Biosensors Fabricated by Laser-Induced Metallization on DLP Composite Resin
Abstract
1. Introduction
2. Materials and Methods
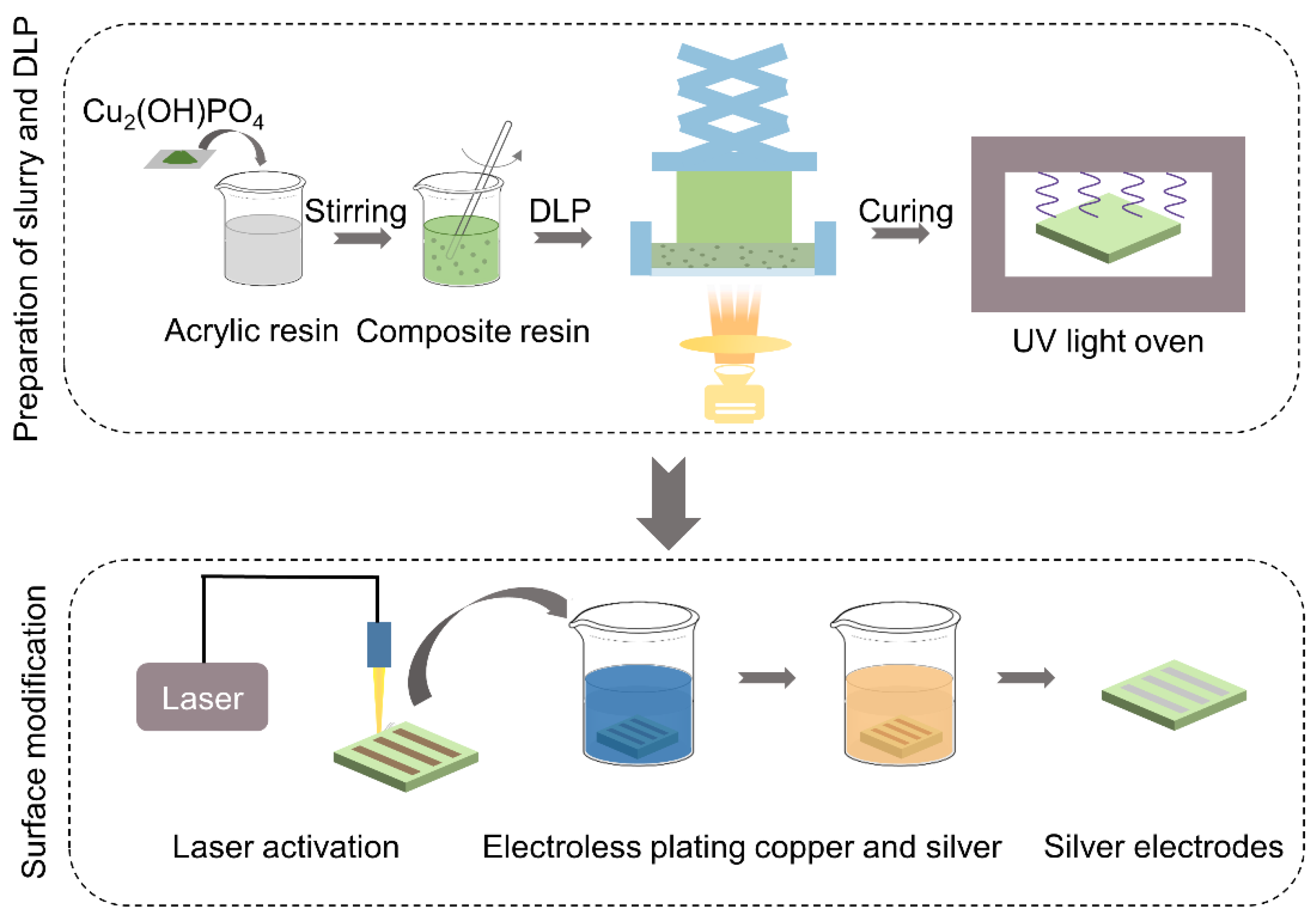
2.1. Preparation of Slurry and Digital Light Processing
2.2. Surface Modification
2.3. Characterization
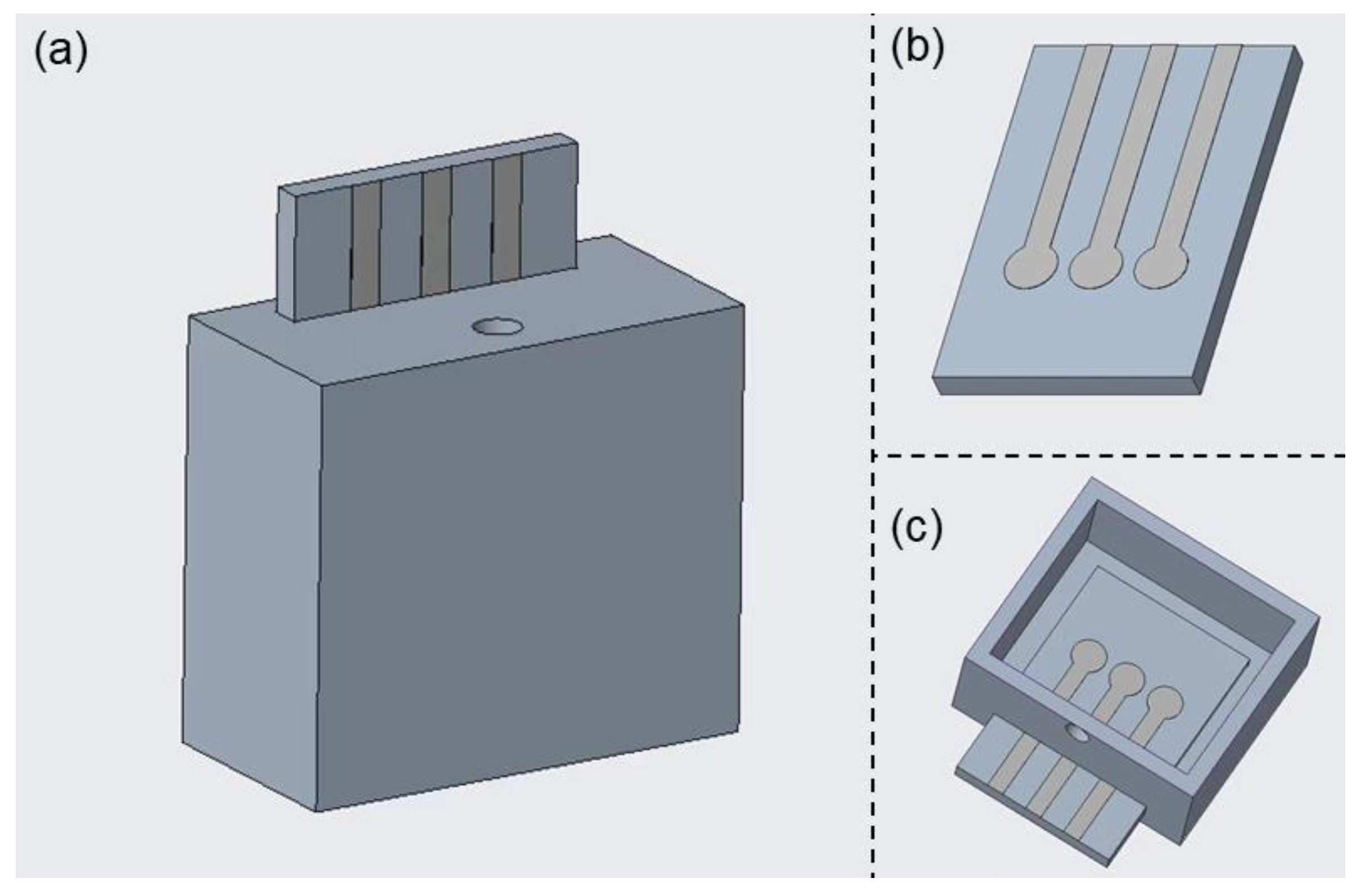
3. Results and Discussion
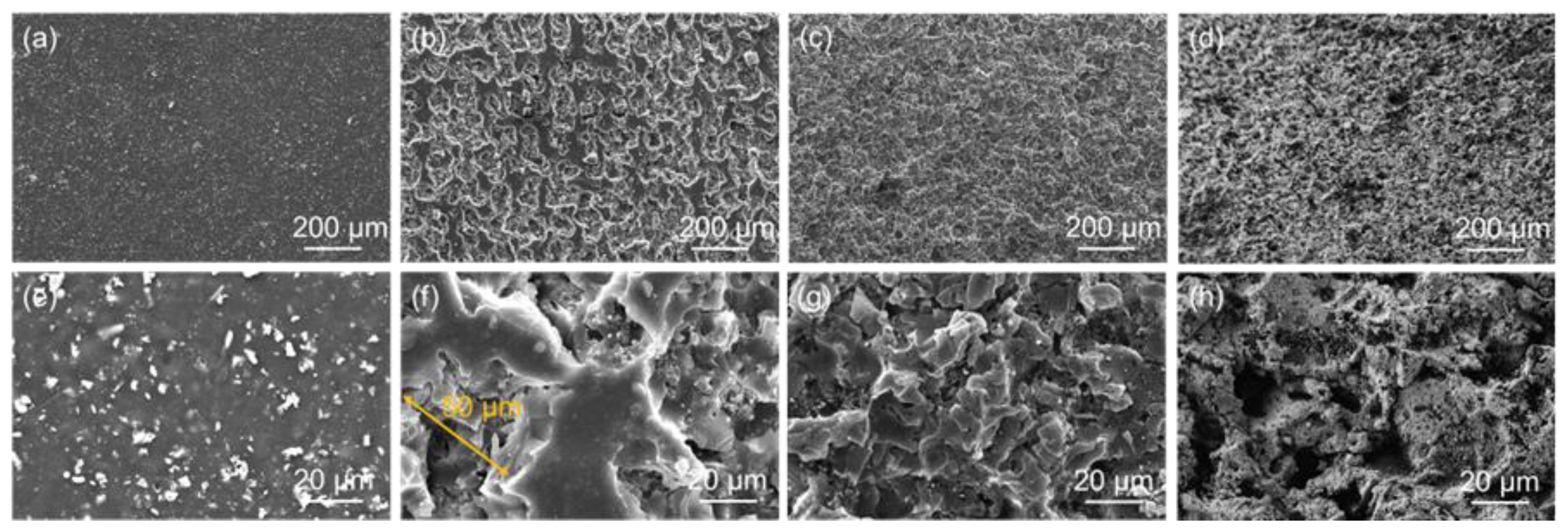
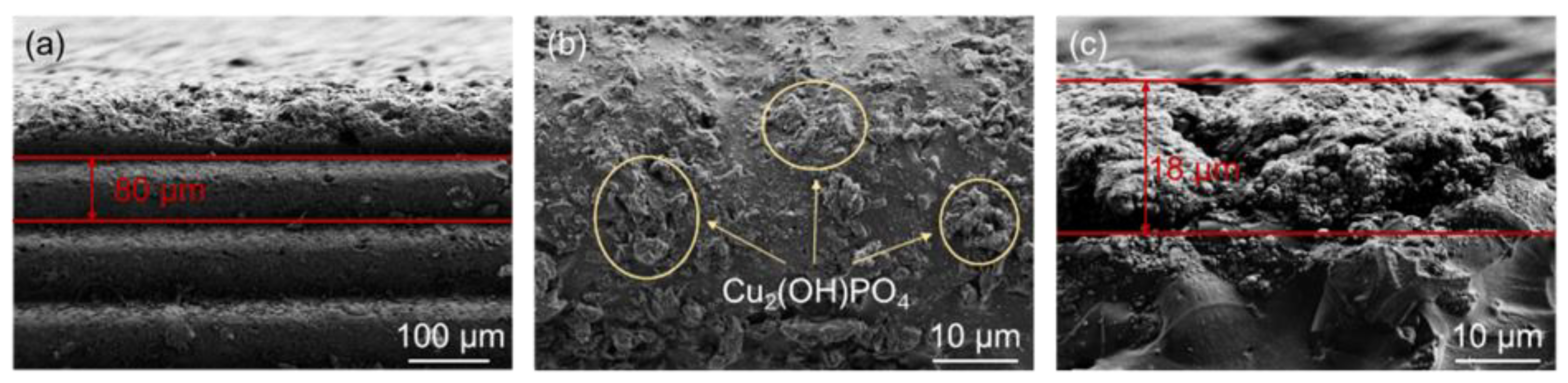
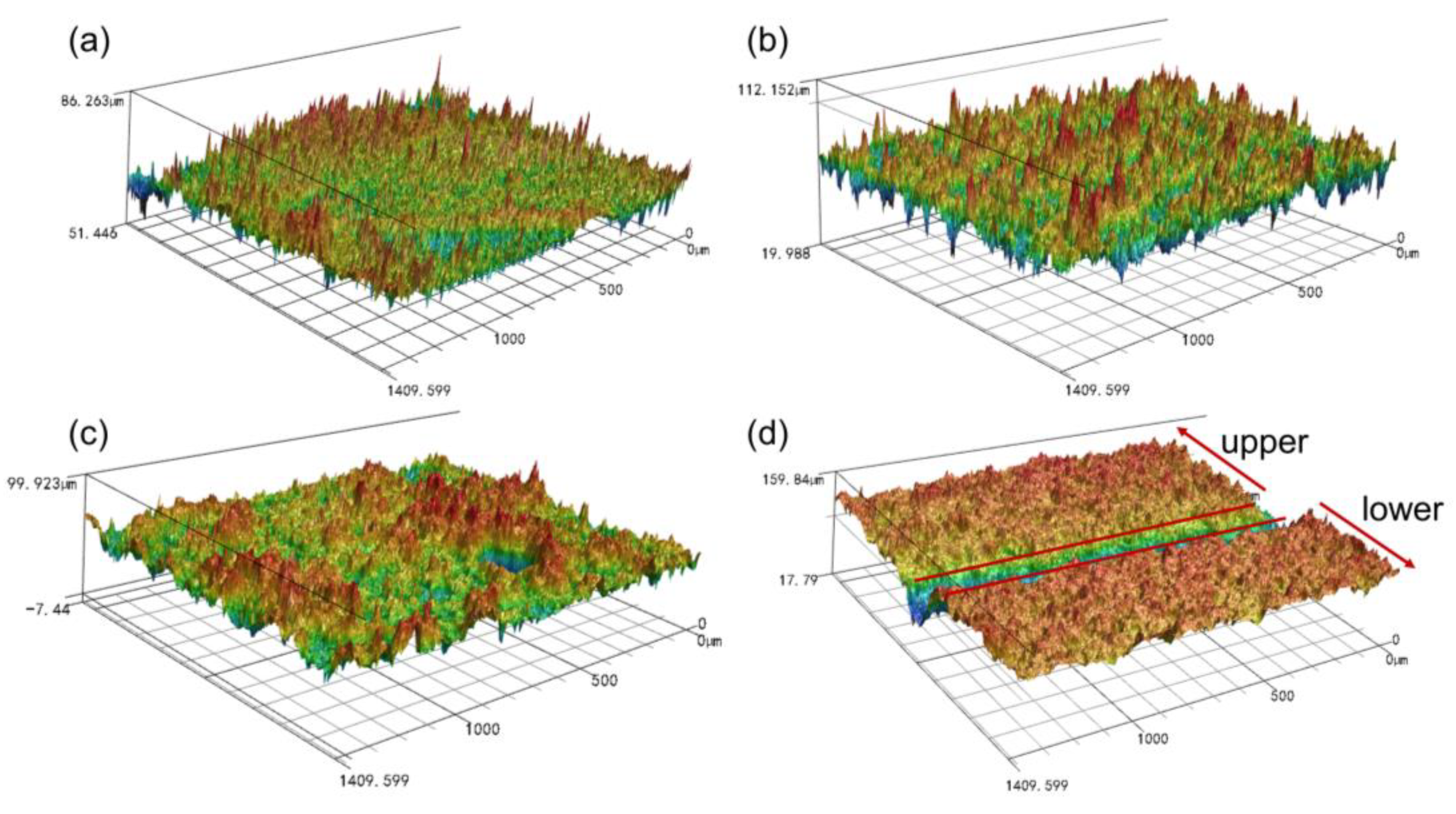
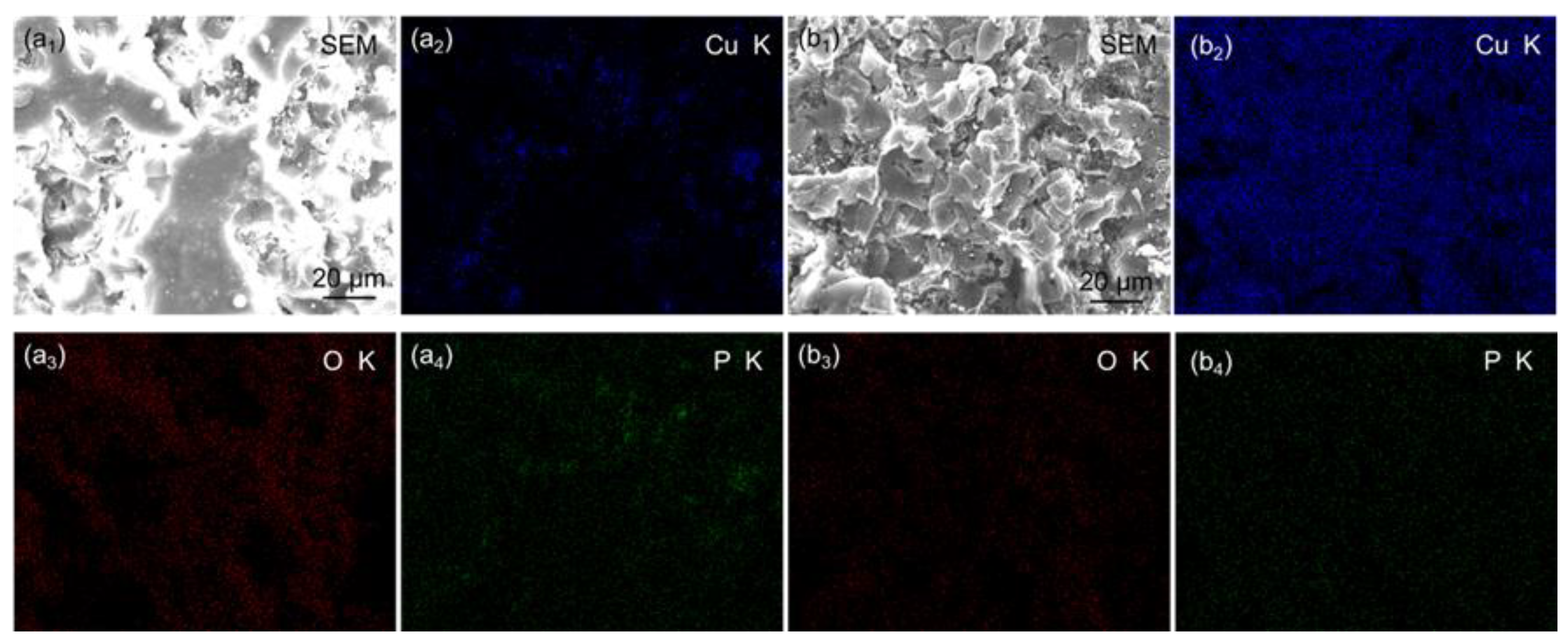
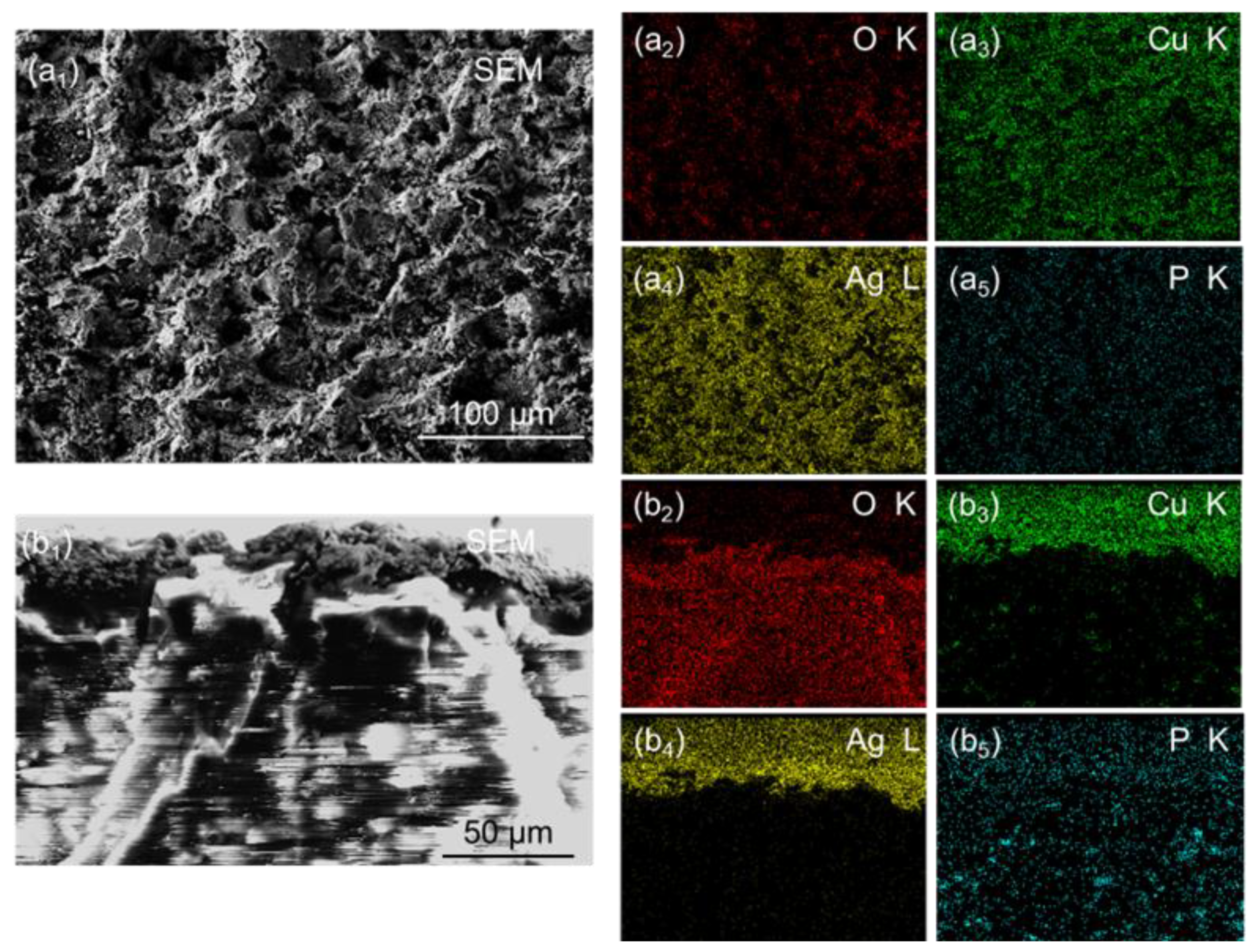
3.1. Surface Morphology
3.2. Chemical Composition
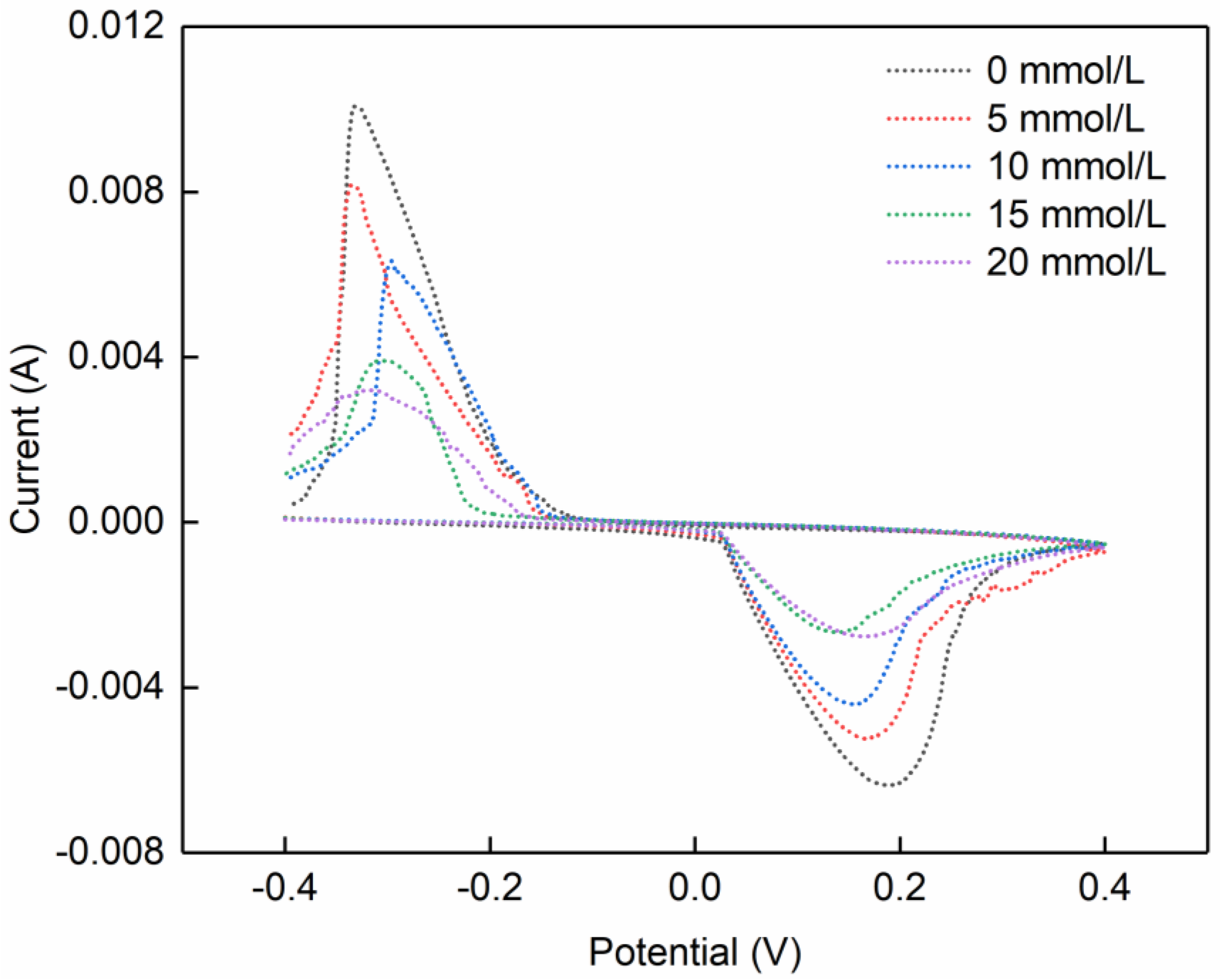
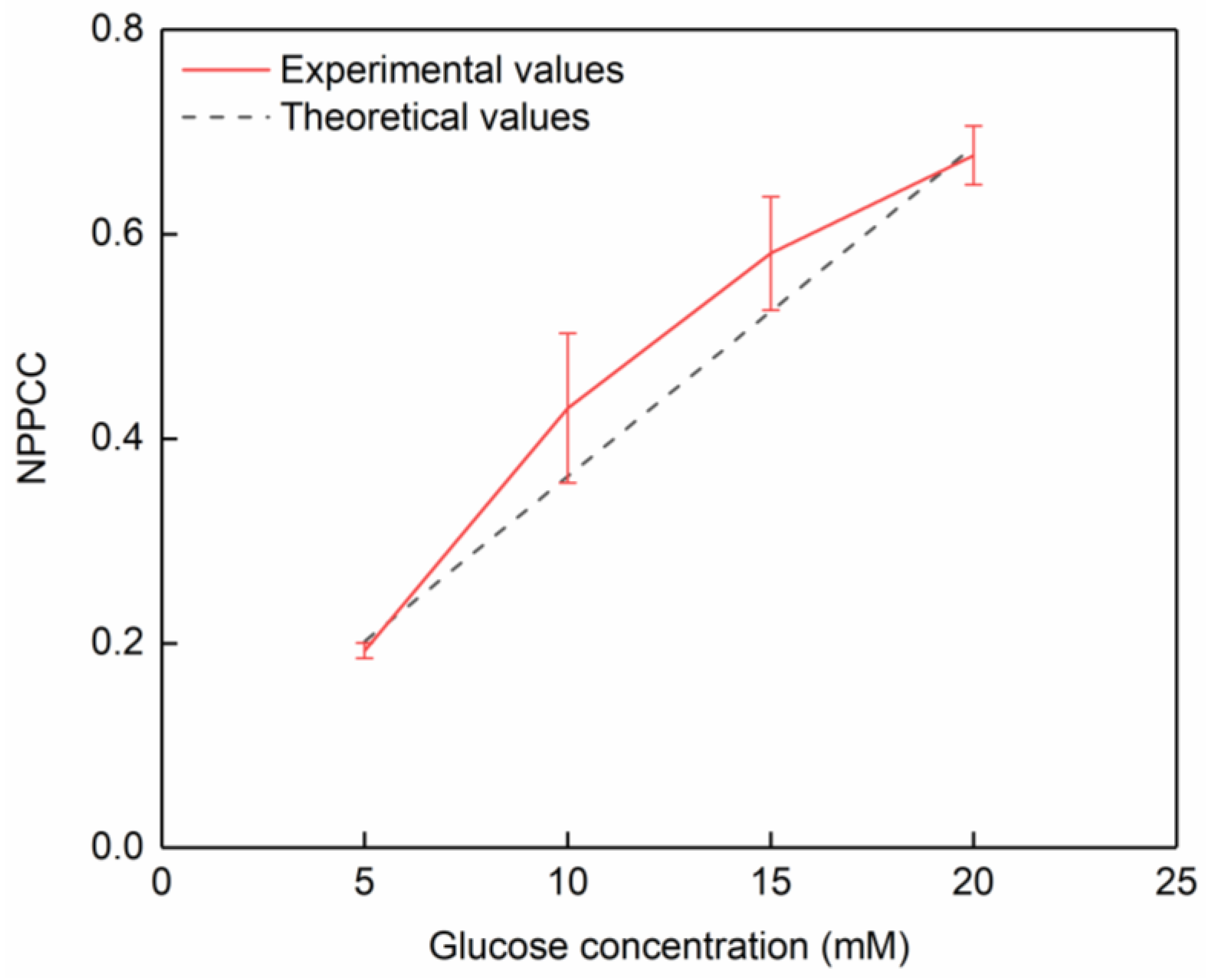
3.3. Cyclic Voltammetry and Applications
3.4. Repeatability and Reproducibility of the Sensor
4. Conclusions
- (1)
- Cu2(OH)PO4 was added to the resin, and the composite resin could be efficiently fabricated for the detection chips and detection kits by DLP.
- (2)
- The composite resin was activated by the Nd: YAG laser to form copper base points on the composite resin surface.
- (3)
- The continuous and conductive copper and silver coatings were prepared by electroless plating. The three-electrode chips were suitable for glucose detection according to the CV and NPPCC.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lunyera, J.; Diamantidis, C.J.; Bosworth, H.B.; Patel, U.D.; Bain, J.; Muehlbauer, M.J.; Ilkayeva, O.; Nguyen, M.; Sharma, B.; Ma, J.Z.; et al. Urine tricarboxylic acid cycle signatures of early-stage diabetic kidney disease. Metabolomics 2022, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Franiek, A.; Sharma, A.; Cockovski, V.; Wishart, D.S.; Zappitelli, M.; Blydt-Hansen, T.D. Urinary metabolomics to develop predictors for pediatric acute kidney injury. Pediatr. Nephrol. 2022, 37, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Khejonnit, V.; Pratumvinit, B.; Reesukumal, K.; Meepanya, S.; Pattanavin, C.; Wongkrajang, P. Optimal criteria for microscopic review of urinalysis following use of automated urine analyzer. Clin. Chim. Acta 2015, 439, 1–4. [Google Scholar] [CrossRef]
- Tantisaranon, P.; Dumkengkhachornwong, K.; Aiadsakun, P.; Hnoonual, A. A comparison of automated urine analyzers cobas 6500, UN 3000-111b and iRICELL 3000 with manual microscopic urinalysis. Pract. Lab. Med. 2021, 24, e203. [Google Scholar] [CrossRef]
- Cai, Y.; You, J.; You, Z.; Dong, F.; Du, S.; Zhang, L. Profuse color-evolution-based fluorescent test paper sensor for rapid and visual monitoring of endogenous Cu2+ in human urine. Biosens. Bioelectron. 2018, 99, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Chao, R.; Ren, Y.; Hu, C.; Xu, Z.; Liu, G.L. Smartphone based portable bacteria pre-concentrating microfluidic sensor and impedance sensing system. Sens. Actuators B Chem. 2014, 193, 653–659. [Google Scholar] [CrossRef]
- Tran, H.V.; Nguyen, T.V.; Nguyen, N.D.; Piro, B.; Huynh, C.D. A nanocomposite prepared from FeOOH and N-doped carbon nanosheets as a peroxidase mimic, and its application to enzymatic sensing of glucose in human urine. Microchim. Acta 2018, 185, 270. [Google Scholar] [CrossRef]
- Zhu, N.; Gu, L.; Wang, J.; Li, X.; Liang, G.; Zhou, J.; Zhang, Z. Novel and Sensitive Chemiluminescence Sensors Based on 2D-MOF Nanosheets for One-Step Detection of Glucose in Human Urine. J. Phys. Chem. C 2019, 123, 9388–9393. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Sezgintürk, M.K.; Dinçkaya, E. A biosensor based on urate oxidase–peroxidase coupled enzyme system for uric acid determination in urine. Talanta 2003, 61, 73–79. [Google Scholar] [CrossRef]
- Kanyong, P.; Pemberton, R.M.; Jackson, S.K.; Hart, J.P. Development of a sandwich format, amperometric screen-printed uric acid biosensor for urine analysis. Anal. Biochem. 2012, 428, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Malode, S.J.; Prabhu K., K.; Shetti, N.P.; Reddy, K.R. Highly sensitive electrochemical assay for selective detection of Aminotriazole based on TiO2/poly(CTAB) modified sensor. Environ. Technol. Innov. 2021, 21, 101222. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Nayak, D.S.; Reddy, K.R. Novel heterostructured Ru-doped TiO2/CNTs hybrids with enhanced electrochemical sensing performance for Cetirizine. Mater. Res. Express 2019, 6, 115085. [Google Scholar] [CrossRef]
- Vernekar, P.R.; Shetti, N.P.; Shanbhag, M.M.; Malode, S.J.; Malladi, R.S.; Reddy, K.R. Novel layered structured bentonite clay-based electrodes for electrochemical sensor applications. Microchem. J. 2020, 159, 105441. [Google Scholar] [CrossRef]
- Morais, A.L.; Rijo, P.; Batanero Hernán, M.B.; Nicolai, M. Biomolecules and Electrochemical Tools in Chronic Non-Communicable Disease Surveillance: A Systematic Review. Biosensors 2020, 10, 121. [Google Scholar] [CrossRef]
- Carneiro, P.; Morais, S.; Do, C.P.M. Biosensors on the road to early diagnostic and surveillance of Alzheimer’s disease. Talanta 2020, 211, 120700. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monitor. 2004, 6, 657–664. [Google Scholar] [CrossRef]
- Zare-Shehneh, N.; Mollarasouli, F.; Ghaedi, M. Recent Advances in Carbon Nanostructure-Based Electrochemical Biosensors for Environmental Monitoring. Crit. Rev. Anal. Chem. 2021, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Du, X. Electrochemical Biosensors for Detection of Foodborne Pathogens. Micromachines 2019, 10, 222. [Google Scholar] [CrossRef]
- Mishra, G.; Barfidokht, A.; Tehrani, F.; Mishra, R. Food Safety Analysis Using Electrochemical Biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef]
- Govindhan, M. Bionanomaterials-based electrochemical biosensing platform for biomedical application. Anal. Methods 2020, 12, 1688–1701. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Skládal, P. Electrochemical biosensors—Principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar] [CrossRef]
- Mach, K.E.; Wong, P.K.; Liao, J.C. Biosensor diagnosis of urinary tract infections: A path to better treatment? Trends Pharmacol. Sci. 2011, 32, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.V.; Marques, A.C.; Fortunato, E.; Sales, M.G.F. Paper-based (bio)sensor for label-free detection of 3-nitrotyrosine in human urine samples using molecular imprinted polymer. Sens. Bio-Sens. Res. 2020, 28, 100333. [Google Scholar] [CrossRef]
- Ianeselli, L.; Grenci, G.; Callegari, C.; Tormen, M.; Casalis, L. Development of stable and reproducible biosensors based on electrochemical impedance spectroscopy: Three-electrode versus two-electrode setup. Biosens. Bioelectron. 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Ramos-Sono, D.; Laureano, R.; Rueda, D.; Gilman, R.H.; La Rosa, A.; Ruiz, J.; León, R.; Sheen, P.; Zimic, M. An electrochemical biosensor for the detection of Mycobacterium tuberculosis DNA from sputum and urine samples. PLoS ONE 2020, 15, e241067. [Google Scholar] [CrossRef]
- Diouf, A.; Motia, S.; El Alami El Hassani, N.; El Bari, N.; Bouchikhi, B. Development and characterization of an electrochemical biosensor for creatinine detection in human urine based on functional molecularly imprinted polymer. J. Electroanal. Chem. 2017, 788, 44–53. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Y.; Guo, M.; Lin, B.; Zhang, L. A sensitive determination of albumin in urine by molecularly imprinted electrochemical biosensor based on dual-signal strategy. Sens. Actuators B Chem. 2019, 288, 564–570. [Google Scholar] [CrossRef]
- Dai, Y.; Somoza, R.A.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor. Angew. Chem. Int. Ed. 2019, 58, 17399–17405. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.D.; Conlan, R.W.; Godwin, M.C.; Moussy, F. Towards a Miniature Implantable In Vivo Telemetry Monitoring System Dynamically Configurable as a Potentiostat or Galvanostat for Two- and Three-Electrode Biosensors. IEEE Trans. Instrum. Meas. 2005, 54, 61–72. [Google Scholar] [CrossRef]
- Ding, L.; Du, D.; Zhang, X.; Ju, H. Trends in Cell-Based Electrochemical Biosensors. Curr. Med. Chem. 2008, 15, 3160–3170. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, H.; Vaithiyanathan, D.; Kumar, H. A Review on Additive Manufactured Sensors. MAPAN 2021, 36, 405–422. [Google Scholar] [CrossRef]
- Maddipatla, D.; Narakathu, B.B.; Atashbar, M. Recent Progress in Manufacturing Techniques of Printed and Flexible Sensors: A Review. Biosensors 2020, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Manzanares Palenzuela, C.L.; Pumera, M. (Bio)Analytical chemistry enabled by 3D printing: Sensors and biosensors. Trends Analyt. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Tan, H.W.; Tran, T.; Chua, C.K. A review of printed passive electronic components through fully additive manufacturing methods. Virtual Phys. Prototyp. 2016, 11, 271–288. [Google Scholar] [CrossRef]
- Sharon, Y.; Khachatryan, B.; Cheskis, D. Towards a low current Hall effect sensor. Sens. Actuator. A Phys. 2018, 279, 278–283. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, M.; Deng, F.; Yang, Y.; Peng, S.; Qi, F.; He, C.; Shen, L.; Liang, H. Forming quality, mechanical properties, and anti-inflammatory activity of additive manufactured Zn-Nd alloy. J. Zhejiang Univ.-Sci. A (Appl. Phys. Eng.) 2020, 21, 876–891. [Google Scholar] [CrossRef]
- Goh, G.L.; Zhang, H.; Chong, T.H.; Yeong, W.Y. 3D Printing of Multilayered and Multimaterial Electronics: A Review. Adv. Electron. Mater. 2021, 7, 2100445. [Google Scholar] [CrossRef]
- Saengchairat, N.; Tran, T.; Chua, C. A review: Additive manufacturing for active electronic components. Virtual Phys. Prototyp. 2017, 12, 31–46. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, P.; Wang, G.; Liu, Y.; Liu, Y.; Li, Q. Selectively Metalizable Stereolithography Resin for Three-Dimensional DC and High-Frequency Electronics via Hybrid Additive Manufacturing. ACS Appl. Mater. Interfaces 2021, 13, 22891–22901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, T.; Wen, L. Selective Metallization Induced by Laser Activation: Fabricating Metallized Patterns on Polymer via Metal Oxide Composite. ACS Appl. Mater. Interfaces 2017, 9, 8996–9005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, J.; Jia, L.; Zhang, H.; Zhang, G.; Sun, S.; Zhou, T. Laser-Induced Selective Metallization on Polymer Substrates Using Organocopper for Portable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 13714–13723. [Google Scholar] [CrossRef]
- Qiang-Qiang, S.; Ya-Nan, Y.; Jin-Jin, L.; Shu-Juan, B. In situ growth of metallic sliver on glucose oxidase for highly sensitive glucose sensor. Rsc Adv. 2015, 5, 34486–34490. [Google Scholar] [CrossRef]
- Liu, F.; Xie, D.; Jiao, C.; Bai, D.; Wu, H.; Shen, L.; Tian, Z.; Zhao, J. Selective metallization on additive manufactured polymer for fabrication of integrated device. J. Mater. Sci. 2022, 57, 1506–1515. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, T.; Wen, L.; Zhang, A. Fabricating Metallic Circuit Patterns on Polymer Substrates through Laser and Selective Metallization. ACS Appl. Mater. Interfaces 2016, 8, 33999–34007. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Ma, X.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M. Cu2(OH)PO4, a Near-Infrared-Activated Photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 4810–4813. [Google Scholar] [CrossRef]

| DLP Processing Parameters | Contents |
|---|---|
| Cured layer thickness | 80 μm |
| Exposure time | 12 s |
| Light intensity | 10,000 mW/cm2 |
| Laser Parameters | Contents |
|---|---|
| Wavelength | 1064 nm |
| Pulse width | 100 ns |
| Spot diameter | 35 μm |
| Hatch distance | 50 μm |
| Scanning speed | 2500 mm |
| Pulse repetition frequency | 20 kHz |
| Laser fluence | 10.4 J/cm2 |
| Electroless copper plating | CuSO4·5H2O | HCHO | HOCH2COOH | C6H15NO3 |
| C6H4SNCSH | C12H8N2·H2O | NaOH | ||
| Time | 30 min | Temperature | 50 °C | |
| Electroless silver plating | AgNO3 | KCN | C₄H₄Na₂O₆ | C₈H₄K₂O12Sb₂ |
| Time | 10 min | Temperature | 40 °C | |
| Elements | Ag | Cu | O | P |
|---|---|---|---|---|
| (wt.%) | 58.87 | 28.99 | 11.99 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, Q.; Chen, Y.; Jiao, C.; Liu, F.; Xu, J.; Zhang, Q.; Zhao, J.; Shen, L.; Wang, C. Biosensors Fabricated by Laser-Induced Metallization on DLP Composite Resin. Electronics 2022, 11, 3254. https://doi.org/10.3390/electronics11193254
Zhang R, Wang Q, Chen Y, Jiao C, Liu F, Xu J, Zhang Q, Zhao J, Shen L, Wang C. Biosensors Fabricated by Laser-Induced Metallization on DLP Composite Resin. Electronics. 2022; 11(19):3254. https://doi.org/10.3390/electronics11193254
Chicago/Turabian StyleZhang, Ran, Qinyi Wang, Ya Chen, Chen Jiao, Fuxi Liu, Junwei Xu, Qiuwei Zhang, Jiantao Zhao, Lida Shen, and Changjiang Wang. 2022. "Biosensors Fabricated by Laser-Induced Metallization on DLP Composite Resin" Electronics 11, no. 19: 3254. https://doi.org/10.3390/electronics11193254
APA StyleZhang, R., Wang, Q., Chen, Y., Jiao, C., Liu, F., Xu, J., Zhang, Q., Zhao, J., Shen, L., & Wang, C. (2022). Biosensors Fabricated by Laser-Induced Metallization on DLP Composite Resin. Electronics, 11(19), 3254. https://doi.org/10.3390/electronics11193254






