Evaluation of Signal Quality from a Wearable Phonocardiogram (PCG) Device and Personalized Calibration
Abstract
:1. Introduction
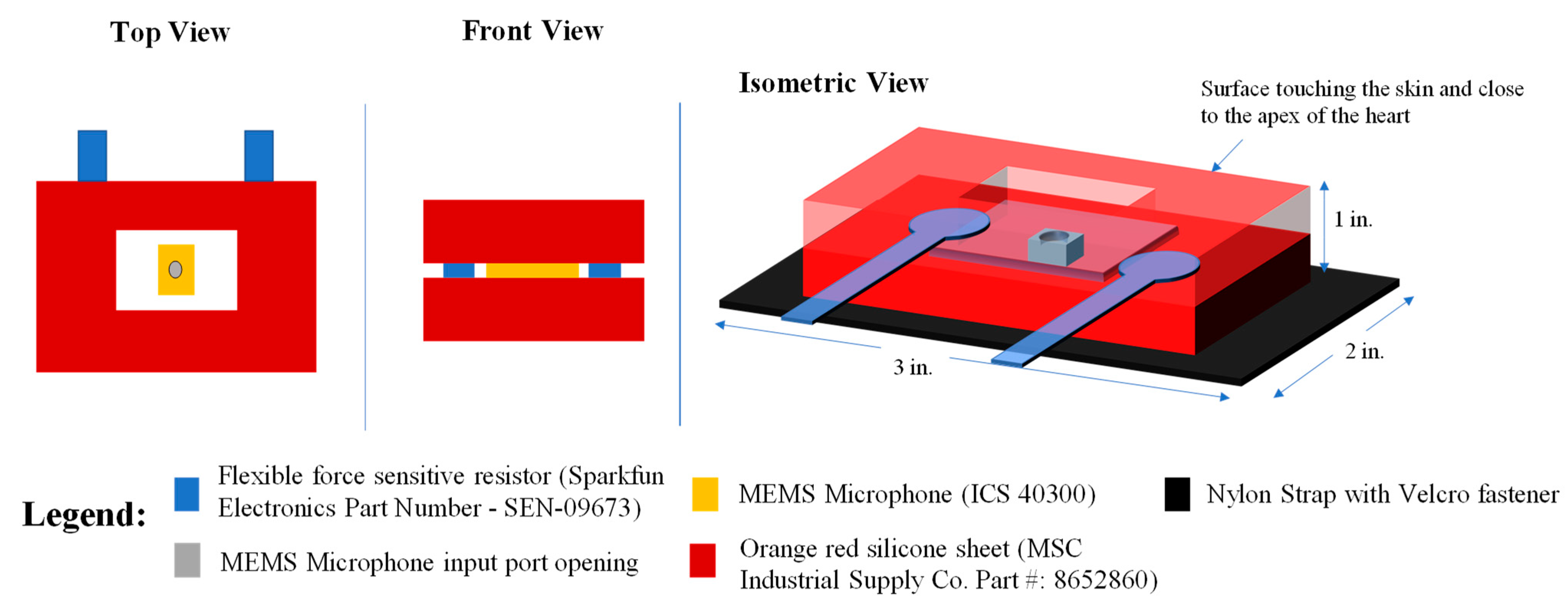
- Belts or Bands that are Adjustable and Incorporate the Sensing Device
- Discrete Hand-Held Devices that can be Placed and Held in Place by a Trained Medical Professional to Ensure Appropriate Positioning of the Heart Sound Sensor
- Sensing Elements that are Incorporated into a Garment
- Adhesives Used to Apply Sensors at Specific Anatomic Locations
- Obtain PCG recordings using a wearable belt-mounted form factor sensor during daily activities to explore noise types introduced by movement and other noise sources.
- Explore how signal characteristics change from subject to subject and depending on how subjects wear the device. The findings are specific to devices that use a belt form factor to apply a heart sound recording system.
2. Materials and Methods
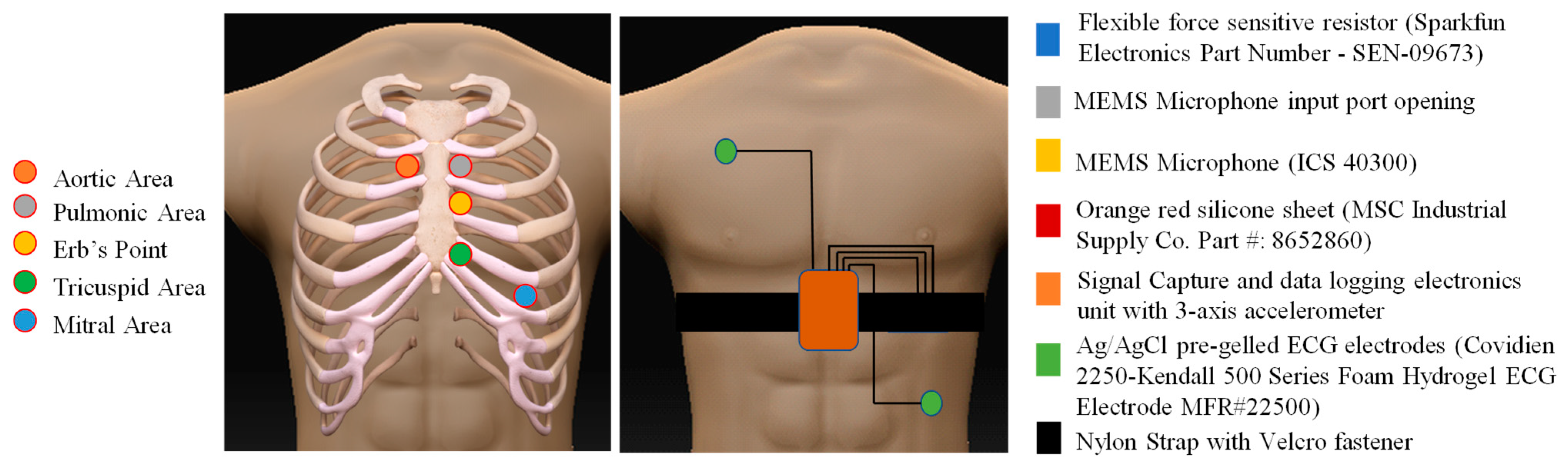
2.1. Device Design and Description
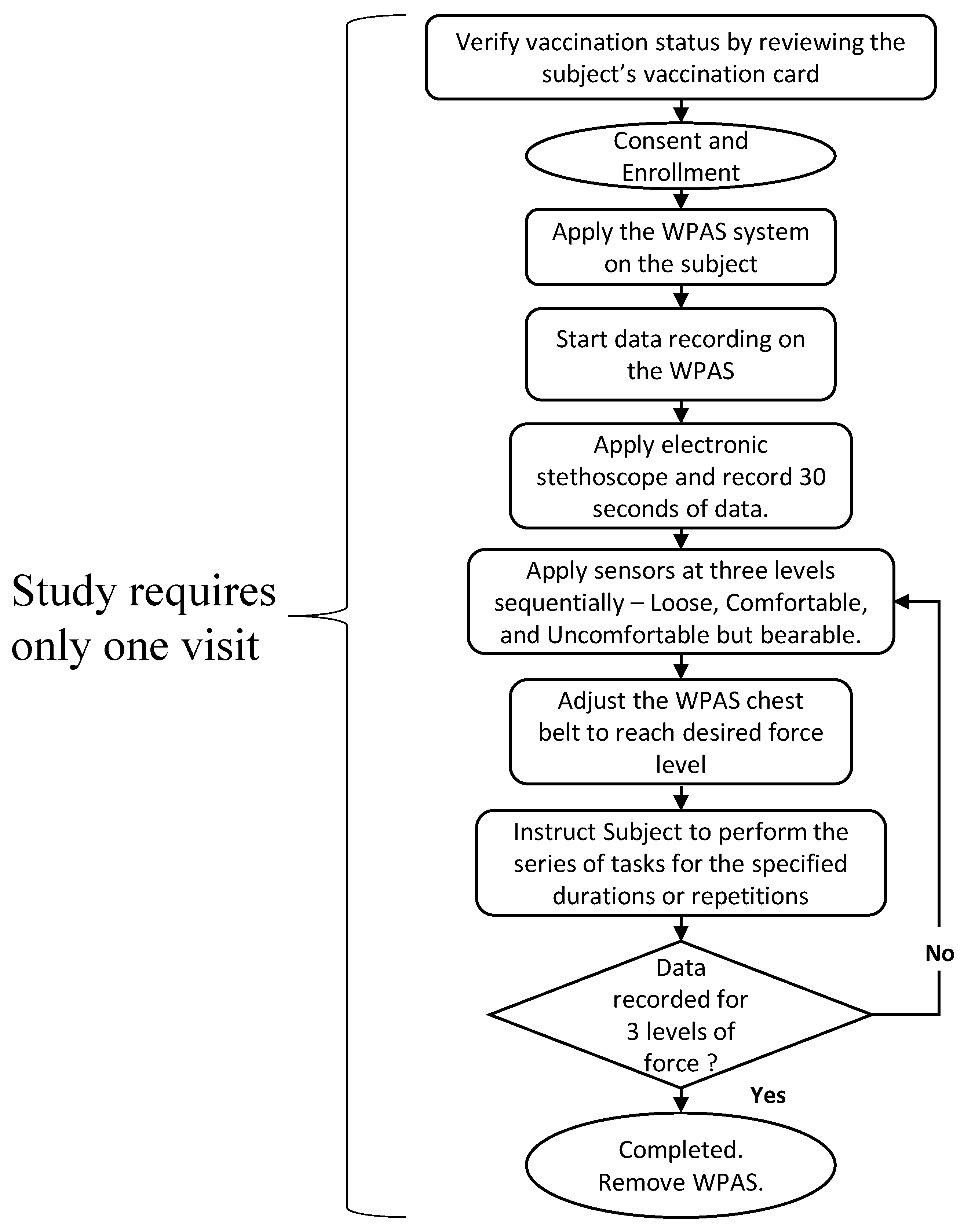
2.2. Protocol for Data Acquisition
- “Loose” when the WPAS sensing element was just touching the skin during Stand Still 1.
- “Comfortable” when the WPAS sensing element was in flush contact with the skin, with the WPAS belt feeling similar to a typical undergarment.
- “Uncomfortable” when the WPAS belt was secured as tightly as possible within tolerable limits, with the subject feeling no discomfort in the form of pain or difficulty performing the activities in Table 2.
2.3. Data Analysis
- Evaluation of the WPAS system for recording PCGs:
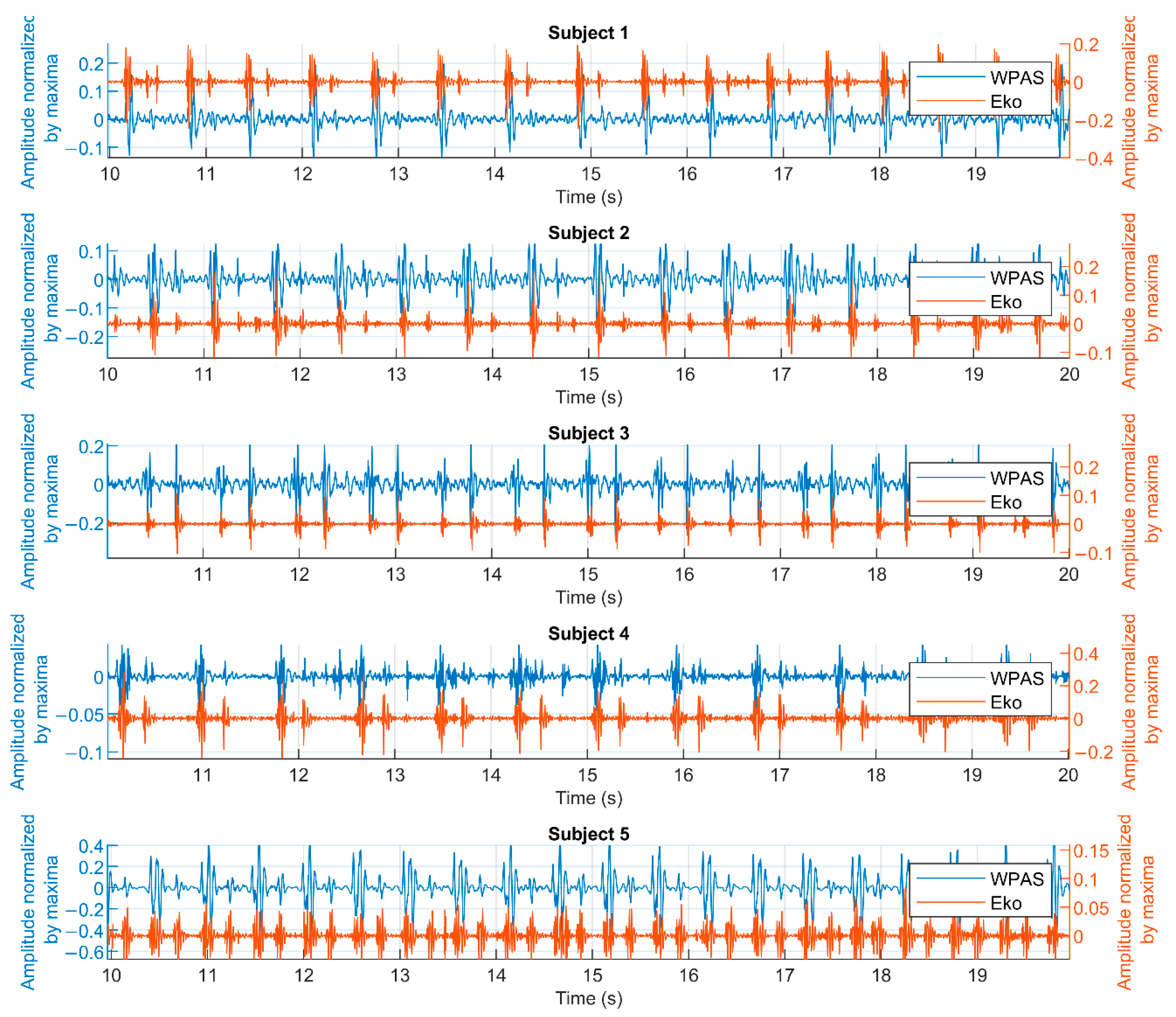
- Comparison of WPAS to a commercial electronic stethoscope to validate that WPAS captures heart sounds.
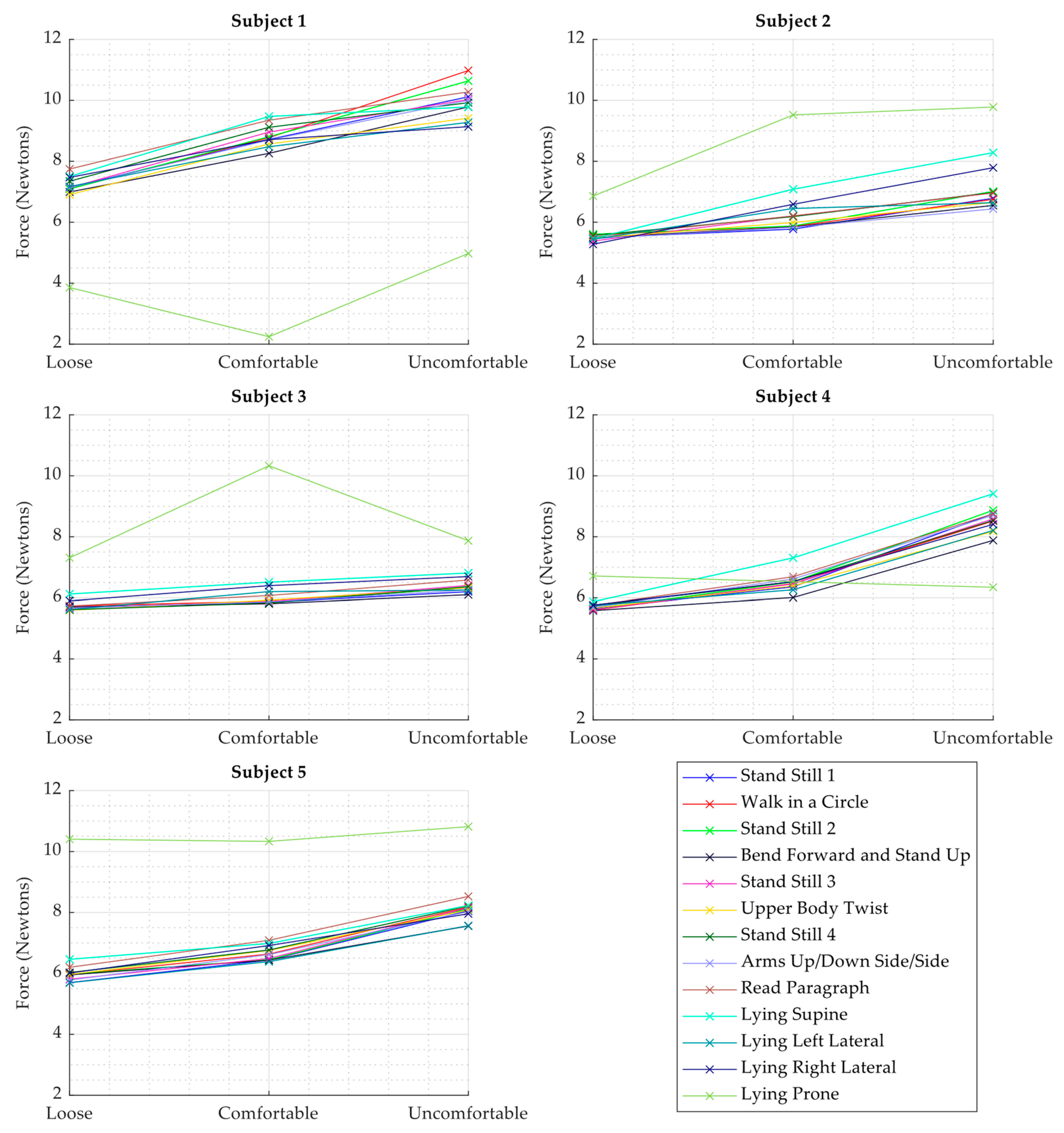
- Assessment of the range of force values observed through the execution of the protocol on each subject.
- Exploration of the relationship between PCG quality and force and activity measured by WPAS:
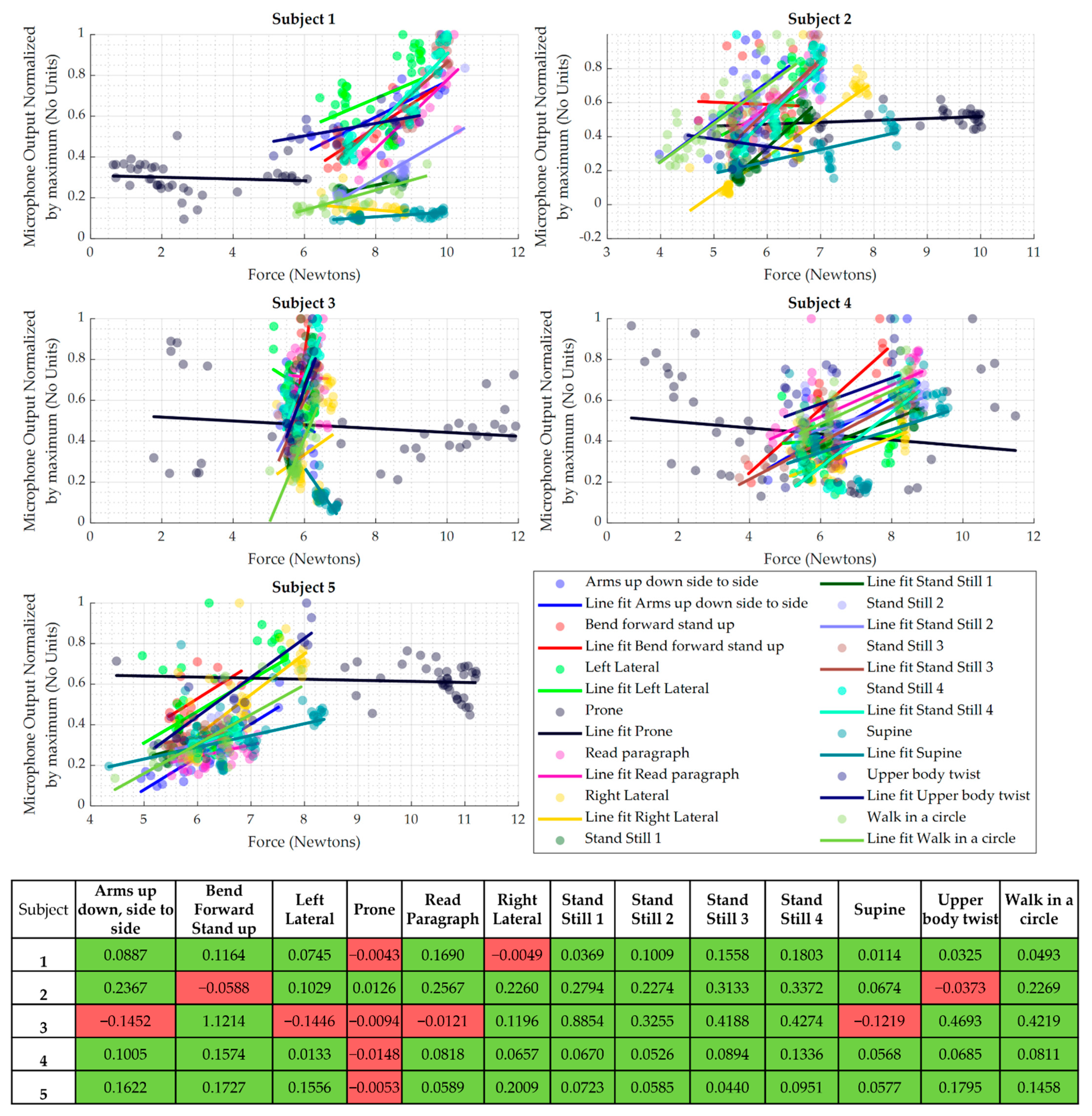
- Analysis of the relationship between loudness of the measured heart sounds and the force values observed by the WPAS sensing element.
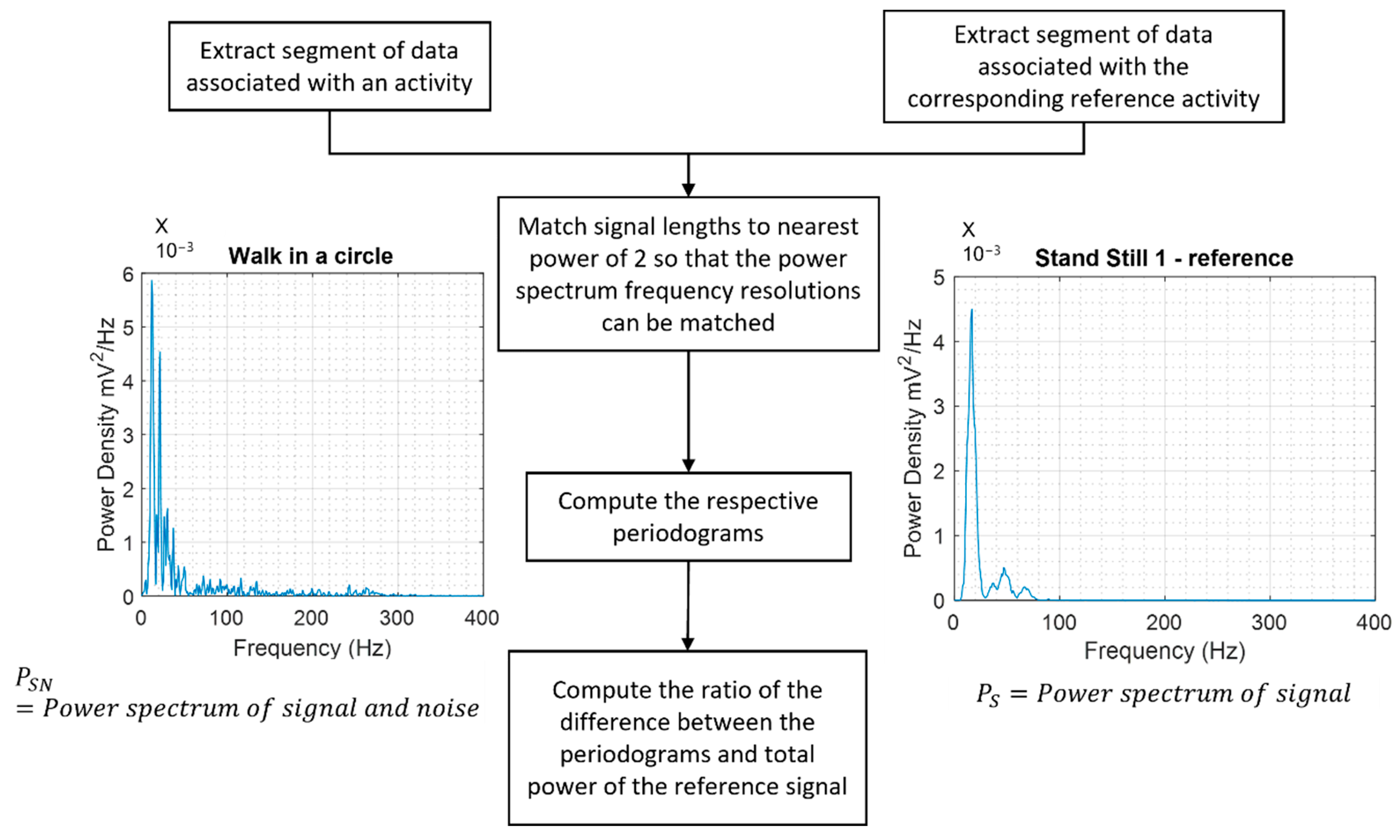
- Analysis of the noise levels in the measured heart sounds during movement compared to the corresponding reference measurements when standing still.
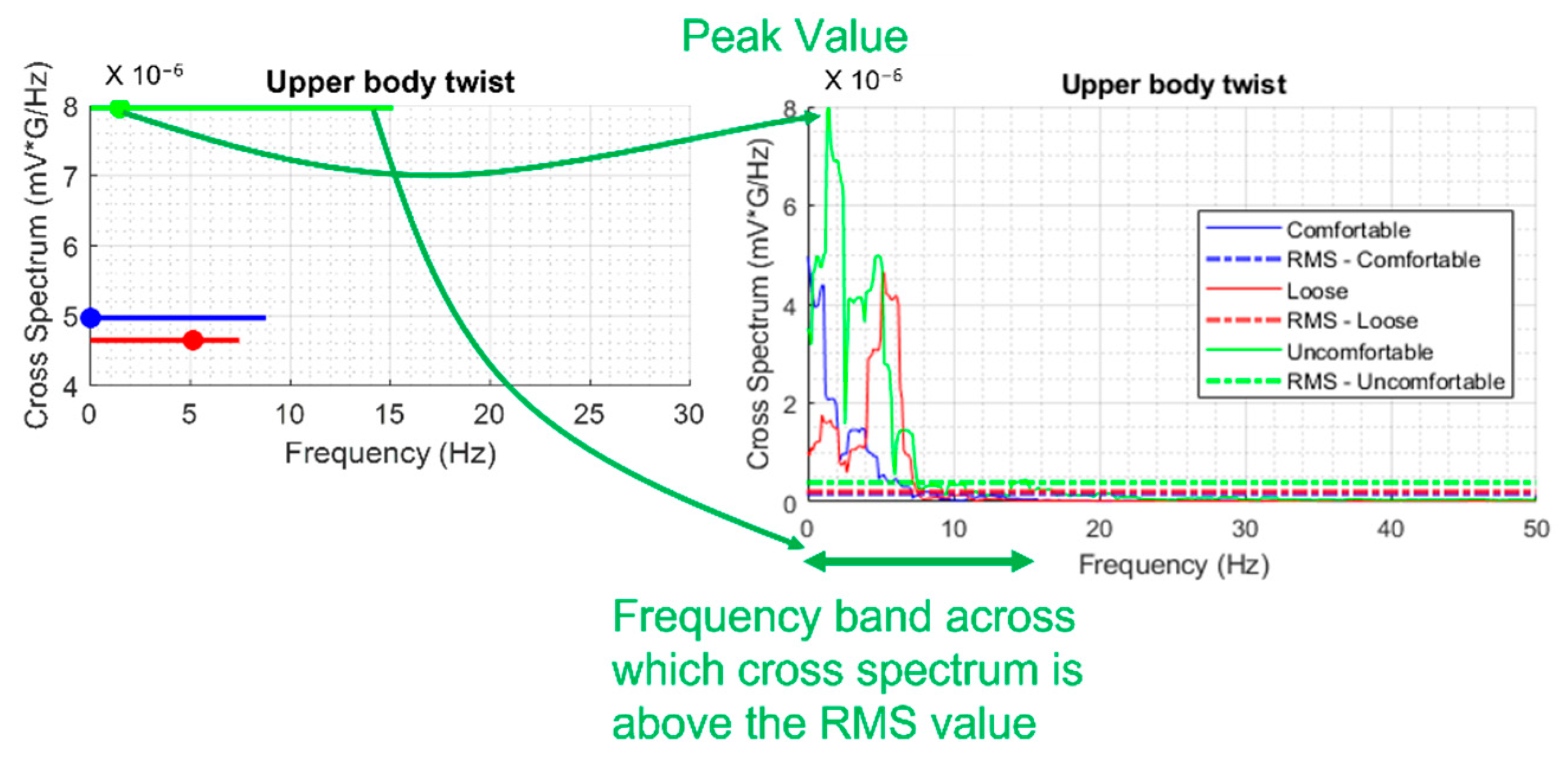
- Analysis of cross-spectral coherence between activity and heart sound for activities involving movements.
- Evaluation of the WPAS system for recording PCGs:
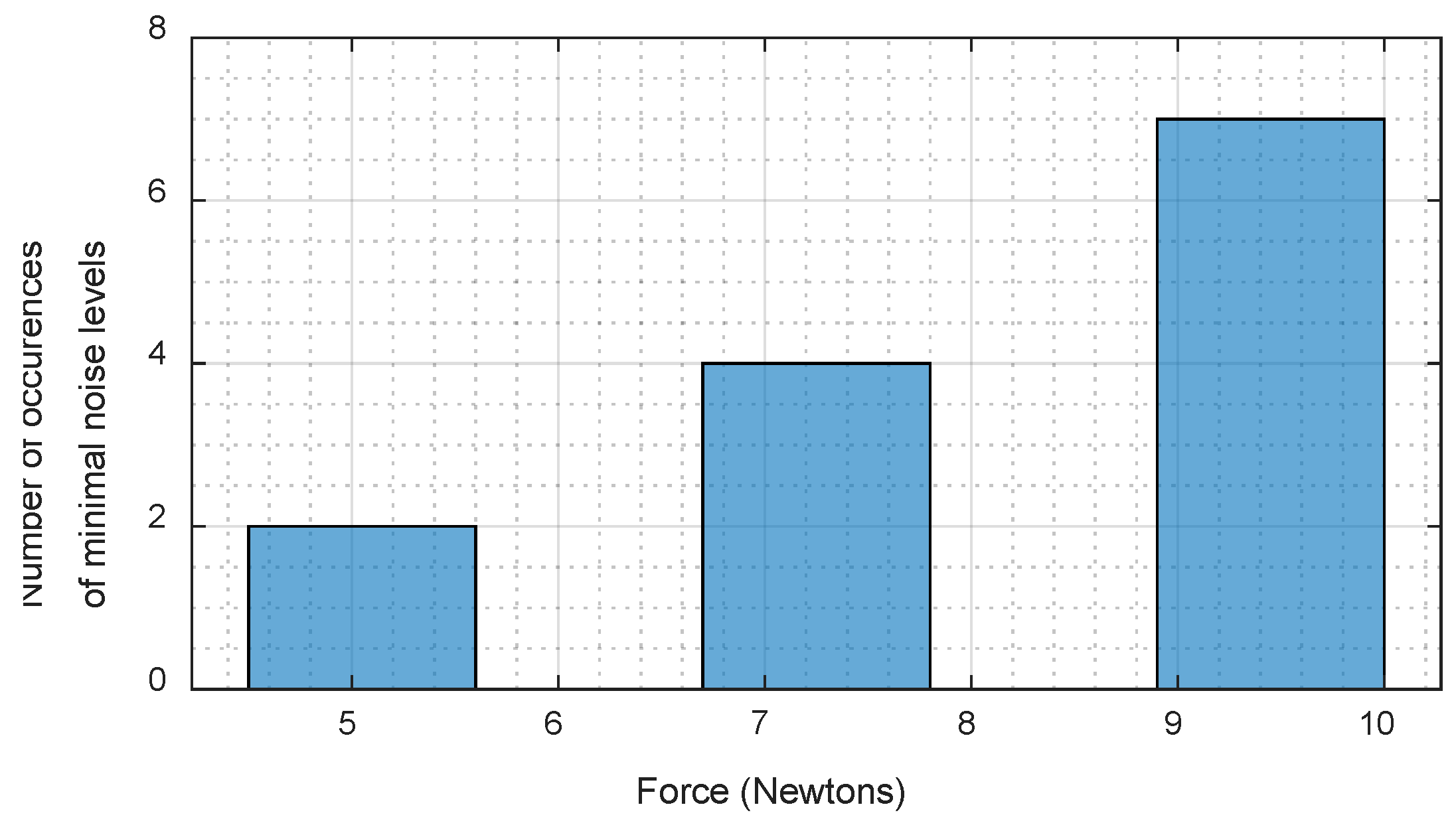
- Search for an individualized optimal force reading to minimize noise levels for each subject.
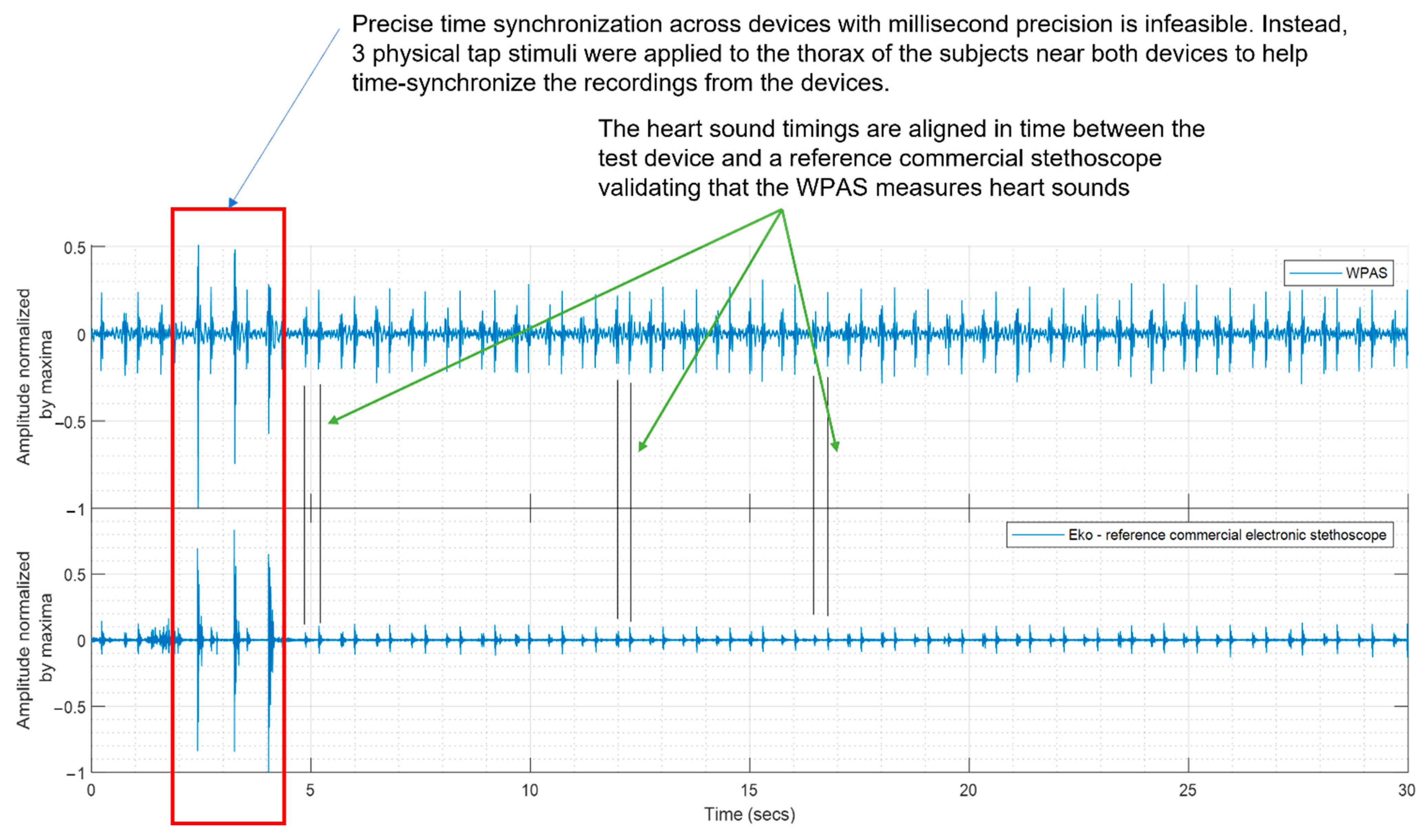
2.3.1. Comparison of WPAS with a Reference Commercial Electronic Stethoscope
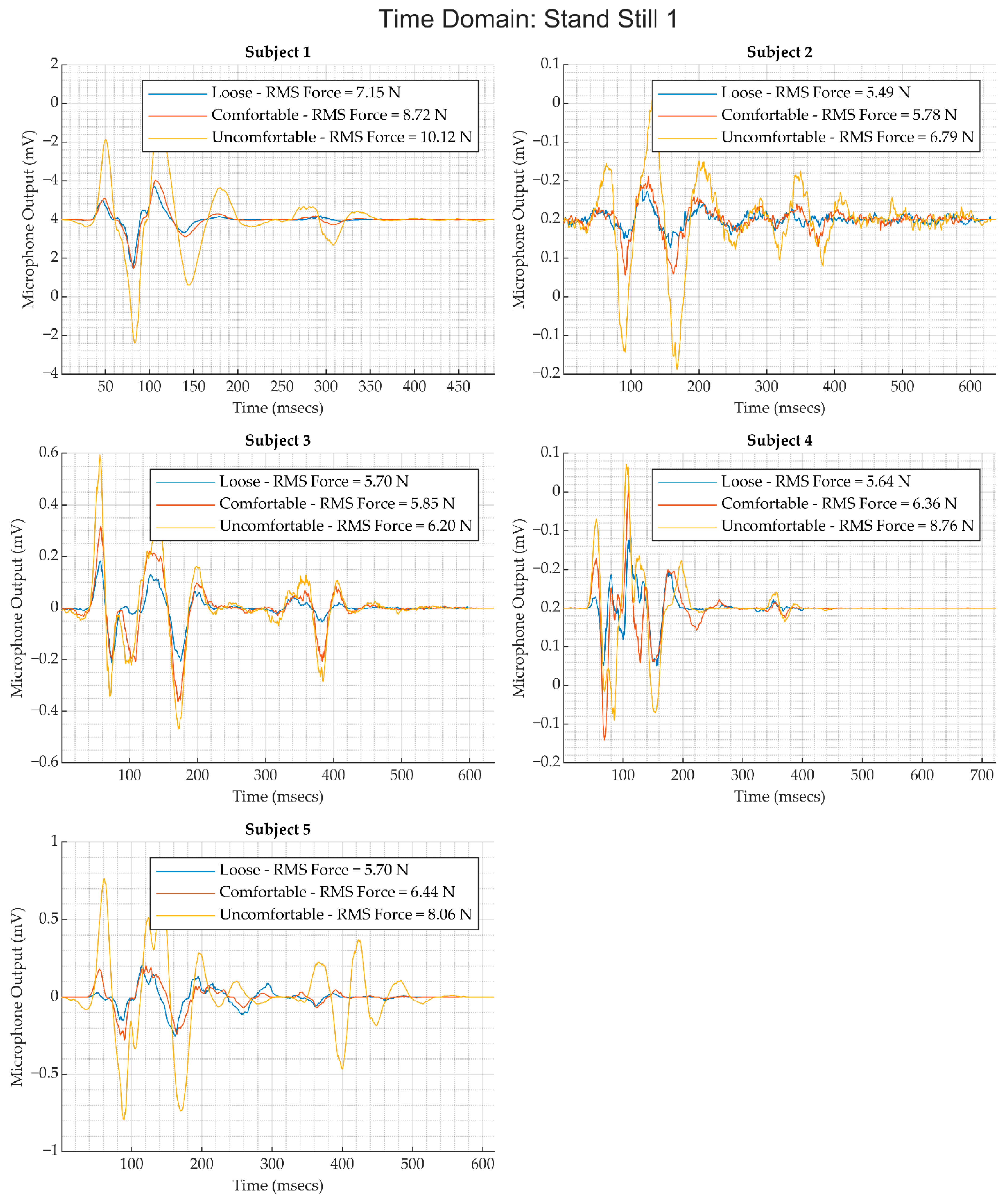
2.3.2. Time-Domain and Frequency Domain Analysis
- For each subject, the heart sound segments associated with each activity were extracted and associated with that subject and activity.
- The heart sound signals were filtered using a second-order Butterworth filter with a passband between 10 and 900 Hz.
- Wavelet denoising has been proposed for removing noise from heart sounds by several researchers [18,19,20]. In this analysis, heart sounds were decomposed to three levels using the ‘coif5′ wavelet; a soft threshold was applied using an adaptive threshold value (Equation (1)) as described by Jain et al. [19]:
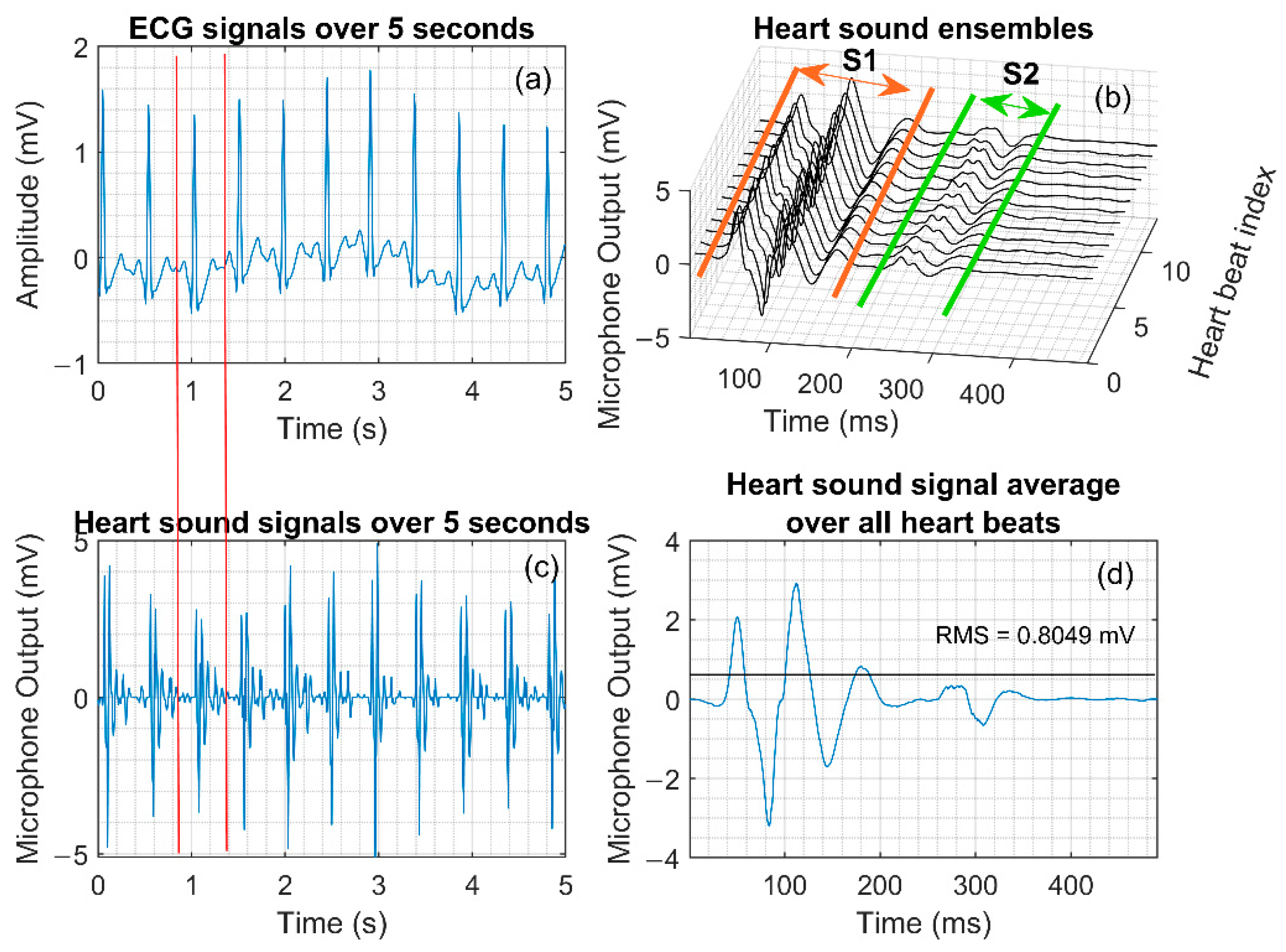
- To calculate of the signal average or ensemble average, we applied the Pan and Tompkins R-peak detection algorithm [21] on the simultaneously acquired ECG data to find the timestamps of R peak occurrence in the ECG. These peaks serve as fiducial points for each heartbeat. The RR intervals associated with each heartbeat were calculated by computing the first-order difference of the timestamp sequence associated with R peaks in the ECG waveform. A delay offset of was applied to the RR interval sequence to obtain a beat-to-beat interval from the p-wave to the subsequent p-wave. The corresponding timestamps of the heart sound signal were then extracted and an ensemble was generated, as shown in Figure 5b. The ensemble median was then used as the representative heart sound signal associated with an activity for a subject.
- For each activity and subject, the corresponding data segments were segmented using the timestamps captured during the execution of the protocol and manual tuning as needed to find the timestamp for the end of an activity. Ensembles were extracted from these segments for each subject and activity over segments 5 s long, and the corresponding RMS value of the heart sound and the force values were calculated.
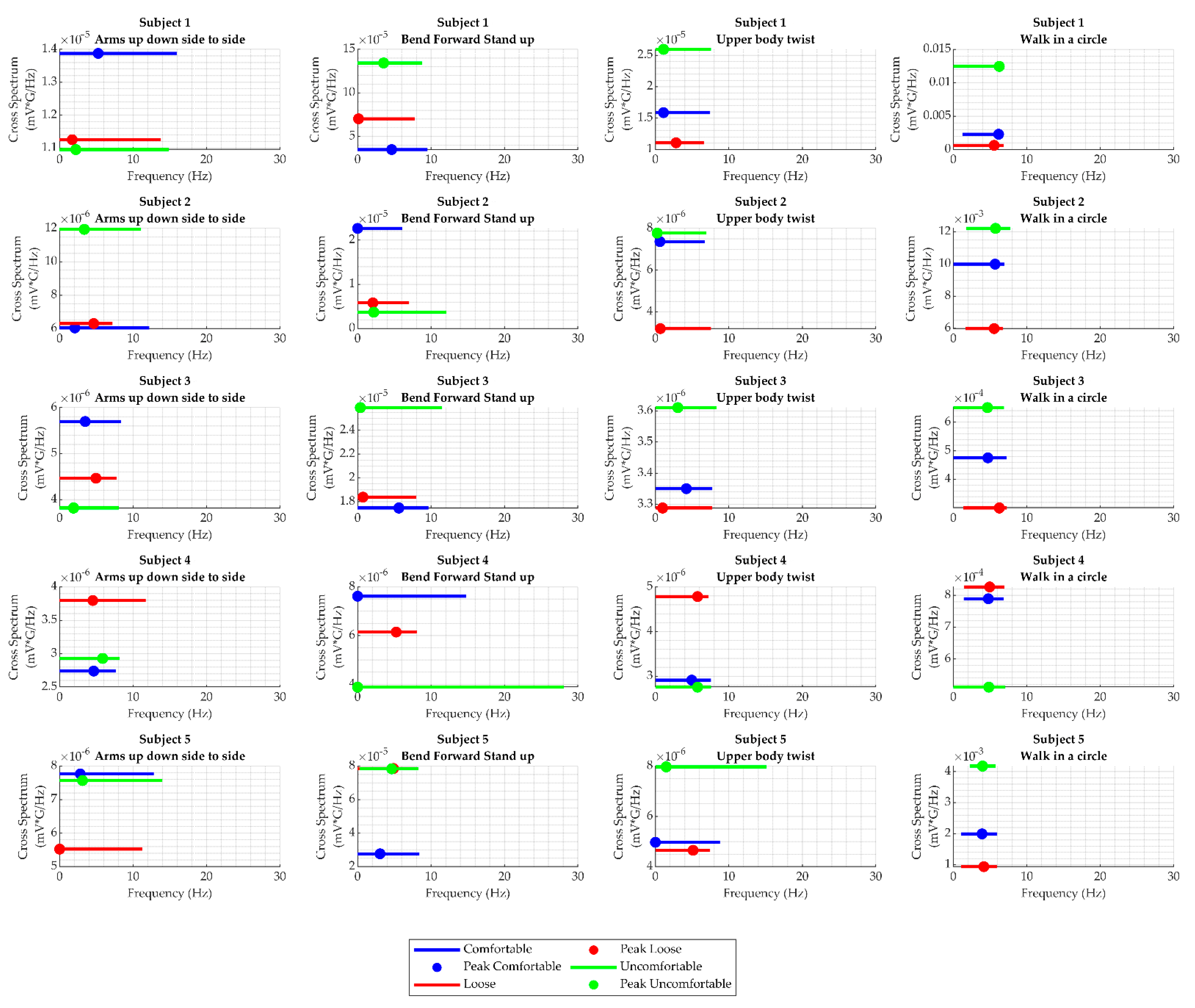
2.3.3. Cross-Spectral Coherence Analysis
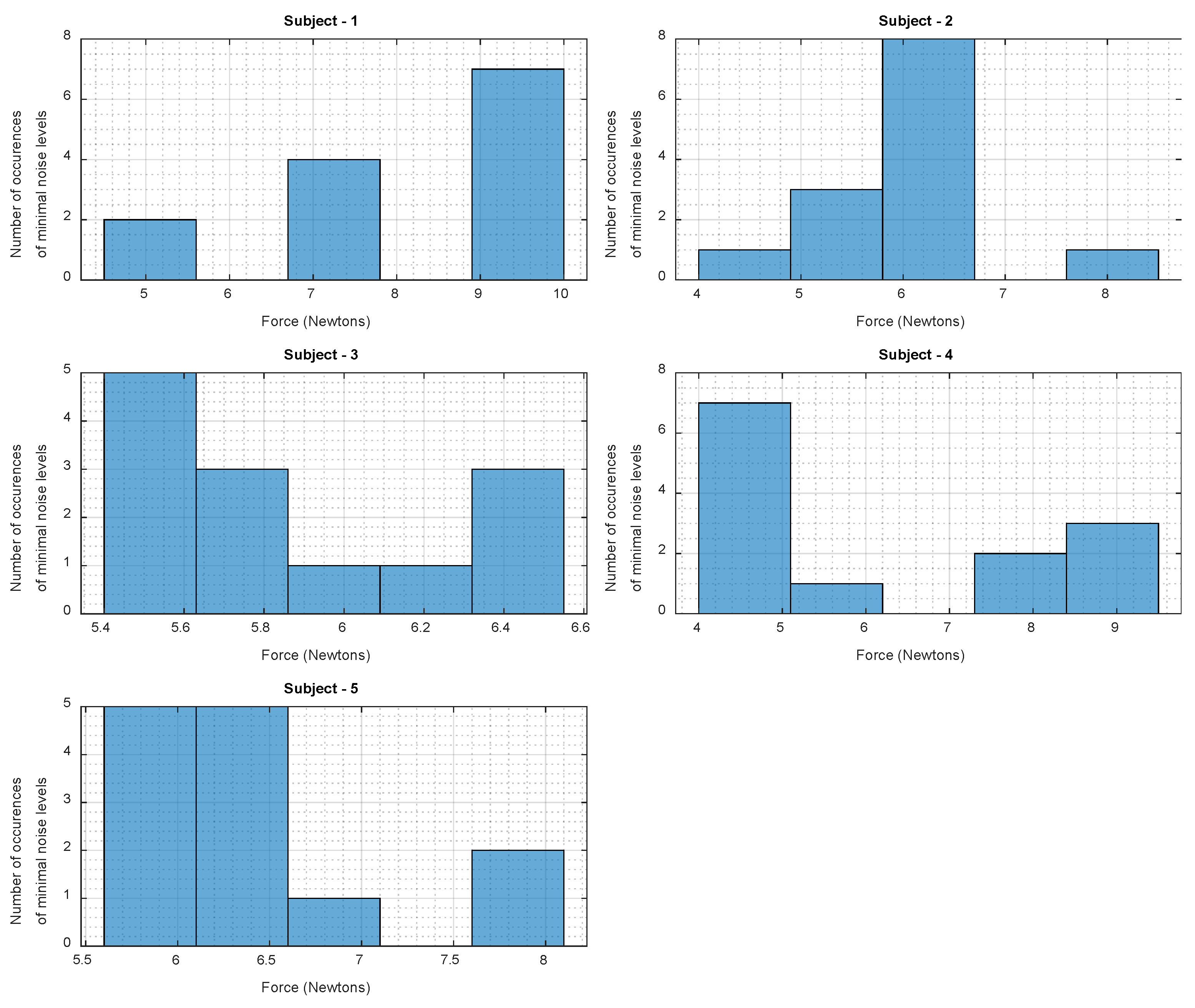
2.3.4. Search for Optimal Force for Each Subject
3. Results
3.1. Comparison of WPAS and Reference Electronic Stethoscope
3.2. Relationship between WPAS Force RMS and Heart Sound Loudness
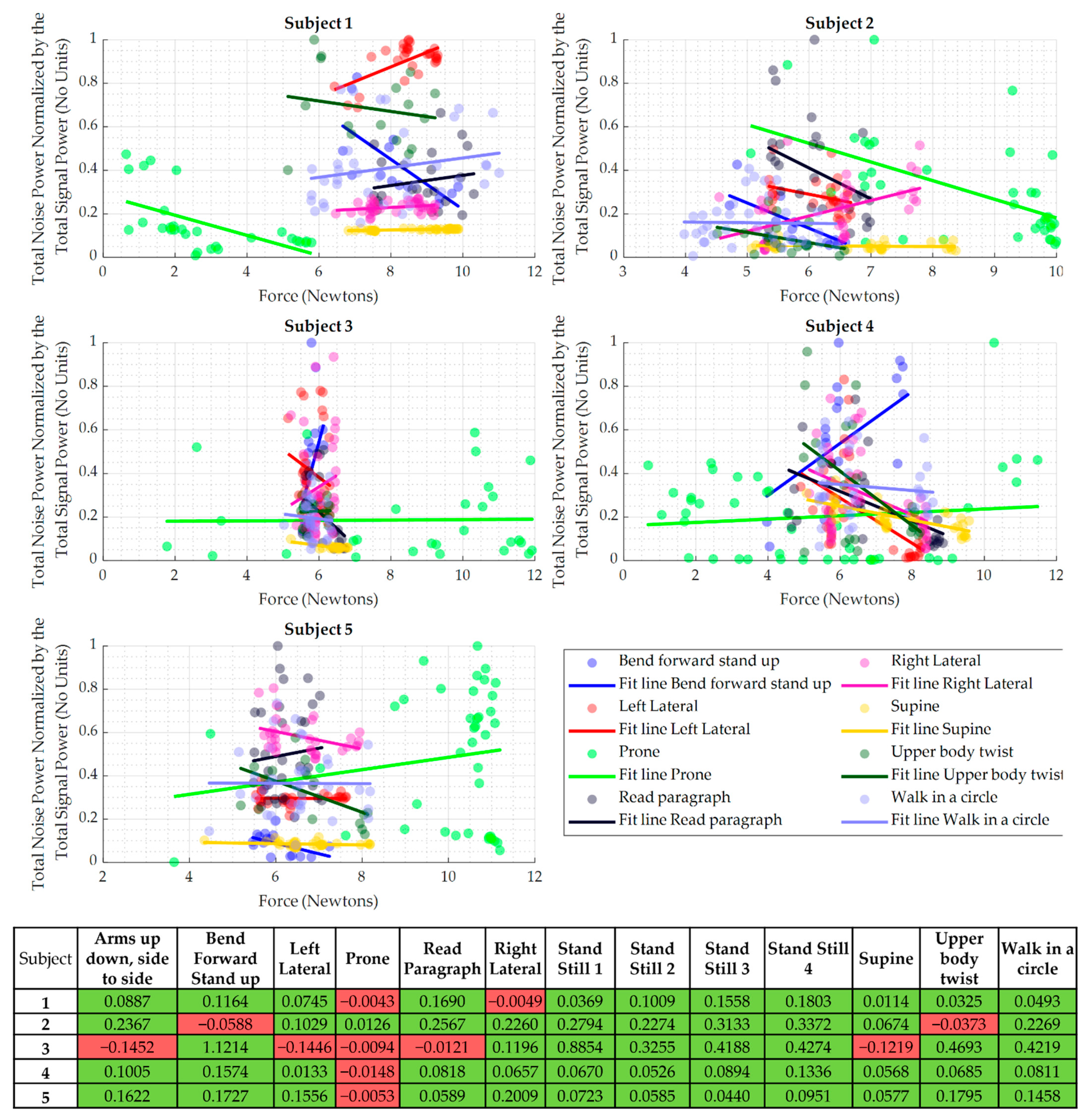
3.3. Relationship between WPAS Force RMS and Noise Levels
3.4. Cross-Spectral Coherence between WPAS Activity and Heart Sounds
3.5. Search for Optimal WPAS Force for Each Subject
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klum, M.; Leib, F.; Oberschelp, C.; Martens, D.; Pielmus, A.G.; Tigges, T.; Penzel, T.; Orglmeister, R. Wearable Multimodal Stethoscope Patch for Wireless Biosignal Acquisition and Long-Term Auscultation. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5781–5785. [Google Scholar]
- Klum, M.; Urban, M.; Tigges, T.; Pielmus, A.-G.; Feldheiser, A.; Schmitt, T.; Orglmeister, R. Wearable Cardiorespiratory Monitoring Employing a Multimodal Digital Patch Stethoscope: Estimation of ECG, PEP, LVET and Respiration Using a 55 mm Single-Lead ECG and Phonocardiogram. Sensors 2020, 20, 2033. [Google Scholar] [CrossRef] [PubMed]
- Behere, S.; Baffa, J.M.; Penfil, S.; Slamon, N. Real-World Evaluation of the Eko Electronic Teleauscultation System. Pediatr. Cardiol. 2019, 40, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sapsanis, C.; Welsh, N.; Pozin, M.; Garreau, G.; Tognetti, G.; Bakhshaee, H.; Pouliquen, P.O.; Mitral, R.; Thompson, W.R.; Andreou, A.G. StethoVest: A simultaneous multichannel wearable system for cardiac acoustic mapping. In Proceedings of the 2018 IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018; pp. 1–4. [Google Scholar]
- Hwang, I.; Kim, H.N.; Seong, M.; Lee, S.-H.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional Smart Skin Adhesive Patches for Advanced Health Care. Adv. Healthc. Mater. 2018, 7, 1800275. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Y. An ultra-sensitive wearable accelerometer for continuous heart and lung sound monitoring. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 694–697. [Google Scholar]
- Shi, W.Y.; Mays, J.; Chiao, J. Wireless stethoscope for recording heart and lung sound. In Proceedings of the 2016 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems (BioWireleSS), Austin, TX, USA, 24–27 January 2016; pp. 1–4. [Google Scholar]
- Chen, Z.; Chen, D.; Xue, L.; Chen, L. A Piezoelectric Heart Sound Sensor for Wearable Healthcare Monitoring Devices. In EAI International Conference on Body Area Networks; Springer: Cham, Switzerland, 2019; pp. 12–23. [Google Scholar]
- Marzorati, D.; Bovio, D.; Salito, C.; Mainardi, L.; Cerveri, P. Chest Wearable Apparatus for Cuffless Continuous Blood Pressure Measurements Based on PPG and PCG Signals. IEEE Access 2020, 8, 55424–55437. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, H.; Chen, Y.; Luo, Q.; Xu, K. All-Silicon Microdisplay Using Efficient Hot-Carrier Electroluminescence in Standard 0.18μm CMOS Technology. IEEE Electron. Device Lett. 2021, 42, 541–544. [Google Scholar] [CrossRef]
- Xu, K. Silicon electro-optic micro-modulator fabricated in standard CMOS technology as components for all silicon monolithic integrated optoelectronic systems. J. Micromech. Microeng. 2021, 31, 054001. [Google Scholar] [CrossRef]
- Rosa, B.G.; Anastasova, S.; Lo, B. Small-form wearable device for long-term monitoring of cardiac sounds on the body surface. In Proceedings of the 2021 IEEE 17th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Athens, Greece, 27–30 July 2021; pp. 1–4. [Google Scholar]
- Wang, T.; Gong, M.; Yu, X.; Lan, G.; Shi, Y. Acoustic-pressure sensor array system for cardiac-sound acquisition. Biomed. Signal Process. Control 2021, 69, 102836. [Google Scholar] [CrossRef]
- Gupta, P.; Moghimi, M.J.; Jeong, Y.; Gupta, D.; Inan, O.T.; Ayazi, F. Precision wearable accelerometer contact microphones for longitudinal monitoring of mechano-acoustic cardiopulmonary signals. NPJ Digit. Med. 2020, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Azaria, M.; Hertz, D. Time delay estimation by generalized cross correlation methods. IEEE Trans. Acoust. Speech Signal Process. 1984, 32, 280–285. [Google Scholar] [CrossRef]
- Gao, J.; Gurbaxani, B.M.; Hu, J.; Heilman, K.J.; Emanuele Ii, V.A.; Lewis, G.F.; Davila, M.; Unger, E.R.; Lin, J.-M.S. Multiscale analysis of heart rate variability in non-stationary environments. Front. Physiol. 2013, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Messer, S.R.; Agzarian, J.; Abbott, D. Optimal wavelet denoising for phonocardiograms. Microelectron. J. 2001, 32, 931–941. [Google Scholar] [CrossRef]
- Jain, P.K.; Tiwari, A.K. An adaptive thresholding method for the wavelet based denoising of phonocardiogram signal. Biomed. Signal Process. Control 2017, 38, 388–399. [Google Scholar] [CrossRef]
- Gradolewski, D.; Redlarski, G. Wavelet-based denoising method for real phonocardiography signal recorded by mobile devices in noisy environment. Comput. Biol. Med. 2014, 52, 119–129. [Google Scholar] [CrossRef]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, A.V.; Schafer, R.W.; Buck, J.R. Discrete-Time Signal Processing, 2nd ed.; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Kay, S.M. Modern Spectral Estimation: Theory and Application; Prentice-Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Baselli, G.; Cerutti, S.; Civardi, S.; Liberati, D.; Lombardi, F.; Malliani, A.; Pagani, M. Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Comput. Biomed. Res. 1986, 19, 520–534. [Google Scholar] [CrossRef]
- Thomas, R.J.; Wood, C.; Bianchi, M.T. Cardiopulmonary coupling spectrogram as an ambulatory clinical biomarker of sleep stability and quality in health, sleep apnea, and insomnia. Sleep 2018, 41, zsx196. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Austin, C.; Legner, C.M.; Pandey, S. Trends in Workplace Wearable Technologies and Connected-Worker Solutions for Next-Generation Occupational Safety, Health, and Productivity. Adv. Intell. Syst. 2022, 4, 2100099. [Google Scholar] [CrossRef]
| Reference | Wearable Device Characteristics | Data Reported for Movement of Activity |
|---|---|---|
| Hu et al., 2012 [6] |
| No protocol described for wearable use scenario; only one snapshot of recording provided |
| Shi W. Y. et al., 2016 [7] |
| Recordings performed in static posture; no protocol described for wearable use scenario |
| Chen Z et al., 2019 [8] |
| No protocol described for wearable use scenario; only one snapshot of recording provided. |
| Klum M et al., 2019 [1] & Klum M et al., 2020 [2] |
| Data reported for supine, lateral, and prone position; no data reported for activity. |
| Marzorati et al., 2020 [9] |
| Recordings performed on subjects in a seated posture; testing intent was to produce a device for blood pressure estimation. |
| This Work |
| Protocol involved movements that emulate daily activities |
| Activity Number | Activity | Duration of Activity (minutes)/Number of Repetitions |
|---|---|---|
| 1 | Stand Still 1 | 1 |
| 2 | Walk around in a circle | 1 |
| 3 | Stand Still 2 | 1 |
| 4 | Bend forward and stand up straight | 10 repetitions |
| 5 | Stand still 3 | 1 |
| 6 | Upper body twist (left to right) | 10 repetitions |
| 7 | Stand Still 4 | 1 |
| 8 | Arms Up/down and Arms Side/side | 10 repetitions |
| 9 | Read Paragraph from Penn State Wikipedia page | One paragraph at conversational loudness. |
| 10 | Supine | 1 |
| 11 | Left Lateral | 1 |
| 12 | Right Lateral | 1 |
| 13 | Prone | 1 |
| Activity | Reference for Activity |
|---|---|
| Walk in a circle | Stand Still 1 |
| Bend forward and stand up | Stand Still 2 |
| Upper Body Twist | Stand Still 3 |
| Arms up/down, and side to side | Stand Still 4 |
| Read Paragraph | Stand Still 4 |
| Lying Supine | Stand Still 4 |
| Lying Left Lateral | Stand Still 4 |
| Lying Right Lateral | Stand Still 4 |
| Lying Prone | Stand Still 4 |
| Subject | Full Range of Force RMS (N) | Optimal Range of Force RMS (N) |
|---|---|---|
| 1 | [5.14, 11.08] | [8.9, 10] |
| 2 | [3.98, 8.37] | [5.8, 6.7] |
| 3 | [5.03, 6.80] | [5.4, 5.63] |
| 4 | [3.97, 9.58] | [4, 5.1] |
| 5 | [4.34, 8.18] | [5.6, 6.6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyam Kumar, P.; Ramasamy, M.; Varadan, V.K. Evaluation of Signal Quality from a Wearable Phonocardiogram (PCG) Device and Personalized Calibration. Electronics 2022, 11, 2655. https://doi.org/10.3390/electronics11172655
Shyam Kumar P, Ramasamy M, Varadan VK. Evaluation of Signal Quality from a Wearable Phonocardiogram (PCG) Device and Personalized Calibration. Electronics. 2022; 11(17):2655. https://doi.org/10.3390/electronics11172655
Chicago/Turabian StyleShyam Kumar, Prashanth, Mouli Ramasamy, and Vijay K. Varadan. 2022. "Evaluation of Signal Quality from a Wearable Phonocardiogram (PCG) Device and Personalized Calibration" Electronics 11, no. 17: 2655. https://doi.org/10.3390/electronics11172655
APA StyleShyam Kumar, P., Ramasamy, M., & Varadan, V. K. (2022). Evaluation of Signal Quality from a Wearable Phonocardiogram (PCG) Device and Personalized Calibration. Electronics, 11(17), 2655. https://doi.org/10.3390/electronics11172655






