-free multiagent reinforcement learning. Science 2022, 378, 990–996. [Google Scholar] [CrossRef]
- Shahid, A.A.; Piga, D.; Braghin, F.; Roveda, L. Continuous control actions learning and adaptation for robotic manipulation through reinforcement learning. Auton. Robot. 2022, 46, 483–498. [Google Scholar] [CrossRef]
- Liu, R.; Nageotte, F.; Zanne, P.; de Mathelin, M.; Dresp, B. Deep Reinforcement Learning for the Control of Robotic Manipulation: A Focussed Mini-Review. Robotics 2021, 10, 22. [Google Scholar] [CrossRef]
- Sallab, A.E.; Abdou, M.; Perot, E.; Yogamani, S.K. Deep Reinforcement Learning framework for Autonomous Driving. Electron. Imaging 2017, 29, 70–76. [Google Scholar] [CrossRef]
- Teikari, P.; Najjar, R.P.; Schmetterer, L.; Milea, D. Embedded deep learning in ophthalmology: Making ophthalmic imaging smarter. Ther. Adv. Ophthalmol. 2018, 11, 7172. [Google Scholar] [CrossRef] [PubMed]
- Hachem, E.; Meliga, P.; Goetz, A.; Rico, P.J.; Viquerat, J.; Larcher, A.; Valette, R.; Sanches, A.F.; Lannelongue, V.; Ghraieb, H.; et al. Reinforcement learning for patient-specific optimal stenting of intracranial aneurysms. Sci. Rep. 2023, 13, 7147. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.; Rajaganapathy, S.; Das, T.; Kim, Y.; Chen, Y.; Dai, Q.; Li, X.; Jiang, X.; Zong, N. Using artificial intelligence to learn optimal regimen plan for Alzheimer’s disease. J. Am. Med. Inform. Assoc. JAMIA 2023, 30, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Lall, A.; Tallur, S.G. Deep reinforcement learning-based pairwise DNA sequence alignment method compatible with embedded edge devices. Sci. Rep. 2023, 13, 2773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Guo, L.; Huang, S.; Wen, B. ReLLIE: Deep Reinforcement Learning for Customized Low-Light Image Enhancement. In Proceedings of the 29th ACM International Conference on Multimedia, Virtual, 20–24 October 2021. [Google Scholar]
- Ghesu, F.C.; Georgescu, B.; Mansi, T.; Neumann, D.; Hornegger, J.; Comaniciu, D. An Artificial Agent for Anatomical Landmark Detection in Medical Images. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Athens, Greece, 17–21 October 2016. [Google Scholar]
- Ghesu, F.C.; Georgescu, B.; Zheng, Y.; Grbic, S.; Maier, A.K.; Hornegger, J.; Comaniciu, D. Multi-Scale Deep Reinforcement Learning for Real-Time 3D-Landmark Detection in CT Scans. IEEE Trans. Pattern Anal. Mach. Intell. 2019, 41, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Kasseroller, K.; Thaler, F.; Payer, C.; Stern, D. Collaborative Multi-agent Reinforcement Learning for Landmark Localization Using Continuous Action Space. In Proceedings of the Information Processing in Medical Imaging, Online, 28–30 June 2021. [Google Scholar]
- Winkel, D.J.; Weikert, T.J.; Breit, H.C.; Chabin, G.; Gibson, E.; Heye, T.; Comaniciu, D.; Boll, D.T. Validation of a fully automated liver segmentation algorithm using multi-scale deep reinforcement learning and comparison versus manual segmentation. Eur. J. Radiol. 2020, 126, 108918. [Google Scholar] [CrossRef]
- Maicas, G.; Carneiro, G.; Bradley, A.P.; Nascimento, J.C.; Reid, I.D. Deep Reinforcement Learning for Active Breast Lesion Detection from DCE-MRI. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Quebec City, QC, Canada, 11–13 September 2017. [Google Scholar]
- Yala, A.; Mikhael, P.G.; Lehman, C.D.; Lin, G.; Strand, F.; Wan, Y.L.; Hughes, K.S.; Satuluru, S.; Kim, T.; Banerjee, I.; et al. Optimizing risk-based breast cancer screening policies with reinforcement learning. Nat. Med. 2021, 28, 136–143. [Google Scholar] [CrossRef]
- Stember, J.N.; Shalu, H. Reinforcement learning using Deep Q networks and Q learning accurately localizes brain tumors on MRI with very small training sets. BMC Med. Imaging 2022, 22, 224. [Google Scholar] [CrossRef]
- Leroy, G.; Rueckert, D.; Alansary, A. Communicative Reinforcement Learning Agents for Landmark Detection in Brain Images. In Machine Learning in Clinical Neuroimaging and Radiogenomics in Neuro-oncology, Proceedings of the Third International Workshop, MLCN 2020, and Second International Workshop, RNO-AI 2020, Held in Conjunction with MICCAI 2020, Lima, Peru, 4–8 October 2020; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Stember, J.N.; Shalu, H. Deep reinforcement learning-based image classification achieves perfect testing set accuracy for MRI brain tumors with a training set of only 30 images. arXiv 2021, arXiv:2102.02895. [Google Scholar]
- Stember, J.N.; Shalu, H. Deep reinforcement learning to detect brain lesions on MRI: A proof-of-concept application of reinforcement learning to medical images. arXiv 2020, arXiv:2008.02708. [Google Scholar]
- Sahba, F.; Tizhoosh, H.R.; Salama, M.M.A. A Reinforcement Learning Framework for Medical Image Segmentation. In Proceedings of the 2006 IEEE International Joint Conference on Neural Network Proceedings, Vancouver, BC, Canada, 16–21 July 2006; pp. 511–517. [Google Scholar]
- Yang, H.; Shan, C.; Kolen, A.F.; de With, P.H.N. Deep Q-Network-Driven Catheter Segmentation in 3D US by Hybrid Constrained Semi-Supervised Learning and Dual-UNet. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Lima, Peru, 4–8 October 2020. [Google Scholar]
- Bae, W.; Lee, S.; Lee, Y.; Park, B.; Chung, M.; Jung, K.H. Resource Optimized Neural Architecture Search for 3D Medical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Shenzhen, China, 13–17 October 2019. [Google Scholar]
- Qin, T.; Wang, Z.; He, K.; Shi, Y.; Gao, Y.; Shen, D. Automatic Data Augmentation Via Deep Reinforcement Learning for Effective Kidney Tumor Segmentation. In Proceedings of the ICASSP 2020–2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Virtual, 4–9 May 2020; pp. 1419–1423. [Google Scholar]
- Yang, D.; Roth, H.R.; Xu, Z.; Milletarì, F.; Zhang, L.; Xu, D. Searching Learning Strategy with Reinforcement Learning for 3D Medical Image Segmentation. arXiv 2019, arXiv:2006.05847. [Google Scholar]
- Yadav, P.; Mishra, A.; Lee, J.; Kim, S. A Survey on Deep Reinforcement Learning-based Approaches for Adaptation and Generalization. arXiv 2022, arXiv:2202.08444. [Google Scholar]
- Hu, M.; Zhang, J.; Matkovic, L.A.; Liu, T.; Yang, X. Reinforcement learning in medical image analysis: Concepts, applications, challenges, and future directions. J. Appl. Clin. Med. Phys. 2022, 24, e13898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Goldberg, A.B. Introduction to Semi-Supervised Learning; Springer Nature: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zhang, Y.; Park, D.S.; Han, W.; Qin, J.; Gulati, A.; Shor, J.; Jansen, A.; Xu, Y.; Huang, Y.; Wang, S.; et al. BigSSL: Exploring the Frontier of Large-Scale Semi-Supervised Learning for Automatic Speech Recognition. IEEE J. Sel. Top. Signal Process. 2021, 16, 1519–1532. [Google Scholar] [CrossRef]
- Shi, Z.; Tonolini, F.; Aletras, N.; Yilmaz, E.; Kazai, G.; Jiao, Y. Rethinking Semi-supervised Learning with Language Models. arXiv 2023, arXiv:2305.13002. [Google Scholar]
- Jiao, R.; Zhang, Y.; Ding, L.; Cai, R.; Zhang, J. Learning with Limited Annotations: A Survey on Deep Semi-Supervised Learning for Medical Image Segmentation. arXiv 2022, arXiv:2207.14191. [Google Scholar]
- Huang, S.C.; Pareek, A.; Jensen, M.E.K.; Lungren, M.P.; Yeung, S.; Chaudhari, A.S. Self-supervised learning for medical image classification: A systematic review and implementation guidelines. NPJ Digit. Med. 2023, 6, 74. [Google Scholar] [CrossRef]
- Gong, M.; Chen, S.; Chen, Q.; Zeng, Y.; Zhang, Y. Generative Adversarial Networks in Medical Image Processing. Curr. Pharm. Des. 2020, 27, 1856–1868. [Google Scholar] [CrossRef]
- LeCun, Y. Self-Supervised Learning. In Proceedings of the 34th AAAI Conference on Artificial Intelligence Invited Speaker Program, Virtual, 9 February 2020. [Google Scholar]
- Xiao, Q.; Wang, J.; Ye, J.; Zhang, H.; Bu, Y.; Zhang, Y.; Wu, H. Self-Supervised Learning for Sleep Stage Classification with Predictive and Discriminative Contrastive Coding. In Proceedings of the ICASSP 2021–2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Virtual, 6–11 June 2021; pp. 1290–1294. [Google Scholar]
- Mohamed, A.; Lee, H.-y.; Borgholt, L.; Havtorn, J.D.; Edin, J.; Igel, C.; Kirchhoff, K.; Li, S.W.; Livescu, K.; Maaløe, L.; et al. Self-Supervised Speech Representation Learning: A Review. IEEE J. Sel. Top. Signal Process. 2022, 16, 1179–1210. [Google Scholar] [CrossRef]
- Dai, Q.; Patil, V.; Hecker, S.; Dai, D.; Gool, L.V.; Schindler, K. Self-supervised Object Motion and Depth Estimation from Video. In Proceedings of the 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Seattle, WA, USA, 14–19 June 2020; pp. 4326–4334. [Google Scholar]
- Li, Y.; Wang, X.; Qi, S.; Huang, C.; Jiang, Z.L.; Liao, Q.; Guan, J.; Zhang, J.J. Self-supervised learning-based weight adaptive hashing for fast cross-modal retrieval. Signal Image Video Process. 2019, 15, 673–680. [Google Scholar] [CrossRef]
- Wang, Y.; He, S.; Xu, X.; Yang, Y.; Li, J.; Shen, H.T. Self-supervised adversarial learning for cross-modal retrieval. In Proceedings of the 2nd ACM International Conference on Multimedia in Asia, Singapore, 7–9 March 2021. [Google Scholar]
- Kragh, M.F.; Rimestad, J.; Lassen, J.T.; Berntsen, J.; Karstoft, H. Predicting Embryo Viability Based on Self-Supervised Alignment of Time-Lapse Videos. IEEE Trans. Med. Imaging 2021, 41, 465–475. [Google Scholar] [CrossRef]
- Bai, W.; Chen, C.; Tarroni, G.; Duan, J.; Guitton, F.; Petersen, S.E.; Guo, Y.; Matthews, P.M.; Rueckert, D. Self-Supervised Learning for Cardiac MR Image Segmentation by Anatomical Position Prediction. arXiv 2019, arXiv:1907.02757. [Google Scholar]
- Lu, Q.; Li, Y.; Ye, C. Volumetric white matter tract segmentation with nested self-supervised learning using sequential pretext tasks. Med. Image Anal. 2021, 72, 102094. [Google Scholar] [CrossRef] [PubMed]
- Srinidhi, C.L.; Kim, S.W.; Chen, F.D.; Martel, A.L. Self-supervised driven consistency training for annotation efficient histopathology image analysis. Med. Image Anal. 2021, 75, 102256. [Google Scholar] [CrossRef] [PubMed]
- Klinghoffer, T.; Morales, P.; Park, Y.G.; Evans, N.B.; Chung, K.; Brattain, L.J. Self-Supervised Feature Extraction for 3D Axon Segmentation. In Proceedings of the 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Seattle, WA, USA, 14–19 June 2020; pp. 4213–4219. [Google Scholar]
- Dietterich, T.G.; Lathrop, R.H.; Lozano-Perez, T. Solving the Multiple Instance Problem with Axis-Parallel Rectangles. Artif. Intell. 1997, 89, 31–71. [Google Scholar] [CrossRef]
- Tian, Y.; Hao, W.; Jin, D.; Chen, G.; Zou, A. A Review of Latest Multi-instance Learning. In Proceedings of the 2020 4th International Conference on Computer Science and Artificial Intelligence, Zhuhai, China, 11–13 December 2020. [Google Scholar]
- Carbonneau, M.A.; Cheplygina, V.; Granger, E.; Gagnon, G. Multiple instance learning: A survey of problem characteristics and applications. arXiv 2016, arXiv:1612.03365. [Google Scholar] [CrossRef]
- Chen, K.; Peng, Z.; Ke, W. Study on image retrieval system base on multi-objective and multi-instance learning. Int. J. Wirel. Mob. Comput. 2013, 6, 158–164. [Google Scholar] [CrossRef]
- Yuan, T.; Wan, F.; Fu, M.; Liu, J.; Xu, S.; Ji, X.; Ye, Q. Multiple Instance Active Learning for Object Detection. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Nashville, TN, USA, 20–25 June 2021; pp. 5326–5335. [Google Scholar]
- Zhou, Z.; Wang, J.; Wang, Y.; Zhu, Z.; Du, J.; Liu, X.; Quan, J. Visual Tracking Using Improved Multiple Instance Learning with Co-training Framework for Moving Robot. KSII Trans. Internet Inf. Syst. 2018, 12, 5496–5521. [Google Scholar]
- Das, D.B.; Birant, D. Human activity recognition based on multi-instance learning. Expert Syst. 2023, 40, e13256. [Google Scholar]
- Wang, B.; Zhao, Y.; Li, X. Multiple Instance Graph Learning for Weakly Supervised Remote Sensing Object Detection. IEEE Trans. Geosci. Remote Sens. 2022, 60, 1–12. [Google Scholar] [CrossRef]
- Lu, P.; Liu, W.; Xu, W.; Li, L.; Zheng, B.; Zhang, J.; Zhang, L. Multi-instance learning for mass retrieval in digitized mammograms. In Proceedings of the Medical Imaging, San Diego, CA, USA, 4–9 February 2012. [Google Scholar]
- Li, C.; Shi, C.; Zhang, H.; Chen, Y.; Zhang, S. Multiple instance learning for computer aided detection and diagnosis of gastric cancer with dual-energy CT imaging. J. Biomed. Inform. 2015, 57, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Quellec, G.; Lamard, M.; Erginay, A.; Chabouis, A.; Massin, P.; Cochener, B.; Cazuguel, G. Automatic detection of referral patients due to retinal pathologies through data mining. Med. Image Anal. 2016, 29, 47–64. [Google Scholar] [CrossRef]
- Quellec, G.; Lamard, M.; Cozic, M.; Coatrieux, G.; Cazuguel, G. Multiple-Instance Learning for Anomaly Detection in Digital Mammography. IEEE Trans. Med. Imaging 2016, 35, 1604–1614. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Xu, H.; Cheng, R.; Wen, X. Deep Multi-Instance Learning with Induced Self-Attention for Medical Image Classification. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020; pp. 446–450. [Google Scholar]
- Zhao, L.; Yuan, L.; Hao, K.; Wen, X. Generalized attention-based deep multi-instance learning. Multimed. Syst. 2022, 29, 275–287. [Google Scholar] [CrossRef]
- Cheplygina, V.; de Bruijne, M.; Pluim, J.P.W. Not-so-supervised: A survey of semi-supervised, multi-instance, and transfer learning in medical image analysis. Med. Image Anal. 2018, 54, 280–296. [Google Scholar] [CrossRef]
- Dertat, A. Applied Deep Learning–Part 3: Autoencoders. 2017. Available online: https://towardsdatascience.com/applied-deep-learning-part-3-autoencoders-1c083af4d798 (accessed on 15 September 2023).
- GeeksforGeeks. Introduction to Convolution Neural Network. 2023. Available online: https://www.geeksforgeeks.org/introduction-convolution-neural-network/ (accessed on 15 September 2023).
- GAN Machine Learning Advanced Courses. Overview of GAN Structure. 2022. Available online: https://developers.google.com/machine-learning/gan/gan_structure (accessed on 15 September 2023).
- Kalita, A.V.D. A Brief Overview of Recurrent Neural Networks (RNN). 2022. Available online: https://www.analyticsvidhya.com/blog/2022/03/a-brief-overview-of-recurrent-neural-networks-rnn/ (accessed on 15 September 2023).
- Douillard, A. Vision Transformers. 2012. Available online: https://arthurdouillard.com/post/visual_transformers/ (accessed on 15 September 2023).
- Chen, S.; Guo, W. Auto-Encoders in Deep Learning—A Review with New Perspectives. Mathematics 2023, 11, 1777. [Google Scholar] [CrossRef]
- Senapati, R.K.; Badri, R.; Kota, A.; Merugu, N.; Sadhul, S. Compression and Denoising of Medical Images Using Autoencoders. In Proceedings of the 2022 International Conference on Recent Trends in Microelectronics, Automation, Computing and Communications Systems (ICMACC), Hyderabad, India, 28–30 December 2022; pp. 466–470. [Google Scholar]
- Liu, J. Review of variational autoencoders model. Appl. Comput. Eng. 2023, 4, 588–596. [Google Scholar] [CrossRef]
- Sagha, H.; Cummins, N.; Schuller, B. Stacked denoising autoencoders for sentiment analysis: A review. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2017, 7, e1212. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv 2015, arXiv:1505.04597. [Google Scholar]
- Radford, A.; Metz, L.; Chintala, S. Unsupervised Representation Learning with Deep Convolutional Generative Adversarial Networks. arXiv 2016, arXiv:1511.06434. [Google Scholar]
- Habijan, M.; Galić, I. Generation of Artificial CT Images using Patch-based Conditional Generative Adversarial Networks. In Proceedings of the 2022 7th International Conference on Smart and Sustainable Technologies (SpliTech), Split / Bol, Croatia, 5–8 July 2022; pp. 1–5. [Google Scholar]
- Giles, C.L.; Kuhn, G.M.; Williams, R.J. Dynamic recurrent neural networks: Theory and applications. IEEE Trans. Neural Netw. Learn. Syst. 1994, 5, 153–156. [Google Scholar] [CrossRef]
- Latif, S.; Usman, M.; Rana, R.; Qadir, J. Phonocardiographic Sensing Using Deep Learning for Abnormal Heartbeat Detection. IEEE Sensors J. 2018, 18, 9393–9400. [Google Scholar] [CrossRef]
- Yu, Y.; Si, X.; Hu, C.; Zhang, J.X. A Review of Recurrent Neural Networks: LSTM Cells and Network Architectures. Neural Comput. 2019, 31, 1235–1270. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.U.; Emebob, O.; Omonhinmin, C.A. Vision Transformers in Medical Imaging: A Review. arXiv 2022, arXiv:2211.10043. [Google Scholar]
- Vaswani, A.; Shazeer, N.M.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention is All you Need. In Proceedings of the NIPS, Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Parvaiz, A.; Khalid, M.A.; Zafar, R.; Ameer, H.; Ali, M.; Fraz, M.M. Vision Transformers in Medical Computer Vision—A Contemplative Retrospection. arXiv 2022, arXiv:2203.15269. [Google Scholar] [CrossRef]
- Ghafar, A.; Sattar, U. Convolutional Autoencoder for Image Denoising. Umt Artif. Intell. Rev. 2021. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ameenudeen, P.E. Bio-medical Image Denoising using Autoencoders. In Proceedings of the 2022 Second International Conference on Next Generation Intelligent Systems (ICNGIS), Virtual, 29–31 July 2022; pp. 1–6. [Google Scholar]
- Kumar, P.; Parmar, A. Versatile Approaches for Medical Image Compression: A Review. Procedia Comput. Sci. 2020, 167, 1380–1389. [Google Scholar] [CrossRef]
- Li, P.; Pei, Y.; Li, J. A comprehensive survey on design and application of autoencoder in deep learning. Appl. Soft Comput. 2023, 138, 110176. [Google Scholar] [CrossRef]
- Kandhro, I.A.; Uddin, M.; Hussain, S.; Chaudhery, T.J.; Shorfuzzaman, M.; Meshref, H.; Albalhaq, M.; Alsaqour, R.A.; Khalaf, O.I. Impact of Activation, Optimization, and Regularization Methods on the Facial Expression Model Using CNN. Comput. Intell. Neurosci. 2022, 2022, 3098604. [Google Scholar] [CrossRef] [PubMed]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2012, 60, 84–90. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.E.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Thakur, S.K.; Singh, D.P.; Choudhary, J. Lung cancer identification: A review on detection and classification. Cancer Metastasis Rev. 2020, 39, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Chaunzwa, T.L.; Hosny, A.; Xu, Y.; Shafer, A.; Diao, N.; Lanuti, M.; Christiani, D.C.; Mak, R.H.; Aerts, H.J. Deep learning classification of lung cancer histology using CT images. Sci. Rep. 2021, 11, 5471. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.N.; Wang, S.; Ali, N.S. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wu, L.; Li, H.; Cai, C.; Wu, Q.; Kong, H.; Liu, R.; Wang, X.; Hou, X.; Liu, Y.; et al. A deep learning system for detecting diabetic retinopathy across the disease spectrum. Nat. Commun. 2021, 12, 3242. [Google Scholar] [CrossRef]
- Xie, Y.; Zaccagna, F.; Rundo, L.; Testa, C.; Agati, R.; Lodi, R.; Manners, D.N.; Tonon, C. Convolutional Neural Network Techniques for Brain Tumor Classification (from 2015 to 2022): Review, Challenges, and Future Perspectives. Diagnostics 2022, 12, 1850. [Google Scholar] [CrossRef]
- Muhammad, K.; Khan, S.; Ser, J.D.; de Albuquerque, V.H.C. Deep Learning for Multigrade Brain Tumor Classification in Smart Healthcare Systems: A Prospective Survey. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 507–522. [Google Scholar] [CrossRef]
- Habijan, M.; Babin, D.; Galic, I.; Leventic, H.; Romic, K.; Velicki, L.U.; Pizurica, A. Overview of the Whole Heart and Heart Chamber Segmentation Methods. Cardiovasc. Eng. Technol. 2020, 11, 725–747. [Google Scholar] [CrossRef]
- Gheflati, B.; Rivaz, H. Vision Transformers for Classification of Breast Ultrasound Images. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 480–483. [Google Scholar]
- Simon, E.; Briassouli, A. Vision Transformers for Brain Tumor Classification. In Proceedings of the 15th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC)/9th International Conference on Bioimaging (BIOIMAGING), Online, 9–11 February 2022; SciTePress: Setubal, Portugal, 2022. [Google Scholar]
- Ren, S.; He, K.; Girshick, R.B.; Sun, J. Faster R-CNN: Towards Real-Time Object Detection with Region Proposal Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2015, 39, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Dasiopoulou, S.; Mezaris, V.; Kompatsiaris, Y.; Papastathis, V.; Strintzis, M.G. Knowledge-assisted semantic video object detection. IEEE Trans. Circuits Syst. Video Technol. 2005, 15, 1210–1224. [Google Scholar] [CrossRef]
- Liu, W.; Anguelov, D.; Erhan, D.; Szegedy, C.; Reed, S.E.; Fu, C.Y.; Berg, A.C. SSD: Single Shot MultiBox Detector. In Proceedings of the European Conference on Computer Vision, Santiago, Chile, 7–13 December 2015. [Google Scholar]
- Gunasekaran, K.P. Leveraging object detection for the identification of lung cancer. arXiv 2023, arXiv:2305.15813. [Google Scholar]
- Prinzi, F.; Insalaco, M.; Orlando, A.A.M.; Gaglio, S.; Vitabile, S. A Yolo-Based Model for Breast Cancer Detection in Mammograms. Cogn. Comput. 2023. [Google Scholar] [CrossRef]
- Chegraoui, H.; Philippe, C.; Dangouloff-Ros, V.; Grigis, A.; Calmon, R.; Boddaert, N.; Frouin, F.; Grill, J.; Frouin, V. Object Detection Improves Tumour Segmentation in MR Images of Rare Brain Tumours. Cancers 2021, 13, 6113. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Zhu, Y.; Papandreou, G.; Schroff, F.; Adam, H. Encoder-Decoder with Atrous Separable Convolution for Semantic Image Segmentation. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 801–818. [Google Scholar] [CrossRef]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R.B. Mask R-CNN. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017; pp. 2980–2988. [Google Scholar]
- Du, S.; Bayasi, N.; Hamarneh, G.; Garbi, R. MDViT: Multi-domain Vision Transformer for Small Medical Image Segmentation Datasets. arXiv 2023, arXiv:2307.02100. [Google Scholar]
- Chen, Y.; Yang, X.H.; Wei, Z.; Heidari, A.A.; Zheng, N.; Li, Z.; Chen, H.; Hu, H.; Zhou, Q.; Guan, Q. Generative Adversarial Networks in Medical Image augmentation: A review. Comput. Biol. Med. 2022, 144, 105382. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.H.; Jakab, A.; Bauer, S.; Kalpathy-Cramer, J.; Farahani, K.; Kirby, J.S.; Burren, Y.; Porz, N.; Slotboom, J.; Wiest, R.; et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans. Med. Imaging 2015, 34, 1993–2024. [Google Scholar] [CrossRef]
- Kao, Y.S.; Yang, J. Deep learning-based auto-segmentation of lung tumor PET/CT scans: A systematic review. Clin. Transl. Imaging 2022, 10, 217–223. [Google Scholar] [CrossRef]
- Bencevic, M.; Galic, I.; Habijan, M.; Babin, D. Training on Polar Image Transformations Improves Biomedical Image Segmentation. IEEE Access 2021, 9, 133365–133375. [Google Scholar] [CrossRef]
- Babin, D.; Pizurica, A.; Velicki, L.U.; Matic, V.; Galic, I.; Leventic, H.; Zlokolica, V.; Philips, W. Skeletonization method for vessel delineation of arteriovenous malformation. Comput. Biol. Med. 2018, 93, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bencevic, M.; Habijan, M.; Galic, I.; Babin, D. Using the Polar Transform for Efficient Deep Learning-Based Aorta Segmentation in CTA Images. In Proceedings of the 2022 International Symposium ELMAR, Zadar, Croatia, 12–14 September 2022; pp. 191–194. [Google Scholar]
- Habijan, M.; Galic, I.; Leventic, H.; Romic, K.; Babin, D. Abdominal Aortic Aneurysm Segmentation from CT Images using Modified 3D U-Net with Deep Supervision. In Proceedings of the 2022 International Symposium ELMAR, Zadar, Croatia, 12–14 September 2022; pp. 123–128. [Google Scholar]
- Habijan, M.; Galic, I.; Romic, K.; Leventic, H. AB-ResUNet+: Improving Multiple Cardiovascular Structure Segmentation from Computed Tomography Angiography Images. Appl. Sci. 2022, 12, 3024. [Google Scholar] [CrossRef]
- Habijan, M.; Galic, I.; Leventic, H.; Romic, K. Whole Heart Segmentation Using 3D FM-Pre-ResNet Encoder–Decoder Based Architecture with Variational Autoencoder Regularization. Appl. Sci. 2021, 11, 3912. [Google Scholar] [CrossRef]
- Habijan, M.; Galic, I.; Leventic, H.; Romic, K.; Babin, D. Segmentation and Quantification of Bi-Ventricles and Myocardium Using 3D SERes-U-Net. In Proceedings of the International Conference on Systems, Signals, and Image Processing, Bratislava, Slovakia, 2–4 June 2021. [Google Scholar]
- Bencevic, M.; Habijan, M.; Galic, I. Epicardial Adipose Tissue Segmentation from CT Images with A Semi-3D Neural Network. In Proceedings of the 2021 International Symposium ELMAR, Zagreb, Croatia, 13–15 September 2021; pp. 87–90. [Google Scholar]
- Leventic, H.; Babin, D.; Velicki, L.U.; Devos, D.; Galic, I.; Zlokolica, V.; Romic, K.; Pizurica, A. Left atrial appendage segmentation from 3D CCTA images for occluder placement procedure. Comput. Biol. Med. 2019, 104, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Leventic, H.; Babin, D.; Velicki, L.; Galic, I.; Zlokolica, V. Semi-automatic left atrial appendage segmentation from 3D CCTA images. In Proceedings of the 2017 International Symposium ELMAR, Zadar, Croatia, 18–20 September 2017; pp. 39–42. [Google Scholar]
- Habijan, M.; Babin, D.; Galic, I.; Leventic, H.; Velicki, L.; Cankovic, M. Centerline Tracking of the Single Coronary Artery from X-ray Angiograms. In Proceedings of the 2022 International Symposium ELMAR, Zadar, Croatia, 12–14 September 2022; pp. 117–121. [Google Scholar]
- Fossen-Romsaas, S.; Storm-Johannessen, A.; Lundervold, A.S. Synthesizing Skin Lesion Images Using CycleGANs—A Case Study. Nor. Inform. 2020. Available online: https://api.semanticscholar.org/CorpusID:231815746 (accessed on 19 September 2023).
- Papadopoulos, D.; Karalis, V.D. Variational Autoencoders for Data Augmentation in Clinical Studies. Appl. Sci. 2023, 13, 8793. [Google Scholar] [CrossRef]
- Dey, R.; Bhattacharjee, D.; Nasipuri, M. Image Denoising Using Generative Adversarial Network. In Intelligent Computing: Image Processing Based Applications; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Wang, T.C.; Liu, M.Y.; Zhu, J.Y.; Tao, A.; Kautz, J.; Catanzaro, B. High-Resolution Image Synthesis and Semantic Manipulation with Conditional GANs. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2017; pp. 8798–8807. [Google Scholar]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Guttag, J.V.; Dalca, A.V. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging 2018, 38, 1788–1800. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H. Deformable medical image registration based on CNN. J. X-ray Sci. Technol. 2022, 31, 85–94. [Google Scholar] [CrossRef]
- Bertinetto, L.; Valmadre, J.; Henriques, J.F.; Vedaldi, A.; Torr, P.H.S. Fully-Convolutional Siamese Networks for Object Tracking. arXiv 2016, arXiv:1606.09549. [Google Scholar]
- Santarossa, M.; Kilic, A.; von der Burchard, C.; Schmarje, L.; Zelenka, C.; Reinhold, S.; Koch, R.; Roider, J. MedRegNet: Unsupervised multimodal retinal-image registration with GANs and ranking loss. In Proceedings of the Medical Imaging, San Diego, CA, USA, 20–24 February 2022. [Google Scholar]
- Rafael-Palou, X.; Aubanell, A.; Bonavita, I.; Ceresa, M.; Piella, G.; Ribas, V.J.; Ballester, M.Á.G. Re-Identification and Growth Detection of Pulmonary Nodules without Image Registration Using 3D Siamese Neural Networks. Med. Image Anal. 2019, 67, 101823. [Google Scholar] [CrossRef]
- Birkemeier, K.L. Imaging of solid congenital abdominal masses: A review of the literature and practical approach to image interpretation. Pediatr. Radiol. 2020, 50, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Yalon, M.; Amawi, A.D.T.; Kelm, Z.S.; Wells, M.L.; Teo, L.L.S.; Heiken, J.P.; Sheedy, S.P.; Torbenson, M.S.; Fidler, J.L.; Venkatesh, S.K. Eosinophilic Disorders of the Gastrointestinal Tract and Associated Abdominal Viscera: Imaging Findings and Diagnosis. RadioGraphics 2022, 42, 1081–1102. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, K. Noninvasive diagnostics of liver diseases—Imaging methods. Vnitr. Lek. 2019, 65, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.R.; Levin, D.; Rao, V.M. Utilization Trends in Abdominal Imaging, 2004–2016. AJR Am. J. Roentgenol. 2020, 215, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Vallejo, V.; Jimenez-Gonzalez, M.; Llop, J.; Reese, T. New Molecular and Functional Imaging Techniques. In Functional Imaging in Oncology: Biophysical Basis and Technical Approaches; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Ma, J.; Zhang, Y.; Gu, S.; Zhu, C.; Ge, C.; Zhang, Y.; An, X.; Wang, C.; Wang, Q.; Liu, X.; et al. AbdomenCT-1K: Is Abdominal Organ Segmentation a Solved Problem? IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 6695–6714. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Reinke, A.; Bakas, S.; Farahani, K.; Kopp-Schneider, A.; Landman, B.A.; Litjens, G.; Menze, B.; Ronneberger, O.; Summers, R.M.; et al. The Medical Segmentation Decathlon. Nat. Commun. 2022, 13, 4128. [Google Scholar] [CrossRef] [PubMed]
- Bilic, P.; Christ, P.F.; Vorontsov, E.; Chlebus, G.; Chen, H.; Dou, Q.; Fu, C.W.; Han, X.; Heng, P.A.; Hesser, J.W.; et al. The Liver Tumor Segmentation Benchmark (LiTS). Med. Image Anal. 2019, 84, 102680. [Google Scholar] [CrossRef] [PubMed]
- Kavur, A.E.; Gezer, N.S.; Baris, M.M.; Conze, P.H.; Groza, V.; Pham, D.D.; Chatterjee, S.; Ernst, P.; Özkan, S.; Baydar, B.; et al. CHAOS Challenge—Combined (CT-MR) Healthy Abdominal Organ Segmentation. Med. Image Anal. 2020, 69, 101950. [Google Scholar] [CrossRef]
- Heller, N.; Isensee, F.; Maier-Hein, K.; Hou, X.; Xie, C.; Li, F.; Nan, Y.; Mu, G.; Lin, Z.; Han, M.; et al. The state of the art in kidney and kidney tumor segmentation in contrast-enhanced CT imaging: Results of the KiTS19 Challenge. Med. Image Anal. 2019, 67, 101821. [Google Scholar] [CrossRef]
- Rister, B.; Yi, D.; Shivakumar, K.; Nobashi, T.W.; Rubin, D. CT-ORG, a new dataset for multiple organ segmentation in computed tomography. Sci. Data 2020, 7, 381. [Google Scholar] [CrossRef]
- Clark, K.W.; Vendt, B.A.; Smith, K.E.; Freymann, J.B.; Kirby, J.S.; Koppel, P.; Moore, S.M.; Phillips, S.R.; Maffitt, D.R.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Landman, B.; Xu, Z.; Igelsias, J.; Styner, M.; Langerak, T.; Klein, A. Multi-atlas labeling beyond the cranial vault-workshop and challenge. In Proceedings of the MICCAI 2015, Munich, Germany, 5–9 October 2015. [Google Scholar]
- Yen, C.; Lin, C.L.; Chiang, M.C. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.A.; Mridha, M.F.; Das, S.C.; Kabir, M.M.; Islam, M.R.; Watanobe, Y. A Comprehensive Survey on the Detection, Classification, and Challenges of Neurological Disorders. Biology 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Babin, D.; Pizurica, A.; Vylder, J.D.; Vansteenkiste, E.; Philips, W. Brain blood vessel segmentation using line-shaped profiles. Phys. Med. Biol. 2013, 58, 8041–8061. [Google Scholar] [CrossRef] [PubMed]
- Rootes-Murdy, K.; Gazula, H.; Verner, E.; Kelly, R.; DeRamus, T.P.; Plis, S.; Sarwate, A.D.; Turner, J.A.; Calhoun, V.D. Federated Analysis of Neuroimaging Data: A Review of the Field. Neuroinformatics 2021, 20, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Mier, W.; Mier, D. Advantages in functional imaging of the brain. Front. Hum. Neurosci. 2015, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Baid, U.; Ghodasara, S.; Bilello, M.; Mohan, S.; Calabrese, E.; Colak, E.; Farahani, K.; Kalpathy-Cramer, J.; Kitamura, F.C.; Pati, S.; et al. The RSNA-ASNR-MICCAI BraTS 2021 Benchmark on Brain Tumor Segmentation and Radiogenomic Classification. arXiv 2021, arXiv:2107.02314. [Google Scholar]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2009, 74, 201–209. [Google Scholar] [CrossRef]
- IXI Dataset|Papers With Code. 2023. Available online: https://paperswithcode.com/dataset/ixi-dataset (accessed on 19 September 2023).
- Mendrik, A.; Vincken, K.L.; Kuijf, H.J.; Breeuwer, M.M.; Bouvy, W.H.; de Bresser, J.; Alansary, A.; de Bruijne, M.; Carass, A.; El-Baz, A.S.; et al. MRBrainS Challenge: Online Evaluation Framework for Brain Image Segmentation in 3T MRI Scans. Comput. Intell. Neurosci. 2015, 2015, 813696. [Google Scholar] [CrossRef]
- Wang, L.; Nie, D.; Li, G.; Puybareau, É.; Dolz, J.; Zhang, Q.; Wang, F.; Xia, J.; Wu, Z.; Chen, J.; et al. Benchmark on Automatic Six-Month-Old Infant Brain Segmentation Algorithms: The iSeg-2017 Challenge. IEEE Trans. Med. Imaging 2019, 38, 2219–2230. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, K.; Wu, Z.; Li, G.; Zong, X.; Lei, Z.; Wei, Y.; Ma, J.; Yang, X.; Feng, X.; et al. Multi-Site Infant Brain Segmentation Algorithms: The iSeg-2019 Challenge. IEEE Trans. Med. Imaging 2021, 40, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Petzsche, M.; de la Rosa, E.; Hanning, U.; Wiest, R.; Valenzuela, W.; Reyes, M.; Meyer, M.; Liew, S.L.; Kofler, F.; Ezhov, I.; et al. ISLES 2022: A multi-center magnetic resonance imaging stroke lesion segmentation dataset. Sci. Data 2022, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- ISLES: Ischemic Stroke Lesion Segmentation Challenge 2022. 2023. Available online: https://www.isles-challenge.org/ (accessed on 5 September 2023).
- Commowick, O.; Istace, A.; Kain, M.; Laurent, B.; Leray, F.; Simon, M.; Pop, S.; Girard, P.; Ameli, R.; Ferré, J.C.; et al. Objective Evaluation of Multiple Sclerosis Lesion Segmentation using a Data Management and Processing Infrastructure. Sci. Rep. 2018, 8, 13650. [Google Scholar] [CrossRef] [PubMed]
- MS Segmentation Challenge Using a Data Management and Processing Infrastructure. 2023. Available online: https://portal.fli-iam.irisa.fr/msseg-2/data/ (accessed on 5 September 2023).
- Taylor, C.R.; Monga, N.; Johnson, C.; Hawley, J.R.; Patel, M. Artificial Intelligence Applications in Breast Imaging: Current Status and Future Directions. Diagnostics 2023, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Steyerová, P.; Burgetová, A. Current imaging techniques and impact on diagnosis and survival —A narrative review. Ann. Breast Surg. 2021, 6, 1–13. [Google Scholar] [CrossRef]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Helbich, T.H.; Baltzer, P.A.T. Contrast-enhanced Mammography versus Contrast-enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Gimenez, F.; Hoogi, A.; Miyake, K.K.; Gorovoy, M.; Rubin, D. A curated mammography data set for use in computer-aided detection and diagnosis research. Sci. Data 2017, 4, 170177. [Google Scholar] [CrossRef]
- Moreira, I.; Amaral, I.; Domingues, I.; Cardoso, A.J.O.; Cardoso, M.J.; Cardoso, J.S. INbreast: Toward a full-field digital mammographic database. Acad. Radiol. 2012, 19, 236–248. [Google Scholar] [CrossRef]
- Suckling, J.; Parker, J.; Dance, D.R.; Astley, S.; Hutt, I.W.; Boggis, C.R.M.; Ricketts, I.W.; Stamatakis, E.A.; Cerneaz, N.; Kok, S.; et al. Mammographic Image Analysis Society (MIAS) Database v1.21. 2015. Available online: https://www.repository.cam.ac.uk/handle/1810/250394 (accessed on 5 September 2023).
- BREAST-DIAGNOSIS—The Cancer Imaging Archive (TCIA) Public Access—Cancer Imaging Archive Wiki. 2023. Available online: https://wiki.cancerimagingarchive.net/display/Public/Breast-Diagnosis (accessed on 19 September 2023).
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and application of artificial intelligence in cardiac imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef]
- Bencevic, M.; Galic, I.; Habijan, M.; Pizurica, A. Recent Progress in Epicardial and Pericardial Adipose Tissue Segmentation and Quantification Based on Deep Learning: A Systematic Review. Appl. Sci. 2022, 12, 5217. [Google Scholar] [CrossRef]
- Habijan, M.; Galić, I. Automating Blood Flow Simulation Through the Aorta in Patient-specific CT Images. In Proceedings of the Hawaii International Conference on System Sciences, Maui, HI, USA, 4–7 January 2022. [Google Scholar]
- Weinstein, A.S.; Bader, A.M.; Urman, R.D.; Hepner, D.; Fox, J.A. Preoperative Cardiac Stress Tests Ordered in the Preoperative Evaluation Clinic: A Retrospective Review of Ordering Patterns. Cardiol. Res. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bernard, O.; Lalande, A.; Zotti, C.; Cervenansky, F.; Yang, X.; Heng, P.A.; Cetin, I.; Lekadir, K.; Camara, O.; Gonzalez Ballester, M.A.; et al. Deep Learning Techniques for Automatic MRI Cardiac Multi-Structures Segmentation and Diagnosis: Is the Problem Solved? IEEE Trans. Med. Imaging 2018, 37, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, S.; Smistad, E.; Pedrosa, J.; Østvik, A.; Cervenansky, F.; Espinosa, F.; Espeland, T.; Berg, E.A.R.; Jodoin, P.M.; Grenier, T.; et al. Deep Learning for Segmentation Using an Open Large-Scale Dataset in 2D Echocardiography. IEEE Trans. Med. Imaging 2019, 38, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; He, B.; Ghorbani, A.; Lungren, M.P.; Ashley, E.A.; Liang, D.H.; Zou, J.Y. EchoNet-Dynamic: A Large New Cardiac Motion Video Data Resource for Medical Machine Learning. In Proceedings of the NeurIPS ML4H Workshop, Vancouver, BC, Canada, 13 December 2019. [Google Scholar]
- Kadish, A.H.; Bello, D.; Finn, J.P.; Bonow, R.O.; Schaechter, A.; Subacius, H.P.; Albert, C.M.; Daubert, J.P.; Fonseca, C.G.; Goldberger, J.J. Rationale and Design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) Trial. J. Cardiovasc. Electrophysiol. 2009, 20, 982–987. [Google Scholar] [CrossRef]
- Bertelsmeier, R.; Dreschler, L.; Heer, I.; Kemen, H.; Neumann, B.; References, B.R.; Nagel, H.H.; Martin, W.; Aggarwal, J.K.; Jain, R.; et al. Design of a Special Interpreter for the Classification of Human Chromosomes. Trans. Pattern Anal. Mach. Intell. 1979, PAMI1, 214–219. [Google Scholar] [CrossRef]
- Zhuang, X.; Shen, J. Multi-scale patch and multi-modality atlases for whole heart segmentation of MRI. Med. Image Anal. 2016, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, A.N.; Skalski, K.A.; Bhatt, A.A. Vascular lesions of the head and neck: An update on classification and imaging review. Insights Imaging 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Berghe, D.T.V.D.; Babin, D.; Chen, D.M.; Callens, D.M.; Brack, D.; Morbée, D.L.; Herregods, P.D.N.; Huysse, D.W.; Jaremko, D.J.L.; Jans, P.D.L. Deep Learning for Detection of Structural Sacroiliac Joint Lesions on Pelvic Computed Tomography: Multicenter Development and Validation. In Proceedings of the 30th Annual Scientific Meeting of the European Society of Musculoskeletal Radiology (ESSR), Bilbao, Spain, 22–24 June 2023. [Google Scholar]
- Berghe, T.V.D.; Babin, D.; Chen, M.; Callens, M.; Brack, D.; Maes, H.; Lievens, J.; Lammens, M.; Sumere, M.V.; Morbée, L.; et al. Neural network algorithm for detection of erosions and ankylosis on CT of the sacroiliac joints: Multicentre development and validation of diagnostic accuracy. Eur. Radiol. 2023. [Google Scholar] [CrossRef]
- Brown, C.P. Advancing musculoskeletal research with nanoscience. Nat. Rev. Rheumatol. 2013, 9, 614–623. [Google Scholar] [CrossRef]
- Neuhaus, M.T.; Zeller, A.N.; Jehn, P.; Lethaus, B.; Gellrich, N.C.; Zimmerer, R. Intraoperative real-time navigation and intraoperative three-dimensional imaging for patient-specific total temporomandibular joint replacement. Int. J. Oral Maxillofac. Surg. 2021, 50, 1342–1350. [Google Scholar] [CrossRef]
- Terhune, E.B.; Polce, E.M.; Williams, J.C. A Novel Fluoroscopic View for Improved Assessment of the Safety of the Posterosuperior Screw in Femoral Neck Fracture Fixation. J. Bone Jt. Surg. 2022, 104, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Koerzdoerfer, G.; Nickel, D.; Mostapha, M.; Nadar, M.S.; Gassenmaier, S.; Kuestner, T.; Othman, A.E. Feasibility and Implementation of a Deep Learning MR Reconstruction for TSE Sequences in Musculoskeletal Imaging. Diagnostics 2021, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Irvin, J.A.; Bagul, A.; Ding, D.Y.; Duan, T.; Mehta, H.; Yang, B.; Zhu, K.; Laird, D.; Ball, R.L.; et al. MURA: Large Dataset for Abnormality Detection in Musculoskeletal Radiographs. arXiv 2017, arXiv:1712.06957. [Google Scholar]
- Abedeen, I.; Rahman, M.A.; Prottyasha, F.Z.; Ahmed, T.; Chowdhury, T.M.; Shatabda, S. FracAtlas: A Dataset for Fracture Classification, Localization and Segmentation of Musculoskeletal Radiographs. Sci. Data 2023, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.; Sánchez, M.B.; May, G.; Loram, I.D. Estimating Full Regional Skeletal Muscle Fibre Orientation from B-Mode Ultrasound Images Using Convolutional, Residual, and Deconvolutional Neural Networks. J. Imaging 2018, 4, 29. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Lu, L.; Lu, Z.; Bagheri, M.; Summers, R.M. ChestX-ray: Hospital-Scale Chest X-ray Database and Benchmarks on Weakly Supervised Classification and Localization of Common Thorax Diseases. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017. [Google Scholar]
- Newbigin, K.; Souza, C.A.; Torres, C.; Marchiori, E.; Gupta, A.; Inácio, J.R.; Armstrong, M.D.; Peña, E. Fat embolism syndrome: State-of-the-art review focused on pulmonary imaging findings. Respir. Med. 2016, 113, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiao, C.; Huang, Y.; Hassan, H.; Huang, B. Deep Learning Applications in Computed Tomography Images for Pulmonary Nodule Detection and Diagnosis: A Review. Diagnostics 2022, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, A.; Kontogianni, K.; Brock, J.M.; Wood, S.A.; Herth, F.J.; Kirby, M. Differentiating COPD and asthma using quantitative CT imaging and machine learning. Eur. Respir. J. 2022, 60, 2103078. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Lam, K.; Le, L.T.; Pham, H.; Tran, D.Q.; Nguyen, D.B.; Le, D.D.; Pham, C.M.; Tong, H.; Dinh, D.H.; et al. VinDr-CXR: An open dataset of chest X-rays with radiologist’s annotations. Sci. Data 2020, 9, 429. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Pollard, T.J.; Berkowitz, S.J.; Greenbaum, N.R.; Lungren, M.P.; Deng, C.Y.; Mark, R.G.; Horng, S. MIMIC-CXR, a de-identified publicly available database of chest radiographs with free-text reports. Sci. Data 2019, 6, 317. [Google Scholar] [CrossRef]
- Bustos, A.; Pertusa, A.; Salinas, J.M.; de la Iglesia-Vayá, M. PadChest: A large chest X-ray image dataset with multi-label annotated reports. Med. Image Anal. 2019, 66, 101797. [Google Scholar] [CrossRef] [PubMed]
- Irvin, J.A.; Rajpurkar, P.; Ko, M.; Yu, Y.; Ciurea-Ilcus, S.; Chute, C.; Marklund, H.; Haghgoo, B.; Ball, R.L.; Shpanskaya, K.S.; et al. CheXpert: A Large Chest Radiograph Dataset with Uncertainty Labels and Expert Comparison. In Proceedings of the AAAI Conference on Artificial Intelligence, Honolulu, HI, USA, 27 January–1 February 2019. [Google Scholar]
- Shiraishi, J.; Katsuragawa, S.; Ikezoe, J.; Matsumoto, T.; Kobayashi, T.; Komatsu, K.; Matsui, M.; Fujita, H.; Kodera, Y.; Doi, K. Development of a digital image database for chest radiographs with and without a lung nodule: Receiver operating characteristic analysis of radiologists’ detection of pulmonary nodules. AJR Am. J. Roentgenol. 2000, 174 1, 71–74. [Google Scholar] [CrossRef]
- JSRT Database | Japanese Society of Radiological Technology. 2023. Available online: http://db.jsrt.or.jp/eng.php (accessed on 9 September 2023).
- Li, D.Q.; Choudhry, N. The future of retinal imaging. Curr. Opin. Ophthalmol. 2020, 31, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Kheradfallah, H.; Sarkar, A.; Balaji, J.J. Automated Detection and Diagnosis of Diabetic Retinopathy: A Comprehensive Survey. J. Imaging 2021, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Bellemo, V.; Xie, Y.; Lee, X.Q.; Yip, M.Y.T.; Ting, D.S.W. Different fundus imaging modalities and technical factors in AI screening for diabetic retinopathy: A review. Eye Vis. 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Likhvantseva, V.G.; Kapkova, S.G.; Gevorgyan, A.S.; Nekrasova, E.Y. Hypermetropia as a Risk Factor for Age-Related Macular Degeneration. Review. Ophthalmol. Russ. 2022, 19, 255–264. [Google Scholar] [CrossRef]
- Stein, J.D.; Khawaja, A.P.; Weizer, J. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Mériaudeau, F.; Guenancia, C.; Germanese, C.; Delcourt, C.; Kawasaki, R.; Cheung, C.Y.; Creuzot-Garcher, C.; Grzybowski, A. Using Artificial Intelligence to Analyse the Retinal Vascular Network: The Future of Cardiovascular Risk Assessment Based on Oculomics? A Narrative Review. Ophthalmol. Ther. 2022, 12, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.H.; Coram, M.; Stumpe, M.C.; Wu, D.J.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.A.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef]
- Wintergerst, M.W.M.; Jansen, L.G.; Holz, F.G.; Finger, R.P. A Novel Device for Smartphone-Based Fundus Imaging and Documentation in Clinical Practice: Comparative Image Analysis Study. JMIR mHealth uHealth 2020, 8, e17480. [Google Scholar] [CrossRef]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef]
- Ong, J.; Zarnegar, A.; Corradetti, G.; Singh, S.R.; Chhablani, J. Advances in Optical Coherence Tomography Imaging Technology and Techniques for Choroidal and Retinal Disorders. J. Clin. Med. 2022, 11, 5139. [Google Scholar] [CrossRef] [PubMed]
- Pachade, S.; Porwal, P.; Thulkar, D.; Kokare, M.; Deshmukh, G.; Sahasrabuddhe, V.; Giancardo, L.; Quellec, G.; Mériaudeau, F. Retinal Fundus Multi-disease Image Dataset (RFMiD): A dataset for multi-disease detection research. Data 2021, 6, 24. [Google Scholar] [CrossRef]
- Machine Vision; Pattern Recognition Laboratory. DiaRetDB1 V2.1—Diabetic Retinopathy Database. Available online: http://www.it.lut.fi/project/imageret/diaretdb1_v2_1/ (accessed on 1 September 2023).
- Staal, J.; Abramoff, M.; Niemeijer, M.; Viergever, M.; van Ginneken, B. Ridge based vessel segmentation in color images of the retina. IEEE Trans. Med. Imaging 2004, 23, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Hoover, A.D.; Kouznetsova, V.; Goldbaum, M. Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans. Med. Imaging 2000, 19, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dashtbozorg, B.; Bekkers, E.J.; Pluim, J.P.W.; Duits, R.; ter Haar Romeny, B.M. Robust Retinal Vessel Segmentation via Locally Adaptive Derivative Frames in Orientation Scores. IEEE Trans. Med. Imaging 2016, 35, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Lee, S.; Yun, I.D.; Lee, K.M. Deep Vessel Segmentation By Learning Graphical Connectivity. Med. Image Anal. 2018, 58, 101556. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Huang, X.; Zhou, J.; Li, Y.; Yan, Y.; Sun, Y.; Zhang, Q.; Wang, Y.; Ye, J. FIVES: A Fundus Image Dataset for Artificial Intelligence based Vessel Segmentation. Sci. Data 2022, 9, 475. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Varoquaux, G.; Cheplygina, V. Machine learning for medical imaging: Methodological failures and recommendations for the future. NPJ Digit. Med. 2021, 5, 48. [Google Scholar] [CrossRef]
- Pulini, A.A.; Kerr, W.T.; Loo, S.K.; Lenartowicz, A. Classification Accuracy of Neuroimaging Biomarkers in Attention-Deficit/Hyperactivity Disorder: Effects of Sample Size and Circular Analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 108–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).