1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia and the sixth leading cause of death in the U.S. [
1]. The major pathological hallmarks of AD are amyloid-beta (
) plaques (A), hyperphosphorylated neurofibrillary tau (T) tangles, and neurodegeneration (N), known as the A/T/N framework, a descriptive classification scheme for AD biomarkers [
2,
3,
4]. Non-invasive neuroimaging has been used for the early detection of A/T/N changes. In particular, positron emission tomography (PET) has been used to image
for detecting AD pathology progression [
5,
6,
7,
8].
Recent works on AD classification are mainly based on 3D CNN models. Based on Ref. [
9], the current 3D model methods are divided into three categories: (1) the 3D regions of interest (ROI) based CNN models, (2) the 3D patch-level CNN models, and (3) the 3D subject-level CNN models. The 3D regions of interest (ROI) based CNN models take ROIs of the 3D brain image as the input to train the 3D CNN model [
10,
11]. This approach is time-consuming because it requires manually drawn ROIs. The 3D patch-level CNN models may include Refs. [
12,
13], which extract the 27 patches of 3D image and independently train 27 3D CNNs for ensemble prediction. It is still a complex model since 27 individual 3D CNN models are trained together. The 3D subject-level CNN models the whole 3D brain images as input of the 3D CNN architectures. For instance, some works [
14,
15] have proposed to forward the whole 3D brain image as input of the 3D CNN architectures, which shows high accuracy classification without the need for manual feature extraction. While these 3D CNN models achieve excellent performance, these methods have shown two limitations. First, compared to 2D-CNN, training the 3D-CNN model is computationally expensive. Second, directly training a deep learning model with a relatively small size of the medical image dataset could lead to overfitting. When working with 2D medical images, this can be addressed by using transfer learning, such as adopting widely used ImageNet pre-trained CNN models [
16]. Unfortunately, such models are not readily available for 3D datasets. These two limitations impede the applications for AD diagnosis and predicting AD progression using 3D imaging data.
Other than the 3D CNN method, researchers also explore using 3D images with 2D CNNs. The initial method is to pick the slice of 3D volumes as the input of the model. Ozsahin et al. [
17] proposed selecting and converting a 2D PET slice into a vector as the input of a multilayer perceptron. Ghaffari et al. [
18] applied the transfer learning approach for MRI image classification. Odusami et al. [
19,
20] proposed a hybrid CNN model by parallelly combining the ResNet18 and DenseNet121 together as feature extractors and concatenating the extracted features for prediction. Another common approach is to use temporal pooling to convert a video clip into a 2D image. The idea is to distill a video into a 2D motion representation that summarizes the whole video clip. Dynamic Image [
21,
22] is one of the most famous methods along this line of research. Ref. [
21] proposed to use an approximate rank pooling (ARP) operation to convert the video frames into a 2D dynamic image. In 3D medical image applications, Liang et al. [
23] combined 2D breast mammography and 3D breast tomosynthesis by using ARP on the 3D volumes. Xing et al. [
24] applied ARP on the 3D brain MRI images for Alzheimer’s disease classification. In general, ARP achieves good performance on various tasks. However, as a fixed function, the weight of each frame in APR is deterministic and is calculated using only the frame index and the total number of frames.
To overcome the limitations, we propose to convert 3D volumes into 2D images with a Learnable Weighted Pooling (LWP) method. The advantage of LWP is that it computes the weighted values of each slide of the 3D image, and provides an overall weighted slice as a fusion image. By converting to a 2D image, we were able to dramatically shorten the training time and apply multiple pre-trained 2D models that are currently not available for 3D CNN, such as VGGNet [
25], ResNet [
26], DenseNet [
27], MobileNet [
28], and EfficientNet [
29]. The benefits of using these pre-trained models will allow us to optimize the choice of the feature extractor for different datasets.
In this study, we applied and compared the results between these widely used and pre-trained 2D-CNN models. We further incorporated an attention module for the classifier, strengthening discriminative feature learning and enhancing the deep learning model performance. We hypothesized that by converting 3D PET- imagery to 2D, we were able to reduce the training time while enhancing the performance for AD prediction. We consider our main contributions as follows:
We proposed a novel learnable weighted pooling module for 3D-to-2D image projection and end-to-end network architecture;
We employed a new dual-attention mechanism module on the top of 2D CNNs to boost the model performance;
Compared with 3D CNNs models, the proposed model gained comparable performance with less training computation cost;
We conducted an intensive evaluation with multiple imaging modalities among different 3D-to-2D modules.
2. Materials and Methods
2.1. Data
We obtained the PET-
(AV45) imaging from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database [
30]. Participants were required to have baseline
imaging biomarkers (from Florbetapir AV45 PET). Each subject in ADNI may have multiple neuroimaging scans at different time points. We used the first-time scan of each subject for the early diagnosis task. The collected PET images are pre-processed from the “Coreg, Avg, Std Img and VoxSiz, Uniform Resolution” category. The PET image size is
.
Table 1 shows the demographics of the CU and AD participants. The dataset in total includes 381 subjects, with 214 CU subjects and 167 AD subjects. The two study groups were balanced in gender, race, and age (CU:
, AD:
,
p-value = 0.1511), but not education. CU overall had longer education (in years) than AD participants. Notably, the groups differed in terms of the expression of the
4 allele of apolipoprotein E (APOE
4), the largest genetic risk factor for Alzheimer’s disease, with the AD group being significantly more likely to carry APOE
4 than CU subjects [
31,
32,
33].
2.2. Architecture
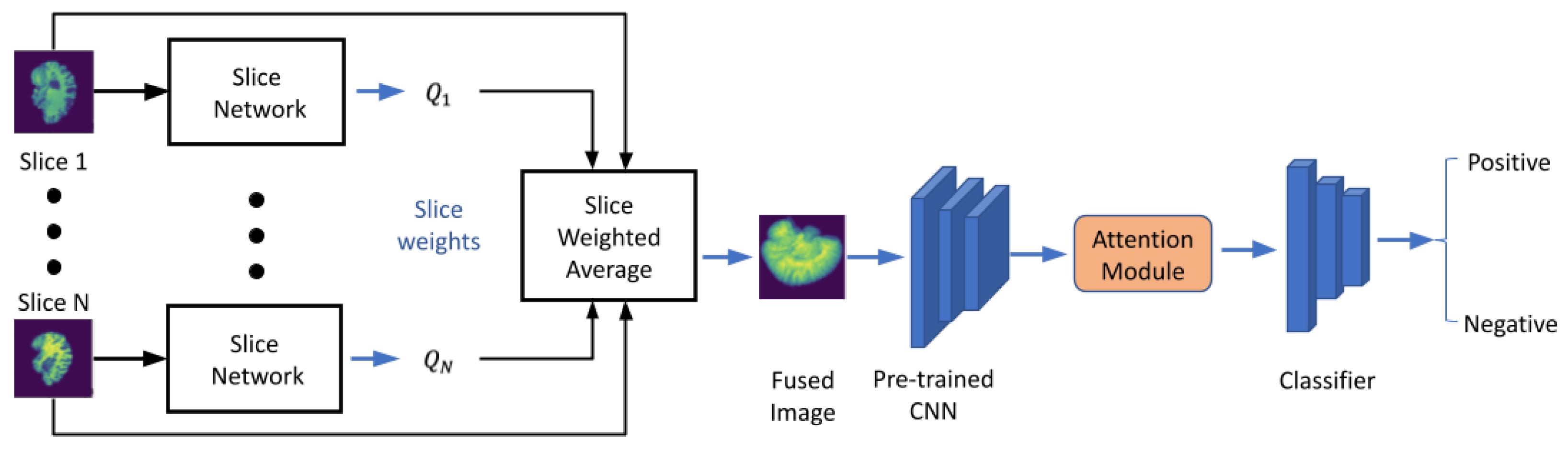
Figure 1 shows the overall workflow of the proposed model. First, each slice of the 3D medical image was passed to the slice network that converts 3D images to a 2D fused image by fusing all the slices using a pooling method. Then, we forwarded the 2D fused image through a pre-trained feature extractor. Afterward, we passed the extracted feature to a dual-attention mechanism to boost our model performance. Finally, the output of the attention module was forwarded to a shallow classifier built by fully connected layers for diagnosis prediction.
2.3. Learnable Weighted Pooling (LWP)
Given a 3D image
, where
is a 2D slice
and
T is equal to the number of slices. A 3D-to-2D projection aims to fuse all slices of the 3D image to get a 2D image
. Inspired by the ARP [
21], we proposed the LWP method. For the LWP operation on a 3D image
V with T slices
, the CNN built slice network
outputs for the corresponding weight of each slice
as
. We use the softmax function
, to normalize the slice weight
between 0 and 1 as Equation (
1). The fused 2D image
is the sum of all weighted slices over a certain view dimension:
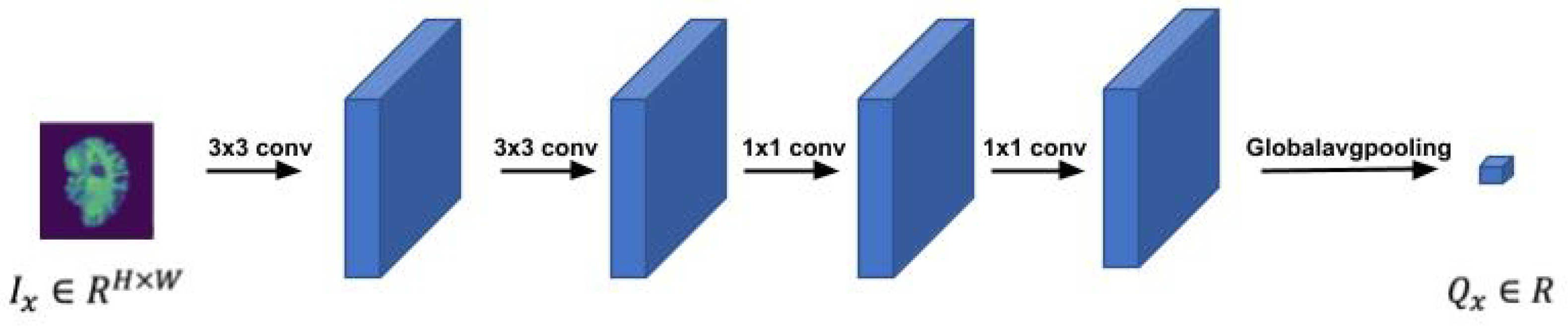
Figure 2 depicts the LWP structure. The value
of a single input slice
is a scalar,
, which means that the temporal rank pooling is on an image level operation. We forward a single slice through a shallow CNN model, which includes four convolutional layers and a global average pooling layer to get a single scalar per slice. The parameters of the slice network were initialized by Kaiming initialization and are fine-tuned during the training.
The whole idea is a 3D-to-2D image-level projection by sum fusion of the weighted slices. There are several significant differences between LWP and ARP. First, the ARP slice weight of each slice is only related to the total slice number T and current slice index , which is fixed and not learnable. Second, ARP applies an average of slices up to slice t on each slice . To address the limitations of ARP, we propose a flexible and trainable fusion module, LWP.
2.4. Attention Module and Classifier
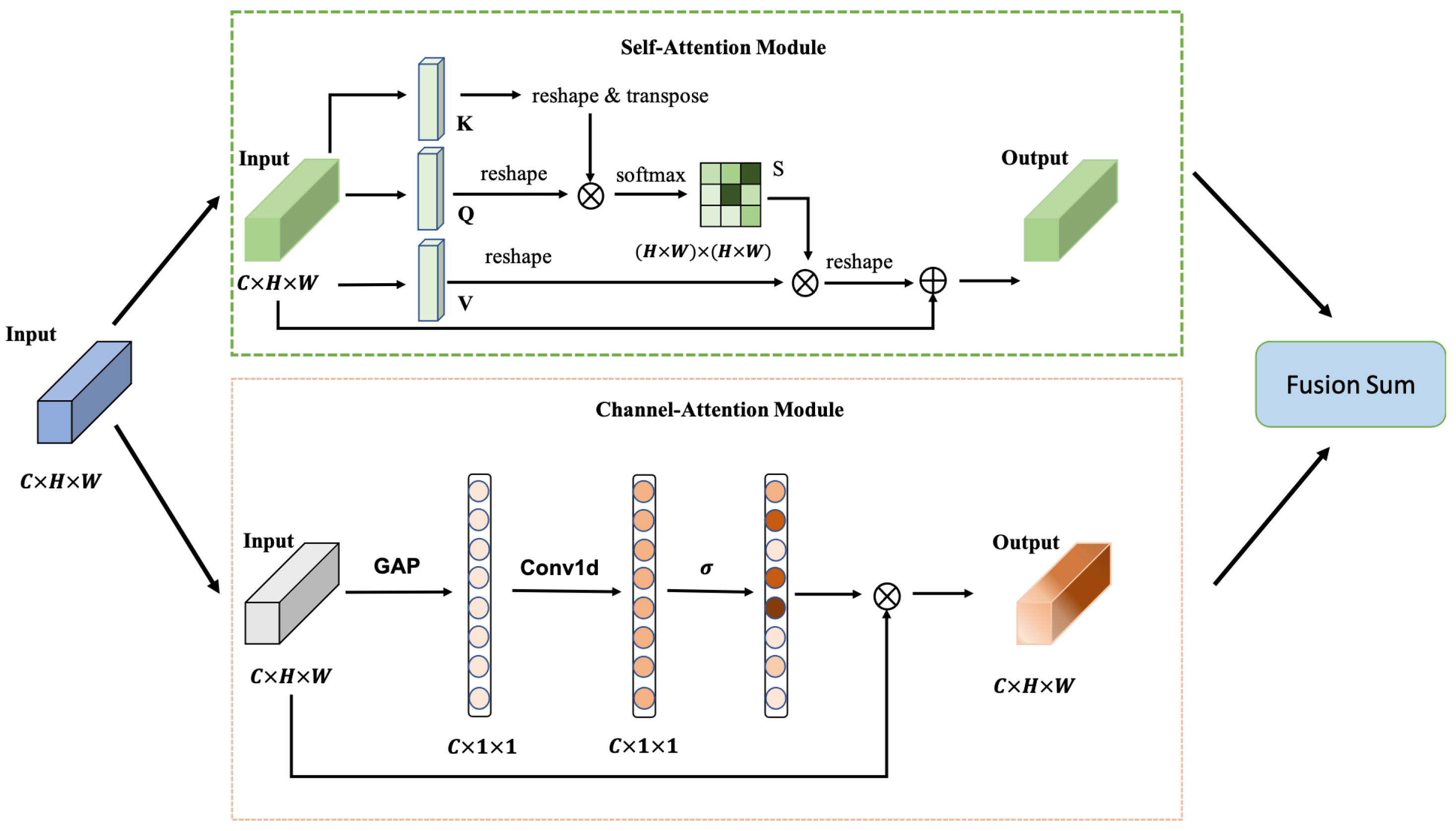
We deploy the attention module to simultaneously refine our extracted feature spatial-wise and channel-wise, so we adopt a dual-attention mechanism architecture. To simplify our model architecture, we employ the dual-attention module on the top of the CNN feature extractor.
Figure 3 depicts the structure of the attention mechanism. The dual-attention module structure is similar to Ref. [
34]. It contains two sub-modules: the self-attention (SA) module [
35] as the position-wise correlation computation and the channel-attention (CA) module [
36] to calculate the channel-wise correlation. The CA module of Ref. [
34] needs quadratic computation cost, but the channel-wise attention of our module is calculated by a Conv1d operation, which is more cost-efficient. We forward the input feature
through the two sub-modules respectively and use sum fusion to merge as the final output feature
.
Our classifier contains three fully connected (FC) layers. The output dimensions of the three FC layers are 512, 64, and 2. Batch normalization and dropout layers are attached after the first two layers. The dropout probability is 0.5.
2.5. Loss Function
In previous AD classification studies, work was mainly concentrated on binary classification. In our work, we did the same for ease of comparison. The overall loss function is weighted binary cross-entropy. For a 3D image
V with label
l and probability prediction
, the loss function is:
where the label
indicates a negative sample and
indicates a positive sample and
and
are loss weights for the positive and negative samples, respectively.
3. Results
To evaluate the proposed method, we carried out several experiments on 3D PET images. The experimental results demonstrated that LWP achieves better performance than the baselines. In the following subsections, we first introduce the implementation details and evaluation metrics, then we report our results on the PET image dataset. Finally, we perform a series of ablation experiments on our dual-attention mechanism.
3.1. Implementation and Metrics
We implemented the CNN models using PyTorch. We trained and tested the models using the 5-fold cross-validation. The feature extractors were pre-trained on ImageNet [
37]. The weights of the classifier were randomly initialized. Both the feature extractor and classifier were fine-tuned during the training. For the 2D models, we set the batch size to 16. Adam optimizer with
,
, and learning rate of
was used during the training. For the 3D CNN models, we followed the parameters setting of Refs. [
14,
15]. We trained all the models for 150 epochs. We computed loss weights for positive (
) and negative (
) classes based on the dataset distribution, by using
,
.
A 3D brain image may be viewed from three directions: axial, coronal, and sagittal. In the implementation, we took the LWP operation on a coronal view for the PET image. More details can be found in
Section 3.2.2.
To evaluate the performance of our model, we used accuracy (Acc), area under the curve of Receiver Operating Characteristics (AUC), F1 score (F1), Precision, Recall, and Average Precision (AP) as our evaluation metrics. We evaluated the training computation cost by the average epoch training time (e-Time). The accuracy is calculated with the following Equation (
4):
where
is the True Positive,
is the True Negative,
is the False Positive, and
is the False Negative.
The
is calculated by the following Equation (
5):
The
is calculated by the following Equation (
6):
The
is calculated by the following Equation (
7):
The AUC curves compare the true positive rate and the false positive rate at different decision thresholds. AP summarizes a precision-recall curve as the weighted mean of precision achieved at each threshold.
3.2. Evaluation
3.2.1. Feature Extractor
High-quality feature extraction is crucial for the final prediction. Different pre-trained CNN models can output different features in terms of size and effective receptive field. Ke et al. [
38] concluded that architecture improvements on ImageNet may not lead to improvement in medical imaging tasks. We conducted five different pre-trained CNNs on the medical dataset to determine which CNN models perform best for our task. To simplify the experiment, we set the PET-AV45 image as the input dataset using LWP and excluded the attention module from the whole experiment.
Table 2 shows different CNN models and the corresponding final metrics. Considering the accuracy and AUC, the ResNet34 delivers comparable performance. In the following experiment, we used ResNet34 as our feature extractor.
3.2.2. Axial vs. Coronal vs. Sagittal
There are three standard views of 3D imaging in medical imaging: sagittal, coronal, and axial. In the main paper, we selected the coronal view for PET empirically. The remainder of this section summarizes the experiment we conducted.
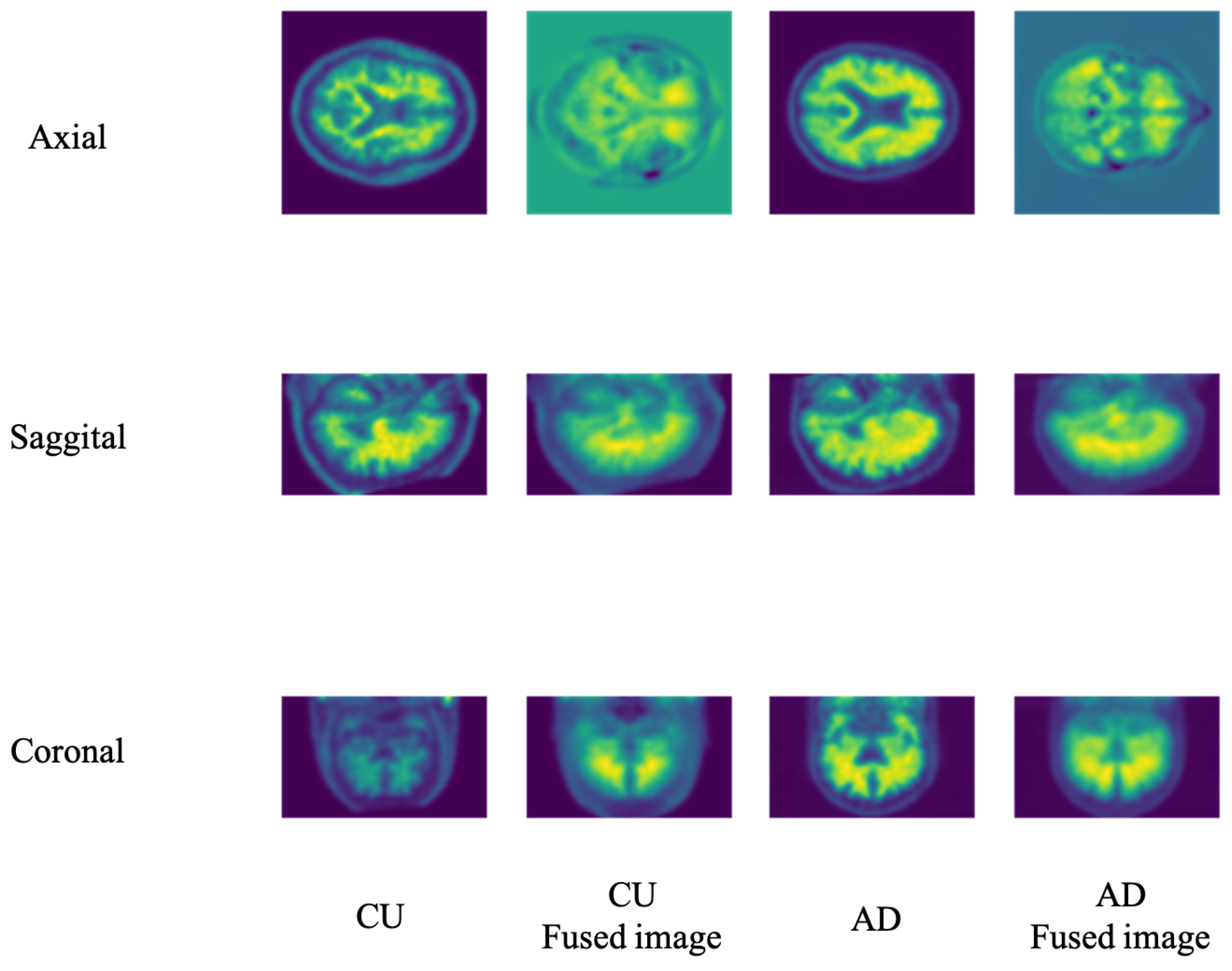
Figure 4 illustrates the different views of the 3D PET images. Considering that the LWP performance may vary due to the different views fusion, we conducted experiments to specify the view influence on our model performance.
Table 3 shows the model performance on the three views under the imaging modality. As a PET image, the coronal view fusion offers better performance than others. Therefore, our following experiments set the fusion views as the default of our LWP model without specific mention.
3.2.3. CU vs. AD
We conducted the binary classification on the CU and AD prediction. To compare the pooling operation, we set three pooling baselines, namely Max pooling (Max.), Average pooling (Avg.), and ARP.
Table 4 presents the experiment results on different models, showing that the proposed 3D-to-2D model outperforms the other CNN models. Compared with the 3D CNN baseline model, the LWP method improves
in accuracy (from 0.84 to 0.88) and
in AUC (from 0.87 to 0.90), respectively. We used the Multiply-Add operations (MADs) and training epoch time (e-time) as the reference, considering the training computation cost.
Table 5 shows the training computation cost of different CNN models. The e-time of the LWP is around
of the 3D baseline model, and the MADs of LWP is around
of the 3D baseline model. We further conducted the t-test on the e-time between LWP and the 3D CNN models, the
p-value is
, proving the significant improvement of the efficient training. Furthermore, we showed the AUC graph as
Figure 5 and the confusion matrix as
Figure 6. In
Figure 6, “Negative” indicates CU subjects, while “Positive” indicates AD subjects.
3.2.4. Attention Mechanism Ablation Study
We evaluated the ablation study to specify our dual-attention module. The baseline (BS) model structure is without an attention mechanism. Since the dual-attention (DA) module contains two sub-attention modules: self-attention (SA) and channel-attention (CA), we conducted four models: BS, BS + SA, BS + CA, and BS + DA.
Table 6 shows the performance of the attention mechanism ablation study. The results show that the dual-attention module performs better than others.
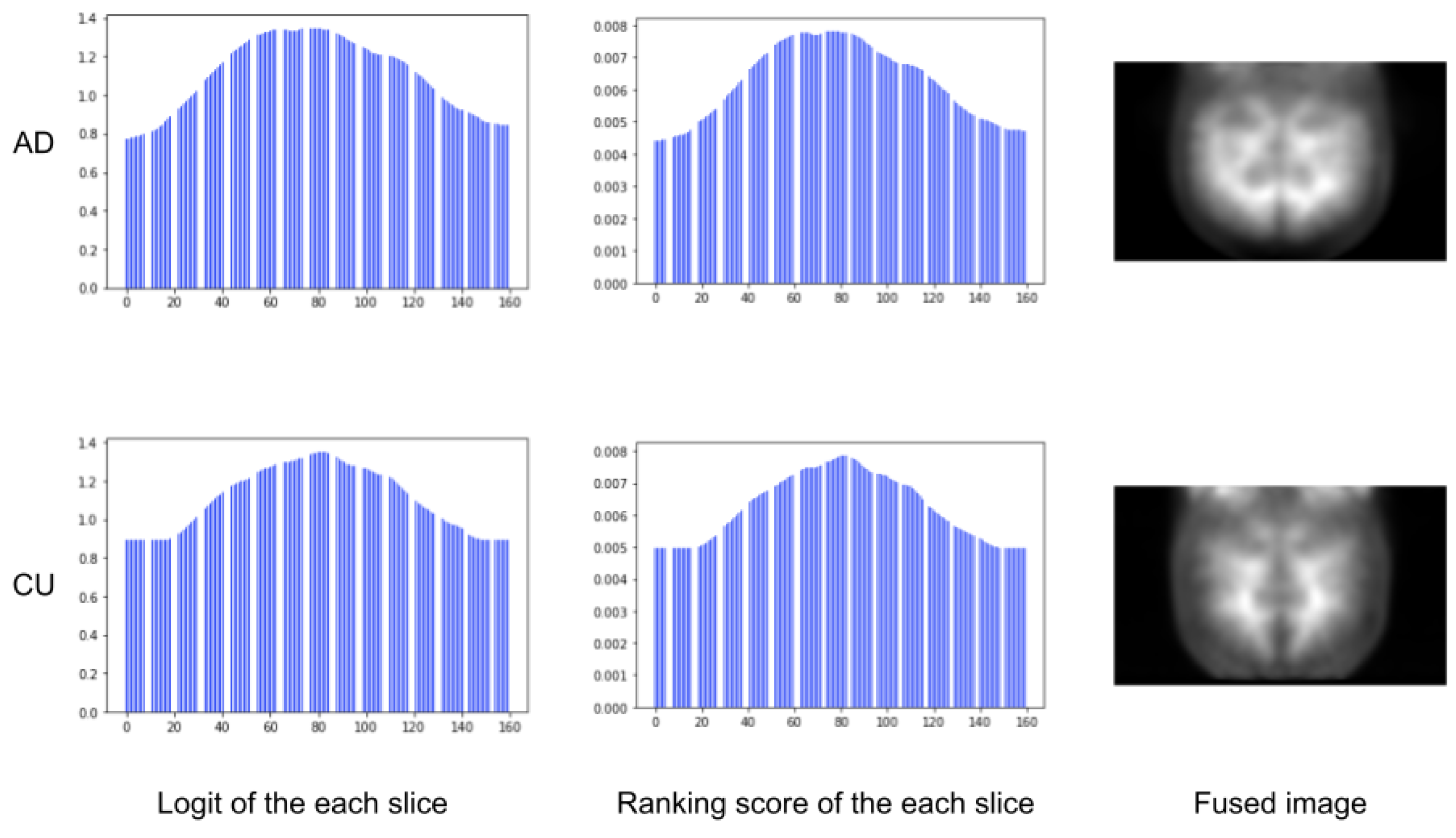
3.2.5. Visualization
Figure 7 shows the results on the slice logits, slice ranking scores, and fused images. We found that central slices of the 3D brain outweighed the surrounding slices. It indicates that brain regions covered by the central slices may play a more important role in revealing AD pathology than those in the lateral slices.
4. Discussion
In this study, we have proposed a novel method for training the 3D image by 2D CNN and end-to-end network architecture. We showed that our newly developed model significantly reduced the processing time while achieving comparable performance compared with traditional 3D CNN models. We also demonstrated several other novel findings, as follows. First, we found that ResNet34 outperforms other 2D CNN backbones as a feature extractor. Second, we demonstrated that different views of 2D images may have different performing outcomes when converting 3D images into 2D. In the current study, we showed that the coronal view performed better than the other two. Third, we showed that the LWP model can effectively convert the 3D to 2D fused images with low training time and computation cost while maintaining high performance. The visualized results further illustrated that the mid-range slices had higher importance than the side-range slices. Fourth, we have proposed a new attention module by paralleling the self-attention module and channel-wise attention module together for better discriminative feature extraction. Our results demonstrated the effectiveness of the new attention module.
Our method is inspired by the ARP method but it is different in implementation. The ARP method fuses the slices of the 3D image with the static weights, but our method is data-driven and learnable by the model itself. Compared with ARP, the LWP can capture more informative features and can gain and boost better performance. Compared with the traditional 3D CNN models, the LWP method saves much more training time and computation cost and achieves comparable or even better performance. Compared with conventional 2D CNN models, the fused image of LWP contains more informative features than the single-slice 2D images, which show better performance than the single-slice input of the 2D CNN. The idea of the LWP method is to record the abnormal variance between the 3D brain slices. In the medical domain, there is severe shrinkage in the structure and different metabolism densities of the brain in an AD patient. The 3D-to-2D projection concentrates on extracting the discriminative image-level information between CU and AD is the critical input of the 2D CNN.
The newly developed 3D-2D model may have profound implications in the future clinical setting for AD early diagnosis. Currently, 3D brain images are not widely available or used in routine clinical diagnosis. One of the major reasons is the unreasonably long processing time needed to get the information timely in daily health care. The proposed model needs fewer computation resources than the traditional 3D models, making the computation faster and less required for the hardware demands, which may be more applicable and affordable to be implemented in the clinical setting, as well as for mobile device use, in the future. Our future work will apply this method to other 3D brain image modalities, such as MRI structural and cerebral blood flow imaging analysis.
A limitation of the study is the lack of the spatial structure information of the 3D image as there was a trade-off between the 3D image spatial information and computation efficiency in our model. Further, our focus in the present study was on the classification of late stage AD versus CU and using PET-AV45 imaging only. In the future, it will be important to apply a similar method to earlier clinical stages (e.g., early mild cognitive impairment) and include other imaging measurements, such as brain atrophy using MRI.
In conclusion, we demonstrated a novel 3D-2D CNN conversion model which significantly increased the efficiency of Alzheimer’s disease classification using PET-AV45 imaging. The method may have important implications for disease diagnosis and medical applications using mobile devices in the future.
Author Contributions
X.X., M.U.R., N.J. and A.-L.L.; Conceptualization. X.X., M.U.R., N.J. and A.-L.L.; Methodology. X.X. and M.U.R.; Software. X.X. and C.W.; Validation. X.X., M.U.R., N.J. and A.-L.L.; Formal analysis. A.-L.L.; Investigation. N.J. and A.-L.L.; Resources. X.X., Y.Z. and C.W.; Data curation. X.X. and A.-L.L.; Writing—original draft. M.U.R., G.L., H.B., Y.Z., C.W., N.J. and A.-L.L.; Review and Editing. X.X. and C.W.; Visualization; N.J. and A.-L.L.; Supervision. A.-L.L.; Project administration and Funding acquisition. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NIH grants R01AG054459 and RF1AG062480 to A.-L.L.
Data Availability Statement
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (
www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflicts of Interest
The authors declare no conflict of interest.
References
- NIH. Alzheimer’s Disease Fact Sheet. 2021. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 7 August 2021).
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.C.; Xing, X.; Wang, C.; Ma, D.; Nho, K.; Crane, P.K.; Elahi, F.; Ziegler, D.A.; Liang, G.; Cheng, Q.; et al. β-amyloid and tau drive early Alzheimer’s disease decline while glucose hypometabolism drives late decline. Commun. Biol. 2020, 3, 352. [Google Scholar] [CrossRef] [PubMed]
- Rabinovici, G.; Rosen, H.; Alkalay, A.; Kornak, J.; Furst, A.; Agarwal, N.; Mormino, E.; O’Neil, J.; Janabi, M.; Karydas, A.; et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011, 77, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cheng, D.; Yan, W.; Alzheimer’s Disease Neuroimaging Initiative. Classification of Alzheimer’s disease by combination of convolutional and recurrent neural networks using FDG-PET images. Front. Neuroinform. 2018, 12, 35. [Google Scholar] [CrossRef]
- Samper-González, J.; Burgos, N.; Bottani, S.; Fontanella, S.; Lu, P.; Marcoux, A.; Routier, A.; Guillon, J.; Bacci, M.; Wen, J.; et al. Reproducible evaluation of classification methods in Alzheimer’s disease: Framework and application to MRI and PET data. NeuroImage 2018, 183, 504–521. [Google Scholar] [CrossRef]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Mari Aparici, C.; et al. A deep learning model to predict a diagnosis of Alzheimer disease by using 18F-FDG PET of the brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef]
- Wen, J.; Thibeau-Sutre, E.; Diaz-Melo, M.; Samper-González, J.; Routier, A.; Bottani, S.; Dormont, D.; Durrleman, S.; Burgos, N.; Colliot, O.; et al. Convolutional neural networks for classification of Alzheimer’s disease: Overview and reproducible evaluation. Med Image Anal. 2020, 63, 101694. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.; Cerasa, A.; Battista, P.; Gilardi, M.C.; Quattrone, A.; Castiglioni, I. Magnetic resonance imaging biomarkers for the early diagnosis of Alzheimer’s disease: A machine learning approach. Front. Neurosci. 2015, 9, 307. [Google Scholar] [CrossRef]
- Lin, W.; Tong, T.; Gao, Q.; Guo, D.; Du, X.; Yang, Y.; Guo, G.; Xiao, M.; Du, M.; Qu, X.; et al. Convolutional neural networks-based MRI image analysis for the Alzheimer’s disease prediction from mild cognitive impairment. Front. Neurosci. 2018, 12, 777. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, M.; Fu, J.; Wang, Y. Classification of MR brain images by combination of multi-CNNs for AD diagnosis. In Proceedings of the Ninth International Conference on Digital Image Processing (ICDIP 2017), Hong Kong, China, 19–22 May 2017; p. 1042042. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, D.; Wang, K.; Wang, Y. Multi-Modality Cascaded Convolutional Neural Networks for Alzheimer’s Disease Diagnosis. Neuroinformatics 2018, 16, 295–308. [Google Scholar] [CrossRef]
- Korolev, S.; Safiullin, A.; Belyaev, M.; Dodonova, Y. Residual and plain convolutional neural networks for 3D brain MRI classification. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, VIC, Australia, 18–21 April 2017; pp. 835–838. [Google Scholar]
- Jo, T.; Nho, K.; Risacher, S.L.; Saykin, A.J. Deep learning detection of informative features in tau PET for Alzheimer’s disease classification. BMC Bioinform. 2020, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, I.; Sekeroglu, B.; Mok, G.S. The use of back propagation neural networks and 18F-Florbetapir PET for early detection of Alzheimer’s disease using Alzheimer’s Disease Neuroimaging Initiative database. PLoS ONE 2019, 14, e0226577. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, H.; Pirzad Jahromi, G. Deep transfer learning–based fully automated detection and classification of Alzheimer’s disease on brain MRI. Br. J. Radiol. 2022, 95, 20211253. [Google Scholar] [CrossRef]
- Odusami, M.; Maskeliūnas, R.; Damaševičius, R. An Intelligent System for Early Recognition of Alzheimer’s Disease Using Neuroimaging. Sensors 2022, 22, 740. [Google Scholar] [CrossRef]
- Odusami, M.; Maskeliūnas, R.; Damaševičius, R.; Misra, S. ResD Hybrid Model Based on Resnet18 and Densenet121 for Early Alzheimer Disease Classification. In Proceedings of the International Conference on Intelligent Systems Design and Applications, Seattle, WA, USA, 13–15 December 2022; pp. 296–305. [Google Scholar]
- Bilen, H.; Fernando, B.; Gavves, E.; Vedaldi, A.; Gould, S. Dynamic Image Networks for Action Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Fernando, B.; Gavves, E.; Oramas, J.; Ghodrati, A.; Tuytelaars, T. Modeling video evolution for action recognition. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 5378–5387. [Google Scholar]
- Liang, G.; Wang, X.; Zhang, Y.; Xing, X.; Blanton, H.; Salem, T.; Jacobs, N. Joint 2d-3d breast cancer classification. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; pp. 692–696. [Google Scholar]
- Xing, X.; Liang, G.; Blanton, H.; Rafique, M.U.; Wang, C.; Lin, A.L.; Jacobs, N. Dynamic image for 3d mri image alzheimer’s disease classification. In Proceedings of the European Conference on Computer Vision, Glasgow, UK, 23–28 August 2020; pp. 355–364. [Google Scholar]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016. [Google Scholar]
- Huang, G.; Liu, Z.; Van Der Maaten, L.; Weinberger, K.Q. Densely connected convolutional networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 4700–4708. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the The IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Salt Lake City, UT, USA, 18–23 June 2018. [Google Scholar]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, PMLR, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar]
- ADNI. 2003. Available online: http://adni.loni.usc.edu/ (accessed on 9 January 2023).
- Yanckello, L.M.; Hoffman, J.D.; Chang, Y.H.; Lin, P.; Nehra, G.; Chlipala, G.; McCulloch, S.D.; Hammond, T.C.; Yackzan, A.T.; Lane, A.N.; et al. Apolipoprotein E genotype-dependent nutrigenetic effects to prebiotic inulin for modulating systemic metabolism and neuroprotection in mice via gut-brain axis. Nutr. Neurosci. 2021, 25, 1669–1679. [Google Scholar] [CrossRef]
- Hammond, T.C.; Xing, X.; Yanckello, L.M.; Stromberg, A.; Chang, Y.H.; Nelson, P.T.; Lin, A.L. Human Gray and White Matter Metabolomics to Differentiate APOE and Stage Dependent Changes in Alzheimer’s Disease. Age 2021, 85, 86–89. [Google Scholar]
- Lin, A.L.; Parikh, I.; Yanckello, L.M.; White, R.S.; Hartz, A.M.; Taylor, C.E.; McCulloch, S.D.; Thalman, S.W.; Xia, M.; McCarty, K.; et al. APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer’s disease. Neurobiol. Dis. 2020, 139, 104834. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, J.; Tian, H.; Li, Y.; Bao, Y.; Fang, Z.; Lu, H. Dual attention network for scene segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Long Beach, CA, USA, 15–20 June 2019; pp. 3146–3154. [Google Scholar]
- Wang, X.; Girshick, R.; Gupta, A.; He, K. Non-local Neural Networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018. [Google Scholar]
- Wang, Q.; Wu, B.; Zhu, P.; Li, P.; Zuo, W.; Hu, Q. ECA-Net: Efficient Channel Attention for Deep Convolutional Neural Networks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 13–19 June 2020. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.F. ImageNet: A Large-Scale Hierarchical Image Database. In Proceedings of the 2009 IEEE Conference on Computer Vision and Pattern Recognition, Miami, FL, USA, 20–25 June 2009. [Google Scholar]
- Ke, A.; Ellsworth, W.; Banerjee, O.; Ng, A.Y.; Rajpurkar, P. CheXtransfer: Performance and Parameter Efficiency of ImageNet Models for Chest X-ray Interpretation. In Proceedings of the Conference on Health, Inference, and Learning, Virtual Event, 8–10 April 2021; Association for Computing Machinery: New York, NY, USA, 2021; pp. 116–124. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).