Microwave-Based Dielectric Properties as an Electrophysiological Biomarker: Future Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Cardiovascular System
3.1. Cardiac Conduction Disorders
3.2. The Current State of the Art in Cardiac EP Mapping
3.3. Potential of Microwave-Based Mapping of Cardiac EP
3.4. The Use of Microwaves for Catheter Ablation
3.5. The Use of Microwaves in Cardiac Medical Devices
4. Nervous System
4.1. Neurological Disorders
4.2. The Current State of the Art in Neuronal EP Mapping
4.3. Potential of Microwave-Based Mapping of Neuronal EP
5. Gastrointestinal System
5.1. Gastrointestinal Disorders
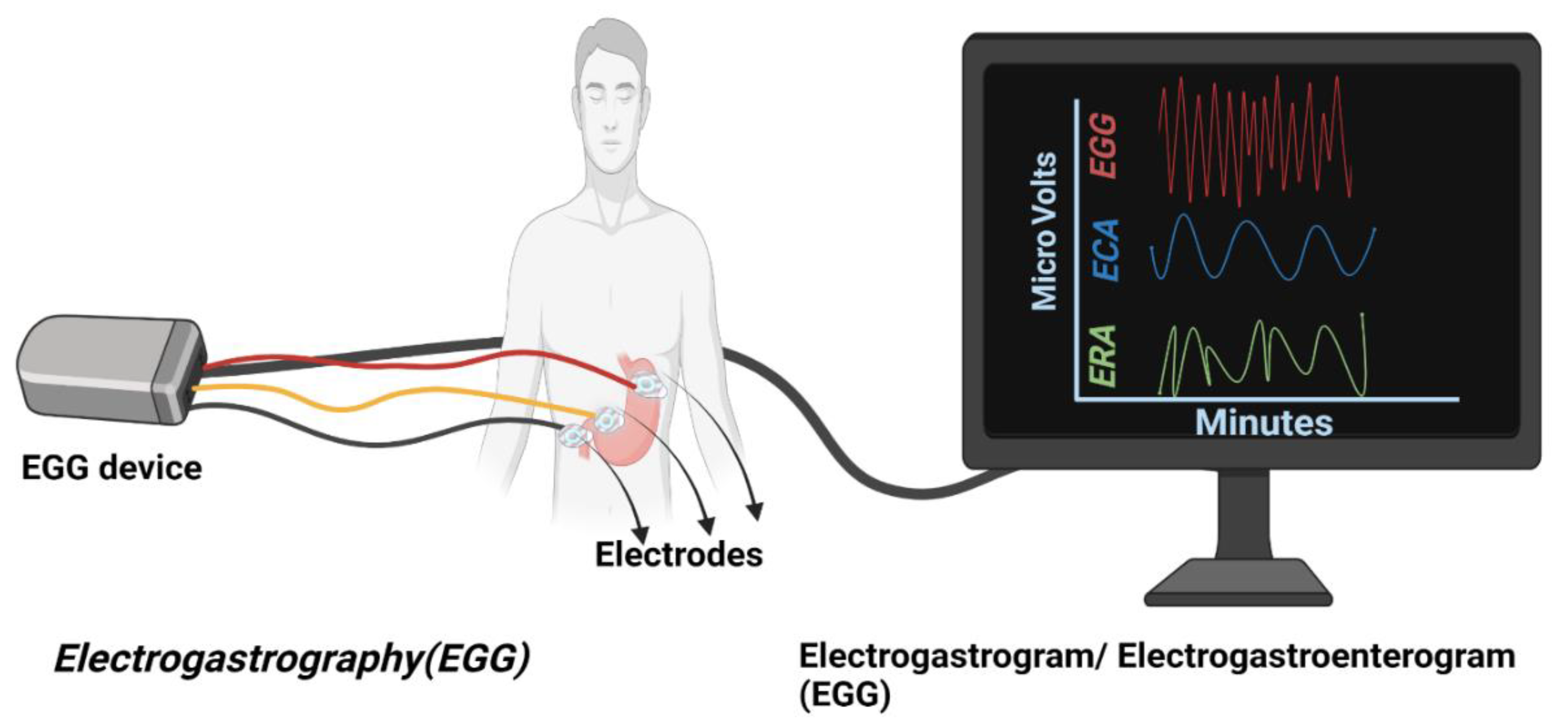
5.2. The Current State of the Art in Gastrointestinal EP Mapping
5.3. Potential of Microwaves-Based Mapping of Gastrointestinal EP
6. Application of Microwaves for Mapping the EP of Other Systems
6.1. Musculoskeletal System
6.2. Urinary System
6.3. Female Reproductive System
6.4. Ophthalmology
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology e-Book; Elsevier Health Sciences: Philadephia, PA, USA, 2020; ISBN 0323640036. [Google Scholar]
- Sperelakis, N. Cell Physiology Source Book: Essentials of Membrane Biophysics; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0080574556. [Google Scholar]
- Kandel, E.R. From nerve cells to cognition: The internal cellular representation required for perception and action. Princ. Neural Sci. 2000, 1, 381–403, ISBN 9780071390118. [Google Scholar]
- Bear, M.; Connors, B.; Paradiso, M.A. Neuroscience: Exploring the Brain, Enhanced Edition: Exploring the Brain; Jones & Bartlett Learning: Burlington, MA, USA, 2020; ISBN 1284211282. [Google Scholar]
- Niedermeyer, E.; da Silva, F.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadephia, PA, USA, 2005; ISBN 0781751268. [Google Scholar]
- Lopes da Silva, F. EEG and MEG: Relevance to neuroscience. Neuron 2013, 80, 1112–1128. [Google Scholar] [CrossRef]
- Baillet, S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017, 20, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.F.; Tatum, W.O.T. Ambulatory EEG Usefulness in Epilepsy Management. J. Clin. Neurophysiol. 2021, 38, 101–111. [Google Scholar] [CrossRef]
- Mohrman, D.E.; Heller, L.J.; Rojas, A.M.G. Fisiología Cardiovascular; McGraw-Hill: México, DF, Mexico, 2007; ISBN 9701061179. [Google Scholar]
- Association, A.H. Electrocardiogram (ECG or EKG); AHA/ASA Journals: Dallas, TX, USA, 2022. [Google Scholar]
- Fogoros, R.N. Electrophysiologic Testing; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118399609. [Google Scholar]
- Scheinman, M.M.; Morady, F. Invasive cardiac electrophysiologic testing: The current state of the art. Circulation 1983, 67, 1169–1173. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Inappropriate interpretation of surface EMG signals and muscle fiber characteristics impedes understanding of the control of neuromuscular function. J. Appl. Physiol. 2015, 119, 1516–1518. [Google Scholar] [CrossRef]
- Josephson, M.E. Clinical Cardiac Electrophysiology: Techniques and Interpretations; Lippincott Williams & Wilkins: Philadephia, PA, USA, 2008; ISBN 0781777399. [Google Scholar]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014; ISBN 0262324067. [Google Scholar]
- Hamill, O.P.; Marty, A.; Neher, E.; Sakmann, B.; Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981, 391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, J.D. Electrogastrography: Methodology, validation and applications. J. Neurogastroenterol. Motil. 2013, 19, 5–17. [Google Scholar] [CrossRef]
- Koch, K.L. Carson DA, O’Grady G, Du P, Gharibans AA, Andrews CN. Body surface mapping of the stomach: New directions for clinically evaluating gastric electrical activity. Neurogastroenterol Mot. 2021;33:e14048. Neurogastroenterol. Motil. 2022, 34, e14254. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Ad, N. The Maze Procedure: Past, Present, and Future, in Manual of Surgical Treatment of Atrial Fibrillation; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 101–110. ISBN 9780470696354; ISBN 9781405140324. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef] [PubMed]
- Pollacco, D.A.; Farina, L.; Wismayer, P.S.; Farrugia, L.; Sammut, C.V. Characterization of the Dielectric Properties of Biological Tissues and their Correlation to Tissue Hydration. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 2191–2197. [Google Scholar] [CrossRef]
- Guo, B.; Li, J.; Zmuda, H.; Sheplak, M. Multifrequency microwave-induced thermal acoustic imaging for breast cancer detection. IEEE Trans. Biomed. Eng. 2007, 54, 2000–2010. [Google Scholar] [CrossRef]
- Wang, X.; Guo, H.; Zhou, C.; Bai, J. High-resolution probe design for measuring the dielectric properties of human tissues. Biomed. Eng. Online 2021, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; La Gioia, A.; Salahuddin, S.; Decker, S.; Shahzad, A.; Elahi, M.A.; O’Halloran, M.; Beyan, O. Minimum information for dielectric measurements of biological tissues (MINDER): A framework for repeatable and reusable data. Int. J. RF Microw. Comput.-Aided Eng. 2018, 28, e21201. [Google Scholar] [CrossRef]
- Kiricuta, I.C., Jr.; Simplaceanu, V. Tissue water content and nuclear magnetic resonance in normal and tumor tissues. Cancer Res. 1975, 35, 1164–1167. [Google Scholar]
- Peyman, A.; Kos, B.; Djokic, M.; Trotovsek, B.; Limbaeck-Stokin, C.; Sersa, G.; Miklavcic, D. Variation in Dielectric Properties Due to Pathological Changes in Human Liver. Bioelectromagnetics 2015, 36, 603–612. [Google Scholar] [CrossRef]
- Meaney, P.M.; Gregory, A.P.; Epstein, N.R.; Paulsen, K.D. Microwave open-ended coaxial dielectric probe: Interpretation of the sensing volume re-visited. BMC Med. Phys. 2014, 14, 3. [Google Scholar] [CrossRef]
- Kaufman, Z.; Paran, H.; Haas, I.; Malinger, P.; Zehavi, T.; Karni, T.; Pappo, I.; Sandbank, J.; Diment, J.; Allweis, T. Mapping breast tissue types by miniature radio-frequency near-field spectroscopy sensor in ex-vivo freshly excised specimens. BMC Med. Imaging 2016, 16, 57. [Google Scholar] [CrossRef][Green Version]
- Sasaki, K.; Porter, E.; Rashed, E.A.; Farrugia, L.; Schmid, G. Measurement and image-based estimation of dielectric properties of biological tissues -past, present, and future-. Phys. Med. Biol. 2022, 67, 14TR01. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Adhikari, A.; Pallipamu, N.; Singh, M.; Nusrat, T.; Gaddam, S.; Samaddar, P.; Rajagopal, A.; Cherukuri, A.S.S.; Yadav, A.; et al. Applications of Microwaves in Medicine Leveraging Artificial Intelligence: Future Perspectives. Electronics 2023, 12, 1101. [Google Scholar] [CrossRef]
- Duck, F. Electrical Properties of Tissue in Physical Properties of Tissue; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Ištuk, N.; Porter, E.; O’loughlin, D.; McDermott, B.; Santorelli, A.; Abedi, S.; Joachimowicz, N.; Roussel, H.; O’halloran, M. Dielectric properties of ovine heart at microwave frequencies. Diagnostics 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C. Studies on microwaves in medicine and biology: From snails to humans. Bioelectromagnetics 2004, 25, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Kao, C. Electrophysiological properties of uterine smooth muscle. In Biology of the Uterus; Springer: Boston, MA, USA, 1989; pp. 403–454. [Google Scholar] [CrossRef]
- Keane, D. New catheter ablation techniques for the treatment of cardiac arrhythmias. Card. Electrophysiol. Rev. 2002, 6, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Choi, S.H.; Weng, L.C.; Wang, E.Y.; Trinquart, L.; Benjamin, E.J.; Ellinor, P.T.; Lubitz, S.A. Frequency of Cardiac Rhythm Abnormalities in a Half Million Adults. Circ. Arrhythm. Electrophysiol. 2018, 11, e006273. [Google Scholar] [CrossRef]
- Whalley, D.W.; Wendt, D.J.; Grant, A.O. Basic concepts in cellular cardiac electrophysiology: Part I: Ion channels, membrane currents, and the action potential. Pacing Clin. Electrophysiol. 1995, 18, 1556–1574. [Google Scholar] [CrossRef]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef]
- Klabunde, R.E. Cardiac electrophysiology: Normal and ischemic ionic currents and the ECG. Adv. Physiol. Educ. 2017, 41, 29–37. [Google Scholar] [CrossRef]
- Saoudi, N.; Cosio, F.; Waldo, A.; Chen, S.A.; Iesaka, Y.; Lesh, M.; Saksena, S.; Salerno, J.; Schoels, W.; Working Group of Arrhythmias of the European of Cardiology; et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 2001, 22, 1162–1182. [Google Scholar] [CrossRef]
- Padeletti, L.; Bagliani, G. General Introduction, Classification, and Electrocardiographic Diagnosis of Cardiac Arrhythmias. Card. Electrophysiol. Clin. 2017, 9, 345–363. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Veenhuyzen, G.D.; Simpson, C.S.; Abdollah, H. Atrial fibrillation. CMAJ 2004, 171, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Waldo, A.L. Mechanisms of atrial flutter and atrial fibrillation: Distinct entities or two sides of a coin? Cardiovasc. Res. 2002, 54, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.; Niwano, S.; Abe, H.; Rudy, Y.; Johnson, N.J.; Waldo, A.L. Mapping the Conversion of Atrial-Flutter to Atrial-Fibrillation and Atrial-Fibrillation to Atrial-Flutter—Insights into Mechanisms. Circ. Res. 1994, 74, 882–894. [Google Scholar] [CrossRef]
- Kadish, A.; Passman, R. Mechanisms and management of paroxysmal supraventricular tachycardia. Cardiol. Rev. 1999, 7, 254–264. [Google Scholar] [CrossRef]
- Kwaku, K.F.; Josephson, M.E. Typical AVNRT—An update on mechanisms and therapy. Card. Electrophysiol. Rev. 2002, 6, 414–421. [Google Scholar] [CrossRef]
- Nesheiwat, Z.G.A.; Jagtap, M. Atrial Fibrillation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cosio, F.G. Atrial Flutter, Typical and Atypical: A Review. Arrhythm. Electrophysiol. Rev. 2017, 6, 55–62. [Google Scholar] [CrossRef]
- Chen, Q.; Kirsch, G.E.; Zhang, D.; Brugada, R.; Brugada, J.; Brugada, P.; Potenza, D.; Moya, A.; Borggrefe, M.; Breithardt, G.; et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998, 392, 293–296. [Google Scholar] [CrossRef]
- Janse, M.J.; Kleber, A.G. Propagation of Electrical-Activity in Ischemic and Infarcted Myocardium as the Basis of Ventricular Arrhythmias. J. Cardiovasc. Electrophysiol. 1992, 3, 77–87. [Google Scholar] [CrossRef]
- Ludhwani, D.; Goyal, A.; Jagtap, M. Ventricular Fibrillation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Y.; Cuculich, P.S.; Zhang, J.; Desouza, K.A.; Vijayakumar, R.; Chen, J.; Faddis, M.N.; Lindsay, B.D.; Smith, T.W.; Rudy, Y. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci. Transl. Med. 2011, 3, 98ra84. [Google Scholar] [CrossRef]
- Friedman, P.A. Novel mapping techniques for cardiac electrophysiology. Heart 2002, 87, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Arentz, T.; Haegeli, L.; Sanders, P.; Weber, R.; Neumann, F.J.; Kalusche, D.; Haissaguerre, M. High-density mapping of spontaneous pulmonary vein activity initiating atrial fibrillation in humans. J. Cardiovasc. Electrophysiol. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Rudy, Y. Noninvasive imaging of cardiac electrophysiology and arrhythmia. Ann. N. Y. Acad. Sci. 2010, 1188, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, A.; Wissner, E.; Metzner, A.; Mironovich, S.; Chaykovskaya, M.; Kalinin, V.; Chmelevsky, M.; Lemes, C.; Kuck, K.H. Mapping of ventricular arrhythmias using a novel noninvasive epicardial and endocardial electrophysiology system. J. Electrocardiol. 2018, 51, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, G.; Luther, V.; Kanagaratnam, P.; Linton, N.W. Arrhythmia Mechanisms Revealed by Ripple Mapping. Arrhythm. Electrophysiol. Rev. 2018, 7, 261–264. [Google Scholar] [CrossRef]
- Tung, R.; Raiman, M.; Liao, H.; Zhan, X.; Chung, F.P.; Nagel, R.; Hu, H.; Jian, J.; Shatz, D.Y.; Besser, S.A.; et al. Simultaneous Endocardial and Epicardial Delineation of 3D Reentrant Ventricular Tachycardia. J. Am. Coll. Cardiol. 2020, 75, 884–897. [Google Scholar] [CrossRef]
- Nademanee, K.; Kosar, E.M. A nonfluoroscopic catheter-based mapping technique to ablate focal ventricular tachycardia. Pacing Clin. Electrophysiol. 1998, 21, 1442–1447. [Google Scholar] [CrossRef]
- Ding, L.; Weng, S.; Zhang, H.; Yu, F.; Qi, Y.; Zhang, S.; Tang, M. Slow-Pathway Visualization by Using Panoramic View: A Novel Ablation Technique for Ablation of Atrioventricular Nodal Reentrant Tachycardia. J. Cardiovasc. Dev. Dis. 2022, 9, 91. [Google Scholar] [CrossRef]
- Abeln, B.G.S.; van den Broek, J.; van Dijk, V.F.; Balt, J.C.; Wijffels, M.; Dekker, L.R.C.; Boersma, L.V.A. Dielectric imaging for electrophysiology procedures: The technology, current state, and future potential. J. Cardiovasc. Electrophysiol. 2021, 32, 1140–1146. [Google Scholar] [CrossRef]
- Fink, T.; Imnadze, G.; Sciacca, V.; Braun, M.; Khalaph, M.; El Hamriti, M.; Guckel, D.; Sommer, P.; Sohns, C. Feasibility of wideband dielectric imaging to guide temperature-controlled atrial fibrillation ablation. Heart Rhythm. 2022, 19, 1473–1474. [Google Scholar] [CrossRef]
- Pongratz, J.; Dorwarth, U.; Riess, L.; Schwartz, Y.; Wankerl, M.; Hoffmann, E.; Straube, F. Catheter Ablation in Complex Atrial Arrhythmias: Pilot Study Evaluating a 3D Wide-Band Dielectric Imaging System. Front. Cardiovasc. Med. 2021, 8, 817299. [Google Scholar] [CrossRef] [PubMed]
- Houmsse, M.; Matto, F.; Sulkin, M.S.; Tomaszewski, D.J.; Shulepov, S.; Glassner, L.; Augostini, R.; Kalbfleisch, S.; Daoud, E.G.; Hummel, J. Feasibility of Assessing Cryoballoon Pulmonary Vein Occlusion with Saline Injection and a Novel Mapping System. JACC Clin. Electrophysiol. 2022, 8, 795–799. [Google Scholar] [CrossRef]
- Gebbard, M.; Gersing, E.; Brockhoff, C.; Schnabel, P.A.; Bretschneider, H. Impedance spectroscopy: A method for surveillance of ischemia tolerance of the heart. Thorac. Cardiovasc. Surg. 1987, 35, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hirose, H.; Sasaki, E.; Bando, M.; Mori, Y.; Murakawa, S. Evaluation of myocardial viability during simple cold storage with the use of electrical properties in broad frequencies. J. Heart Lung Transplant. 1996, 15, 1005–1011. [Google Scholar] [PubMed]
- Schaefer, M.; Gross, W.; Ackemann, J.; Gebhard, M.M. The complex dielectric spectrum of heart tissue during ischemia. Bioelectrochemistry 2002, 58, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Gross, W.; Preuss, M.; Ackemann, J.; Gebhard, M.M. Monitoring of water content and water distribution in ischemic hearts. Bioelectrochemistry 2003, 61, 85–92. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Tatsis, G.P. Microwave spectroscopy of myocardial ischemia and infarction. 1. Experimental study. Ann. Biomed. Eng. 2000, 28, 48–54. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Bulyshev, A.E.; Souvorov, A.E.; Nazarov, A.G.; Sizov, Y.E.; Posukh, V.G.; Pavlovsky, A.; Tatsis, G.P. Microwave spectroscopy of myocardial ischemia and infarction. 2. Biophysical reconstruction. Ann. Biomed. Eng. 2000, 28, 55–60. [Google Scholar] [CrossRef]
- Awan, M.F.; Perez-Simbor, S.; Garcia-Pardo, C.; Kansanen, K.; Cardona, N. Experimental Phantom-Based Security Analysis for Next-Generation Leadless Cardiac Pacemakers. Sensors 2018, 18, 4327. [Google Scholar] [CrossRef]
- Johansson, A.J. Simulation and verification of pacemaker antennas. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No. 03CH37439), Cancun, Mexico, 17–21 September 2003. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Per. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- Kiourti, A.; Psathas, K.A.; Nikita, K.S. Implantable and ingestible medical devices with wireless telemetry functionalities: A review of current status and challenges. Bioelectromagnetics 2014, 35, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [PubMed]
- Grider, M.H.; Kabir, R. Physiology, Action Potential. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J. EEG in the diagnosis, classification, and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S2), ii2–ii7. [Google Scholar] [CrossRef]
- Boison, D. The adenosine kinase hypothesis of epileptogenesis. Prog. Neurobiol. 2008, 84, 249–262. [Google Scholar] [CrossRef]
- DeLorenzo, R.J.; Sun, D.A.; Blair, R.E.; Sombati, S. An in vitro model of stroke-induced epilepsy: Elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int. Rev. Neurobiol. 2007, 81, 59–84. [Google Scholar] [CrossRef]
- Taylor, C.P.; Angelotti, T.; Fauman, E. Pharmacology and mechanism of action of pregabalin: The calcium channel α2–δ (alpha2–delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007, 73, 137–150. [Google Scholar] [CrossRef]
- Lee, L.N.; Huang, C.S.; Chuang, H.H.; Lai, H.J.; Yang, C.K.; Yang, Y.C.; Kuo, C.C. An electrophysiological perspective on Parkinson’s disease: Symptomatic pathogenesis and therapeutic approaches. J. Biomed. Sci. 2021, 28, 85. [Google Scholar] [CrossRef]
- Gionfriddo, M.R.; Greenberg, A.J.; Wahegaonkar, A.L.; Lee, K.H. Pathways of translation: Deep brain stimulation. Clin. Transl. Sci. 2013, 6, 497–501. [Google Scholar] [CrossRef]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schafer, H.; Botzel, K.; Daniels, C.; Deutschlander, A.; Dillmann, U.; Eisner, W.; et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef]
- Kogan, M.; McGuire, M.; Riley, J. Deep Brain Stimulation for Parkinson Disease. Neurosurg. Clin. N. Am. 2019, 30, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2012, 28 (Suppl. S1), 8–14. [Google Scholar] [CrossRef] [PubMed]
- England, J.D.; Gronseth, G.S.; Franklin, G.; Miller, R.G.; Asbury, A.K.; Carter, G.T.; Cohen, J.A.; Fisher, M.A.; Howard, J.F.; Kinsella, L.J.; et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005, 64, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J., Jr.; Kyle, R.A.; Miles, J.M.; Dyck, P.J. Osteosclerotic myeloma and peripheral neuropathy. Neurology 1983, 33, 202–210. [Google Scholar] [CrossRef]
- Holzbaur, E.L.; Scherer, S.S. Microtubules, axonal transport, and neuropathy. N. Engl. J. Med. 2011, 365, 2330–2332. [Google Scholar] [CrossRef]
- Donofrio, P.D.; Albers, J.W. AAEM minimonograph #34: Polyneuropathy: Classification by nerve conduction studies and electromyography. Muscle Nerve 1990, 13, 889–903. [Google Scholar] [CrossRef]
- Boutros, N.N. Electrophysiology: Inexpensive brain probing. CNS Spectr. 1999, 4, 16. [Google Scholar] [CrossRef][Green Version]
- Selvitelli, M.F.; Walker, L.M.; Schomer, D.L.; Chang, B.S. The relationship of interictal epileptiform discharges to clinical epilepsy severity: A study of routine electroencephalograms and review of the literature. J. Clin. Neurophysiol. 2010, 27, 87–92. [Google Scholar] [CrossRef]
- Rosenow, F.; Luders, H. Presurgical evaluation of epilepsy. Brain 2001, 124 Pt 9, 1683–1700. [Google Scholar] [CrossRef]
- Staba, R.J.; Stead, M.; Worrell, G.A. Electrophysiological biomarkers of epilepsy. Neurotherapeutics 2014, 11, 334–346. [Google Scholar] [CrossRef]
- Knowlton, R.C.; Shih, J. Magnetoencephalography in epilepsy. Epilepsia 2004, 45 (Suppl. S4), 61–71. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, B.B.; Costa, V.D. Motivational neural circuits underlying reinforcement learning. Nat. Neurosci. 2017, 20, 505–512. [Google Scholar] [CrossRef]
- Stefan, H.; Trinka, E. Magnetoencephalography (MEG): Past, current and future perspectives for improved differentiation and treatment of epilepsies. Seizure 2017, 44, 121–124. [Google Scholar] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Computational Intelligence and Neuroscience, 2011. Brainstorm: A user-friendly application for meg/eeg analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- van Graan, L.A.; Lemieux, L.; Chaudhary, U.J. Methods and utility of EEG-fMRI in epilepsy. Quant. Imaging Med. Surg. 2015, 5, 300. [Google Scholar]
- Bagic, A.I.; Knowlton, R.C.; Rose, D.F.; Ebersole, J.S.; Committee, A.C.P.G. American Clinical Magnetoencephalography Society Clinical Practice Guideline 1: Recording and analysis of spontaneous cerebral activity. J. Clin. Neurophysiol. 2011, 28, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Worrell, G.; Gotman, J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: Clinical studies. Biomark. Med. 2011, 5, 557–566. [Google Scholar] [CrossRef]
- Young, G.S.; Kimbrell, V.; Seethamraju, R.; Bubrick, E.J. Clinical 7T MRI for epilepsy care: Value, patient selection, technical issues, and outlook. J. Neuroimaging 2022, 32, 377–388. [Google Scholar] [CrossRef]
- Rondinoni, C.; Magnun, C.; Vallota da Silva, A.; Heinsen, H.M.; Amaro, E. Epilepsy under the scope of ultra-high field MRI. Epilepsy Behav. 2021, 121, 106366. [Google Scholar] [CrossRef]
- Balchandani, P.; Naidich, T.P. Ultra-High-Field MR Neuroimaging. AJNR Am. J. Neuroradiol. 2015, 36, 1204–1215. [Google Scholar] [CrossRef]
- Karamat, M.I.; Darvish-Molla, S.; Santos-Diaz, A. Opportunities and Challenges of 7 Tesla Magnetic Resonance Imaging: A Review. Crit. Rev. Biomed. Eng. 2016, 44, 73–89. [Google Scholar] [CrossRef]
- Mallik, A.; Weir, A.I. Nerve conduction studies: Essentials and pitfalls in practice. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S2), ii23–ii31. [Google Scholar] [CrossRef] [PubMed]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice; Oxford University Press: Oxford, NY, USA, 2013. [Google Scholar] [CrossRef]
- Chung, T.; Prasad, K.; Lloyd, T.E. Peripheral neuropathy: Clinical and electrophysiological considerations. Neuroimaging Clin. N. Am. 2014, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Geng, Z.; Li, X.; Peng, L.; Kang, B.; Zheng, C. Microwave transmission approach for dynamic dielectric detection at brain functional site. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017. [Google Scholar] [CrossRef]
- Wang, J.K.; Jiang, X.; Peng, L.; Li, X.M.; An, H.J.; Wen, B.J. Detection of Neural Activity of Brain Functional Site Based on Microwave Scattering Principle. IEEE Access 2019, 7, 13468–13475. [Google Scholar] [CrossRef]
- Beason, R.C.; Semm, P. Responses of neurons to an amplitude modulated microwave stimulus. Neurosci. Lett. 2002, 333, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Meaney, P.M.; Fanning, M.W.; Li, D.; Poplack, S.P.; Paulsen, K.D. A clinical prototype for active microwave imaging of the breast. IEEE Trans. Microw. Theory Tech. 2000, 48, 1841–1853. [Google Scholar] [CrossRef]
- Persson, M.; McKelvey, T.; Fhager, A.; Lui, H.S.; Shirvany, Y.; Chodoroski, A.; Mahmood, Q.; Edelvik, F.; Thordstein, M.; Hedström, A.; et al. Advances in neuro diagnostic based on microwave technology, transcranial magnetic stimulation and EEG source localization. In Proceedings of the Asia-Pacific Microwave Conference 2011, Melbourne, Australia, 5–8 December 2011. [Google Scholar] [CrossRef]
- Ireland, D.; Bialkowski, M.E. Microwave head imaging for stroke detection. Prog. Electromagn. Res. M 2011, 21, 163–175. [Google Scholar] [CrossRef]
- Mohammed, B.J.; Abbosh, A.M.; Henin, B.H.; Sharpe, P.C. Head phantom for testing microwave systems for head imaging. In Proceedings of the 2012 Cairo International Biomedical Engineering Conference (CIBEC), Giza, Egypt, 20–22 December 2012; pp. 191–193. [Google Scholar] [CrossRef]
- Parittotokkaporn, S.; Varghese, C.; O’Grady, G.; Svirskis, D.; Subramanian, S.; O’Carroll, S.J. Non-invasive neuromodulation for bowel, bladder and sexual restoration following spinal cord injury: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105822. [Google Scholar] [CrossRef]
- Fu, T.; Lineaweaver, W.C.; Zhang, F.; Zhang, J. Role of shortwave and microwave diathermy in peripheral neuropathy. J. Int. Med. Res. 2019, 47, 3569–3579. [Google Scholar] [CrossRef]
- Harid, V.; Kim, H.; Li, B.Z.; Lei, T. A method for non-destructive microwave focusing for deep brain and tissue stimulation. PLoS ONE 2023, 18, e0278765. [Google Scholar] [CrossRef]
- Coffey, R.J. Deep brain stimulation devices: A brief technical history and review. Artif. Organs 2009, 33, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Lai, E.T.; Yeo, J.M.; Tse, V.; Wong, S.H. Mechanisms of Electrical Activation and Conduction in the Gastrointestinal System: Lessons from Cardiac Electrophysiology. Front. Physiol. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Manabat, M.L. Medscape. Intestinal Motility Disorders. 2020. Available online: https://emedicine.medscape.com/article/179937-overview (accessed on 30 March 2023).
- Henze, D.A.; Buzsaki, G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience 2001, 105, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.T.; Johnson, C.K.; Agmon, A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J. Neurosci. 2001, 21, 2699–2710. [Google Scholar] [CrossRef]
- Murakami, H.; Matsumoto, H.; Ueno, D.; Kawai, A.; Ensako, T.; Kaida, Y.; Abe, T.; Kubota, H.; Higashida, M.; Nakashima, H.; et al. Current status of multichannel electrogastrography and examples of its use. J. Smooth Muscle Res. 2013, 49, 78–88. [Google Scholar] [CrossRef]
- Szurszewski, J. Physiology of the gastrointestinal tract. Electrical basis for gastrointestinal motility. N. Y. Raven 1987, 383, 422. [Google Scholar]
- Parkman, H.P.; Hasler, W.L.; Barnett, J.L.; Eaker, E.Y. Electrogastrography: A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol. Motil. 2003, 15, 89–102. [Google Scholar] [CrossRef]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The migrating motor complex: Control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef]
- Patel, K.S.; Thavamani, A. Physiology, Peristalsis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tse, G.; Lai, E.T.; Lee, A.P.; Yan, B.P.; Wong, S.H. Electrophysiological Mechanisms of Gastrointestinal Arrhythmogenesis: Lessons from the Heart. Front. Physiol. 2016, 7, 230. [Google Scholar] [CrossRef]
- Keller, J.; Layer, P. Intestinal and anorectal motility and functional disorders. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 407–423. [Google Scholar] [CrossRef]
- Johnson, A.C.; Louwies, T.; Ligon, C.O.; Greenwood-Van Meerveld, B. Enlightening the frontiers of neurogastroenterology through optogenetics. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 319, G391–G399. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; O’Grady, G.; Cheng, L.K.; Pullan, A.J. A Multiscale Model of the Electrophysiological Basis of the Human Electrogastrogram. Biophys. J. 2010, 99, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Russo, F.; Indrio, F. Electrogastrography in adults and children: The strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. BioMed Res. Int. 2013, 2013, 282757. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lin, Z.; McCallum, R.W. Artifact reduction in electrogastrogram based on empirical mode decomposition method. Med. Biol. Eng. Comput. 2000, 38, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Akbarali, H.; Hawkins, E.G.; Ross, G.R.; Kang, M. Ion channel remodeling in gastrointestinal inflammation. Neurogastroenterol. Motil. 2010, 22, 1045–1055. [Google Scholar] [CrossRef]
- Gharibans, A.A.; Kim, S.; Kunkel, D.; Coleman, T.P. High-Resolution Electrogastrogram: A Novel, Noninvasive Method for Determining Gastric Slow-Wave Direction and Speed. IEEE Trans. Biomed. Eng. 2017, 64, 807–815. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.D. Pacing the gut in motility disorders. Curr. Treat. Options Gastroenterol. 2006, 9, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Familoni, B.O.; Abell, T.L.; Nemoto, D.; Voeller, G.; Johnson, B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig. Dis. Sci. 1997, 42, 892–897. [Google Scholar] [CrossRef]
- Daram, S.R.; Tang, S.J.; Abell, T.L. Video: Temporary gastric electrical stimulation for gastroparesis: Endoscopic placement of electrodes (ENDOstim). Surg. Endosc. 2011, 25, 3444–3445. [Google Scholar] [CrossRef]
- Rao, S.; Dubey, S.; Deb, S.; Hughes, Z.; Seo, Y.-S.; Nguyen, M.Q.; Tang, S.-J.; Abell, T.; Lahr, C.; Chiao, J.-C. Wireless gastric stimulators. In Proceedings of the Texas Symposium on Wireless and Microwave Circuits and Systems, Waco, TX, USA, 3–4 April 2014. [Google Scholar] [CrossRef]
- Patel, R.; Kulkarni, P. The Enterra device and the future of gastric electrical pacing. J. Gastric Disord. Ther. 2016, 2, 2–5. [Google Scholar]
- Wang, R.; Abukhalaf, Z.; Javan-Khoshkholgh, A.; Wang, T.H.; Sathar, S.; Du, P.; Angeli, T.R.; Cheng, L.K.; O’Grady, G.; Paskaranandavadivel, N.; et al. A Miniature Configurable Wireless System for Recording Gastric Electrophysiological Activity and Delivering High-Energy Electrical Stimulation. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.T.; Choi, J.; Dexheimer, C.A.; Verhaalen, M.A.; Javan-Khoshkholgh, A. An Inductively Powered Implantable System to Study the Gastrointestinal Electrophysiology in Freely Behaving Rodents. Bioengineering 2022, 9, 530. [Google Scholar] [CrossRef]
- Savage, M.; Avci, R.; Aghababaie, Z.; Matthee, A.; Chamani, F.; Prakash, P.; Cheng, L.K.; Angeli-Gordon, T.R. A computational model of radiofrequency ablation in the stomach, an emerging therapy for gastric dysrhythmias. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021. [Google Scholar] [CrossRef]
- Aghababaie, Z.; Paskaranandavadivel, N.; Amirapu, S.; Chan, C.H.A.; Du, P.; Asirvatham, S.J.; Farrugia, G.; Beyder, A.; O’Grady, G.; Cheng, L.K.; et al. Gastric ablation as a novel technique for modulating electrical conduction in the in vivo stomach. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G573–G585. [Google Scholar] [CrossRef]
- Aghababaie, Z.; Chan, C.-H.A.; Paskaranandavadivel, N.; Beyder, A.; Farrugia, G.; Asirvatham, S.; O’Grady, G.; Cheng, L.K.; Angeli, T.R. Feasibility of high-resolution electrical mapping for characterizing conduction blocks created by gastric ablation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar] [CrossRef]
- McHale, N.G.; Hollywood, M.A.; Sergeant, G.P.; Shafei, M.; Thornbury, K.T.; Ward, S.M. Organization and function of ICC in the urinary tract. J. Physiol. 2006, 576 Pt 3, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Kadima, S.; Drumm, B.; Hollywood, M.A.; Thornbury, K.D.; McHale, N.G.; Sergeant, G.P. Novel excitatory effects of adenosine triphosphate on contractile and pacemaker activity in rabbit urethral smooth muscle. J. Urol. 2010, 183, 801–811. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Catterall, W.A.; Wisedchaisri, G.; Zheng, N. The chemical basis for electrical signaling. Nat. Chem. Biol. 2017, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Plomp, J.J.; Morsch, M.; Phillips, W.D.; Verschuuren, J.J. Electrophysiological analysis of neuromuscular synaptic function in myasthenia gravis patients and animal models. Exp. Neurol. 2015, 270, 41–54. [Google Scholar] [CrossRef]
- Subasi, A. Classification of EMG signals using PSO optimized SVM for diagnosis of neuromuscular disorders. Comput. Biol. Med. 2013, 43, 576–586. [Google Scholar] [CrossRef]
- Clarys, J.P. Electromyography in sports and occupational settings: An update of its limits and possibilities. Ergonomics 2000, 43, 1750–1762. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Boulyshev, A.E.; Souvorov, A.E.; Borisov, V.Y.; Sizov, Y.; Starostin, A.N.; Dezern, K.R.; Tatsis, G.P.; Baranov, V.Y. Microwave tomography: Two-dimensional system for biological imaging. IEEE Trans. Biomed. Eng. 1996, 43, 869–877. [Google Scholar] [CrossRef]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [PubMed]
- Feeney, M.M.; Rosenblum, N.D. Urinary tract pacemaker cells: Current knowledge and insights from nonrenal pacemaker cells provide a basis for future discovery. Pediatr. Nephrol. 2014, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Rolle, U.; Piotrowska, A.P.; Nemeth, L.; Puri, P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch. Pathol. Lab. Med. 2002, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.S.; Fowke, J.; Dmochowski, R. The Burden of Overactive Bladder on US Public Health. Curr. Bladder Dysfunct. Rep. 2016. 11, 8–13. [CrossRef]
- Haeberlin, A.; Schurch, K.; Niederhauser, T.; Sweda, R.; Schneider, M.P.; Obrist, D.; Burkhard, F.; Clavica, F. Cardiac electrophysiology catheters for electrophysiological assessments of the lower urinary tract-A proof of concept ex vivo study in viable ureters. Neurourol. Urodyn. 2019, 38, 87–96. [Google Scholar] [CrossRef]
- Kinder, M.V.; Gommer, E.D.; Janknegt, R.A.; van Waalwijk van Doorn, E.S. A method for the electromyographic mapping of the detrusor smooth muscle. Arch. Physiol. Biochem. 1997, 105, 673–690. [Google Scholar] [CrossRef]
- Porter, E.; Raterink, A.; Farshkaran, A. Microwave-Based Detection of the Bladder State as a Support Tool for Urinary Incontinence [Bioelectromagnetics]. IEEE Antennas Propag. Mag. 2022, 64, 112–122. [Google Scholar] [CrossRef]
- Cao, C.; Nie, L.; Lou, C.; Xing, D. The feasibility of using microwave-induced thermoacoustic tomography for detection and evaluation of renal calculi. Phys. Med. Biol. 2010, 55, 5203–5212. [Google Scholar] [CrossRef]
- Snow, B.W.; Taylor, M.B. Non-invasive vesicoureteral reflux imaging. J. Pediatr. Urol. 2010, 6, 543–549. [Google Scholar] [CrossRef]
- Nakao, K.; Inoue, Y.; Okabe, K.; Kawarabayashi, T.; Kitamura, K. Oxytocin enhances action potentials in pregnant human myometrium—A study with microelectrodes. Am. J. Obstet. Gynecol. 1997, 177, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.C.; Tribe, R.M.; Smith, R.; Taggart, M.J. Computational modeling reveals key contributions of KCNQ and hERG currents to the malleability of uterine action potentials underpinning labor. PLoS ONE 2014, 9, e114034. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; Hamid, R. The initiation, continuation, and termination of spontaneous episodes of circus movements in the pregnant myometrium of the rat. Am. J. Obstet. Gynecol. 1998, 179 Pt 1, 1515–1526. [Google Scholar] [CrossRef]
- Popescu, L.M.; Vidulescu, C.; Curici, A.; Caravia, L.; Simionescu, A.A.; Ciontea, S.M.; Simion, S. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur. J. Pharmacol. 2006, 546, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.; Caldeyro, R. Contractility of the human uterus recorded by new methods. Surg. Gynecol. Obstet. 1950, 91, 1–13. [Google Scholar] [PubMed]
- Wolfs, G.M.; van Leeuwen, M. Electromyographic observations on the human uterus during labour. Acta Obstet. Gynecol. Scand. Suppl. 1979, 58 (Suppl. S90), 1–61. [Google Scholar] [CrossRef]
- McNamara, H.M. Problems and challenges in the management of preterm labour. BJOG 2003, 110 (Suppl. S20), 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rabotti, C.; Mischi, M. Propagation of electrical activity in uterine muscle during pregnancy: A review. Acta Physiol. (Oxf.) 2015, 213, 406–416. [Google Scholar] [CrossRef]
- Zia, G.; Sebek, J.; Prakash, P. Temperature-dependent dielectric properties of human uterine fibroids over microwave frequencies. Biomed. Phys. Eng. Express 2021, 7, 065038. [Google Scholar] [CrossRef]
- Liu, L.; Wang, T.; Lei, B. Ultrasound-guided Microwave Ablation in the Management of Symptomatic Uterine Myomas: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 1982–1992. [Google Scholar] [CrossRef]
- Hood, D.C.; Birch, D.G. The A-wave of the human electroretinogram and rod receptor function. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2070–2081. [Google Scholar]
- Lai, T.Y.; Chan, W.M.; Lai, R.Y.; Ngai, J.W.; Li, H.; Lam, D.S. The clinical applications of multifocal electroretinography: A systematic review. Surv. Ophthalmol. 2007, 52, 61–96. [Google Scholar] [CrossRef] [PubMed]
- Atilla, H.; Tekeli, O.; Ornek, K.; Batioglu, F.; Elhan, A.H.; Eryilmaz, T. Pattern electroretinography and visual evoked potentials in optic nerve diseases. J. Clin. Neurosci. 2006, 13, 55–59. [Google Scholar] [CrossRef]
- Reeser, F.; Weinstein, G.W.; Feiock, K.B.; Oser, R.S. Electro-Oculography as a Test of Retinal Function: The Normal and supernormal EOG. Am. J. Ophthalmol. 1970, 70, 505–514. [Google Scholar] [CrossRef]
- Kaneko, M.; Machida, S.; Hoshi, Y.; Kurosaka, D. Alterations of photopic negative response of multifocal electroretinogram in patients with glaucoma. Curr. Eye Res. 2015, 40, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.; Zrenner, E. Electrophysiology in the investigation of acquired retinal disorders. Surv. Ophthalmol. 2000, 45, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Kiiski, H.S.; Ni Riada, S.; Lalor, E.C.; Goncalves, N.R.; Nolan, H.; Whelan, R.; Lonergan, R.; Kelly, S.; O’Brien, M.C.; Kinsella, K.; et al. Delayed P100-like Latencies in Multiple Sclerosis: A Preliminary Investigation Using Visual Evoked Spread Spectrum Analysis. PLoS ONE 2016, 11, e0146084. [Google Scholar] [CrossRef]
- Ferreiro, A.S.; Bellido, L.M. Ocular electrophysiology. Arch. Soc. Esp. Oftalmol. 2012, 87, 415–416. [Google Scholar] [CrossRef]
- Zhang, H.M.; Ren, M.Y.; Zhang, S.X.; Liu, J.Q.; Qin, H. Microwave-induced thermoacoustic imaging for biomedical applications. Phys. Scr. 2023, 98, 032001. [Google Scholar] [CrossRef]
- Carmeliet, E. Electrophysiology on the molecular way. Verh. K. Acad. Geneeskd. Belg. 1993, 55, 5–26. [Google Scholar]
- Ireland, D.; Bialkowski, K.; Abbosh, A. Microwave imaging for brain stroke detection using Born iterative method. IET Microw. Antennas Propag. 2013, 7, 909–915. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Svenson, R.H.; Bulyshev, A.E.; Souvorov, A.E.; Nazarov, A.G.; Sizov, Y.E.; Posukh, V.G.; Pavlovsky, A.V.; Repin, P.N.; Tatsis, G.P. Spatial resolution of microwave tomography for detection of myocardial ischemia and infarction-experimental study on two-dimensional models. IEEE Trans. Microw. Theory Tech. 2000, 48, 538–544. [Google Scholar] [CrossRef]
- Tajik, D.; Trac, J.; Nikolova, N.K. Spatial Resolution Evaluation of a Microwave System for Breast Cancer Screening. In Proceedings of the 2019 13th European Conference on Antennas and Propagation (EuCAP), Krakow, Poland, 31 March–5 April 2019. [Google Scholar]
- Ahmed, S.S.; Schiessl, A.; Gumbmann, F.; Tiebout, M.; Methfessel, S.; Schmidt, L.P. Advanced Microwave Imaging. IEEE Microw. Mag. 2012, 13, 26–43. [Google Scholar] [CrossRef]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A feasibility study on microwave imaging for brain stroke monitoring. Prog. Electromagn. Res. B 2012, 40, 305–324. [Google Scholar] [CrossRef]
- Scapaticci, R.; Bellizzi, G.G.; Cavagnaro, M.; Lopresto, V.; Crocco, L. Exploiting Microwave Imaging Methods for Real-Time Monitoring of Thermal Ablation. Int. J. Antennas Propag. 2017, 2017, 5231065. [Google Scholar] [CrossRef]
- Hirata, A.; Diao, Y.L.; Onishi, T.; Sasaki, K.; Ahn, S.; Colombi, D.; De Santis, V.; Laakso, I.; Giaccone, L.; Joseph, W.; et al. Assessment of Human Exposure to Electromagnetic Fields: Review and Future Directions. IEEE Trans. Electromagn. Compat. 2021, 63, 1619–1630. [Google Scholar] [CrossRef]
- Wang, T.-W.; Lin, T.-T. Electromagnetic Compatibility Issues in Medical Devices. In Recent Topics in Electromagnetic Compatibility; IntechOpen: London, UK, 2022; ISBN 978-1-83969-668-8; ISBN 978-1-83969-669-5. [Google Scholar] [CrossRef]
- Koos, K.; Olah, G.; Balassa, T.; Mihut, N.; Rozsa, M.; Ozsvar, A.; Tasnadi, E.; Barzo, P.; Farago, N.; Puskas, L.; et al. Automatic deep learning-driven label-free image-guided patch clamp system. Nat. Commun. 2021, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N.A.; Popescu, D.M.; Shade, J.K. Machine Learning in Arrhythmia and Electrophysiology. Circ. Res. 2021, 128, 544–566. [Google Scholar] [CrossRef]
- Edwards, K.; Khoshdel, V.; Asefi, M.; LoVetri, J.; Gilmore, C.; Jeffrey, I. A Machine Learning Workflow for Tumour Detection in Breasts Using 3D Microwave Imaging. Electronics 2021, 10, 674. [Google Scholar] [CrossRef]
- Shao, W.; Du, Y. Microwave Imaging by Deep Learning Network: Feasibility and Training Method. IEEE Trans. Antennas Propag. 2020, 68, 5626–5635. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherukuri, A.S.S.; Modi, V.K.; Baraskar, B.; Sood, S.; Reguram, R.; Palvia, D.; Gopalakrishnan, K.; Damani, D.N.; Gaddam, S.; Samaddar, P.; et al. Microwave-Based Dielectric Properties as an Electrophysiological Biomarker: Future Perspectives. Electronics 2023, 12, 3276. https://doi.org/10.3390/electronics12153276
Cherukuri ASS, Modi VK, Baraskar B, Sood S, Reguram R, Palvia D, Gopalakrishnan K, Damani DN, Gaddam S, Samaddar P, et al. Microwave-Based Dielectric Properties as an Electrophysiological Biomarker: Future Perspectives. Electronics. 2023; 12(15):3276. https://doi.org/10.3390/electronics12153276
Chicago/Turabian StyleCherukuri, Akhila Sai Sree, Vaishnavi Kalpesh Modi, Bhavana Baraskar, Shubham Sood, Reshma Reguram, Divyanshi Palvia, Keerthy Gopalakrishnan, Devanshi N. Damani, Sunil Gaddam, Poulami Samaddar, and et al. 2023. "Microwave-Based Dielectric Properties as an Electrophysiological Biomarker: Future Perspectives" Electronics 12, no. 15: 3276. https://doi.org/10.3390/electronics12153276
APA StyleCherukuri, A. S. S., Modi, V. K., Baraskar, B., Sood, S., Reguram, R., Palvia, D., Gopalakrishnan, K., Damani, D. N., Gaddam, S., Samaddar, P., Katukuri, N., Shivaram, S., Dey, S., Mitra, D., Roy, S., Linden, D. R., Beyder, A., Kulkarni, K., & Arunachalam, S. P. (2023). Microwave-Based Dielectric Properties as an Electrophysiological Biomarker: Future Perspectives. Electronics, 12(15), 3276. https://doi.org/10.3390/electronics12153276





