Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review
Abstract
1. Introduction
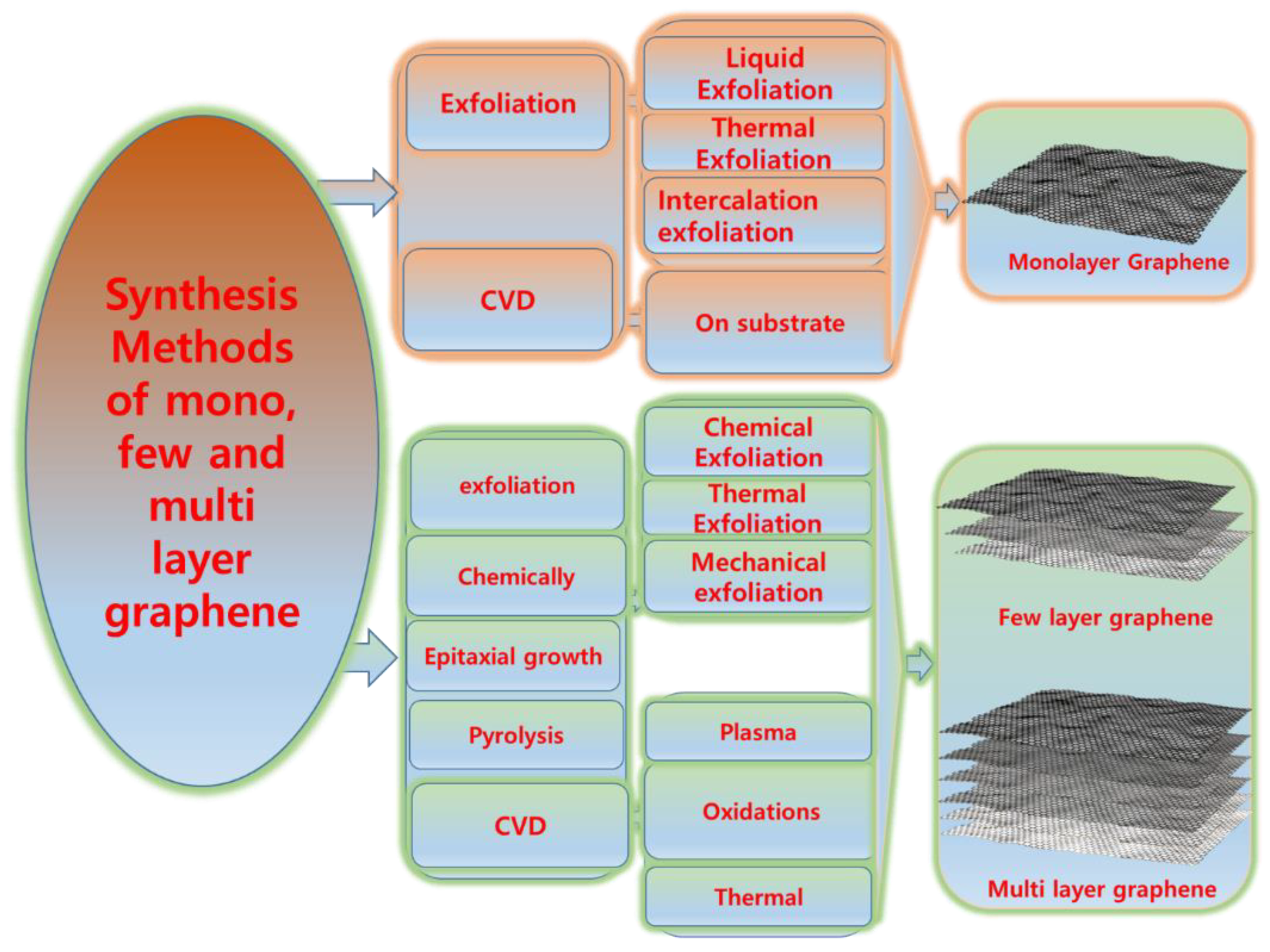
2. Production of Mono-, Few-, and Multi-Layer Graphene
2.1. Synthesis of Few- to Multi-Layer Graphene
2.1.1. Exfoliation of Graphite
- (a)
- There are several ways to overcome these weak interactions, and the most promising among them is the sonication of graphite in different solvents; however, the yields of multi-layer and monolayer graphene [52] are very poor in this process.
- (b)
- Another promising strategy to obtain graphene from graphite involves oxidizing graphite by various methods, such as Hummer’s method, and reducing it chemically or thermally to obtain a large-scale yield [53]. However, the redox results of graphite into graphene mostly provide high yields of few-layer graphene or multi-layer graphene.
- (c)
- Another method of producing few- and multi-layer graphene involves the exfoliation of graphite via graphite intercalation [54,55]. Different types of chemicals can be inserted to graphite interlayer space, thereby increasing the interlayer distance of adjacent graphene sheets in graphite. This phenomenon also changes the properties of graphene, since the increase in interlayer spacing affects electronic coupling between adjacent graphene sheets in graphite [56].
- (d)
- Another way to exfoliate graphite into few- and multi-layer graphene is via ball milling [57]. This is a way of exfoliating graphite via mechanical exfoliation. Ball milling has been extensively used in the past to reduce the particle size of a material [58]. Scientists thus propose ball milling as a way to mechanically exfoliate graphite in small-size nano-graphite, increasing the mixing time to obtain few- or multi-layer graphene. Thus, ball milling is a promising technique for exfoliating graphite into graphene. The advantage of using ball milling to produce graphene is its low production cost, its easy handling, and its ability to produce graphene at large scale.
- (e)
- The plasma synthesis method is another significant way to produce graphene with few-to-multiple layers. Microwave plasmas produced by surface waves at a stimulation frequency of 2.45 GHz and under atmospheric pressure conditions were successfully used to produce highly structured and stable self-standing graphene sheets [16]. There were also investigations into how the addition of hydrogen affects the density of the carbon precursor (C2, C) and the structural soundness of synthetic graphene sheets. Changes in the sp3/sp2 ratio and the C2 and C number densities were shown to be correlated [59]. Microwave-driven plasmas were used to control oxygen functions and the sp2/sp3 carbon ratio (~15) to a high degree [60].
2.1.2. Synthesis of Monolayer Graphene
- (a)
- In 2004, for the first time, Geim and Novoselov developed a method of synthesizing graphene using micromechanical cleavage as “scotch tape” via mechanical exfoliation [18]. This was for the first time in history that any scientist experimentally synthesized monolayer graphene. After synthesizing the monolayer graphene, these scientists further demonstrated its outstanding properties [61]. However, due to the uneven thickness of the graphene flakes and its high production costs, the mechanical exfoliation method was not suitable for the mass production of graphene that might be used to study graphene-based devices.
- (b)
- Another method of producing monolayer graphene is the chemical vapour deposition method [62]. Monolayer graphene can be grown epitaxially on a silicon carbide substrate, and can be used for various applications, such as transistors. The size of the monolayer graphene grown depends on the size of the silicon wafer. The surface of the silicon wafer also influences the properties of the synthesized monolayer graphene.
3. Properties of Mono-, Few-, and Multi-Layer Graphene
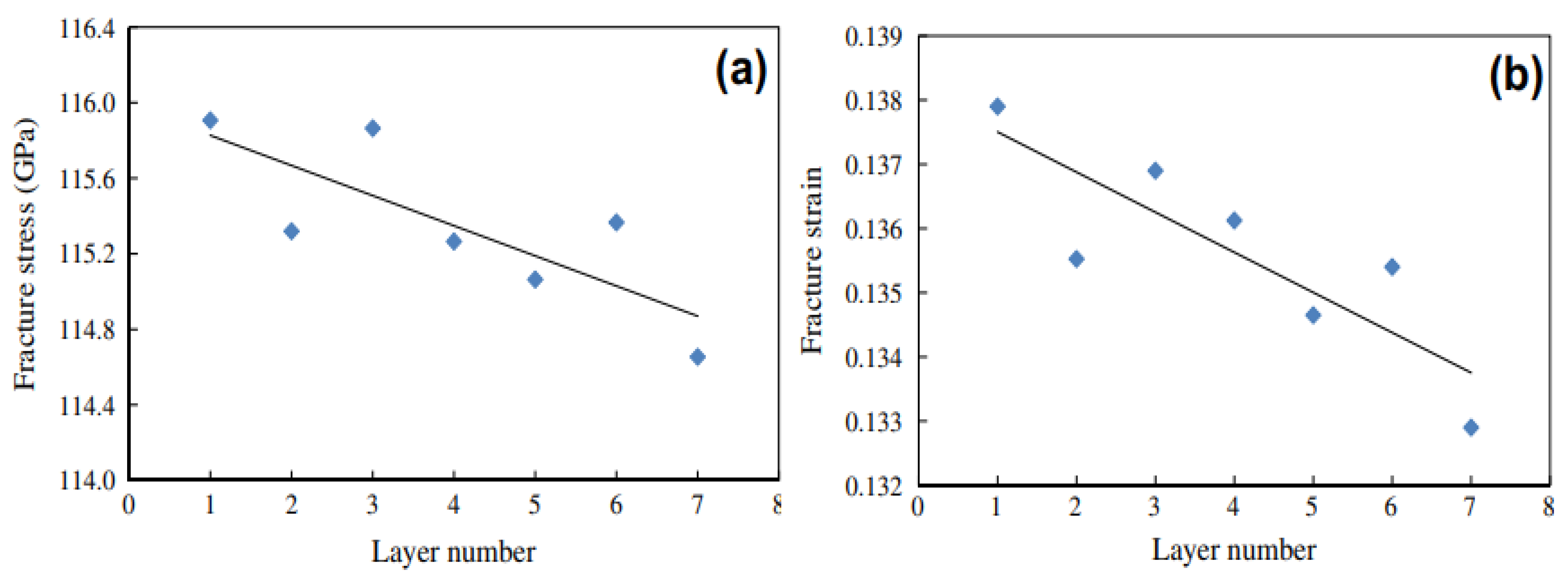
3.1. Mechanical Properties of Mono-, Few-, and Multi-Layer Graphene
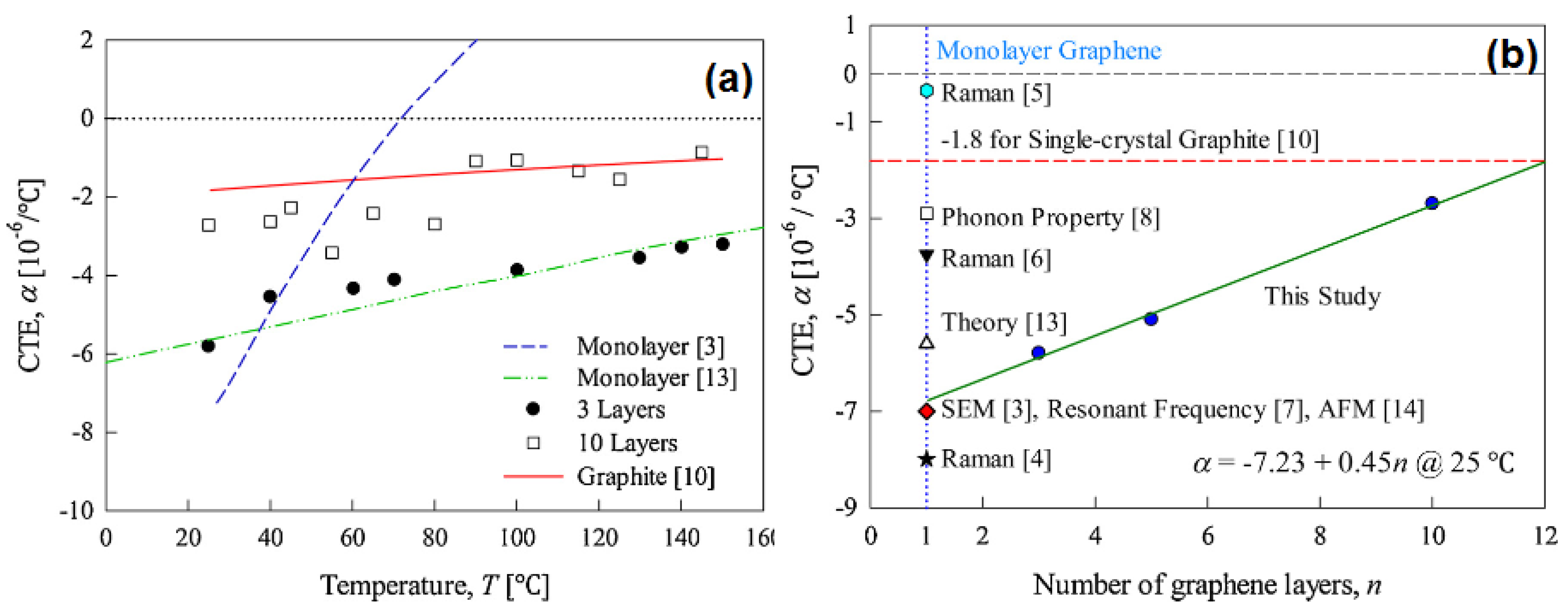
3.2. Thermal Properties of Mono-, Few-, and Multi-Layer Graphene
3.3. Optical Properties of Mono-, Few-, and Multi-Layer Graphene
4. Determination of Mono-, Few-, and Multi-Layer Graphene
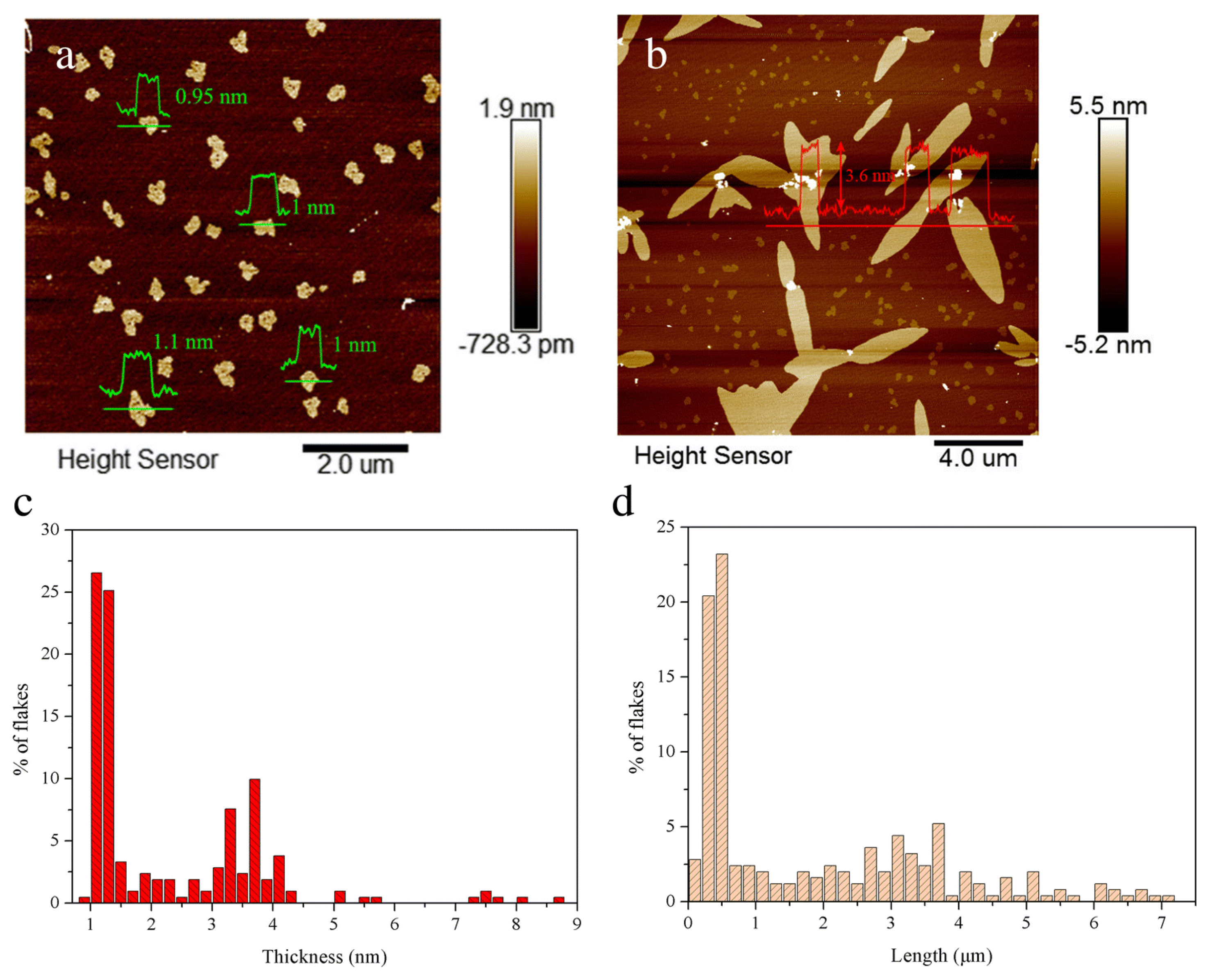
4.1. Atomic Force Microscopy (AFM)
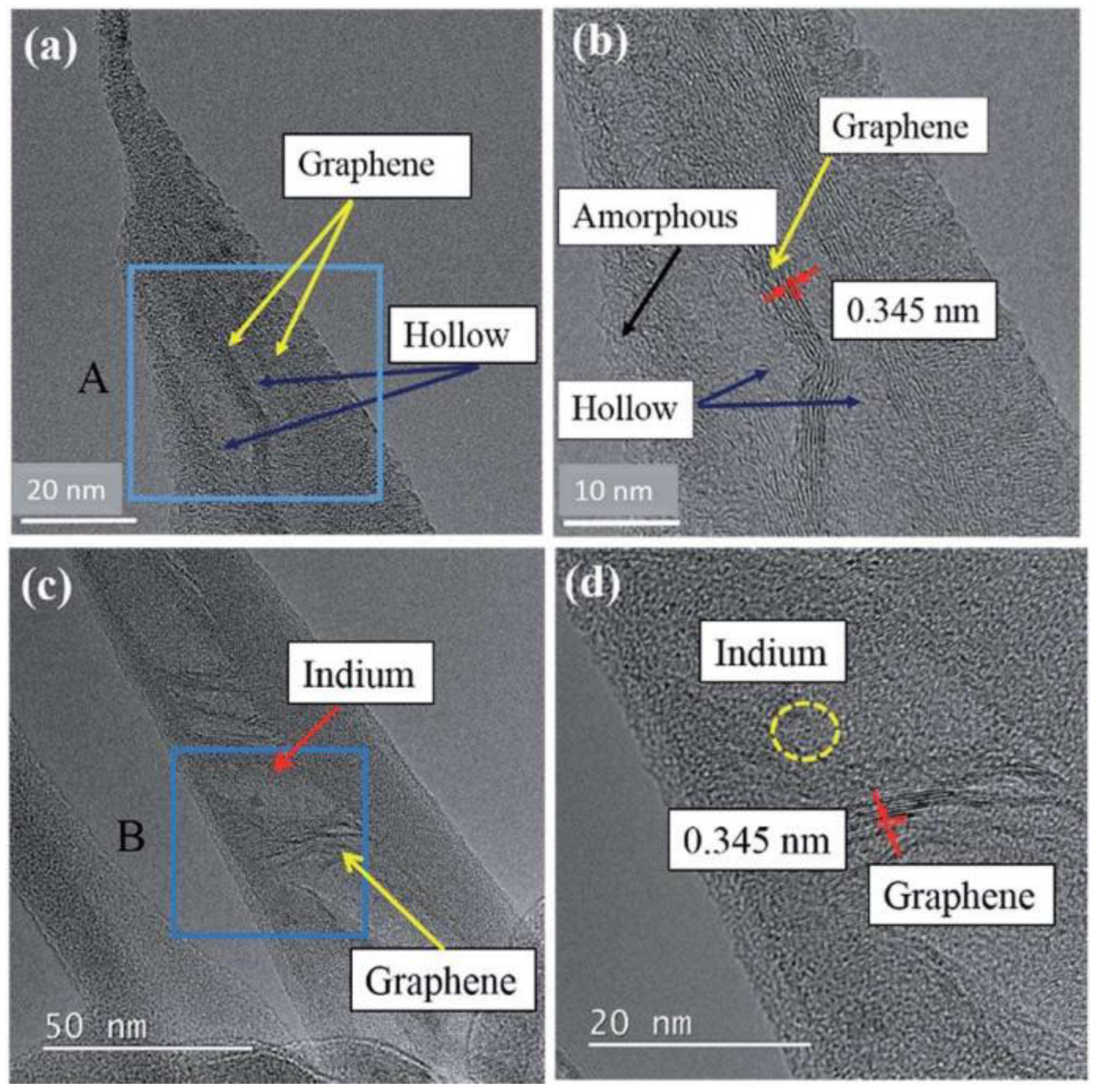
4.2. Transmission Electron Microscopy (TEM)
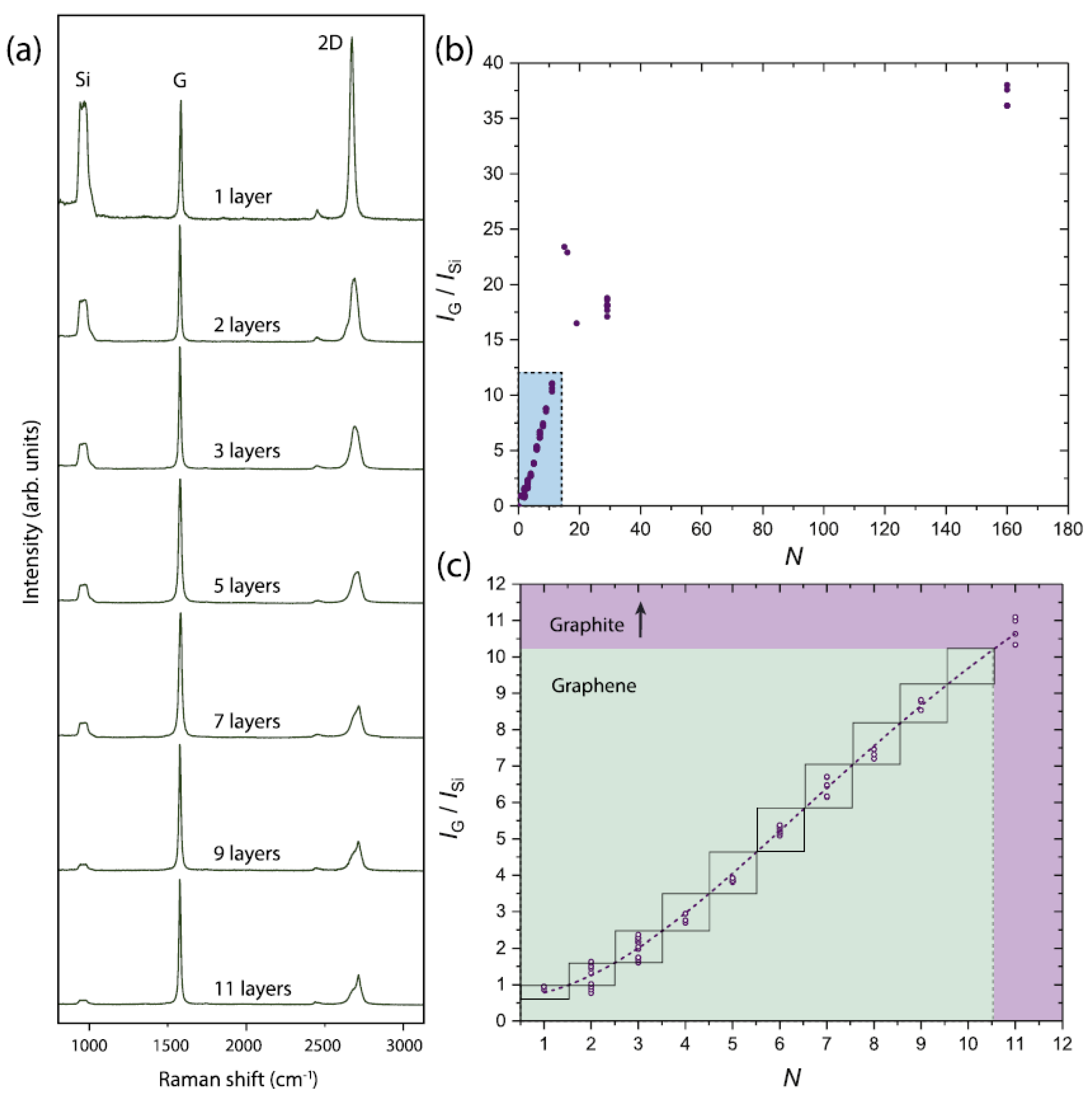
4.3. Raman Spectra
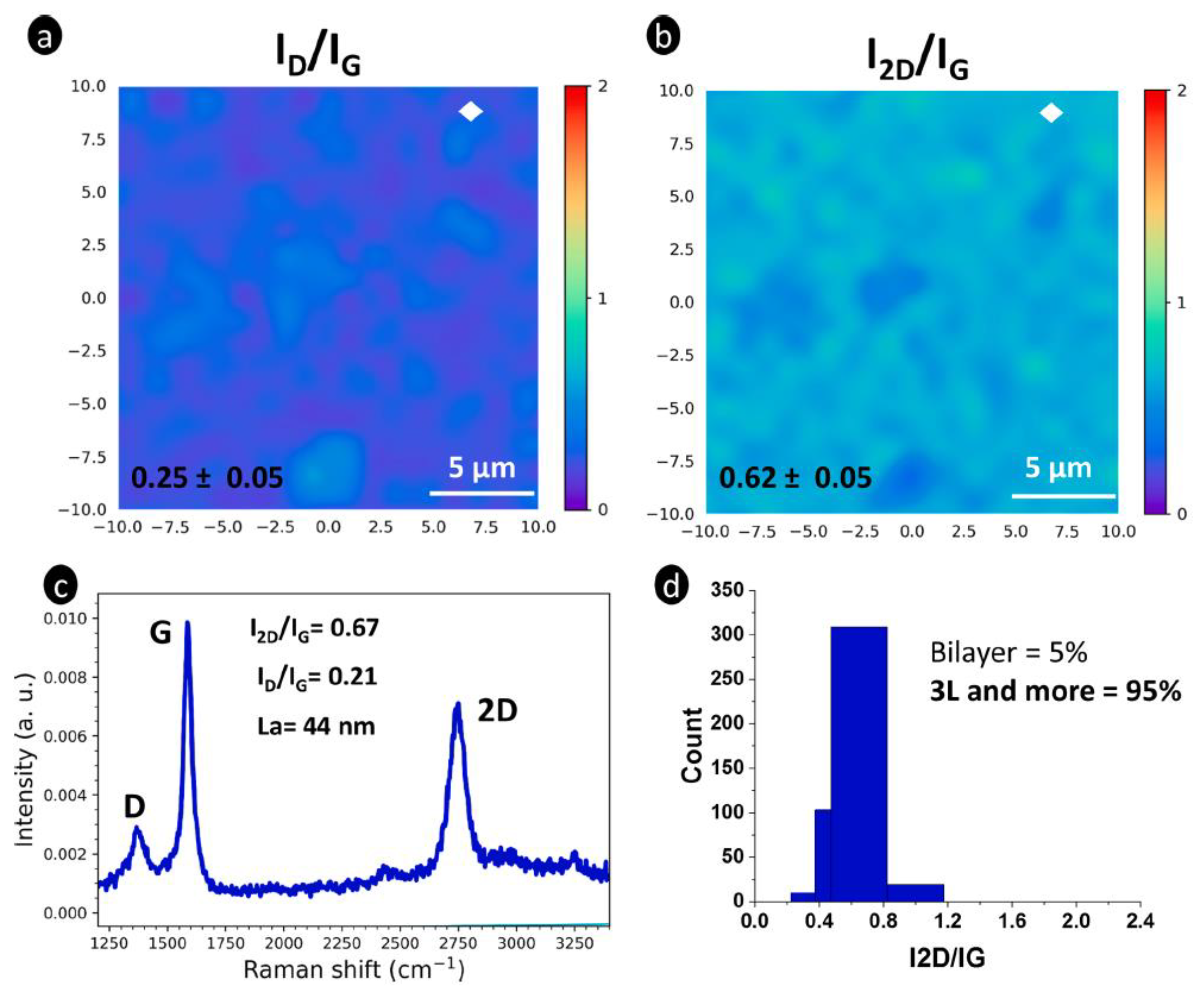
4.4. Raman Mapping
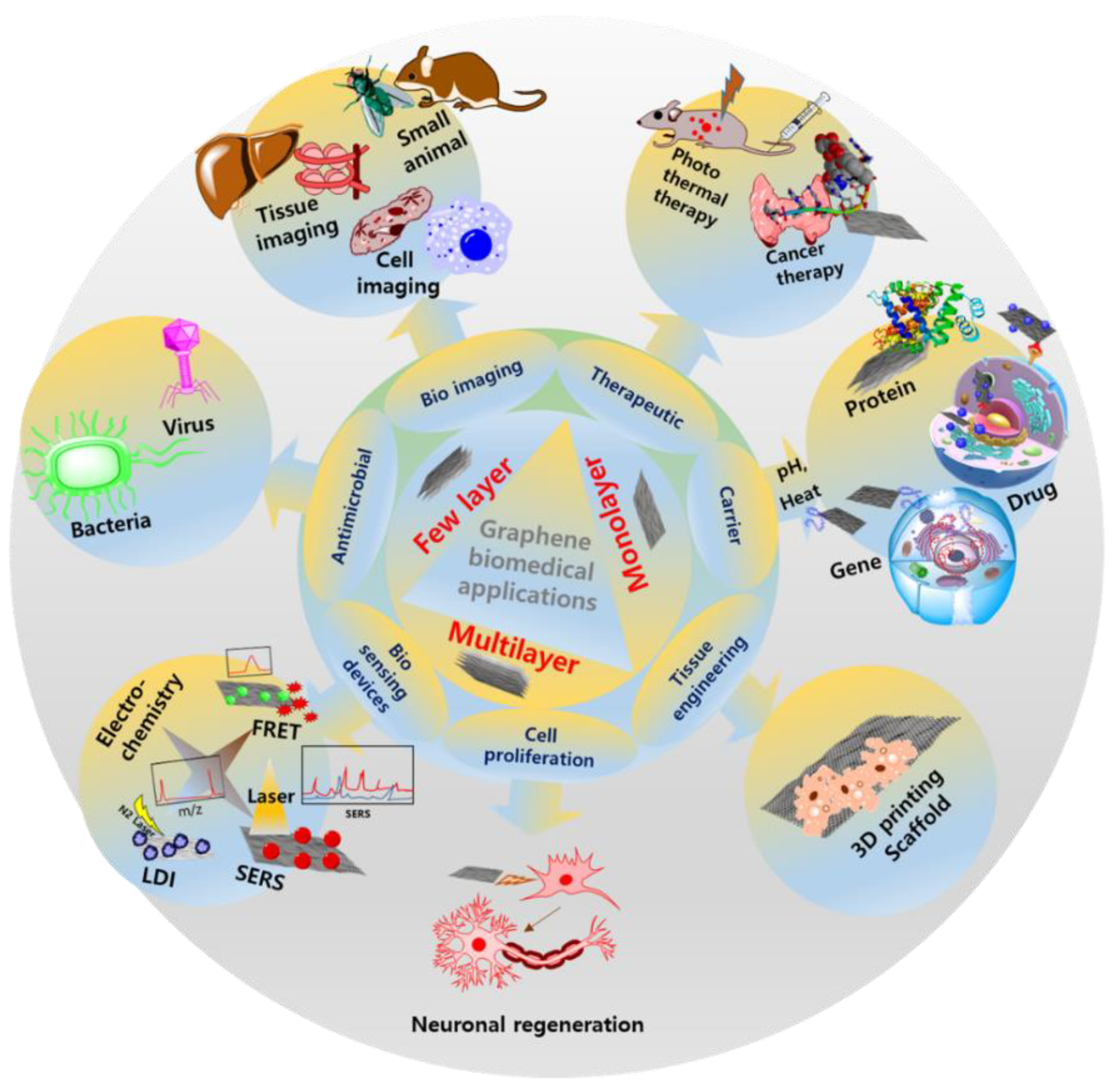
5. Biomedical Devices and Other Biomedical Applications
5.1. Use as a Biomedical Device
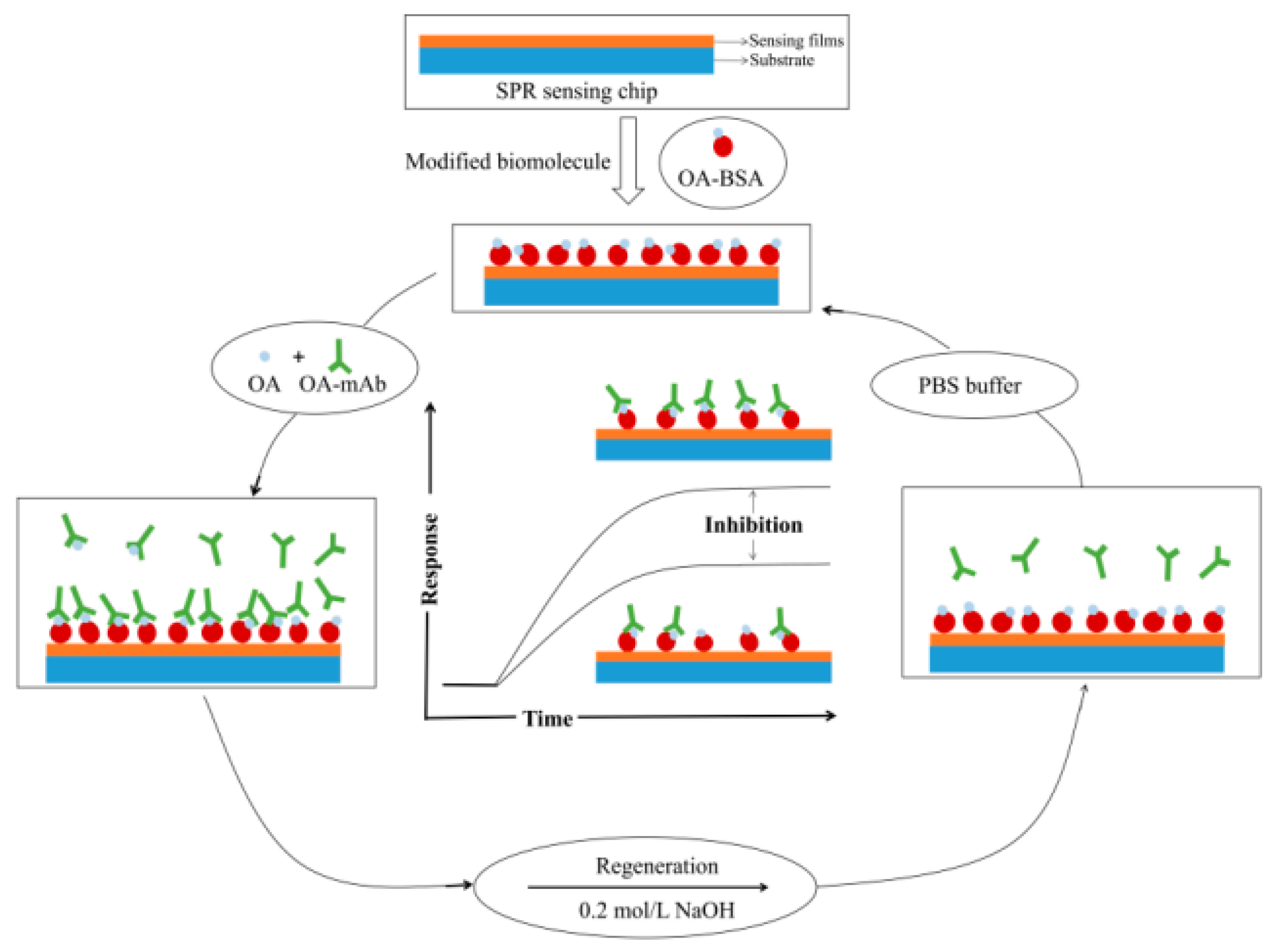
5.2. Use as a Sensor Device
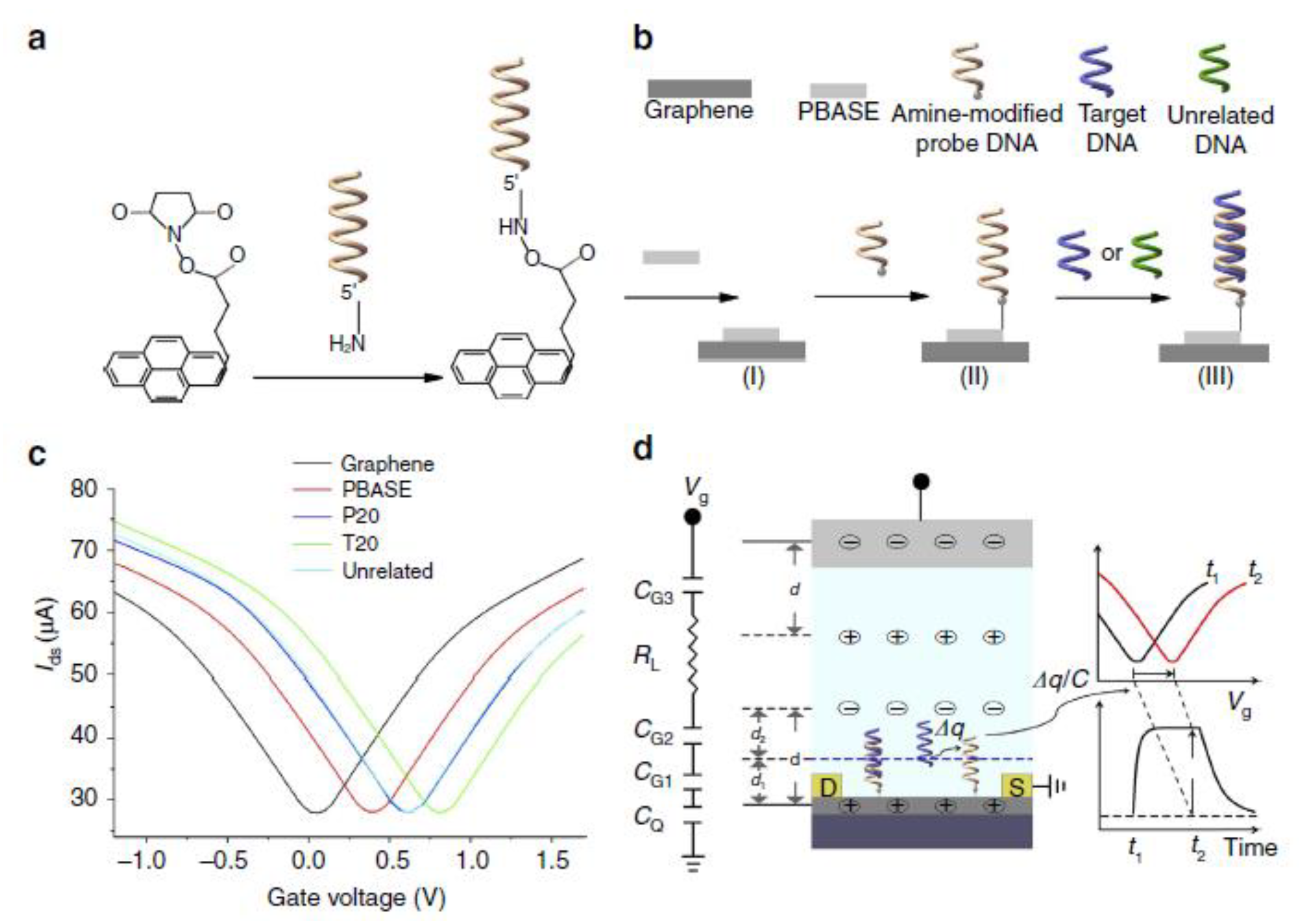
5.2.1. Nucleic Acids Sensor Device
5.2.2. Mammalian Cell Sensor Device
5.2.3. Monolayer Graphene and Immune Sensor Devices
5.2.4. The Development of Imaging Devices for Biomedical Purposes
5.3. Use in Other Biomedical Applications
5.3.1. As a Delivery Carrier and in Treatment
Ligand-Based Drug Delivery
Stimuli Response Delivery
Gene Delivery
5.3.2. Therapeutic Applications
5.3.3. Tissue Engineering for Cell Growth
Monolayer Graphene and Scaffold Formation for Fibroblasts
Few-Layer and Multi-Layer Graphene and Scaffold Formation
Differentiation of Stem Cells
5.3.4. Anti-Microbial Effects
5.3.5. Digital and Analog Devices and Equipment
6. Conclusions, Current Trends, and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Dong, H.; Zhang, Z.; Feng, Z.; Kang, J.; Wu, D.; Wang, Q.; Li, J.; Su, R. Origins of low lattice thermal conductivity in 2D carbon allotropes. J. Mater. Res. Technol. 2021, 11, 1982–1990. [Google Scholar] [CrossRef]
- Liu, W.-D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.-G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800. [Google Scholar] [CrossRef]
- Mailian, A.; Panosyan, Z.; Yengibaryan, Y.; Mailian, M. Identification of Carbon Allotropes in Tribolayers Obtained by Rubbing of Graphite. Mater. Today Proc. 2017, 4, 6842–6848. [Google Scholar] [CrossRef]
- Kabir, H.; Zhu, H.; May, J.; Hamal, K.; Kan, Y.; Williams, T.; Echeverria, E.; McIlroy, D.N.; Estrada, D.; Davis, P.H.; et al. The sp2-sp3 carbon hybridization content of nanocrystalline graphite from pyrolyzed vegetable oil, comparison of electrochemistry and physical properties with other carbon forms and allotropes. Carbon 2019, 144, 831–840. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Fu, J.; Zhao, J. New metallic carbon: Three dimensionally carbon allotropes comprising ultrathin diamond nanostripes. Carbon 2018, 126, 601–610. [Google Scholar] [CrossRef]
- Zhang, X.; Schneider, R.; Müller, E.; Gerthsen, D. Practical aspects of the quantification of sp2-hybridized carbon atoms in diamond-like carbon by electron energy loss spectroscopy. Carbon 2016, 102, 198–207. [Google Scholar] [CrossRef]
- Theye, M.-L.; Paret, V. Spatial organization of the sp2-hybridized carbon atoms and electronic density of states of hydrogenated amorphous carbon films. Carbon 2002, 40, 1153–1166. [Google Scholar] [CrossRef]
- Mandal, T.K.; Hou, Y.; Gao, Z.Y.; Ning, H.; Yang, W.S.; Gao, M.Y. Graphene oxide-based sensor for ultrasensitive visual detection of fluoride. Adv. Sci. 2016, 3, 1600217. [Google Scholar] [CrossRef]
- Bin Hamid, M.A.; Chan, K.T.; Raymond Ooi, C.H.; Zainuddin, H.; Mohd Shah, N.; Shahrol Nidzam, N.N. Structural stability and electronic properties of graphene/germanene heterobilayer. Results Phys. 2021, 28, 104545. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Gao, Q.; Song, J.; Han, G. A review on microstructures and properties of graphene-reinforced aluminum matrix composites fabricated by friction stir processing. J. Manuf. Process. 2021, 68, 126–135. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Shao, J.-L.; Zhan, H. Damage and self-healing characteristics of monolayer graphene enhanced Cu under ballistic impact. Mech. Mater. 2021, 155, 103736. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, S.; Gao, N.; Cheng, L.; Liu, Y.; Zhang, Y.; Feng, D. Thermosetting CFRP interlaminar toughening with multi-layers graphene and MWCNTs under mode I fracture. Compos. Sci. Technol. 2019, 183, 107829. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Gu, Y.T. Mechanical properties of graphene: Effects of layer number, temperature and isotope. Comput. Mater. Sci. 2013, 71, 197–200. [Google Scholar] [CrossRef]
- Mag-isa, A.E.; Kim, S.-M.; Kim, J.-H.; Oh, C.-S. Variation of thermal expansion coefficient of freestanding multilayer pristine graphene with temperature and number of layers. Mater. Today Commun. 2020, 25, 101387. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Henriques, J.; Felizardo, E.; Dias, A.; Abrashev, M.; Kissovski, J.; Botelho do Rego, A.M.; Ferraria, A.M.; Tatarova, E. Simultaneous Synthesis and Nitrogen Doping of Free-Standing Graphene Applying Microwave Plasma. Materials 2020, 13, 4213. [Google Scholar] [CrossRef]
- Mukri, M.-R.; Elias, M.S.; Aziz, M.; Tanemura, M.; Mohd Yusop, M.Z. Structural Modification of Pristine Graphene Network Towards Nanoporous Graphene Membrane: A Review. J. Appl. Membr. Sci. Technol. 2018, 22. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology; Macmillan Publishers Ltd.: London, UK, 2009; pp. 11–19. [Google Scholar]
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385. [Google Scholar] [CrossRef]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Urban, K.W. The challenges of graphene. Nat. Mater. 2011, 10, 165–166. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, Y.H.; Roh, K.C.; Kim, K.-B. Effect of thermally decomposable spacers on graphene microsphere structure and restacking of graphene sheets during electrode fabrication. Carbon 2019, 150, 128–135. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Zhao, P. Facile fabrication of honeycomb-like restacking-inhibited graphene architecture with superior electrochemical performance for energy storage. Mater. Lett. 2018, 225, 93–96. [Google Scholar] [CrossRef]
- Kota, M.; Park, H.S. Restacking-inhibited nitrogen-incorporated mesoporous reduced graphene oxides for high energy supercapacitors. Ceram. Int. 2018, 44, 3195–3200. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, J.; Geng, X.; Dou, H.; Chen, L.; Yan, X.; Zhu, H. Caterpillar-like graphene confining sulfur by restacking effect for high performance lithium sulfur batteries. Chem. Eng. J. 2017, 322, 454–462. [Google Scholar] [CrossRef]
- Tambe, P. Synthesis and characterization of acid treated reduced graphene oxide. Mater. Today Proc. 2022, 49, 1294–1297. [Google Scholar] [CrossRef]
- Fu, H.; Gao, B.; Liu, Z.; Liu, W.; Wang, Z.; Wang, M.; Li, J.; Feng, Z.; Reza Kamali, A. Electrochemical performance of honeycomb graphene prepared from acidic graphene oxide via a chemical expansion method. J. Electroanal. Chem. 2022, 920, 116545. [Google Scholar] [CrossRef]
- Hauquier, F.; Alamarguy, D.; Viel, P.; Noël, S.; Filoramo, A.; Huc, V.; Houzé, F.; Palacin, S. Conductive-probe AFM characterization of graphene sheets bonded to gold surfaces. Appl. Surf. Sci. 2012, 258, 2920–2926. [Google Scholar] [CrossRef]
- Agarwal, P.B.; Paulchowdhury, P.; Mukherjee, A.; Lohani, P.; Thakur, N.K. Optimization of oxygen plasma based etching of single layered graphene through Raman and FESEM characterization. Mater. Today Proc. 2022, 48, 616–618. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Ivanov, I.G.; Pliatsikas, N.; Iakimov, T.; Jamnig, A.; Sarakinos, K.; Yakimova, R. Probing the uniformity of silver-doped epitaxial graphene by micro-Raman mapping. Phys. B Condens. Matter 2020, 580, 411751. [Google Scholar] [CrossRef]
- Pelaez-Fernandez, M.; Bermejo, A.; Benito, A.M.; Maser, W.K.; Arenal, R. Detailed thermal reduction analyses of graphene oxide via in-situ TEM/EELS studies. Carbon 2021, 178, 477–487. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Zhao, Z. Silk fibroin hydrogel encapsulated graphene filed-effect transistors as enzyme-based biosensors. Microchem. J. 2021, 169, 106585. [Google Scholar] [CrossRef]
- GV, Y.D.; Prabhu, A.; Anil, S.; Venkatesan, J. Preparation and characterization of dexamethasone loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102624. [Google Scholar] [CrossRef]
- Karimi, S.; Namazi, H. Fe3O4@PEG-coated dendrimer modified graphene oxide nanocomposite as a pH-sensitive drug carrier for targeted delivery of doxorubicin. J. Alloys Compd. 2021, 879, 160426. [Google Scholar] [CrossRef]
- Sharif, S.; Ahmad, K.S.; Rehman, F.; Bhatti, Z.; Thebo, K.H. Two-dimensional graphene oxide based membranes for ionic and molecular separation: Current status and challenges. J. Environ. Chem. Eng. 2021, 9, 105605. [Google Scholar] [CrossRef]
- Ikram, R.; Jan, B.M.; Ahmad, W. Advances in synthesis of graphene derivatives using industrial wastes precursors; prospects and challenges. J. Mater. Res. Technol. 2020, 9, 15924–15951. [Google Scholar] [CrossRef]
- Rotte, N.K.; Naresh, V.; Muduli, S.; Reddy, V.; Srikanth, V.V.S.; Martha, S.K. Microwave aided scalable synthesis of sulfur, nitrogen co-doped few-layered graphene material for high-performance supercapacitors. Electrochim. Acta 2020, 363, 137209. [Google Scholar] [CrossRef]
- Salussolia, G.; Barbieri, E.; Pugno, N.M.; Botto, L. Micromechanics of liquid-phase exfoliation of a layered 2D material: A hydrodynamic peeling model. J. Mech. Phys. Solids 2020, 134, 103764. [Google Scholar] [CrossRef]
- Hwang, G.; Kim, T.; Shin, J.; Shin, N.; Hwang, S. Machine learnings for CVD graphene analysis: From measurement to simulation of SEM images. J. Ind. Eng. Chem. 2021, 101, 430–444. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Guo, J.; Mao, B.; Li, J.; Wang, X.; Yang, X. Rethinking the reaction pathways of chemical reduction of graphene oxide. Carbon 2021, 171, 963–967. [Google Scholar] [CrossRef]
- Barkauskas, J.; Gaidukevič, J.; Niaura, G. Thermal reduction of graphite oxide in the presence of nitrogen-containing dyes. Carbon Lett. 2021, 31, 1097–1110. [Google Scholar] [CrossRef]
- Wadekar, P.H.; Ghosh, A.; Khose, R.V.; Pethsangave, D.A.; Mitra, S.; Some, S. A novel chemical reduction/co-precipitation method to prepare sulfur functionalized reduced graphene oxide for lithium-sulfur batteries. Electrochim. Acta 2020, 344, 136147. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, P.; Rodríguez-Escudero, M.A.; Burgos, F.J.; Llorente, I.; Caballero-Calero, O.; González, M.M.; Fernández, R.; García-Alonso, M.C. Synthesis of Cu/rGO composites by chemical and thermal reduction of graphene oxide. J. Alloys Compd. 2019, 800, 379–391. [Google Scholar] [CrossRef]
- Demetriou, G.; Biancalana, F.; Abraham, E.; Ji, W.; Wang, Y.; Kar, A.K. Direct observation of an irradiance dependent nonlinear refraction in CVD single layer graphene. Opt. Commun. 2021, 481, 126535. [Google Scholar] [CrossRef]
- Jiang, X.; Song, J.; Chen, S.; Su, Y.; Fan, H.; Zhang, Y.; Hu, L. In-situ fabricated bulk metallic glass/graphite composites with a 3D lubricating layer: Tribological properties under dry sliding and in seawater. Tribol. Int. 2020, 148, 106301. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, M.; Chen, J.; Liu, G.; Zhang, L. Microstructure and properties characterization of Ti-containing Ni60/Graphite self-lubricating composite coatings applied on 300 M ultra-high strength steel by laser cladding. Mater. Chem. Phys. 2021, 266, 124554. [Google Scholar] [CrossRef]
- Ferreira, M.P.; da Nova Mussel, W.; Dutra, P.R.; Ângela de Barros Correia Menezes, M.; Pedrosa, T.A. Physical-chemical exfoliation of pristine graphite flakes. Radiat. Phys. Chem. 2021, 188, 109652. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Viteri, E.; Maldonado, A.; Pisarra, M.; Sindona, A.; Vacacela Gomez, C.; Bellucci, S. Calibration of Fermi Velocity to Explore the Plasmonic Character of Graphene Nanoribbon Arrays by a Semi-Analytical Model. Nanomaterials 2022, 12, 2028. [Google Scholar] [CrossRef] [PubMed]
- Aixart, J.; Díaz, F.; Llorca, J.; Rosell-Llompart, J. Increasing reaction time in Hummers’ method towards well exfoliated graphene oxide of low oxidation degree. Ceram. Int. 2021, 47, 22130–22137. [Google Scholar] [CrossRef]
- Rozmanowski, T.; Krawczyk, P. Methanol electrooxidation at NiCl2–FeCl3–graphite intercalation compound affected by ozone treatment. J. Phys. Chem. Solids 2021, 157, 110223. [Google Scholar] [CrossRef]
- Yamamoto, H.; Matsumoto, K.; Hagiwara, R. Stage-number dependence of intercalated species for fluorosilicate graphite intercalation compounds: Pentafluorosilicate vs. hexafluorosilicate. J. Fluor. Chem. 2021, 242, 109714. [Google Scholar] [CrossRef]
- Cahen, S.; Vangelisti, R. Chemical vapor transport for intercalation reactions: Synthesis of a 1st stage DyCl3 graphite intercalation compound. J. Solid State Chem. 2021, 299, 122185. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Binitha, N.N. Graphene preparation by jaggery assisted ball-milling of graphite for the adsorption of Cr(VI). Mater. Today Proc. 2020, 25, 236–240. [Google Scholar] [CrossRef]
- Tenorio Gonzalez, F.N.; Barajas Rosales, I.R.; Vera Serna, P.; Sánchez de Jesus, F.; Bolarin Miró, A.M.; Garrido Hernández, A.; Kusý, M. Reducing the crystallite and particle size of SrFe12O19 with PVA by high energy ball milling. J. Alloys Compd. 2019, 771, 464–470. [Google Scholar] [CrossRef]
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Dias, A.; Henriques, J.; Rego, A.; Ferraria, A.; Abrashev, M.V.; Dias, F.M.; Luhrs, C.C.; et al. On the plasma-based growth of ‘flowing’ graphene sheets at atmospheric pressure conditions. Plasma Sources Sci. Technol. 2016, 25, 015013. [Google Scholar] [CrossRef]
- Tatarova, E.; Dias, A.; Henriques, J.; Abrashev, M.; Bundaleska, N.; Kovacevic, E.; Bundaleski, N.; Cvelbar, U.; Valcheva, E.; Arnaudov, B.; et al. Towards large-scale in free-standing graphene and N-graphene sheets. Sci. Rep. 2017, 7, 10175. [Google Scholar] [CrossRef]
- Zhao, C.; Hong, Y.; Chu, X.; Dong, Y.; Hu, Z.; Sun, X.; Yan, S. Enhanced ferroelectric properties of P(VDF-TrFE) thin film on single-layer graphene simply adjusted by crystallization condition. Mater. Today Energy 2021, 20, 100678. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Komvopoulos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89. [Google Scholar] [CrossRef]
- Depine, R.A. Graphene Optics: Electromagnetic Solution of Canonical Problems; IOP Publishing: Bristol, UK, 2016; ISBN 978-1-6817-4309-7. [Google Scholar]
- Hanson, G.W. Dyadic Green’s functions and guided surface waves for a surface conductivity model of graphene. J. Appl. Phys. 2008, 103, 064302. [Google Scholar] [CrossRef]
- Xu, X.; Sun, S.; Luo, J.; Ma, R.; Lin, J.; Fang, L.; Zhang, P.; Chen, Y. Few-layer graphene prepared via microwave irradiation of black sesame for supercapacitor applications. Chem. Eng. J. 2021, 425, 130664. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Zhu, L.; Chen, W.; Dong, C.; Wan, Z.; Cao, W.; Zhang, X.; Peng, R.; Wang, M. Sb2S3-based bulk/nano planar heterojunction film solar cells with graphene/polymer composite layer as hole extracting interface. Mater. Lett. 2021, 300, 130190. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Wu, C.; Ji, J. Efficient Production of High-Quality Few-Layer Graphene Using a Simple Hydrodynamic-Assisted Exfoliation Method. Nanoscale Res. Lett. 2018, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Schilirò, E.; Lo Nigro, R.; Panasci, S.E.; Gelardi, F.M.; Agnello, S.; Yakimova, R.; Roccaforte, F.; Giannazzo, F. Aluminum oxide nucleation in the early stages of atomic layer deposition on epitaxial graphene. Carbon 2020, 169, 172–181. [Google Scholar] [CrossRef]
- Gao, B.; Hu, C.; Fu, H.; Sun, Y.; Li, K.; Hu, L. Preparation of single-layer graphene based on a wet chemical synthesis route and the effect on electrochemical properties by double layering surface functional groups to modify graphene oxide. Electrochim. Acta 2020, 361, 137053. [Google Scholar] [CrossRef]
- Yuan, S.-J.; Dong, B.; Dai, X.-H. Facile and scalable synthesis of high-quality few-layer graphene from biomass by a universal solvent-free approach. Appl. Surf. Sci. 2021, 562, 150203. [Google Scholar] [CrossRef]
- Bhuiyan, A.G.; Terai, T.; Katsuzaki, T.; Takeda, N.; Hashimoto, A. Growth of single crystalline Si on graphene using RF-MBE: Orientation control with an AlN interface layer. Appl. Surf. Sci. 2021, 548, 149295. [Google Scholar] [CrossRef]
- Araby, M.I.; Rosmi, M.S.; Vishwakarma, R.; Sharma, S.; Wakamatsu, Y.; Takahashi, K.; Kalita, G.; Kitazawa, M.; Tanemura, M. Graphene formation at 150 °C using indium as catalyst. RSC Adv. 2017, 7, 47353–47356. [Google Scholar] [CrossRef]
- Long, C.; Xie, X.; Fu, J.; Wang, Q.; Guo, H.; Zeng, W.; Wei, N.; Wang, S.; Xiong, Y. Supercapacitive brophene-graphene aerogel as elastic-electrochemical dielectric layer for sensitive pressure sensors. J. Colloid Interface Sci. 2021, 601, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Navik, R.; Gai, Y.; Wang, W.; Zhao, Y. Curcumin-assisted ultrasound exfoliation of graphite to graphene in ethanol. Ultrason. Sonochem. 2018, 48, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, H.; Yu, H. Graphene nanofluids based on one-step exfoliation and edge-functionalization. Carbon 2021, 171, 29–35. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Campanelli, D.A.; Menezes, J.W.; Valsecchi, C.; Zegarra, L.B.R.; Jacinto, C.; Armas, L.E.G. Interaction between Yb3+ doped glasses substrates and graphene layers by raman spectroscopy. Thin Solid Films 2020, 712, 138315. [Google Scholar] [CrossRef]
- Wang, T.; Huntzinger, J.-R.; Bayle, M.; Roblin, C.; Decams, J.-M.; Zahab, A.-A.; Contreras, S.; Paillet, M.; Landois, P. Buffer layers inhomogeneity and coupling with epitaxial graphene unravelled by Raman scattering and graphene peeling. Carbon 2020, 163, 224–233. [Google Scholar] [CrossRef]
- Silva, D.L.; Campos, J.L.E.; Fernandes, T.F.D.; Rocha, J.N.; Machado, L.R.P.; Soares, E.M.; Miquita, D.R.; Miranda, H.; Rabelo, C.; Vilela Neto, O.P.; et al. Raman spectroscopy analysis of number of layers in mass-produced graphene flakes. Carbon 2020, 161, 181–189. [Google Scholar] [CrossRef]
- Huang, S.; Li, S.; Hsu, K.-J.; Villalobos, L.F.; Agrawal, K.V. Systematic design of millisecond gasification reactor for the incorporation of gas-sieving nanopores in single-layer graphene. J. Membr. Sci. 2021, 637, 119628. [Google Scholar] [CrossRef]
- Bouhafs, C.; Pezzini, S.; Geisenhof, F.R.; Mishra, N.; Mišeikis, V.; Niu, Y.; Struzzi, C.; Weitz, R.T.; Zakharov, A.A.; Forti, S.; et al. Synthesis of large-area rhombohedral few-layer graphene by chemical vapor deposition on copper. Carbon 2021, 177, 282–290. [Google Scholar] [CrossRef]
- Bleu, Y.; Bourquard, F.; Michalon, J.-Y.; Lefkir, Y.; Reynaud, S.; Loir, A.-S.; Barnier, V.; Garrelie, F.; Donnet, C. Transfer-free graphene synthesis by nickel catalyst dewetting using rapid thermal annealing. Appl. Surf. Sci. 2021, 555, 149492. [Google Scholar] [CrossRef]
- Lebioda, M.; Pawlak, R.; Szymański, W.; Kaczorowski, W.; Jeziorna, A. Laser Patterning a Graphene Layer on a Ceramic Substrate for Sensor Applications. Sensors 2020, 20, 2134. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Rodriguez-Losada, N.; Aguirre, J. The impact of graphene on neural regenerative medicine. Neural Regen. Res. 2017, 12, 1071. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Kowsari, E.; Haddadi-Asl, V.; Khoobi, M.; Bazri, B.; Aryafard, M.; Lee, J.H.; Kadumudi, F.B.; Talebian, S.; Kamaly, N.; et al. An innovative and eco-friendly modality for synthesis of highly fluorinated graphene by an acidic ionic liquid: Making of an efficacious vehicle for anti-cancer drug delivery. Appl. Surf. Sci. 2020, 515, 146071. [Google Scholar] [CrossRef]
- Potsi, G.; Bourlinos, A.B.; Mouselimis, V.; Poláková, K.; Chalmpes, N.; Gournis, D.; Kalytchuk, S.; Tomanec, O.; Błoński, P.; Medveď, M.; et al. Intrinsic photoluminescence of amine-functionalized graphene derivatives for bioimaging applications. Appl. Mater. Today 2019, 17, 112–122. [Google Scholar] [CrossRef]
- Gu, H.; Tang, H.; Xiong, P.; Zhou, Z. Biomarkers-based Biosensing and Bioimaging with Graphene for Cancer Diagnosis. Nanomaterials 2019, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, E.; Lesongeur, P.; Hogan, B.T.; Baldycheva, A. Multi-layer graphene as a selective detector for future lung cancer biosensing platforms. Nanoscale 2019, 11, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Gao, J.; Yin, X.; Qi, H.; Wang, L.; Jiao, H. Angle-Insensitive Broadband Absorption Enhancement of Graphene Using a Multi-Grooved Metasurface. Nanoscale Res. Lett. 2019, 14, 105. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Gu, P.; Yan, Z.; Tang, C.; Xu, Z.; Liu, B.; Liu, Z. Electrically modulating and switching infrared absorption of monolayer graphene in metamaterials. Carbon 2020, 162, 187–194. [Google Scholar] [CrossRef]
- Divya, J.; Selvendran, S.; Raja, A.S.; Sivasubramanian, A. Graphene-Au-Coated Plasmonic Sensor Based on D-Shaped Bezier Polygonal Hollow Core Photonic Crystal Fiber. Braz. J. Phys. 2021, 51, 1314–1323. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Serban, B.-C.; Cobianu, C.; Buiu, O.; Bumbac, M.; Dumbravescu, N.; Avramescu, V.; Nicolescu, C.M.; Brezeanu, M.; Radulescu, C.; Craciun, G.; et al. Quaternary Oxidized Carbon Nanohorns—Based Nanohybrid as Sensing Coating for Room Temperature Resistive Humidity Monitoring. Coatings 2021, 11, 530. [Google Scholar] [CrossRef]
- Kumar Manoharan, A.; Jayavel, R.; Shanmugam, M.; Sengottaiyan, C.; Chinnathambi, S.; Mohankumar, N. A Compact Sensory Platform Based pH Sensor Using Graphene Field Effect Transistor. J. Nanosci. Nanotechnol. 2021, 21, 3299–3305. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jiang, X.; Wang, C.; Kalsoom, U.E.; Wang, B.; Khan, J.; Muhammad, Y.; Duan, Y.; Zhu, H.; Ren, X.; et al. An Insightful Picture of Nonlinear Photonics in 2D Materials and their Applications: Recent Advances and Future Prospects. Adv. Opt. Mater. 2021, 9, 2001671. [Google Scholar] [CrossRef]
- Hu, Z.; Shao, Q.; Moloney, M.G.; Xu, X.; Zhang, D.; Li, J.; Zhang, C.; Huang, Y. Nondestructive Functionalization of Graphene by Surface-Initiated Atom Transfer Radical Polymerization: An Ideal Nanofiller for Poly(p-phenylene benzobisoxazole) Fibers. Macromolecules 2017, 50, 1422–1429. [Google Scholar] [CrossRef]
- Devi, N.A.; Swain, B.P. Investigation of Metal-Oxide/Reduced Graphene-Oxide Nanocomposites for Gas Sensor Applications. In Nanostructured Materials and Their Applications; Springer: Singapore, 2021; pp. 211–227. [Google Scholar]
- Nurrohman, D.T.; Chiu, N.-F. A Review of Graphene-Based Surface Plasmon Resonance and Surface-Enhanced Raman Scattering Biosensors: Current Status and Future Prospects. Nanomaterials 2021, 11, 216. [Google Scholar] [CrossRef]
- Wenjuan, Y.; Le Goff, A.; Spinelli, N.; Holzinger, M.; Diao, G.-W.; Shan, D.; Defrancq, E.; Cosnier, S. Electrogenerated trisbipyridyl Ru(II)-/nitrilotriacetic-polypyrene copolymer for the easy fabrication of label-free photoelectrochemical immunosensor and aptasensor: Application to the determination of thrombin and anti-cholera toxinantibody. Biosens. Bioelectron. 2013, 42, 556–562. [Google Scholar] [CrossRef]
- Cai, H.; Wang, M.; Wu, Z.; Liu, J.; Wang, X. Performance Enhancement of SPR Biosensor Using Graphene–MoS2 Hybrid Structure. Nanomaterials 2022, 12, 2219. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Qazi, U.Y.; Mansha, A.; Bhatti, I.A.; Javaid, R.; Abbas, Q.; Nadeem, N.; Rehan, Z.A.; Noreen, S.; Zahid, M. Recent developments for antimicrobial applications of graphene-based polymeric composites: A review. J. Ind. Eng. Chem. 2021, 100, 40–58. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Iliut, M.; Zhou, M.; Moffat, J.; Elsawy, M.A.; Pinheiro, W.A.; Hoyland, J.A.; Miller, A.F.; Vijayaraghavan, A.; Saiani, A. Designing Peptide/Graphene Hybrid Hydrogels through Fine-Tuning of Molecular Interactions. Biomacromolecules 2018, 19, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.; Allabush, F.; Tabaei, S.R.; Male, L.; Dafforn, T.R.; Tucker, J.H.R.; Mendes, P.M. Förster Resonance Energy Transfer Nanoplatform Based on Recognition-Induced Fusion/Fission of DNA Mixed Micelles for Nucleic Acid Sensing. ACS Nano 2021, 15, 8517–8524. [Google Scholar] [CrossRef] [PubMed]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K. Fabricating versatile cell supports from nano- and micro-sized graphene oxide flakes. J. Mech. Behav. Biomed. Mater. 2020, 103, 103594. [Google Scholar] [CrossRef] [PubMed]
- Koren, K.; Zieger, S.E. Optode Based Chemical Imaging—Possibilities, Challenges, and New Avenues in Multidimensional Optical Sensing. ACS Sens. 2021, 6, 1671–1680. [Google Scholar] [CrossRef]
- Parvin, N.; Mandal, T.; Roy, P. Soluble Nanoparticles Versatile Tools for Plant Tissue Imaging. J. Bionanosci. 2013, 7, 256–259. [Google Scholar] [CrossRef]
- Kim, S.; Moriya, S.; Maruki, S.; Fukaminato, T.; Ogata, T.; Kurihara, S. Adsorption and release on three-dimensional graphene oxide network structures. R. Soc. Open Sci. 2021, 8, 201585. [Google Scholar] [CrossRef]
- Driscoll, J.; Moirangthem, A.; Yan, I.K.; Patel, T. Fabrication and Characterization of a Biomaterial Based on Extracellular-Vesicle Functionalized Graphene Oxide. Front. Bioeng. Biotechnol. 2021, 9, 686510. [Google Scholar] [CrossRef]
- Moody, A.S.; Dayton, P.A.; Zamboni, W.C. Imaging methods to evaluate tumor microenvironment factors affecting nanoparticle drug delivery and antitumor response. Cancer Drug Resist. 2021, 4, 382. [Google Scholar] [CrossRef]
- Jakubovic, R.; Ramjist, J.; Gupta, S.; Guha, D.; Sahgal, A.; Foster, F.S.; Yang, V.X.D. High-Frequency Micro-Ultrasound Imaging and Optical Topographic Imaging for Spinal Surgery: Initial Experiences. Ultrasound Med. Biol. 2018, 44, 2379–2387. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Huynh, T.-C.; Manivasagan, P.; Mondal, S.; Oh, J. An Up-To-Date Review on Biomedical Applications of Palladium Nanoparticles. Nanomaterials 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Wychowaniec, J.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Jin, Q.; Wei, Y.; Yu, R.; Zheng, B.; Huang, L.; Zhang, Y.; Wang, L.; Zhang, H.; Gao, M.; et al. Few-Layer Graphdiyne Nanosheets Applied for Multiplexed Real-Time DNA Detection. Adv. Mater. 2017, 29, 201606755. [Google Scholar] [CrossRef]
- Dolatkhah, M.; Hashemzadeh, N.; Barar, J.; Adibkia, K.; Aghanejad, A.; Barzegar-Jalali, M.; Omidi, Y. Graphene-based multifunctional nanosystems for simultaneous detection and treatment of breast cancer. Colloids Surf. B Biointerfaces 2020, 193, 111104. [Google Scholar] [CrossRef]
- Jang, S.-C.; Kang, S.-M.; Lee, J.Y.; Oh, S.Y.; Vilian, A.E.; Lee, I.; Han, Y.-K.; Park, J.H.; Cho, W.-S.; Roh, C.; et al. Nano-graphene oxide composite for in vivo imaging. Int. J. Nanomed. 2018, 13, 221–234. [Google Scholar] [CrossRef]
- Matsumoto, K.; Mitchell, J.B.; Krishna, M.C. Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 2021, 26, 1614. [Google Scholar] [CrossRef]
- Sheth, D.B.; Gratzl, M. Electrochemical mapping of oxygenation in the three-dimensional multicellular tumour hemi-spheroid. Proc. R. Soc. A Math. Phys. Eng. Sci. 2019, 475, 20180647. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Shankar, R.A.; Zweier, J.L. In vivo measurement of arterial and venous oxygenation in the rat using 3D spectral-spatial electron paramagnetic resonance imaging. Phys. Med. Biol. 1998, 43, 1837–1844. [Google Scholar] [CrossRef]
- Khramtsov, V.V.; Bobko, A.A.; Tseytlin, M.; Driesschaert, B. Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Anal. Chem. 2017, 89, 4758–4771. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef]
- Boś-Liedke, A.; Walawender, M.; Woźniak, A.; Flak, D.; Gapiński, J.; Jurga, S.; Kucińska, M.; Plewiński, A.; Murias, M.; Elewa, M.; et al. EPR Oximetry Sensor—Developing a TAM Derivative for In Vivo Studies. Cell Biochem. Biophys. 2018, 76, 19–28. [Google Scholar] [CrossRef]
- Mrówczyński, R.; Coy, L.E.; Scheibe, B.; Czechowski, T.; Augustyniak-Jabłokow, M.; Jurga, S.; Tadyszak, K. Electron Paramagnetic Resonance Imaging and Spectroscopy of Polydopamine Radicals. J. Phys. Chem. B 2015, 119, 10341–10347. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Valenti, V.; Tripoli, A.; Illari, S.I.; Molino, L.; Parisi, S.; Cacciola, A.; Lillo, S.; Giuffrida, D.; Pergolizzi, S. Lattice or Oxygen-Guided Radiotherapy: What If They Converge? Possible Future Directions in the Era of Immunotherapy. Cancers 2021, 13, 3290. [Google Scholar] [CrossRef]
- Hirai, N.; Maeda, Y.; Hashimoto, K.; Andriana, B.B.; Matsuyoshi, H.; Sato, H. Coherent Anti-Stokes Raman Scattering Spectroscopy Using a Double-Wavelength-Emission Electronically Tuned Ti:Sapphire Laser. Appl. Spectrosc. 2021, 75, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Pence, I.J.; Goss, A.; Fast, A.; Brinkmann, M.; Hellwig, T.; Fallnich, C.; Evans, C.L. Development of a clinical coherent Raman imaging system for in vivo drug monitoring. In Proceedings of the Biophotonics Congress 2021; Optica Publishing Group: Washington, DC, USA; 2021; p. NTu3C.4. [Google Scholar] [CrossRef]
- Yeh, P.-C.; Yoon, S.; Kurniawan, D.; Chung, Y.G.; Chiang, W.-H. Unraveling the Fluorescence Quenching of Colloidal Graphene Quantum Dots for Selective Metal Ion Detection. ACS Appl. Nano Mater. 2021, 4, 5636–5642. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Huang, P. Graphene-Based Nanomaterials in Bioimaging. In Biomedical Applications of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–287. [Google Scholar]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Liang, L.; Peng, X.; Sun, F.; Kong, Z.; Shen, J.-W. A review on the cytotoxicity of graphene quantum dots: From experiment to simulation. Nanoscale Adv. 2021, 3, 904–917. [Google Scholar] [CrossRef]
- Samanta, S.; Banerjee, S.L.; Bhattacharya, K.; Singha, N.K. Graphene Quantum Dots-Ornamented Waterborne Epoxy-Based Fluorescent Adhesive via Reversible Addition–Fragmentation Chain Transfer-Mediated Miniemulsion Polymerization: A Potential Material for Art Conservation. ACS Appl. Mater. Interfaces 2021, 13, 36307–36319. [Google Scholar] [CrossRef]
- Bapli, A.; Chatterjee, A.; Gautam, R.K.; Jana, R.; Seth, D. Modulation of the Protein–Ligand Interaction in the Presence of Graphene Oxide: A Detailed Spectroscopic Study. Langmuir 2021, 37, 5034–5048. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, K. Poly(Ethylene Glycol) Functionalized Graphene Oxide in Tissue Engineering: A Review on Recent Advances. Int. J. Nanomed. 2020, 15, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, T.; Kumar, V.; Kumar, V. Hydrophilic graphene quantum dots as turn-off fluorescent nanoprobes for toxic heavy metal ions detection in aqueous media. Chemosphere 2021, 282, 131019. [Google Scholar] [CrossRef]
- A Ocsoy, M.; Yusufbeyoglu, S.; Ildiz, N.; Ulgen, A.; Ocsoy, I. DNA Aptamer-Conjugated Magnetic Graphene Oxide for Pathogenic Bacteria Aggregation: Selective and Enhanced Photothermal Therapy for Effective and Rapid Killing. ACS Omega 2021, 6, 20637–20643. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cai, Y.; Zhang, X.; Liu, J.; Liu, Z.; Li, B.; Wong, H.; Xu, F.; Sheng, L.; Sun, D.; et al. Aligned Graphene Mesh-Supported Double Network Natural Hydrogel Conduit Loaded with Netrin-1 for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.D.; Kim, J.; Lee, D.H.; Kim, J.H.; Jang, C.W.; Kim, S.; Choi, S.-H. Structural and optical characteristics of graphene quantum dots size-controlled and well-aligned on a large scale by polystyrene-nanosphere lithography. J. Phys. D Appl. Phys. 2016, 49, 025308. [Google Scholar] [CrossRef]
- Borandeh, S.; Alimardani, V.; Abolmaali, S.S.; Seppälä, J. Graphene Family Nanomaterials in Ocular Applications: Physicochemical Properties and Toxicity. Chem. Res. Toxicol. 2021, 34, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]
- Wang, F.; Duo, T.; Wang, Y.; Xiao, Z.; Xu, A.; Liu, R. Novel Polyethyleneimine/κ-Carrageenan Composite from Facile One-Step Fabrication for the Removal of Copper Ion from Aqueous Solution. J. Polym. Environ. 2022, 30, 1001–1011. [Google Scholar] [CrossRef]
- Oh, J.-S.; Park, J.-S.; Lee, E.-J. Enhanced Effect of Polyethyleneimine-Modified Graphene Oxide and Simvastatin on Osteogenic Differentiation of Murine Bone Marrow-Derived Mesenchymal Stem Cells. Biomedicines 2021, 9, 501. [Google Scholar] [CrossRef]
- Jonoush, Z.A.; Farahani, M.; Bohlouli, M.; Niknam, Z.; Golchin, A.; Hatamie, S.; Rezaei-Tavirani, M.; Omidi, M.; Zali, H. Surface Modification of Graphene and its Derivatives for Drug Delivery Systems. Mini-Rev. Org. Chem. 2021, 18, 78–92. [Google Scholar] [CrossRef]
- Olson, E.; Plaut, J.S.; Barnhill, S.A.; Sabuncu, S.; Dambacher, C.M.; Speese, S.D.; Ranganathan, S.V.; Branchaud, B.P.; Yildirim, A. Enzyme-Instructed Formation of β-Sheet-Rich Nanoplatelets for Label-Free Protease Sensing. ACS Appl. Nano Mater. 2021, 4, 7800–7810. [Google Scholar] [CrossRef]
- Pontrelli, G.; Toniolo, G.; McGinty, S.; Peri, D.; Succi, S.; Chatgilialoglu, C. Mathematical modelling of drug delivery from pH-responsive nanocontainers. Comput. Biol. Med. 2021, 131, 104238. [Google Scholar] [CrossRef]
- Ren, M.-X.; Wang, Y.-Q.; Lei, B.-Y.; Yang, X.-X.; Hou, Y.-L.; Meng, W.-J.; Zhao, D.-L. Magnetite nanoparticles anchored on graphene oxide loaded with doxorubicin hydrochloride for magnetic hyperthermia therapy. Ceram. Int. 2021, 47, 20686–20692. [Google Scholar] [CrossRef]
- Yoon, J.; Lim, J.; Shin, M.; Lee, S.-N.; Choi, J.-W. Graphene/MoS2 Nanohybrid for Biosensors. Materials 2021, 14, 518. [Google Scholar] [CrossRef]
- Lemus, L.M.R.; Azamar-Barrios, J.A.; Ortiz-Vazquez, E.; Quintana-Owen, P.; Freile-Pelegrín, Y.; Perera, F.G.; Madera-Santana, T.J. Development and physical characterization of novel bio-nanocomposite films based on reduced graphene oxide, agar and melipona honey. Carbohydr. Polym. Technol. Appl. 2021, 2, 100133. [Google Scholar] [CrossRef]
- Mathew, T.; Sree, R.A.; Aishwarya, S.; Kounaina, K.; Patil, A.G.; Satapathy, P.; Hudeda, S.P.; More, S.S.; Muthucheliyan, K.; Kumar, T.N.; et al. Graphene-based functional nanomaterials for biomedical and bioanalysis applications. FlatChem 2020, 23, 100184. [Google Scholar] [CrossRef]
- Işıklan, N.; Hussien, N.A.; Türk, M. Synthesis and drug delivery performance of gelatin-decorated magnetic graphene oxide nanoplatform. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126256. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Teimouri, M.; Pooladi, M. The Synergistic Anticancer Traits of Graphene Oxide Plus Doxorubicin Against BT474 and MCF7 Breast Cancer Stem Cells In Vitro. Appl. Biochem. Biotechnol. 2021, 193, 3586–3601. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Luo, T.; Qu, J.; Chen, W.R. Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials 2021, 265, 120421. [Google Scholar] [CrossRef]
- Mudaliar, P.; Pradeep, P.; Abraham, R.; Sreekumar, E. Targeting cap-dependent translation to inhibit Chikungunya virus replication: Selectivity of p38 MAPK inhibitors to virus-infected cells due to autophagy-mediated down regulation of phospho-ERK. J. Gen. Virol. 2021, 102, 001629. [Google Scholar] [CrossRef]
- Xie, L.; Chen, F.; Du, H.; Zhang, X.; Wang, X.; Yao, G.; Xu, B. Graphene oxide and indole-3-acetic acid cotreatment regulates the root growth of Brassica napus L. via multiple phytohormone pathways. BMC Plant Biol. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K. Dually emissive P,N-co-doped carbon dots for fluorescent and photoacoustic tissue imaging in living mice. Microchim. Acta 2017, 184, 1117–1125. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, L.; Huang, X.; Li, Y.; Qiao, M.; Liu, W.; Zhang, T.; Qi, Y.; Wang, W.; et al. An ultrasensitive, homogeneous fluorescence quenching immunoassay integrating separation and detection of aflatoxin M1 based on magnetic graphene composites. Microchim. Acta 2021, 188, 59. [Google Scholar] [CrossRef]
- Vasilopoulos, V.; Pitou, M.; Fekas, I.; Papi, R.; Ouranidis, A.; Pavlidou, E.; Patsalas, P.; Choli-Papadopoulou, Τ. Graphene-Wrapped Copper Nanoparticles: An Antimicrobial and Biocompatible Nanomaterial with Valuable Properties for Medical Uses. ACS Omega 2020, 5, 26329–26334. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.; Tang, Y.; Huo, P. An environmentally friendly nanocomposite polypyrrole@silver/reduced graphene oxide with high catalytic activity for bacteria and antibiotics. J. Mater. Sci. Mater. Electron. 2021, 32, 15211–15225. [Google Scholar] [CrossRef]
- Mandal, T.K.; Lee, Y.R.; Parvin, N. Red phosphorus Decorating Graphene oxide Nanosheets: Label-free DNA detection. Biomater. Sci. 2020, 8, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-R.; Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Behaviors of NIH-3T3 Fibroblasts on Graphene/Carbon Nanotubes: Proliferation, Focal Adhesion, and Gene Transfection Studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Dubey, S.K.; Bahadur, P. Graphene nanosheets as reinforcement and cell-instructive material in soft tissue scaffolds. Adv. Colloid Interface Sci. 2020, 281, 102167. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Kim, H.; Han, Y.; Cho, Y.-W.; Son, H.; Luo, Z.; Kim, T.-H. Enhancing osteogenesis of adipose-derived mesenchymal stem cells using gold nanostructure/peptide-nanopatterned graphene oxide. Colloids Surf. B Biointerfaces 2021, 204, 111807. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Duan, L.; Weng, W.; Cheng, K.; Wang, D.; Ouyang, H. Light-induced osteogenic differentiation of BMSCs with graphene/TiO2 composite coating on Ti implant. Colloids Surf. B Biointerfaces 2021, 207, 111996. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Kwak, S.-Y.; Yang, J.-K.; Lee, Y.-S.; Kim, J.-H.; Kim, H.D.; Hwang, N.S. Graphene oxide film guided skeletal muscle differentiation. Mater. Sci. Eng. C 2021, 126, 112174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Li, Y.; Jin, W.; Huang, X. Construction of halogenated graphenes by halogenation of hydrogenated graphene. Compos. Commun. 2021, 25, 100771. [Google Scholar] [CrossRef]
- Feng, X.; Wang, J.; Cai, P.; Yang, Z.; Shen, J.; Zhang, Y.; Zhang, X. Graphene/protamine assembled hybrid paper with antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126977. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Zhou, Q.; Hu, X.; Wu, J.; Li, G.; Li, G.; Hou, F.; Wu, Y. Graphene oxide enters the rice roots and disturbs the endophytic bacterial communities. Ecotoxicol. Environ. Saf. 2020, 192, 110304. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.-P.; Bunker, C.E. Graphene Oxide: A Nonspecific Enhancer of Cellular Growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef]
- Abdalla, O.; Wahab, M.A.; Abdala, A. Fabrication of Graphene Oxide-Based Membranes and their Applications in Water Treatment. Curr. Pharm. Biotechnol. 2021, 22, 1686–1704. [Google Scholar] [CrossRef]
- Haddadan, F.; Soroosh, M.; Alaei-Sheini, N. Designing an electro-optical encoder based on photonic crystals using the graphene–Al2O3 stacks. Appl. Opt. 2020, 59, 2179. [Google Scholar] [CrossRef]
- Jalali Azizpour, M.R.; Soroosh, M.; Dalvand, N.; Seifi-Kavian, Y. All-Optical Ultra-Fast Graphene-Photonic Crystal Switch. Crystals 2019, 9, 461. [Google Scholar] [CrossRef]
- Haddadan, F.; Soroosh, M.; Alaei-Sheini, N. Cross-talk reduction in a graphene-based ultra-compact plasmonic encoder using an Au nano-ridge on a silicon substrate. Appl. Opt. 2022, 61, 3209. [Google Scholar] [CrossRef]
- Haddadan, F.; Soroosh, M. Design and simulation of a subwavelength 4-to-2 graphene-based plasmonic priority encoder. Opt. Laser Technol. 2023, 157, 108680. [Google Scholar] [CrossRef]
- Bagheri, F.; Soroosh, M.; Haddadan, F.; Seifi-Kavian, Y. Design and simulation of a compact graphene-based plasmonic D flip-flop. Opt. Laser Technol. 2022, 155, 108436. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. https://doi.org/10.3390/electronics11203345
Parvin N, Kumar V, Joo SW, Park S-S, Mandal TK. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics. 2022; 11(20):3345. https://doi.org/10.3390/electronics11203345
Chicago/Turabian StyleParvin, Nargish, Vineet Kumar, Sang Woo Joo, Sang-Shin Park, and Tapas Kumar Mandal. 2022. "Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review" Electronics 11, no. 20: 3345. https://doi.org/10.3390/electronics11203345
APA StyleParvin, N., Kumar, V., Joo, S. W., Park, S.-S., & Mandal, T. K. (2022). Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics, 11(20), 3345. https://doi.org/10.3390/electronics11203345







