Abstract
For decades, projects have been carried out in various countries to assess the developmental status of children and adolescents using anthropometry and specific kinesiological measurements. There is a need for the ability to evaluate this developmental status using a sufficiently simple method or a calculation to be applicable in practice. The most commonly used feature for this purpose is currently body mass index (BMI). From recent experience, this feature may cause problems if used indiscriminately in the developmental phase of life. Therefore, we aimed to find a more suitable feature set. We used data from Artos, the national program monitoring school children and adolescents in Slovenia. The data was analyzed using machine learning (ML) tools to find the most important features to predict a motor efficiency index (MEI), which has been shown to correlate strongly with a person’s health prospects. After data preparation and training a baseline model, a feature selection process was performed, which promoted some features as candidates to predict the motor efficiency index sufficiently. By implementing a hyperparameter optimization, we tuned the ML model to improve its generalization and present the feature interaction more elaborately. We show that besides the single feature’s importance, the features’ interaction should be considered. In the case of MEI, we find that the skin fold thicknesses can complement BMI and contribute to a better development status assessment of children and adolescents.
1. Introduction
Several countries have established regular campaigns to examine the growth and development of the young population—children and adolescents. The National Health and Nutrition Examination Survey is performed yearly in the USA, and in Holland, The Amsterdam Growth and Health Longitudinal Study is conducted [1]. In Slovenia, the Faculty of Sport at the University of Ljubljana initiated a pioneering project called Artos in the decade of 1960, which is still ongoing today [2]. The project investigates the interconnection between children’s motor development and their functional capacities and bodily, emotional, mental, and social status dimensions, as well as the impact of the environment. All these features combined define quality of life and health in a broad sense. The measurements consist of assessing various motoric tasks and measurements of body dimensions. Additionally, the participants complete two surveys. All the anthropometry is performed noninvasively; no samples of body liquids or tissues are collected. The lengths, widths, and circumferences of limbs and torso are measured using meters, scales, calipers, and special compasses. These measurements as a whole are termed bio-psycho-social properties. Project Artos has been conducted to the present and continues.
In the scientific literature, most attention among the anthropometric data has been paid to the body mass index (BMI), defined as body mass divided by the square of the body height (kg/m2). Practitioners have simple tools at their disposal to assess the status of an individual in the form of growth charts that are percentile based and drawn from population surveys over many years. However, some doubts about the use of BMI arise because there are still some problems with scientific evidence [3]. In addition to overweight and obesity, thinness also poses health problems [4]. Other indicators have been proposed, e.g., the Slaughter formula [5], which is not ideal either.
Hence, our aim is to:
- Find more conclusive evidence of the role that BMI plays in the healthy development of children and adolescents or to
- Find other features that could substitute or amend it.
The method presented in this study used to find such features among the collected data is feature selection, specifically the greedy feature selection (GFS) method [6]. This method selects the most important feature first and then adds other features that improve prediction the most. The research question is about which features influence the motor efficiency index (MEI) the most, so the GFS is a natural choice for this task.
We then use graphic tools to show the model decisions. Such tools are needed because the selected machine learning method acts like a black box—the model has too many parameters to be easily comprehensible. These tools can help us to better tune model hyperparameters into the desired direction suitable for our goals.
Studies [7,8] show that high blood lipid levels can be linked to both BMI and insufficient physical activity. Studies also show that it is possible to reduce health risks by increasing physical activity. It is shown that children with high BMI and a high level of aerobic effectiveness have a lower risk of metabolic syndrome than those with high BMI and lower aerobic capacity [9]. It has also been shown that the epidemic dimension of obesity can be primarily attributed to physical inactivity rather than excessive caloric intake. As a preventive and corrective measure, it is suggested that persons with high BMI should increase physical activity and, consequently, their fitness, which can improve their health prospects for later life [10]. In this sense, we try to analyze the Artos data and find its relationship with MEI. It is defined as a function of the results of motoric tests according to EUROFIT [11] and SLOfit [12] protocols, which are also part of the Artos data. This index is an indicator of fitness. It serves as the predicted variable, and predictions are made from other features.
In the Experiment section, we present the necessary steps to preprocess the data and the initial machine learning tests. Then, in the Feature Selection and Evaluation section, we present intermediate results. In the Tuning by Optimization section, we develop the process of tuning the model parameters to obtain clearer insights into the data. Additionally, in the Spearman’s Rank Coefficient as a Source of Feature Importance Information section, we present another view of the data through their statistical properties. In the Discussion section, we present the current study’s limitations, compare our work with related publications, and envision possible directions for future work. Finally, in the Conclusions, we summarize the important findings.
2. Materials and Methods
2.1. Data Pre-Processing
We analyzed the Artos data from the last completed measurement campaign from the years 2013 and 2014. The data were obtained in another study following protocol [2] and under the supervision of their original authors; therefore, explicit ethical approval was not needed. The children and adolescents were selected from various primary and secondary schools so that a nationally representative sample was obtained with regard to sex, age, size of city or town, geographical region, and, in the case of secondary schools, program. All further details about the sample are available in the study protocol [2].
The first step in the preparation of the Artos data was to divide the dataset in two groups based on sex. The reason for this division is that the development in the studied age period is different for each sex. The feature set is also different for each sex. All the data preparatory activities were performed separately for each sex.
The motor efficiency index was calculated in three components following the same principle as defined in [13]:
- Neuromuscular Fitness (NMF),
- Muscular Fitness (MF), and
- Cardiorespiratory Fitness (CRF).
Each MEI component is dependent on a subset of the physical performance features. These features were discretized by centiles inside the age group. Each MEI component was obtained by averaging the centile values of their respective constitutive features analogously to the physical fitness index calculation in [13]. The constitution of MEI components is listed in Table 1.

Table 1.
MEI components and their constitutive features.
For each model training run, only one of the MEI components was added to the dataset as the output value. After adding that MEI component to the dataset, all the features constituting any of the MEI components were removed from the dataset. All other anthropometric features were also discretized by centiles inside the age group.
Finally, there remained 704 features on 2514 samples from the boys (M) and 705 features on 2444 samples from the girls (F).
2.2. Basic Model Training on Whole Datasets
The machine learning task was performed using the Scikit-learn Python package, version 0.24.2 [14]. Initially, we compared prediction scores obtained from various estimators (RandomForestRegressor—RFR, ExtraTreesRegressor—ETR, GradientBoostingRegressor—GBR). These estimators have the bagging mechanism in common [15]. This gives us overfitting protection without cross-validation, which would take a longer computation time proportionally with the number of folds.
For the results to be comparable, we used the mean squared error score in the cross-validation procedure for all the experiments because it is appropriate with all of the estimators we used.
With all the estimators, the hyperparameter space was defined following the procedure defined in [6] and is as follows:
- max_features: 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 705;
- n_estimators: 100, 121, 144, 169, 196, 225, 256, 289;
- max_depth: 4, 9, 16, 25, 36, 49.
For the gradient boosting estimator, we showed additionally the reduced hyperparameter space, which also allowed us to gain a very high score in a short time:
- max_features: 1, 2, 4, 8, 16;
- n_estimators: 32, 64, 128;
- max_depth: 4, 9, 16.
3. Results
3.1. Basic Model Training Evaluation
The results obtained on the complete set of features are presented in Table 2. Note that the results from the full range of the hyperparameters will nevertheless be used in the rest of the experiments for the results to be fully comparable.

Table 2.
Prediction results from tested regressors.
Among these basic predictions of MEI components, the gradient boosting estimator achieved the best score, even with very low hyperparameter values, and was obtained in a drastically shorter time. Because of this, all the rest of the experiments were performed using this estimator.
We can also stress that the gradient boosting estimator achieved a high score even though the distributions of the MEI components’ values are not uniform, and a very small number of samples represents the values on both sides of their range (tails) (see Figure 1).
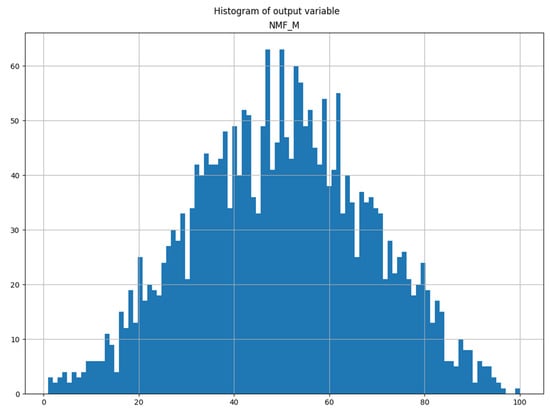
Figure 1.
Histogram of neuro-muscular fitness (NMF) output variable.
These results serve only as a baseline and as a support for the choice of the estimator, and we will not present them in any more detail.
3.2. Feature Selection and Evaluation of Selected Features
The feature selection process was performed using the GFS method [6]. Initially, the hyperparameter space was retained as with the basic training process.
In the following, for the presentation to remain concise, the NMF as the MEI component will be analyzed as an example.
The first selected feature was predominantly the BMI. The relation between BMI and NMF, as modeled in the first step of the feature selection process, is displayed in Figure 2. Besides the partial dependence plot in blue superimposed over the red samples, we also show the moving average of the predicted values over ±1% of the feature values range in orange. The partial dependence plot reveals extensive jumps in prediction for even a small change in the selected feature value, which motivated us to find a smoother solution.
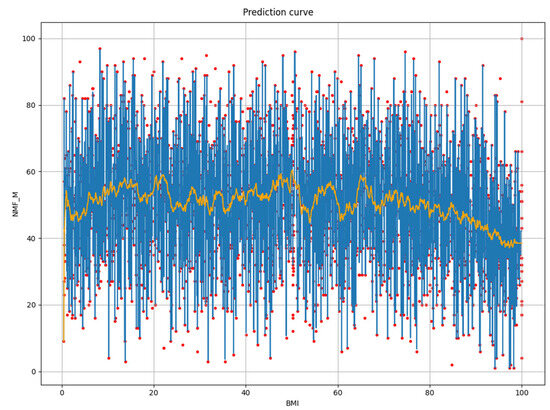
Figure 2.
Prediction of NMF from body mass index (BMI) at first step (red—samples, blue—prediction, orange—windowed average ± 1% of range).
It is known from the theoretical foundations of gradient boosting that by tuning the number of nodes in the trees used in the ensemble, it is possible to improve the prediction of the estimator given specific data [16] (see Section 10.11). Generally, the higher values of the parameter J (implemented as max_depth in the software used) improve the prediction score. However, higher values mean large trees, which cause more computation, so it should be set around 4–8 and finetuned to achieve the best predictions. We take advantage of the fact that lower values limit the interaction between features, and in the case of value J = 2, the estimator uses only one feature in each tree.
Ensemble estimators are of a black-box nature, and for them to be more understandable, one of the explainability techniques must be used. A simple technique is visual and involves plotting a partial dependence plot, which presents the predicted value in relation to the value of a certain feature. The plot can be three-dimensional (3D) and display the interaction of a pair of features so that the prediction appears in the third dimension. A partial dependence plot in 3D becomes a partial dependence surface. Such a plot can be flattened to 2 dimensions and encode the third dimension in a color scheme.
Displaying the partial dependence plots for different, especially lower, values of hyperparameters shows the change in the behavior of the estimator. By limiting the hyperparameter space to lower values of the max_depth parameter, we can observe that the partial dependence curve flattens (see Figure 3).
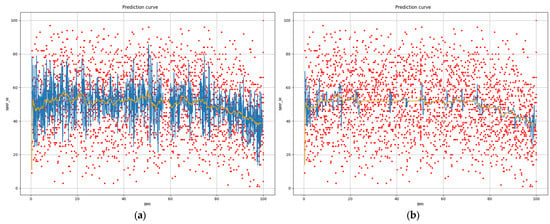
Figure 3.
Prediction of NMF from BMI at first step at (a) max_depth = 6; (b) max_depth = 2 (red—samples, blue—prediction, orange—windowed average ± 1% of range).
At the value max_depth = 2, the predictions stay close to the average line. In this case, the relation between the BMI and predicted NMF is almost constant in the prevalent part of the range, except at percentiles lower than six and higher than 74.
The partial dependence plot at lower values of the max_depth parameter is close to the median curve of the samples, as plotted using the ExpectileGAM method, shown in Figure 4. In Python, this method from the family of Generalized Additive Models (GAM) [16] is implemented in the package pygam (the version used was 0.8.0) [17].
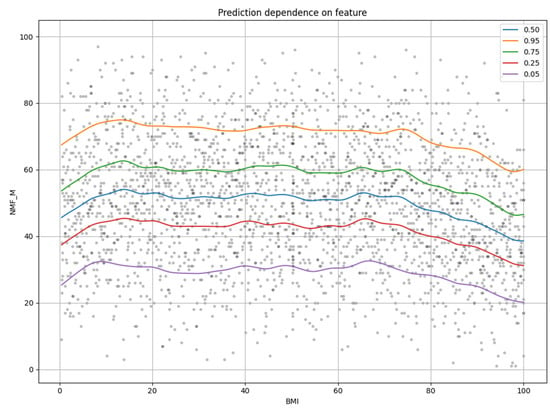
Figure 4.
ExpectileGAM curves for NMF in relation to BMI (0.50—median).
We show the prediction score achieved on the first step of the feature selection process because the first selected feature has the biggest prediction power. In the subsequent steps of the process, the score grows further, but the increments are smaller. Higher values of the first step score also imply fewer features needed to achieve prediction comparable to the baseline estimator. Among the score functions that are commonly used, we chose the mean squared error (MSE) because it is more intuitive than others (e.g., R2). It is implemented as negated in the Scikit-learn package to comply with the principle that a higher value is better.
Note that larger values of the hyperparameters mentioned incur more computation time.
We can see on the PDP of two features selected in the first two steps (BMI, V3teza) that MEI depends on both. V3teza is weight at birth. The PDP of these two features is shown in Figure 5, both in 2D and 3D versions. We can notice roughly three areas where lower values are predicted: at highest values of both features, high V3teza and low BMI, and low values of both features.
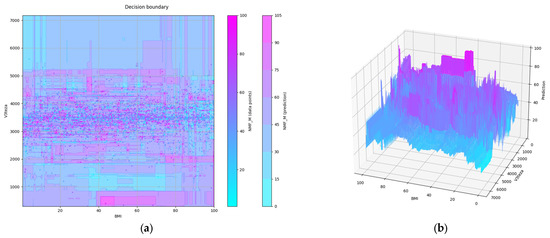
Figure 5.
Partial dependence plot of a pair of features: (a) in 2D; (b) in 3D.
All the figures, 3D included, are obtained using the matplotlib [18] package, version 3.6.1. 3D figures can, at runtime, be tilted and rotated utilizing mouse dragging to understand the dependence contour’s shape better. They are all shown from the same perspective for comparison (elevation = 25°, azimuth = 110°, roll = 0°).
Other selected features show less similarity between the partial dependence plot and the median curve. Their contribution to the overall prediction is complementary—they add information through feature interaction. This conclusion also stems from the fact that the prevalent part of the score gain is obtained in the first two or three steps of the GFS process. We can indeed assume that the excellent predictions of the baseline estimator, which used all the features, are due to the multiple feature interactions and, to some extent, to overfitting.
3.3. Tuning by Optimization
Increasing the n_estimators parameter to a very high level can compensate for lower prediction scores of the limited max_depth parameter, as seen in Table 3. Decreasing this parameter helps to smooth the partial dependence plot and to get it closer to the sliding average line, but not monotonously. A minimum exists, past which the partial dependence plot departs substantially from the sliding average line. To optimize the n_estimators parameter value, we implemented a criterion function that sums the mean squared difference between the prediction for a given n_estimators parameter value and the sliding average line from GFS predictions. We performed optimization using this criterion function for all features selected in the GFS process. Because the minima for different features differ and the estimator can only take one value, we had to make a compromise, choosing the maximal one to avoid overly large errors. A typical plot of the prediction error depending on the n_estimators parameter value for the BMI is shown in Figure 6.

Table 3.
Prediction scores and selected features at the first step depending on hyperparameter values.
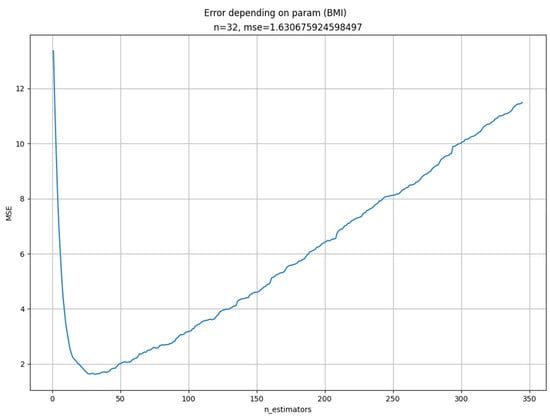
Figure 6.
Prediction error depending on the n_estimators parameter value.
The smoothed prediction of NMF from BMI using the optimized n_estimators parameter value compared to the sliding average line is shown in Figure 7.
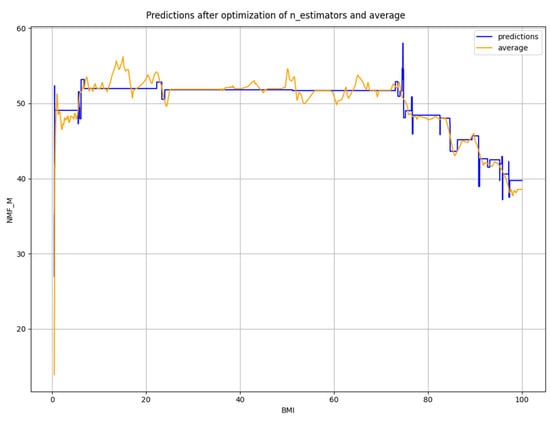
Figure 7.
Smoothed prediction of NMF from BMI using the optimized value of n_estimators.
Using a limited max_depth and optimized n_estimators parameter value is also beneficial in estimating the joint influence of selected features on the predictions. In the following, we show the 3D plots of predicted values after the limiting and optimization for comparison with the one from before this process.
It is easier to discern a feature influence from a smoothed plot in Figure 8 than from the original one in Figure 5. Here, the feature interaction is more obvious and less obstructed by excessive detail.
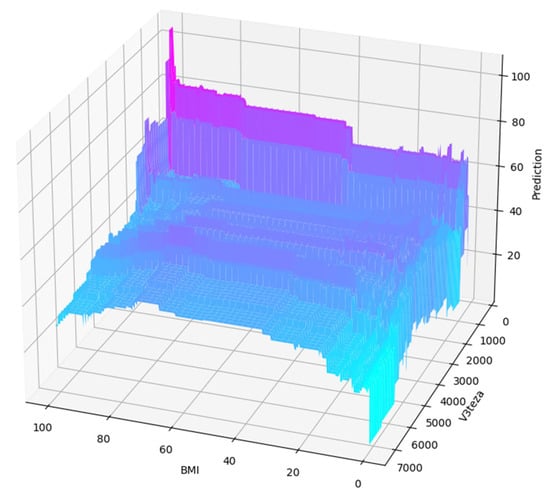
Figure 8.
Prediction related to two features after limiting and optimization.
Despite limiting the tree depth to 2, by which the individual tree depends on a single feature only, we can still observe the feature interaction because multiple trees vote for a common prediction. By optimizing the number of trees, we prevent the ensemble from adapting to detail too much.
To verify our results, we plotted the feature interaction using the ExpectileGAM method, this time in 3D. The GAM terms were set to: s(0) + s(1) + te(0, 1). This means that an additional tensor with interactions is employed in addition to linear functions of single features. We show the surface plot in Figure 9.
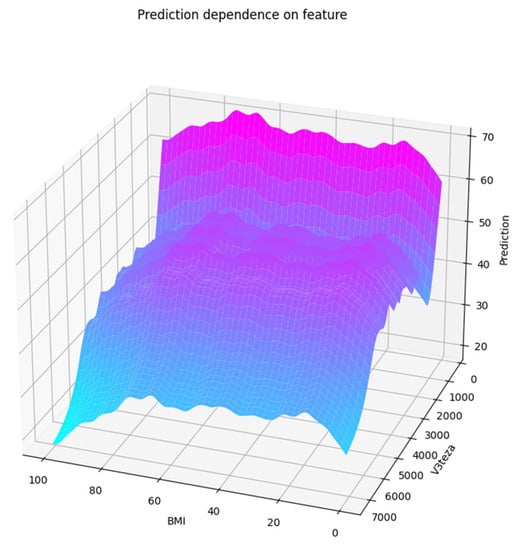
Figure 9.
Generalized additive model (GAM) plot of predictions related to two features.
As observed, the gradient boosting method provides a more thorough shape of the dependence surface compared to the GAM plot. Namely, the cross-section of the GAM surface at various V3teza values has the same shape, with only minor difference in the inclination. However, its coarse shape is similar to our optimized GBR plot. This weakness is due to the fact that most GAM parameters were left at their default values; the only modification was that n_splines was increased to 200 with the s term and 30 with the te term. To improve the alignment, a deeper analysis of all the possible tuning options of the GAM models might be needed.
3.4. Spearman’s Rank Coefficient as a Source of Feature Importance Information
Calculating the Spearman’s rank coefficient between each feature and the NMF component of MEI gives us an introspection of their relatedness. The features most correlated with the output NMF are listed in Table 4. Interestingly, all the skin fold thickness measurements (AKG…) are near the top. They are part of the Slaughter formula [5]. Features AO… are limbs and trunk circumferences. The features whose names begin with Q…, V… and SDQ… contain results from various surveys. The position of BMI varies; in the case of NMF, it comes even at the 14th place.

Table 4.
Features with the highest absolute value of Spearman’s rank coefficient in relation to the MEI components.
Combining the BMI with all the skin fold thickness measurements manually into a smaller feature set and performing a similar analysis as above gives us the following results. The two most important features that bring in the majority of prediction score are BMI and AKGBb. Their initial 3D partial dependence plot is shown in Figure 10.
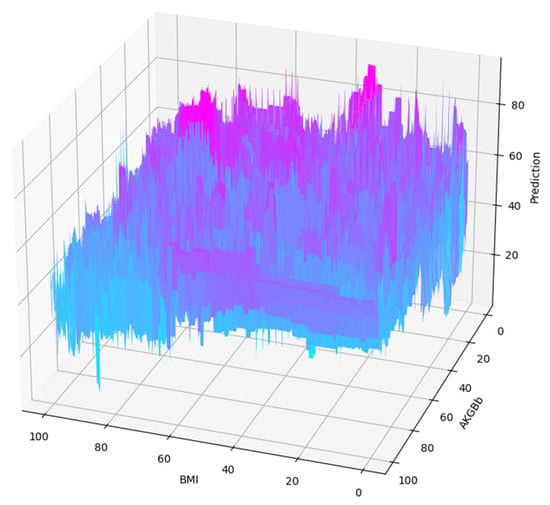
Figure 10.
Partial dependence plot of a pair of features from the smaller set in 3D.
The partial dependence plot obtained after limiting the max_depth hyperparameter and optimization of the n_estimators hyperparameter is shown in Figure 11, both in 2D and 3D. Obviously, the lowest predicted values of NMF are those of samples with both high BMI and high AKGBb. According to the model, a high BMI with low values of AKGBb does not mean so much of an NMF reduction. High values of AKGBb in samples with the lowest BMI would also mean some reduction in NMF, but in practice, the number of samples with such a combination of values is low.
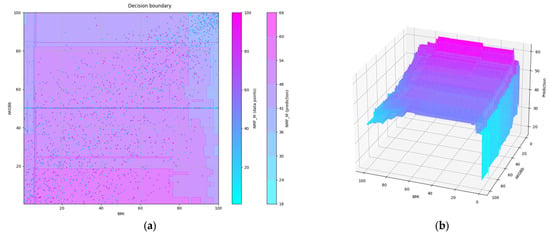
Figure 11.
Partial dependence plot of a pair of features from the smaller set after hyperparameter limiting and optimization: (a) in 2D; (b) in 3D.
In fact, this feature selection is more in line with our initial goal to find features that could be a measure of the current physical status of a person and also indicate fat mass in proportion to the whole-body mass. To predict MEI, it is therefore necessary to consider both the BMI and skin fold thickness.
The GAM model of these two features is shown in Figure 12. Similarly, this plot confirms roughly the main properties of the interaction between the two features. However, it cannot capture all the interaction details as the one from the optimized gradient boosting estimator did.
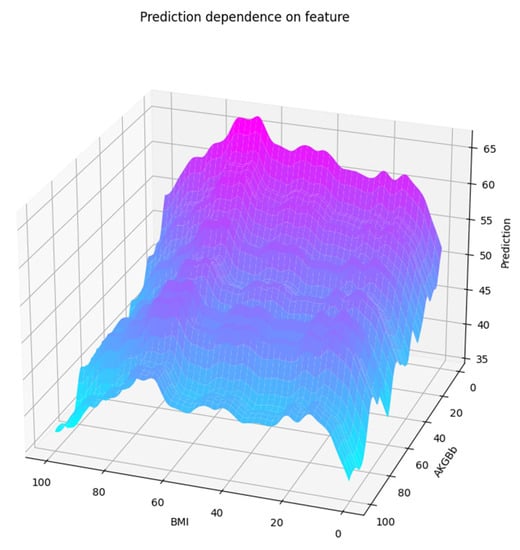
Figure 12.
GAM plot of predictions related to two features from the smaller set.
4. Discussion
As only data from a single Artos measurement campaign was analyzed, the sample is rather small, which poses a problem for the machine learning algorithms. More data would mean more reliable results, as is true for the statistical methods in general. The quantity of data could be improved by combining data from several Artos measurement campaigns. Combining the data from multiple Artos campaigns would change the cross-sectional approach used in our study to a longitudinal one, requiring different methods than described here. Note that this would not constitute the typical cohort study where specific individuals are followed up. Namely, the Artos campaigns are conceived so that the nationally representative sample of school children is examined every ten years. For primary school pupils, this means that the selected individuals could not be examined twice, as the schooling period only lasts nine years (8 years before the reform in the 2000s). Special attention should also be paid to such a combined study due to its retrospective character to minimize potential confounding and bias.
As similar campaigns in other countries do not assess the same features, combining the data from different countries would mean that only a subset of features occurring in all countries could be analyzed. As shown in [3,4], some differences between populations from different parts of the world should be accounted for.
Feature interactions are of special interest in genomic studies. Here, we briefly list a few to put our work into a broader perspective.
In gene association networks, a graph is constructed by means of calculating a paCor matrix, which defines edges in the graph. If an element of the paCor matrix is nonzero, then the edge exists between respective nodes determined by matrix element indices. Study [19] develops a paCor matrix calculation method avoiding linearity and normality assumptions but still discusses a problem of feature scaling in calculations, which generally affects linear methods. The nonlinear methods like GBR which we used are not susceptible to this effect.
Study [20] describes, among others, the Multifactor-dimensionality reduction as a method of detecting gene–gene and gene–environment interactions. Its weaknesses are that it is computationally intensive, its results are difficult to interpret due to feature engineering, and the data must be balanced. Neither of these are present with the GBR method.
Another method shown to be able to uncover feature interactions is random forests. However, study [21] warns against its indiscriminate use because of the inherent problems of overlooking interactions in cases where interacting features have no marginal effect. The study lists approaches to overcome this problem known at the time of writing. We might propose the GBR as an additional candidate for this purpose.
As our method is greedy, it does not discover interactions between features that exhibit low importance in the first step of GFS. This problem was mentioned in [22]. However, in our method, interactions of these features can still influence the subsequent steps if they are related to the already selected features.
A recent review of the scientific literature in the field of gene–environment interactions shows an extensive interest in studying interactions among disparate sources of information to understand complex causal relationships better than would be possible by studying them separately [23].
5. Conclusions
In this work, we analyzed the bio-psycho-social data of children and adolescents. The data come from a long-term project, Artos, conducted in Slovenia from the decade of 1960 and still ongoing. This data have already been analyzed by the authors who conducted the project in the past. Similar campaigns are performed in other countries with similar but not identical goals and gather different sets of features.
The research question in our study was which features have the biggest influence on the MEI, by which it is possible to assess an individual’s health prospects. The most common feature used for this purpose is still BMI; however, it has been shown that BMI is not always appropriate.
To obtain a set of the most important features, we performed GFS using an ensemble estimator, in our case, the gradient boosting regressor. As this estimator’s predictions are hard to explain from the estimator parameters (black box), we used a graphical tool, partial dependence plots, to present them. These helped us to tune the estimator hyperparameters towards better generalizability. We achieve this with two measures. The first is limiting the number of nodes in the tree to two, consequently constraining the influence on predictions to a single feature. The other is by optimizing the number of trees in the ensemble on the difference between predictions and the median of feature values. Both of these can be regarded as a form of regularization. In addition to single feature importances, we show that feature interactions are a key factor in a model’s decision process. They enhance our understanding of the features and their involvement in the modeling and prediction.
Combining contributions from the most important features, we obtain a set of features that are most interesting for further study and practical work on suggestions to individuals about possible measures to improve their future well-being. Besides the body mass index, which is most frequently used currently and does not distinguish between fat and lean mass, we show that skin fold thickness measurements are the candidate features to complement it.
Author Contributions
Conceptualization, F.D. and G.S.; methodology, F.D.; software, F.D.; validation, G.S., G.J. and A.K.; formal analysis, F.D.; investigation, F.D. and G.S.; resources, F.D. and G.S.; data curation, F.D. and G.S.; writing—original draft preparation, F.D.; writing—review and editing, A.K. and M.P.; visualization, F.D.; supervision, A.K., G.J. and M.P.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly supported by the Slovenian Research and Innovation Agency within the research program P2-0425, “Decentralized solutions for the digitalization of industry and smart cities and communities”.
Data Availability Statement
The data used in the analysis are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wijnstok, N.J.; Hoekstra, T.; Van Mechelen, W.; Kemper, H.C.; Twisk, J.W. Cohort Profile: The Amsterdam Growth and Health Longitudinal Study. Int. J. Epidemiol. 2013, 42, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Starc, G.; Kovač, M.; Strel, J.; Pajek, M. The ACDSi 2014—A decennial study on adolescents’ somatic, motor, psycho-social development and healthy lifestyle: Study protocol. Anthropol. Noteb. 2015, 21, 107–123. [Google Scholar]
- Cole, T.J. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Horlick, M.; Berenson, G.S. A comparison of the Slaughter skinfold-thickness equations and BMI in predicting body fatness and cardiovascular disease risk factor levels in children. Am. J. Clin. Nutr. 2013, 98, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Drobnič, F.; Kos, A.; Pustišek, M. On the Interpretability of Machine Learning Models and Experimental Feature Selection in Case of Multicollinear Data. Electronics 2020, 9, 761. [Google Scholar] [CrossRef]
- Reuter, C.P.; da Silva, P.T.; Renner, J.D.P.; de Mello, E.D.; de Moura Valim, A.R.; Pasa, L.; da Silva, R.; Burgos, M.S. Dyslipidemia is Associated with Unfit and Overweight-Obese Children and Adolescents. Arq. Bras. Cardiol. 2016, 106, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Okosun, I.S.; Boltri, J.M.; Lyn, R.; Davis-Smith, M. Continuous Metabolic Syndrome Risk Score, Body Mass Index Percentile, and Leisure Time Physical Activity in American Children. J. Clin. Hypertens. 2010, 12, 636–644. [Google Scholar] [CrossRef] [PubMed]
- DuBose, K.D.; Eisenmann, J.C.; Donnelly, J.E. Aerobic Fitness Attenuates the Metabolic Syndrome Score in Normal-Weight, at-Risk-for-Overweight, and Overweight Children. Pediatrics 2007, 120, e1262–e1268. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B. Diet vs exercise for the prevention of pediatric obesity: The role of exercise. Int. J. Obes. 2011, 35, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.; Klissouras, V.; Ravazzolo, M.; Renson, R.; Tuxworth, W.; Kemper, H.C.; van Mechelen, W.; Hlobil, H.; Beunen, G.; Levarlet-Joye, H. EUROFIT—European Test of Physical Fitness, 2nd ed; Council of Europe, Committee for the Development of Sport: Strasbourg, France, 1993. [Google Scholar]
- SLOfit. Available online: https://en.slofit.org/ (accessed on 26 June 2023).
- Praprotnik, M.; Gantar, I.S.; Krivec, U.; Lucovnik, M.; Berlot, J.R.; Starc, G. Physical fitness trajectories from childhood to adolescence in extremely preterm children: A longitudinal cohort study. Pediatr. Pulmonol. 2023, 58, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; Vanderplas, J.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 2011, 2825–2830. [Google Scholar]
- Scikit-Learn: Out of Bag Estimates. Available online: https://scikit-learn.org/stable/modules/grid_search.html#out-of-bag-estimates (accessed on 26 June 2023).
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2009. [Google Scholar]
- Servén, D.C.; Brummitt, H.A. Hlink, Dswah/pyGAM: v0.8.0; Zenodo: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Andrei, A.; Kendziorski, C. An efficient method for identifying statistical interactors in gene association networks. Biostatistics 2009, 10, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guo, M.; Wang, C.; Liu, X.; Zou, Q. An overview of SNP interactions in genome-wide association studies. Brief. Funct. Genom. 2015, 14, 143–155. [Google Scholar] [CrossRef] [PubMed]
- MWright, N.; Ziegler, A.; König, I.R. Do little interactions get lost in dark random forests? BMC Bioinform. 2016, 17, 145. [Google Scholar] [CrossRef]
- Cordell, H.J. Detecting gene–gene interactions that underlie human diseases. Nat. Rev. Genet. 2009, 10, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, S.J.; VonHandorf, A.; Viel, K.C.M.F.; Weirauch, M.T.; Kottyan, L.C. Gene–environment interactions and their impact on human health. Genes Immun. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).