Abstract
The use of telerehabilitation systems has shown a significant growth in the past years, demonstrating their crucial relevance in the time of the COVID-19 pandemic. Many devices and sensors have been proposed to analytically measure parameters for patient assessment, with limitations due to costs or feasibility. In this paper, we present a motor telerehabilitation system with computer vision-assisted markerless measures for patients with Rett syndrome. Twenty-one RTT (Rett syndrome) patients, with ages ranging from age 4 to 31 (Median: 12.50; IQR (interquartile range): 9.50–17.25) were recruited. The study follows a pre-test–post-test design, where the patients were submitted to a pre-test, treatment, post-test 1, treatment, post-test 2 procedure. Progress in patient outcomes was assessed by measuring joint passive range of movement (PRoM). Results show the reliability of our system, and the feasibility of a telerehabilitation treatment for RTT patients, with significant improvements in shoulder mobility and in elbow flexion and extension. Limited results in lower limbs suggest that home treatment should be fostered to reduce sedentary time.
1. Introduction
Rett syndrome (RTT) is a severe neurodevelopmental disease caused, in most cases, by a mutation in the MECP2 gene [1,2]. The condition affects about 1/10,000 females and a few males, although some researchers have reported a higher incidence rate [3,4,5]. Cognitive, communication, and motor regression are the hallmarks of RTT, occurring after a seemingly normal prenatal and perinatal period. People with RTT typically show repetitive hand movements, muscle tone abnormalities, and movement disorders such as ataxia and apraxia, causing limited gross and fine motor function [6,7,8,9,10,11,12,13,14]. The neuromuscular impairment and limited mobility facilitate the development of musculoskeletal abnormalities. RTT syndrome frequently affects feet and spine, but all body joints can be affected [15].
Although body joint musculoskeletal abnormalities showed a prevalence between 36 and 60% in the population with RTT [16,17], these conditions received little attention in the literature. Range of motion (RoM) limitations of body joints negatively affect the subject’s active and passive mobility, complicating the execution of movements and daily care provision by caregivers (such as dressing, undressing, and personal hygiene). Several nonsurgical intervention strategies are available for improving joint RoM (see [18,19] for reviews). However, to the authors’ knowledge, when looking at the population with RTT, only one physiotherapy intervention concerning joint RoM improvement was proposed and tested [20]. It referred to an individualized, remotely supervised intervention based on the performance of motor activities and postural strategies to contrast with the RoM limitations of the joints. However, although conducted remotely, this intervention was based on an in vivo evaluation of the participants, requiring the professionals and experts to visit the person’s house. Moreover, as the intervention was supervised through videoconferences, the researchers could not evaluate the performance of the provided activities in real time.
Remotely provided physiotherapy interventions referred to the field of telerehabilitation. Telerehabilitation refers to using various technologies to provide rehabilitation and habilitation services remotely [21], from assessing a person’s condition to individualized intervention development. Moreover, using telerehabilitation strategies allows improved access to rehabilitation services and specialists, preventing unnecessary delays in care and support [22].
In the last decade, the literature has been enriched with publications exploring new technologically based therapeutic strategies for evaluating and treating the cognitive, communicative, and motor disabilities produced by RTT [23,24]. In all these fields, technology-supported strategies effectively support skill acquisition or improvement. However, while high-level technological tools were proposed for cognitive and communication areas (such as eye tracking and face recognition systems [25,26]), the available motor remote rehabilitation interventions for people with RTT are based on videoconferencing (a low technological level strategy).
Recent technical developments in computer vision provide the potential for markerless human motion capture for biomechanical and clinical applications. The use of such technology in rehabilitation has rapidly grown in the last decade [27,28,29,30,31,32,33,34,35,36,37], allowing continuous rehabilitation training at home with similar effects compared to conventional therapies. Markerless motion capture based on devices that include dedicated pose-estimating software and standard cameras working with separate software such as OpenPose were used to evaluate and train people with neuromotor disorders of various etiologies. The use of these tools for evaluation purposes was successfully proposed for assessing gait spatiotemporal parameters (such as walking speed and cadence [38,39]) and upper- and lower-body joint kinematics and dynamics during mobility tests and tasks [40,41,42]. The data retrieved were used to estimate motor function classifications (e.g., Gross Motor Function Classification System and Gait Deviation Index [31]), diagnostic analysis (e.g., general movement assessment in children for early detection of cerebral palsy [43,44]), disease progression monitoring [45,46], and fall prevention in older adults [47].
Despite the growing body of literature concerning the clinical use of markerless motion capture technologies, its application for the passive range of motion (PRoM) assessment of joints was rarely proposed, even though an excellent agreement with goniometer readings and small measurement standard error was reported when assessing joint PRoM [48,49]. In the past, methods based on inertial sensors [50,51,52] and 3D marker-based motion capture [53] have been shown to be very accurate for measuring RoM. However, these methods require a specific setup and are not as user-friendly as markerless motion capture [31]. Moreover, as far as the authors know, no attempts to use markerless motion capture systems were made to assess the joint PRoM of people with severe and multiple disabilities, such as those with RTT. The authors previously developed the Telerehabilitation, Counseling, and Training in Rett Syndrome (TCTRS) system [54], an integrated remote rehabilitation platform for people with RTT. The TCTRS enables remote cognitive, communication, and motor rehabilitation intervention through a rehabilitation platform developed explicitly for rehabilitating people with RTT, which integrates videoconference software, eye-tracking technology, and a markerless body motion capture system, allowing rehabilitation professionals to conduct evaluation and intervention remotely through consumer-grade devices and communication technologies, whereas in other cases, specific approaches were exploited to guarantee adequate communication performances [55]. In particular, the TCTRS introduces a digital goniometer function based on markerless motion capture technology, allowing remote PRoM assessment.
Therefore, the current study aims to estimate the reliability of the digital goniometer included in the TCTRS system. A second aim is to evaluate the effect of an individualized physical therapy intervention, remotely provided through the TCTRS system, on improving the shoulder, elbow, and knee PRoM of people with RTT, using the TCTRS digital goniometer as an outcome measure. Therefore, the overall research question is to investigate if the motor telerehabilitation setting provided by the TCTRS project is suitable and adequate for patients with multiple disabilities, and specifically for RETT patients, given their peculiarities.
2. Materials and Methods
2.1. Participants
The participants are the same as a previously published work on the development of cognitive abilities [23]. Twenty one RTT patients were recruited in the study by the Italian Rett Association (www.airett.it, last accessed on 29 November 2022). Their ages ranged from 4 to 31 (Median: 12.50; IQR: 9.50–17.25). RTT patients were classified as clinical stage III or IV, following criteria defined by Neul et al. [9]. All the participants manifested hand stereotypies and used to attend schools or socio-educational centers. Syndrome severity was assessed for each patient using the neuropsychiatric reference. Levels of severity ranged from 5 to 20, where level 5 refers to mild severity, while level 20 refers to severe severity, according to the standardization of the subscale “clinical severity” of the RARS [56]. Table 1 shows the attributes of the groups.

Table 1.
Characteristics of participants (this table comes from work Fabio et al. [23]).
2.2. Study Design
A pre-test, post-test 1, post-test 2 study design was employed, with a group composed of 21 young girls and women with RTT syndrome (Figure 1).
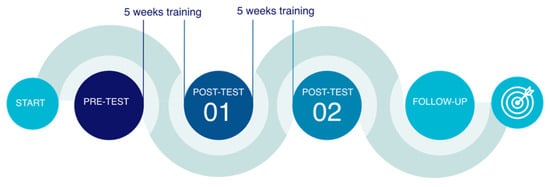
Figure 1.
Phases of the study [23].
Subjects were evaluated by means of a telerehabilitation system, equipped with enhanced tools to acquire a digitized version of the patient’s skeleton superimposed on the video depicting the patient. Patients were evaluated in the pre-test phase, measuring joint angles. Evaluations were repeated after a first treatment step (5th week) and after the conclusion of treatment (10th week). Measurements obtained in the different phases were compared to assess the efficacy of the intervention. Outcomes in the different phases were registered by blinded investigators to prevent subjective bias (Cohen’s kappa > 0.86).
2.3. The Telerehabilitation System Architecture
The telerehabilitation system contemplated two types of workstations, namely patient side (local) and specialist side (remote), as Figure 2 shows.
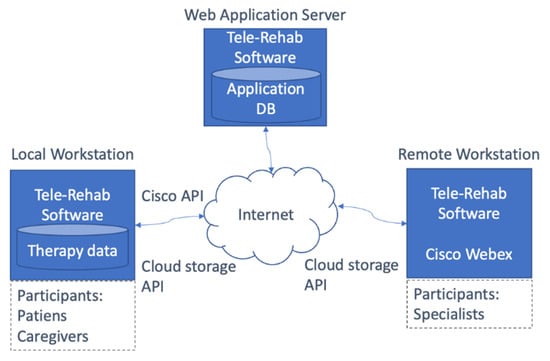
Figure 2.
The telerehabilitation system architecture [54].
Patient and caregiver could communicate with therapists both for a cognitive or motor rehabilitation session. Note that, in the case of RTT syndrome, patients could not be autonomous in this operation, since they always need the presence of a caregiver. Patient side, the system used a laptop with a built-in eye-tracker, a stereo camera, a webcam and a headset. The software was implemented as a Web Application, exploiting CISCO Webex API for videocalls.
2.3.1. Skeleton Model
The measurement system of our telerehabilitation software is based on the ZED OpenPose library (https://github.com/stereolabs/zed-openpose (accessed on 29 November 2022)) an open-source 3D version of the open-source library, OpenPose [57]. OpenPose is a well-known AI-based software that allows the detection of the body skeleton of a person in a single 2D image or in each frame of a video. As the inferred skeleton keypoints are inherently 2D, the output of OpenPose was not sufficient to produce a suitable measurement of joint angles in space. As a consequence, a 3D skeleton detection approach was needed. In our experimental setup, a 3D camera, based on a stereo camera and a stereo-to-3D reconstruction software library made available by the producer of the camera, was exploited. The 3D camera produced a dense 3D cloud of points for each acquired video frame. The keypoints of the 2D skeleton detected by the OpenPose software were then matched with the 3D points and for each 2D keypoint, the closest 3D point was selected as the corresponding 3D skeleton keypoint. The matching was performed by comparing the (x, y) coordinates of each 2D skeleton keypoint with the (x, y) coordinates of the 3D points. If an exact match was missing, then the Euclidean Distance was used to select the nearest 3D point, taking into consideration the z coordinates of nearby, already selected, 3D skeleton points. Noticeably, as the stereo rig baseline was only 12cm wide, the 3D point cloud did not contain points that were not visible in the 2D image analyzed by the OpenPose software. As a consequence, for each 2D keypoint, one 3D point at most could be selected by the matching process and no ambiguities were possible. By using the described approach, the ZED OpenPose software produced a set of 3D skeleton keypoints for each person detected in the stereo video.
In Figure 3, an example of the skeleton is shown, with the keypoint labeling and planes.
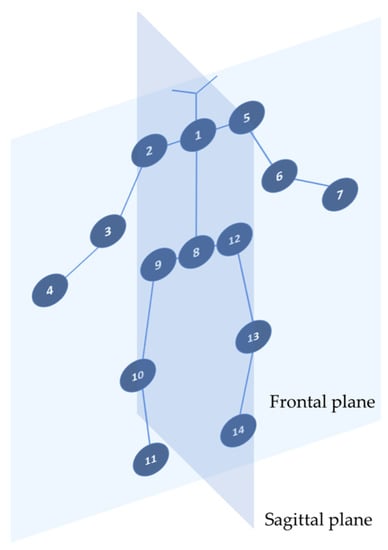
Figure 3.
Skeleton model and keypoints used for joint measurements: left shoulder flexion: 1-5-6 (sagittal plane); right shoulder flexion: 1-2-3 (sagittal plane); left shoulder abduction: 1-5-6 (frontal plane); right shoulder abduction 1-2-3 (frontal plane); left elbow flexion: 5-6-7 (sagittal plane); right elbow flexion: 2-3-4 (sagittal plane); left knee extension: 12-13-14 (sagittal plane); right knee extension: 9-10-11 (sagittal plane).
2.3.2. Data
Data were collected during videoconference calls by the local workstation (patient side), composed of a laptop with a connected stereo ZED camera and equipped with our telerehabilitation software. During telerehabilitation sessions, the specialist could remotely instruct the local workstation to record video sequences and text notes without distracting the local caregiver, by typing specific text commands in the video call chat. For example, writing Start recording began the recording event, while Start zed triggered the acquiring of skeleton data. All these annotations were recorded in a JSON file that contained all the session’s data and metadata (see Figure 4). Metadata refers to text annotations, date and time of the session, references to the other session files (video recordings). Data were about the 3D coordinates of the keypoints detected by the ZED OpenPose library during the session.
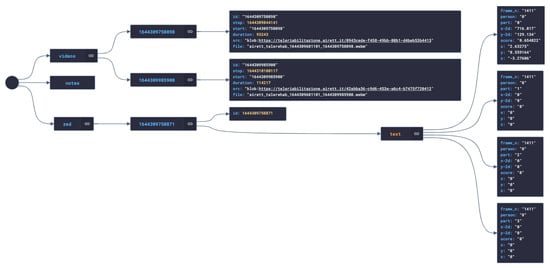
Figure 4.
A graph representation of the JSON file storing data and metadata about a therapy session.
Hence, at the end of each session, two types of files were locally stored, namely a JSON file for data and metadata, and one or more video recordings (in WebM format), and shared by the patient/caregiver through a Google Drive cloud storage service, which was embedded in the telerehabilitation software. Each patient/caregiver user accessed her/his cloud folder via OAuth 2.0 protocol and shared the folder with the therapists, so that those data were available both to the patient/caregivers and the therapists.
It is worth noting that the stored files contained only the 2D and 3D coordinates of the joints (keypoints) that would be sufficient to infer the joint angles described in Figure 3. The process of 3D angle calculation was performed as follows (2D angles are similarly calculated). Keypoint coordinates of two connected bones forming a joint were acquired as described above (see section Skeleton model and Figure 3). Each keypoint was defined as a triple in the 3D space as follows:
Pm (xm, ym, zm)
Pn (xn, yn, zn)
Po (xo, yo, zo)
Additionally, the skeleton bones defined by the segments PmPn and PnPo form an angle in Pn. These segments were normalized and translated to the axes origin and were represented as two vectors, defined by their coordinates, as follows (see Figure 5):
u = (u1, u2, u3)
v = (v1, v2, v3)
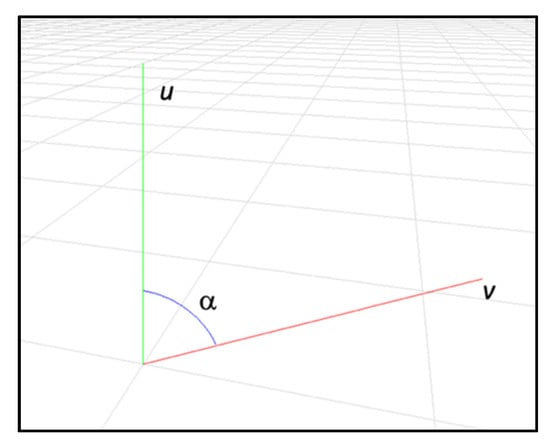
Figure 5.
The angle α between two 3D vectors representing two skeleton keypoints forming a joint.
Then, the angle αmno formed between the 3D vectors u and v was calculated as follows:
where is the scalar product of the vectors and
represents the norm of the vectors.
As a consequence, data about angles were represented as a list of triples:
where subi, i ∊ {0,1} represents the detected subject and αmno is the angle relative to the joint referring to skeleton keypoints Pm, Pn, Po, and plane ∊ {frontal, sagittal}. It is worth noting that the system could acquire more than one skeleton. This is a very important feature since RTT patients are not autonomous and needed the assistance of a caregiver to perform rehabilitation tasks. Hence, the therapist could discriminate the patient’s skeleton from the software interface (see next section), avoiding mistakes in the joint angle data.
<subi, αmno, plane>
2.3.3. Therapist’s Interface
The software was developed within the project and has both client-side and server-side components. The server-side component was used essentially to manage user accounts, with different access levels (namely therapist, patient/caregiver, and administrator). The user logged in to the system through a Cisco Webex account, via OAtuh 2.0 protocol, since the Webex API was used to perform video calls.
The therapist’s interface allowed the therapist to analyze the data of the telerehabilitation sessions, recorded as discussed above. Through the software interface, therapists could analyze the acquired data both for the cognitive and motor session. In the specific case of motor rehabilitation, the therapist could synoptically analyze 2D and 3D skeletons, together with contextual annotations and joint angle measurements. During the telerehabilitation session, the therapist guided the caregiver on which exercise to do, gave suggestions, annotated text, or triggered data or video acquisition, but the patient evaluation was an offline task (see section Data). However, during the online session, the skeleton overlapped on the patient video, so that the therapist could notice if the data acquisition was not optimal, for instance because of obstructions or poor light conditions.
In Figure 6, a screenshot of the interface is shown.
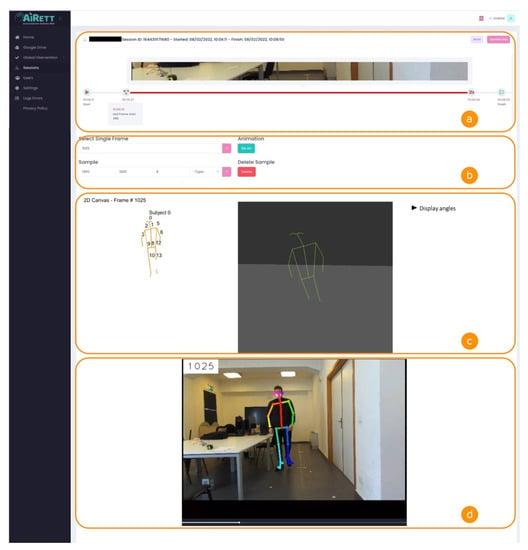
Figure 6.
Telerehabilitation software interface for patient assessment. In (a) thumbnails of the telerehabilitation session video recordings are shown; (b) contains the form to select the frame number that the therapist wants to analyze; in (c) 2D and 3D skeleton are depicted, and measurements about joints’ angles are shown; (d) displays the video recordings with skeleton, frame number and therapist’s comments overlayed.
This tool had a very important role, as it provided objectivity and confirmed the therapist’s observation.
The interface was composed of four sections (named from a to d in Figure 6) and a menu on the left side. In section a, the interface shows some metadata about the therapy session, such as the patient’s name, session ID, and the start and end date and time of the session. Moreover, in section a, there is a timeline that shows a thumbnail of the video recordings and text annotations taken by the therapist during the session, displayed at the correct position in the timeline. Those annotations are also overlayed on the video shown in section d at the correct time.
In section b, the interface shows a form where the therapist can choose either a single frame to display skeleton data (section c), or a frame interval to see the skeleton animation between those frames. The frame interval was not used in joint angle calculations, since it is a new feature that we want to use in future for gait analysis, and so is not part of the present study.
In section c, there are two skeleton representations, in 2D and 3D. While the 2D is used to label the joints, the 3D skeleton is used to understand the position of the patient in space. In the same section, the therapist can display the angles referring to the selected frame.
Section d shows the video recording of the session. The video has three overlayed layers. The first shows the frame number and helps the therapist choose the desired frame in section b. The second layer shows the acquired skeleton, so that the therapist can notice if the data acquisition is not optimal in a specific frame, for instance because of obstructions or poor light conditions. The third layer shows text annotations of the therapist at the time in which they were taken.
As a further improvement, we plan to integrate a decision support system in the software, to better help therapists in data analysis and add functionalities, for instance for gait analysis.
2.4. Procedure
Motor evaluation carried out through TCTRS allowed for the assessment joint PRoM. Joint mobility was evaluated before (pre-test—T1) the intervention and both five (post-test 1—T2) and 10 (post-test 2—T3) weeks after it. The procedure flow diagram is depicted in Figure 1. During the assessment, the patient sat on a chair with her back resting on a backrest and her feet on the ground. The 3D ZED camera was placed in front of the subject to evaluate ProM on the frontal plane, and to her side to evaluate ProM on the sagittal plane. The body segments related to the evaluated joint were passively and gently moved until the end of the allowed movement; at the end of the movement, a sample measurement was acquired. Each evaluation was conducted by the participant’s primary caregivers, who passively moved the subject’s joints in front of the 3D ZED camera. A trained therapist, expert in carrying out such evaluation, with expertise in rehabilititating RTT patients, followed the evaluation using the live coverage, guiding the procedure and ensuring the correctness of the data collection. The PRoM of shoulder flexion and abduction, elbow flexion and extension, and knee extension were collected for both sides of the participant’s body. Shoulder flexion and abduction PRoM consisted of the angle formed by the arm segment and the line connecting the shoulder and hip virtual joints. For shoulder flexion, the measurement was collected on the sagittal plane, and for the abduction, on the frontal plane. Elbow flexion and extension were measured on the sagittal plane and corresponded to the angle created by the arm and forearm segments measured from the crook side of the elbow. Knee extension was also measured on the sagittal plane and referred to the angle between the thigh and leg segments measured from the crook side of the knee. Moreover, to obtain an index of the subject’s spontaneous trunk alignment in sitting position, the angle formed by the pelvis segment and median axis of the trunk was calculated. The trunk alignment measurement was collected on the frontal plane after two minutes of maintaining the position without any external intervention allowing the patient to settle in her preferred natural position.
Motor activities were planned individually for each participant, considering the subject’s motor functioning and musculoskeletal conditions. The living environment and habit characteristics of participants were also included in the planning process of therapeutic exercises. Therefore, due to the high variability of the enrolled participants’ clinical and personal aspects, therapeutic interventions differed one from the other. However, they all followed the same approach. After the evaluation of the participant’s PRoM limitations and motor functioning, a list of activities that required the subject to use (thus strengthening) the muscles that opposed the movement limitation were planned (e.g., if a subject exhibited a reduction in right shoulder flexion, she was asked to use her right arm to grasp an object she liked placed on a high shelf, so that she spontaneously used her shoulder flexor muscles, thus counteracting the limitation of movement). Together with these active strategies, caregivers were taught passive postures to position the participants so that the muscles responsible for the reductions in joint mobility could stretch. These passive postures were identified to be easily learned by caregivers and used in the participants’ daily routines and environments. Each rehabilitation program consisted of three or four active exercises and two or three passive postures. The active exercises were performed every week within three thirty-minute sessions for the duration of the intervention (three months). These sessions were conducted by participants’ primary caregivers (who were together with the girls) with the live supervision of a therapist experienced in the motor treatment of people with RTT. Live supervisions were provided through the videoconference platform included in the TCTRS and aimed to guide the execution of the activities, clarify caregivers’ doubts, solve problems, and set new activities if needed. The performances of the passive postures were also monitored through videoconference once a week by the same therapist. Caregivers were asked to have participants maintain passive postures during the times of the day when they wanted to rest, at least three times a week for 20 min for each posture. In this way, the participants were more likely to relax, allowing the muscle to stretch, and no time was spent on the passive posturing program during the remotely supervised therapy sessions.
3. Results
The reliability of the conversion of 2D skeleton measures to the 3D skeleton measures and to the goniometer therapist measures was estimated, and the results were put in relation with the mobility of shoulder abduction and flection, elbow flection and extension, and knee extension. We choose to compare our system with a universal two-axis goniometer, since it is the most commonly clinical tool to measure joint angles, even if in some cases an electronic inclinometer has also proven to be a highly reliable tool [58].
With regard to the mobility of the shoulder, we considered that the range of physiology varies from 162° to 189° of flexion and from 140° to 172° of abduction. The elbow range varies from −12.5° to 20° of extension and from 129.5° to 165.5° of flexion. Knee extension range of motion varies from −6.5° to 18°.
To estimate the reliability of the conversion between 2D skeleton measures, 3D skeleton measures and the goniometer therapist measures, we applied Pearson correlations and the intraclass correlation coefficient to assess the strength of the relationships among the three methods. For each measure (shoulder abduction and flection, elbow flection and extension and knee extension), the comparison between 3D skeleton measures and the goniometer therapist measures are highly correlated (with r ranging from 0.62 to 0.89, p < 0.01) and the reliability of the ratings for clusters is also high (with ICC ranging from 0.78 to 0.92). With reference to the comparison between 2D skeleton measures and the goniometer therapist measures, they are lower but still reliable (with r ranging from 0.45 to 0.77, p < 0.05 and ICC ranging from 0.68 to 0.81). Moreover, the sensitivity to intervention was analyzed. With reference to the range of passive left shoulder flexion and right shoulder flexion, Table 1 shows means, standard deviation, and range for each phase. Comparisons between T1 and T2, T1 and T3, and T2 and T3 with their p-values related to Wilcoxon test are also shown.
As we can see, girls with Rett syndrome significantly increased the shoulder joint mobility after the treatment. Table 2 also shows shoulder abduction. This parameter was significantly wider at post-treatment evaluation compared to baseline. No effect was found between T1 and T2 in both right and left shoulder abduction (left shoulder p = 0.92 and right shoulder p = 0.26). Comparisons between T1 and T3 (left shoulder p = 0.021 and right shoulder p = 0.005) and between T2 and T3 show a significant improvement (left shoulder p = 0.005 and right shoulder p = 0.0018) in shoulder abduction. These results mean that girls with Rett syndrome need more time to improve shoulder abduction range of motion.

Table 2.
Measurements and estimated p-value from the passive left shoulder and right shoulder movement sessions.
With reference to elbow flexion, data analysis showed that there was a substantial and significant improvement at the end of the 10-week intervention period (T1 to T3) and also when comparing pre-treatment scores to scores after five weeks of treatment (T1 to T2); this means that girls with Rett syndrome obtained better results with elbow flexion in the first period of time and then maintained the level acquired for the remaining training period.
A significant improvement was detected in right elbow extension between T1 and T2 and between T1 and T3, but not between T2 and T3.
The difference between the greater improvement in elbow flexion and in elbow extension could be due to the type of exercises suggested to the girls. Functional exercises were preferred during the intervention and no passive treatment, such as stretching, was performed by the caregivers who participated in the program. Moreover, 20° was found to be the minimum angle of elbow extension required to perform activities of daily living (ADL) tasks. At the beginning of the intervention, the angle of the girls’ left elbow extension was 14.99 (SD ± 14.75) and right elbow extension was 16.33 (SD ± 11.09).
The results showed statistically significant differences which are also likely to be clinically significant.
4. Discussion
The 3D skeleton measure has proved to be a highly reliable instrument for measuring mobility in patients with RTT. This opens new possibilities in this field, due to the greater reliability and user-friendliness of this instrument, which allow measurements to be taken very quickly. We demonstrated how our computer vision-based markerless system is robust compared with the linear goniometer, a tool that therapists normally use in the presence of the patient. Moreover, we learned that a remote telerehabilitation program, where professionals can constantly monitor the patient, getting quantitative measures, such as joint angles, can result in sensible improvements in patients’ mobility. The simplicity of the setting and the reliability of the system can then reduce some of the limitations and costs associated with other systems, such as those based on wearable sensors. In fact, wearable sensors (such as an accelerometer or gyroscope in the case of PRoM measures [50]) may be expensive, potentially difficult to bear by a RTT patient, and hard to manage by a caregiver. Moreover, patients performed the rehabilitation tasks in a familiar and ecological environment, even during the monitoring sessions, where the only devices were a laptop and a camera, leaned on the table in the room, without interfering with the patient’s daily routine. This is a key issue that caregivers reported as a strength in the project, since they could help patients (often their daughters) to perform tasks they were already trained to do. From the therapists’ point of view, the system added objectivity to their observations in video calls, and their evaluations could be corroborated by measured data.
However, our system showed some limitations, mainly because it cannot measure all movements and we calculated its reliability with a small sample. To overcome these limitations, we plan to test some easy-to-use wearable devices such as cuffs or wristbands in combination with computer vision algorithms that can improve data quality and extend the set of monitored movements. Additionally, we plan to test the system reliability with a larger sample. Moreover, since our system exploits computer vision algorithms, having good data depends on the setting, for example light conditions or obstructions. These difficulties could appear simple to overcome, but in the context of a family dealing with a relative affected by a multidisability, even finding a corner in the house suitable for a telerehabilitation session can be tricky.
Despite its limitations, new studies should be performed on its reliability in different joints and movements.
In future interventions it could be necessary to associate an active rehabilitation program to have a greater improvement of RoM.
Significant improvements were found almost only in the upper limbs. This could be due to the greater use of upper limbs during the daily activities, while an improvement in lower limbs (i.e., knee extension) would need a greater dose of motor activity such as longer walks and uptime activities. Girls with Rett syndrome have low levels of daily physical activity and high levels of sedentary time. This intervention might not have enough impact on reducing sedentary time; an important focus for interventions to address long-term health and quality of life in RTT could be enhancing activities such as standing and walking for longer time [59].
The results showed statistically significant differences which are likely to be also clinically significant.
5. Conclusions
Telerehabilitation systems have shown their critical relevance during the COVID-19 pandemic, when several patients lost precious therapy interventions because of the pandemic restrictions. However, for many patients suffering from rare diseases, accessing targeted therapies is often very difficult, due to the lack of specialized centers and professionals in their proximity.
The proposed telerehabilitation system aims at reducing discomfort that patients and families suffer to reach a far specialized center, giving an easy tool to therapists and patients to remotely monitor motor therapies.
The main goals of the present study are the following: (i) analyzing the reliability of our vision-based markerless system compared to the linear goniometer for joint angle measurement; (ii) demonstrating the feasibility of a motor telerehabilitation program for Rett patients, to improve their mobility. Despite the limitations discussed above, we observed a significant improvement in the mobility of patients involved in the study.
We plan to apply several system enhancements, for instance adding an automatic subject recognition system to discriminate whether the acquired data are related to the patient or the caregiver, for instance embedding a face recognition algorithm.
Moreover, as part of the future work, we are working on adding a gait analysis system, so that therapists can better promote mobility and reduce sedentary time for Rett patients.
In conclusion, we think that computer vision can play a significant role where more precise tools are unavailable, not only to provide a simple and ecological tool to remotely assess patient condition, but also to serve as an alternative interaction medium for impaired people, for instance exploiting movement recognition in a virtual environment.
Author Contributions
Conceptualization, R.A.F. and A.N.; methodology, R.A.F., A.N. and G.I.; software, A.N. and G.I.; validation, R.A.F. and A.N.; formal analysis, R.A.F. and A.N.; investigation, R.A.F., A.R. and M.P.; data curation, R.A.F., A.N. and M.P.; writing—original draft preparation, R.A.F., A.N., A.R. and M.P.; writing—review and editing, A.N., G.I. and R.A.F.; visualization, R.A.F. and A.N.; supervision, R.A.F. and A.N.; project administration, R.A.F.; funding acquisition, R.A.F., A.N. and G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by Rotary Foundation (GG 1978856).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of Clinical and Experimental Medicine Department, University of Messina (protocol code PSY/2020/32).
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van Den Veyver, I.B.; Schultz, R.; Malicki, D.M.; Tran, C.Q.; Dahle, E.J.; Philippi, A.; Timar, L.; Percy, A.K.; Mo-til, K.J.; et al. Influence of Mutation Type and X Chromosome Inactivation on Rett Syndrome Phenotypes. Ann. Neurol. 2000, 47, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E.; Simmons, H.; Ford, T.; Meltzer, H.; Goodman, R. Prevalence of pervasive developmental disorders in the British nationwide survey of child mental health. Int. Rev. Psychiatry 2003, 15, 158–165. [Google Scholar] [CrossRef]
- Skjeldal, O.H.; von Tetzchner, S.; Aspelund, F.; Herder, G.A.; Lofterød, B. Rett syndrome: Geographic variation in prevalence in Norway. Brain Dev. 1997, 19, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Pini, G.; Milan, M.; Zappella, M. Rett syndrome in Northern Tuscany (Italy): Family tree studies. Clin. Genet. 1996, 50, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.G. Rett Syndrome--Clinical and Biological Aspects: Studies on 130 Swedish Females; Cambridge University Press: Cambridge, UK, 1995; Volume 52, ISBN 0521412838. [Google Scholar]
- Humphreys, P.; Barrowman, N. The Incidence and Evolution of Parkinsonian Rigidity in Rett Syndrome: A Pilot Study. Can. J. Neurol. Sci. J. Can. des Sci. Neurol. 2016, 43, 567–573. [Google Scholar] [CrossRef]
- Hagberg, B. Clinical manifestations and stages of rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 61–65. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Katz, D.M.; Bird, A.; Coenraads, M.; Gray, S.J.; Menon, D.U.; Philpot, B.D.; Tarquinio, D.C. Rett Syndrome: Crossing the Threshold to Clinical Translation. Trends Neurosci. 2016, 39, 100–113. [Google Scholar] [CrossRef]
- Lane, J.B.; Lee, H.S.; Smith, L.W.; Cheng, P.; Percy, A.K.; Glaze, D.G.; Neul, J.L.; Motil, K.J.; Barrish, J.O.; Skinner, S.A.; et al. Clinical severity and quality of life in children and adolescents with Rett syndrome. Neurology 2011, 77, 1812–1818. [Google Scholar] [CrossRef]
- Lee, J.; Leonard, H.; Piek, J.; Downs, J. Early development and regression in Rett syndrome. Clin. Genet. 2013, 84, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Lotan, M.; Ben-Zeev, B. Rett Syndrome. A Review with Emphasis on Clinical Characteristics and Intervention. Sci. World J. 2006, 6, 1517–1541. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.D.M.; Savelsbergh, G.; Smorenburg, A.; Graciani, Z.; Torriani-Pasin, C.; de Abreu, L.C.; Valenti, V.; Kok, F. Quantification of functional abilities in Rett syndrome: A comparison between stages III and IV. Neuropsychiatr. Dis. Treat. 2014, 10, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Smeets, E.E.; Schrander-Stumpel, C.T.R.M. Rett Syndrome. In Management of Genetic Syndromes, 3d ed.; Department of Clinical Genetics, Academic Hospital Maastricht: Maastricht, The Netherlands, 2010; pp. 677–691. ISBN 9780470191415. [Google Scholar]
- Vignoli, A.; La Briola, F.; Peron, A.; Turner, K.; Savini, M.; Cogliati, F.; Russo, S.; Canevini, M.P. Medical care of adolescents and women with Rett syndrome: An Italian study. Am. J. Med. Genet. Part A 2011, 158A, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Halbach, N.; Smeets, E.; Steinbusch, C.; Maaskant, M.; van Waardenburg, D.; Curfs, L. Aging in Rett syndrome: A longitudinal study. Clin. Genet. 2012, 84, 223–229. [Google Scholar] [CrossRef]
- Michlovitz, S.L.; Harris, B.A.; Watkins, M.P. Therapy interventions for improving joint range of motion: A systematic review. J. Hand Ther. 2004, 17, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Svane, C.; Nielsen, J.B.; Lorentzen, J. Nonsurgical Treatment Options for Muscle Contractures in Individuals with Neurologic Disorders: A Systematic Review With Meta-Analysis. Arch. Rehabilit. Res. Clin. Transl. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Romano, A.; Di Rosa, G.; Tisano, A.; Fabio, R.A.; Lotan, M. Effects of a remotely supervised motor rehabilitation program for individuals with Rett syndrome at home. Disabil. Rehabilit. 2021, 44, 5898–5908. [Google Scholar] [CrossRef] [PubMed]
- Richmond, T.; Peterson, C.; Cason, J.; Billings, M.; Terrell, E.A.; Lee, A.C.W.; Towey, M.; Parmanto, B.; Saptano, A.; Cohn, E.R.; et al. American Telemedicine Association’s Principles for Delivering Telerehabilitation Services. Int. J. Telerehabilit. 2017, 9, 63–68. [Google Scholar] [CrossRef]
- Cason, J.; Cohn, E.R. Telepractice: An Overview and Best Practices. Perspect. Augment. Altern. Commun. 2014, 23, 4–17. [Google Scholar] [CrossRef]
- Fabio, R.A.; Semino, M.; Giannatiempo, S.; Caprì, T.; Iannizzotto, G.; Nucita, A. Comparing Advanced with Basic Telerehabilitation Technologies for Patients with Rett Syndrome—A Pilot Study on Behavioral Parameters. Int. J. Environ. Res. Public Health 2022, 19, 507. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Greenspoon, D.; Hunt, A.; McAdam, L. Rehabilitation interventions in Rett syndrome: A scoping review. Dev. Med. Child Neurol. 2020, 62, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, F.; Iannizzotto, G.; Lo Bello, L. A Person Authentication System Based on RFID Tags and a Cascade of Face Recognition Algorithms. IEEE Trans. Circuits Syst. Video Technol. 2016, 27, 1676–1690. [Google Scholar] [CrossRef]
- Iannizzotto, G.; Lo Bello, L.; Nucita, A.; Grasso, G.M. A Vision and Speech Enabled, Customizable, Virtual Assistant for Smart Environments. In Proceedings of the 2018 11th International Conference on Human System Interaction (HSI), Gdansk, Poland, 4–6 July 2018; pp. 50–56. [Google Scholar] [CrossRef]
- Knippenberg, E.; Verbrugghe, J.; Lamers, I.; Palmaers, S.; Timmermans, A.; Spooren, A. Markerless motion capture systems as training device in neurological rehabilitation: A systematic review of their use, application, target population and efficacy. J. Neuroeng. Rehabilit. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prima, O.D.; Imabuchi, T.; Ono, Y.; Murata, Y.; Ito, H.; Nishimura, Y. Single Camera 3D Human Pose Estimation for Tele-rehabilitation. In Proceedings of the eTELEMED 2019: The Eleventh International Conference on eHealth, Telemedicine, and Social Medicine, Athens, Greece, 24–28 February 2019. [Google Scholar]
- Prima, O.D.; Ono, Y.; Murata, Y.; Ito, H.; Imabuchi, T.; Nishimura, Y. Evaluation of Joint Range of Motion Measured by Vision Cameras. Int. J. Adv. Life Sci. 2019, 11, 128–137. [Google Scholar]
- Lam, W.W.T.; Fong, K.N.K. The application of markerless motion capture (MMC) technology in rehabilitation programs: A systematic review and meta-analysis. Virtual Real. 2022, 1–16. [Google Scholar] [CrossRef]
- Scott, B.; Seyres, M.; Philp, F.; Chadwick, E.K.; Blana, D. Healthcare Applications of Single Camera Markerless Motion Cap-ture: A Scoping Review. PeerJ 2022, 10, e13517. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, S.; Nuzzi, C.; Covre, N.; Luchetti, A.; Maule, L.; Serpelloni, M.; Lancini, M. Validation of Marker-Less System for the Assessment of Upper Joints Reaction Forces in Exoskeleton Users. Sensors 2020, 20, 3899. [Google Scholar] [CrossRef]
- Vilas-Boas, M.D.C.; Rocha, A.P.; Choupina, H.M.P.; Cardoso, M.N.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. Portable RGB-D Camera-Based System for Assessing Gait Impairment Progression in ATTRv Amyloidosis. Appl. Sci. 2022, 12, 10203. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Y.; Guo, M.; Chen, Y. Deep Learning Methods for 3D Human Pose Estimation under Different Supervision Paradigms: A Survey. Electronics 2021, 10, 2267. [Google Scholar] [CrossRef]
- Cerfoglio, S.; Ferraris, C.; Vismara, L.; Amprimo, G.; Priano, L.; Pettiti, G.; Galli, M.; Mauro, A.; Cimolin, V. Kinect-Based Assessment of Lower Limbs during Gait in Post-Stroke Hemiplegic Patients: A Narrative Review. Sensors 2022, 22, 4910. [Google Scholar] [CrossRef]
- Reimer, L.M.; Kapsecker, M.; Fukushima, T.; Jonas, S.M. Evaluating 3D Human Motion Capture on Mobile Devices. Appl. Sci. 2022, 12, 4806. [Google Scholar] [CrossRef]
- Rybarczyk, Y.; Medina, J.L.P.; Leconte, L.; Jimenes, K.; González, M.; Esparza, D. Implementation and Assessment of an Intelligent Motor Tele-Rehabilitation Platform. Electronics 2019, 8, 58. [Google Scholar] [CrossRef]
- Vilas-Boas, M.D.C.; Rocha, A.P.; Choupina, H.M.P.; Cardoso, M.N.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients. Sensors 2019, 19, 4929. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Sandroff, B.; Motl, R.W. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.; Needham, L.; McGuigan, P.; Bilzon, J. Applications and Limitations of Current Markerless Motion Capture Meth-ods for Clinical Gait Biomechanics. PeerJ 2022, 10, e12995. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.; Paterson, K.; Bower, K.; McGinley, J.; Miller, K.; Pua, Y.-H.; Clark, R.A. Quantifying Individual Components of the Timed Up and Go Using the Kinect in People Living with Stroke. Neurorehabilit. Neural Repair 2014, 29, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Scano, A.; Caimmi, M.; Malosio, M.; Tosatti, L.M. Using Kinect for upper-limb functional evaluation in home rehabilitation: A comparison with a 3D stereoscopic passive marker system. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Sao Paulo, Brazil, 12–15 August 2014; pp. 561–566. [Google Scholar] [CrossRef]
- Tsuji, T.; Nakashima, S.; Hayashi, H.; Soh, Z.; Furui, A.; Shibanoki, T.; Shima, K.; Shimatani, K. Markerless Measurement and Evaluation of General Movements in Infants. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Støen, R.; on behalf of the CIMA Norway Study Group; Songstad, N.T.; Silberg, I.E.; Fjørtoft, T.; Jensenius, A.R.; Adde, L. Computer-based video analysis identifies infants with absence of fidgety movements. Pediatr. Res. 2017, 82, 665–670. [Google Scholar] [CrossRef]
- Vilas-Boas, M.D.C.; Rocha, A.P.; Choupina, H.M.P.; Cardoso, M.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. TTR-FAP Progression Evaluation Based on Gait Analysis Using a Single RGB-D Camera. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019; pp. 5494–5497. [Google Scholar] [CrossRef]
- Han, J.J.; Kurillo, G.; Abresch, R.T.; de Bie, E.; Nicorici, A.; Bajcsy, R. Reachable workspace in facioscapulohumeral muscular dystrophy (FSHD) by kinect. Muscle Nerve 2014, 51, 168–175. [Google Scholar] [CrossRef]
- Stone, E.E.; Skubic, M. Fall Detection in Homes of Older Adults Using the Microsoft Kinect. IEEE J. Biomed. Health Inform. 2014, 19, 290–301. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, C.; Chung, S.G.; Kim, H.C.; Kwak, Y.; Park, H.-W.; Kim, K. Measurement of Shoulder Range of Motion in Patients with Adhesive Capsulitis Using a Kinect. PLoS ONE 2015, 10, e0129398. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Affandi, M.; Basah, S.N.; Din, M.Y. Evaluating Lower Limb Joint Flexion by Computerized Visual Tracking System and Compared with Electrogoniometer and Universal Goniometer. J. Telecommun. Electr. Comput. Eng. 2018, 10, 9–14. [Google Scholar]
- Díaz, S.; Stephenson, J.B.; Labrador, M.A. Use of Wearable Sensor Technology in Gait, Balance, and Range of Motion Analysis. Appl. Sci. 2019, 10, 234. [Google Scholar] [CrossRef]
- Walmsley, C.P.; Williams, S.A.; Grisbrook, T.; Elliott, C.; Imms, C.; Campbell, A. Measurement of Upper Limb Range of Mo-tion Using Wearable Sensors: A Systematic Review. Sports Med. Open 2018, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Agustín, R.M.-S.; García-Vidal, J.A.; Cánovas-Ambit, G.; Vecchia, A.A.-D.; López-Nicolás, M.; Medina-Mirapeix, F. Validity and Reliability of a New Optoelectronic System for Measuring Active Range of Motion of Upper Limb Joints in Asymptomatic and Symptomatic Subjects. J. Clin. Med. 2019, 8, 1851. [Google Scholar] [CrossRef]
- Ropars, M.; Cretual, A.; Thomazeau, H.; Kaila, R.; Bonan, I. Volumetric definition of shoulder range of motion and its correlation with clinical signs of shoulder hyperlaxity. A motion capture study. J. Shoulder Elb. Surg. 2015, 24, 310–316. [Google Scholar] [CrossRef]
- Caprì, T.; Fabio, R.A.; Iannizzotto, G.; Nucita, A. The TCTRS Project: A Holistic Approach for Telerehabilitation in Rett Syndrome. Electronics 2020, 9, 491. [Google Scholar] [CrossRef]
- Carpenzano, A.; Caponetto, R.; Bello, L.L.; Mirabella, O. Fuzzy traffic smoothing: An approach for real-time communication over Ethernet networks. In Proceedings of the 4th IEEE International Workshop on Factory Communication Systems, Vasteras, Sweden, 28–30 August 2002; pp. 241–248. [Google Scholar] [CrossRef]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Construction and standardization of the RARS (rett assessment rating scale) tool. [Costruzione e standardizzazione dello strumento RARS (Rett Assessment Rating Scale)]. Life Span Disabil. 2005, 8, 34–45. [Google Scholar]
- Cao, Z.; Simon, T.; Wei, S.E.; Sheikh, Y. Realtime Multi-Person 2D Pose Estimation Using Part Affinity Fields. In Proceedings of the 30th IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 1302–1310. [Google Scholar] [CrossRef]
- Herrero, P.; Carrera, P.; García, E.; Gómez-Trullén, E.M.; Blazquez, B.O. Reliability of goniometric measurements in children with cerebral palsy: A comparative analysis of universal goniometer and electronic inclinometer. A pilot study. BMC Musculoskelet. Disord. 2011, 12, 155. [Google Scholar] [CrossRef]
- Stahlhut, M.; Downs, J.; Wong, K.; Bisgaard, A.-M.; Nordmark, E. Feasibility and Effectiveness of an Individualized 12-Week “Uptime” Participation (U-PART) Intervention in Girls and Women with Rett Syndrome. Phys. Ther. 2019, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).