A Novel Seismocardiogram Mathematical Model for Simplified Adjustment of Adaptive Filter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Related Works
2.2. Mathematical Modeling
- —seismocardiogram signal fiducial points values aria;
- TMC,AO—time between the aorta opening and closing;
- TA0,AC—time between the aorta opening and closing;
- TMC,MO—time between the closing and opening of the mitral valves;
- TAC,MO—time between the closure of the aorta and the opening of the mitral valve;
- TRBE—duration of systole;
- TRBF—duration of diastole.
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.K.; Gupta, R. Artificial intelligence methods for analysis of electrocardiogram signals for cardiac abnormalities: State-of-the-art and future challenges. Artif. Intell. Rev. 2022, 55, 1519–1565. [Google Scholar] [CrossRef]
- Maršánová, L.; Ronzhina, M.; Smíšek, R.; Vítek, M.; Němcová, A.; Smital, L.; Nováková, M. ECG features and methods for automatic classification of ventricular premature and ischemic heartbeats: A comprehensive experimental study. Sci. Rep. 2017, 7, 11239. [Google Scholar] [CrossRef] [Green Version]
- Miramontes, R.; Aquino, R.; Flores, A.; Rodríguez, G.; Anguiano, R.; Ríos, A.; Edwards, A. PlaIMoS: A Remote Mobile Healthcare Platform to Monitor Cardiovascular and Respiratory Variables. Sensors 2017, 17, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Mello; Skoric; Xu; Roche; Lortie; Gagnon; Plant Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors 2019, 19, 3472. [CrossRef] [PubMed] [Green Version]
- Mehrang, S.; Jafari Tadi, M.; Lahdenoja, O.; Kaisti, M.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Pankaala, M.; Koivisto, T. Machine Learning Based Classification of Myocardial Infarction Conditions Using Smartphone-Derived Seismo- and Gyrocardiography. In Proceedings of the Computing in Cardiology; IEEE Computer Society, Maastricht, The Netherlands, 23–26 September 2018; Volume 45. [Google Scholar]
- Andreozzi, E.; Centracchio, J.; Punzo, V.; Esposito, D.; Polley, C.; Gargiulo, G.D.; Bifulco, P. Respiration Monitoring via Forcecardiography Sensors. Sensors 2021, 21, 3996. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Vaini, E.; Castiglioni, P.; Merati, G.; Meriggi, P.; Parati, G.; Faini, A.; Rizzo, F. Wearable seismocardiography: Towards a beat-by-beat assessment of cardiac mechanics in ambulant subjects. Auton. Neurosci. Basic Clin. 2013, 178, 50–59. [Google Scholar] [CrossRef]
- Polley, C.; Jayarathna, T.; Gunawardana, U.; Naik, G.; Hamilton, T.; Andreozzi, E.; Bifulco, P.; Esposito, D.; Centracchio, J.; Gargiulo, G. Wearable Bluetooth Triage Healthcare Monitoring System. Sensors 2021, 21, 7586. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Thakkar, H.K.; Lin, W.Y.; Chang, P.C.; Lee, M.Y. On the design of an efficient cardiac health monitoring system through combined analysis of ECG and SCG signals. Sensors 2018, 18, 379. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-N.; Nguyen, T.-H. Deep Learning Framework with ECG Feature-Based Kernels for Heart Disease Classification. Elektron. Elektrotechnika 2021, 27, 48–59. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.; Bomar, A.; Sandler, R.; Mansy, H. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.K.; Tiwari, A.K. Heart monitoring systems-A review. Comput. Biol. Med. 2014, 54, 1–13. [Google Scholar] [CrossRef]
- Aboltins, A.; Pikulins, D.; Grizans, J.; Tjukovs, S. Piscivorous Bird Deterrent Device Based on a Direct Digital Synthesis of Acoustic Signals. Elektron. Elektrotechnika 2021, 27, 42–48. [Google Scholar] [CrossRef]
- Conn, N.J.; Schwarz, K.Q.; Borkholder, D.A. In-home cardiovascular monitoring system for heart failure: Comparative study. JMIR mHealth uHealth 2019, 7, e12419. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Thakkar, H.K.; Lee, M.Y. A cardiac early warning system with multi channel SCG and ECG monitoring for mobile health. Sensors 2017, 17, 711. [Google Scholar] [CrossRef] [PubMed]
- Leitão, F.; Moreira, E.; Alves, F.; Lourenço, M.; Azevedo, O.; Gaspar, J.; Rocha, L.A. High-Resolution Seismocardiogram Acquisition and Analysis System. Sensors 2018, 18, 3441. [Google Scholar] [CrossRef] [Green Version]
- Andreozzi, E.; Fratini, A.; Esposito, D.; Naik, G.; Polley, C.; Gargiulo, G.D.; Bifulco, P. Forcecardiography: A Novel Technique to Measure Heart Mechanical Vibrations onto the Chest Wall. Sensors 2020, 20, 3885. [Google Scholar] [CrossRef]
- Andreozzi, E.; Gargiulo, G.D.; Esposito, D.; Bifulco, P. A Novel Broadband Forcecardiography Sensor for Simultaneous Monitoring of Respiration, Infrasonic Cardiac Vibrations and Heart Sounds. Front. Physiol. 2021, 12, 725716. [Google Scholar] [CrossRef]
- Holcik, J.; Moudr, J. Mathematical Model of Seismocardiogram. In World Congress on Medical Physics and Biomedical Engineering 2006; Springer: Berlin/Heidelberg, Germany, 2007; Volume 14, pp. 3415–3418. [Google Scholar]
- Casas, B.; Lantz, J.; Viola, F.; Cedersund, G.; Bolger, A.F.; Carlhäll, C.J.; Karlsson, M.; Ebbers, T. Bridging the gap between measurements and modelling: A cardiovascular functional avatar. Sci. Rep. 2017, 7, 6214. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, K.; Schmidt, S.E.; Jensen, A.S.; Søgaard, P.; Struijk, J.J. Definition of Fiducial Points in the Normal Seismocardiogram. Sci. Rep. 2018, 8, 15455. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, Z.; Elfadel, I.; Rasras, M. Monolithic Multi Degree of Freedom (MDoF) Capacitive MEMS Accelerometers. Micromachines 2018, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Centracchio, J.; Andreozzi, E.; Esposito, D.; Gargiulo, G.D.; Bifulco, P. Detection of Aortic Valve Opening and Estimation of Pre-Ejection Period in Forcecardiography Recordings. Bioengineering 2022, 9, 89. [Google Scholar] [CrossRef]
- Andreozzi, E.; Centracchio, J.; Esposito, D.; Bifulco, P. A Comparison of Heart Pulsations Provided by Forcecardiography and Double Integration of Seismocardiogram. Bioengineering 2022, 9, 167. [Google Scholar] [CrossRef]
- Shi, W.; Chew, M.-S. Mathematical and physical models of a total artificial heart. In Proceedings of the 2009 IEEE International Conference on Control and Automation, Christchurch, New Zealand, 9–11 December 2009; pp. 637–642. [Google Scholar] [CrossRef]
- Guidoboni, G.; Sala, L.; Enayati, M.; Member, S.; Sacco, R.; Szopos, M.; Keller, J.M.; Fellow, L.; Popescu, M.; Member, S.; et al. Cardiovascular Function and Ballistocardiogram: A Relationship Interpreted via Mathematical Modeling. IEEE Trans. Biomed. Eng. 2019, 66. [Google Scholar] [CrossRef] [Green Version]
- Htet, Z.L.; Aye, T.P.P.; Singhavilai, T.; Naiyanetr, P. Hemodynamics during Rotary Blood Pump support with speed synchronization in heart failure condition: A modelling study. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3307–3310. [Google Scholar] [CrossRef]
- Pockevicius, V.; Markevicius, V.; Cepenas, M.; Andriukaitis, D.; Navikas, D. Blood Glucose Level Estimation Using Interdigital Electrodes. Electron. Electr. Eng. 2013, 19. [Google Scholar] [CrossRef]
- Abdolrazaghi, M.; Navidbakhsh, M.; Hassani, K. Mathematical modelling and electrical analog equivalent of the human cardiovascular system. Cardiovasc. Eng. 2010, 10, 45–51. [Google Scholar] [CrossRef]
- Yang, X.; Leandro, J.S.; Cordeiro, T.D.; Lima, A.M.N. An Inverse Problem Approach for Parameter Estimation of Cardiovascular System Models. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Virtual Conference, 1–5 November 2021; pp. 5642–5645. [Google Scholar] [CrossRef]
- Jain, K.; Patra, A.; Maka, S. Modeling of the Human Cardiovascular System for Detection of Atherosclerosis. IFAC PapersOnLine 2018, 51, 545–550. [Google Scholar] [CrossRef]
- Zia, J.; Kimball, J.; Hersek, S.; Inan, O.T. Modeling Consistent Dynamics of Cardiogenic Vibrations in Low-Dimensional Subspace. IEEE J. Biomed. Health Informatics 2020, 24, 1887–1898. [Google Scholar] [CrossRef]
- Uskovas, G.; Valinevicius, A.; Zilys, M.; Navikas, D.; Frivaldsky, M.; Prauzek, M.; Konecny, J.; Andriukaitis, D. Driver Cardiovascular Disease Detection Using Seismocardiogram. Electronics 2022, 11, 484. [Google Scholar] [CrossRef]
- Prauzek, M.; Konecny, J. Optimizing of Q-Learning Day/Night Energy Strategy for Solar Harvesting Environmental Wireless Sensor Networks Nodes. Elektron. Elektrotechnika 2021, 27, 50–56. [Google Scholar] [CrossRef]
- Skovierova, H.; Pavelek, M.; Okajcekova, T.; Palesova, J.; Strnadel, J.; Spanik, P.; Halašová, E.; Frivaldsky, M.; Foresta, F. The Biocompatibility of Wireless Power Charging System on Human Neural Cells. Appl. Sci. 2021, 11, 3611. [Google Scholar] [CrossRef]
- Hrbac, R.; Kolar, V.; Bartlomiejczyk, M.; Mlcak, T.; Orsag, P.; Vanc, J. A Development of a Capacitive Voltage Divider for High Voltage Measurement as Part of a Combined Current and Voltage Sensor. Elektron. Elektrotechnika 2020, 26, 25–31. [Google Scholar] [CrossRef]
- Surgailis, T.; Valinevicius, A.; Markevicius, V.; Navikas, D.; Andriukaitis, D. Avoiding Forward Car Collision using Stereo Vision System. Electron. Electr. Eng. 2012, 18. [Google Scholar] [CrossRef] [Green Version]
- Soni, N.; Malekian, R.; Andriukaitis, D.; Navikas, D. Internet of Vehicles Based Approach for Road Safety Applications Using Sensor Technologies. Wirel. Pers. Commun. 2019, 105, 1257–1284. [Google Scholar] [CrossRef] [Green Version]
- Ieremeiev, O.; Lukin, V.; Okarma, K.; Egiazarian, K. Full-Reference Quality Metric Based on Neural Network to Assess the Visual Quality of Remote Sensing Images. Remote Sens. 2020, 12, 2349. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, Q. A Novel Fuzzy Optimized CNN-RNN Method for Facial Expression Recognition. Elektron. Elektrotechnika 2021, 27, 67–74. [Google Scholar] [CrossRef]
- Semmlow, J.L.; Griffel, B. Biosignal and Medical Image Processing MATLAB-Based Application, 3rd ed.; Taylor & Francis Group: New York, NY, USA, 2014; ISBN 9781466567368. [Google Scholar]
- Humaidi, A.J.; Ibraheem, I.K.; Ajel, A.R. A novel adaptive LMS algorithm with genetic search capabilities for system identification of adaptive FIR and IIR filters. Information 2019, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Mora, N.; Cocconcelli, F.; Matrella, G.; Ciampolini, P. Detection and Analysis of Heartbeats in Seismocardiogram Signals. Sensors 2020, 20, 1670. [Google Scholar] [CrossRef] [Green Version]
- Sotner, R.; Domansky, O.; Jerabek, J.; Herencsar, N.; Petrzela, J.; Andriukaitis, D. Integer-and Fractional-Order Integral and Derivative Two-Port Summations: Practical Design Considerations. Appl. Sci. 2019, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, T.; Bhuyan, M.K.; Sharma, L.N. Orthogonal subspace projection based framework to extract heart cycles from SCG signal. Biomed. Signal Process. Control 2019, 50, 45–51. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Mitra, M. R-peak detection algorithm for ECG using double difference and RR interval processing peer-review under responsibility of C3IT. Procedia Technol. 2012, 4, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Paterova, T.; Prauzek, M. Estimating Harvestable Solar Energy from Atmospheric Pressure Using Deep Learning. Elektron. Elektrotechnika 2021, 27, 18–25. [Google Scholar] [CrossRef]
- Peksinski, J.; Zeglinski, G.; Mikolajczak, G.; Kornatowski, E. Estimation of BER Bit Error Rate Using Digital Smoothing Filters. Elektron. Elektrotechnika 2021, 27, 75–83. [Google Scholar] [CrossRef]
- Peric, Z.H.; Denic, B.D.; Savic, M.S.; Vucic, N.J.; Simic, N.B. Binary Quantization Analysis of Neural Networks Weights on MNIST Dataset. Elektron. Elektrotechnika 2021, 27, 55–61. [Google Scholar] [CrossRef]
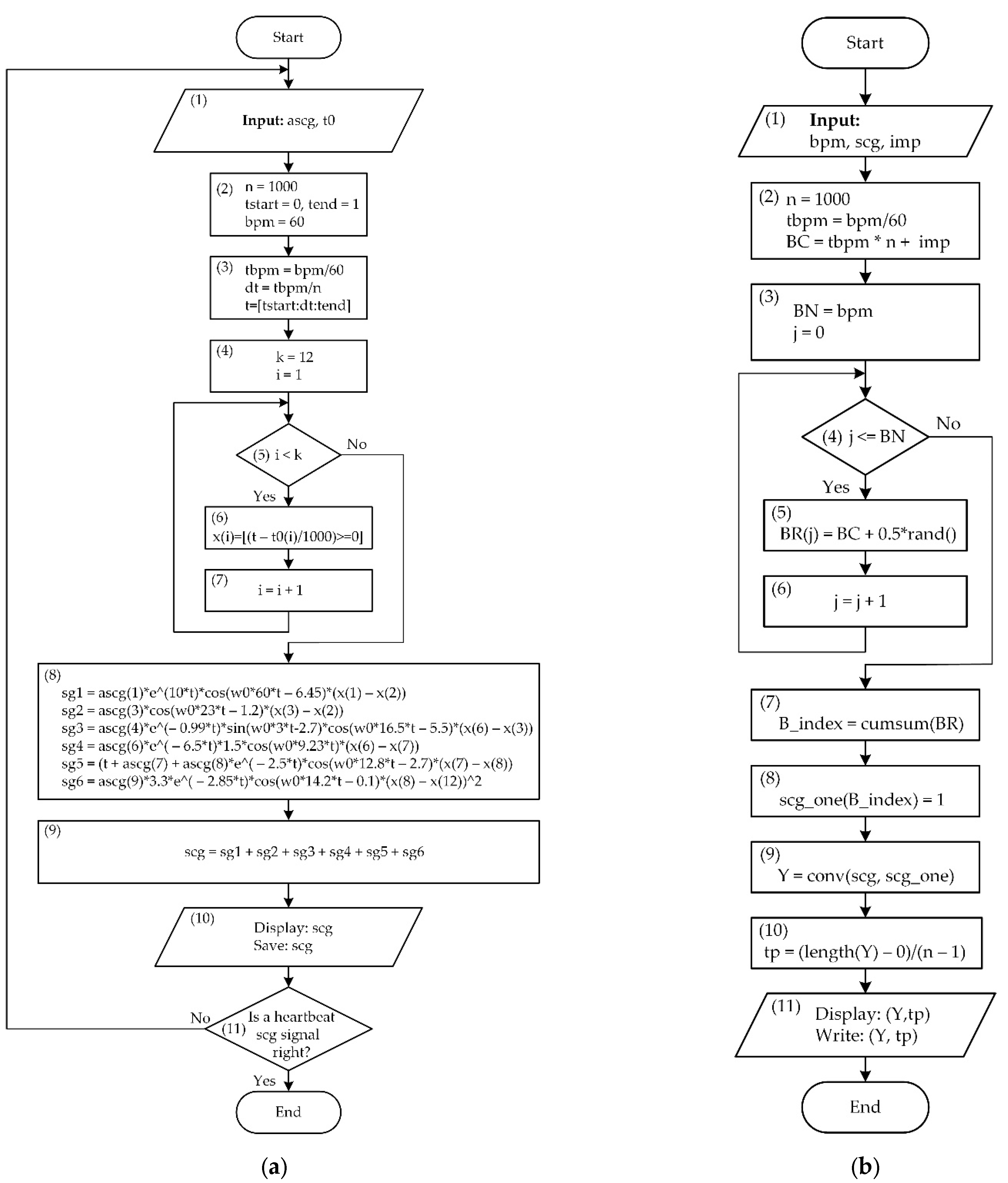
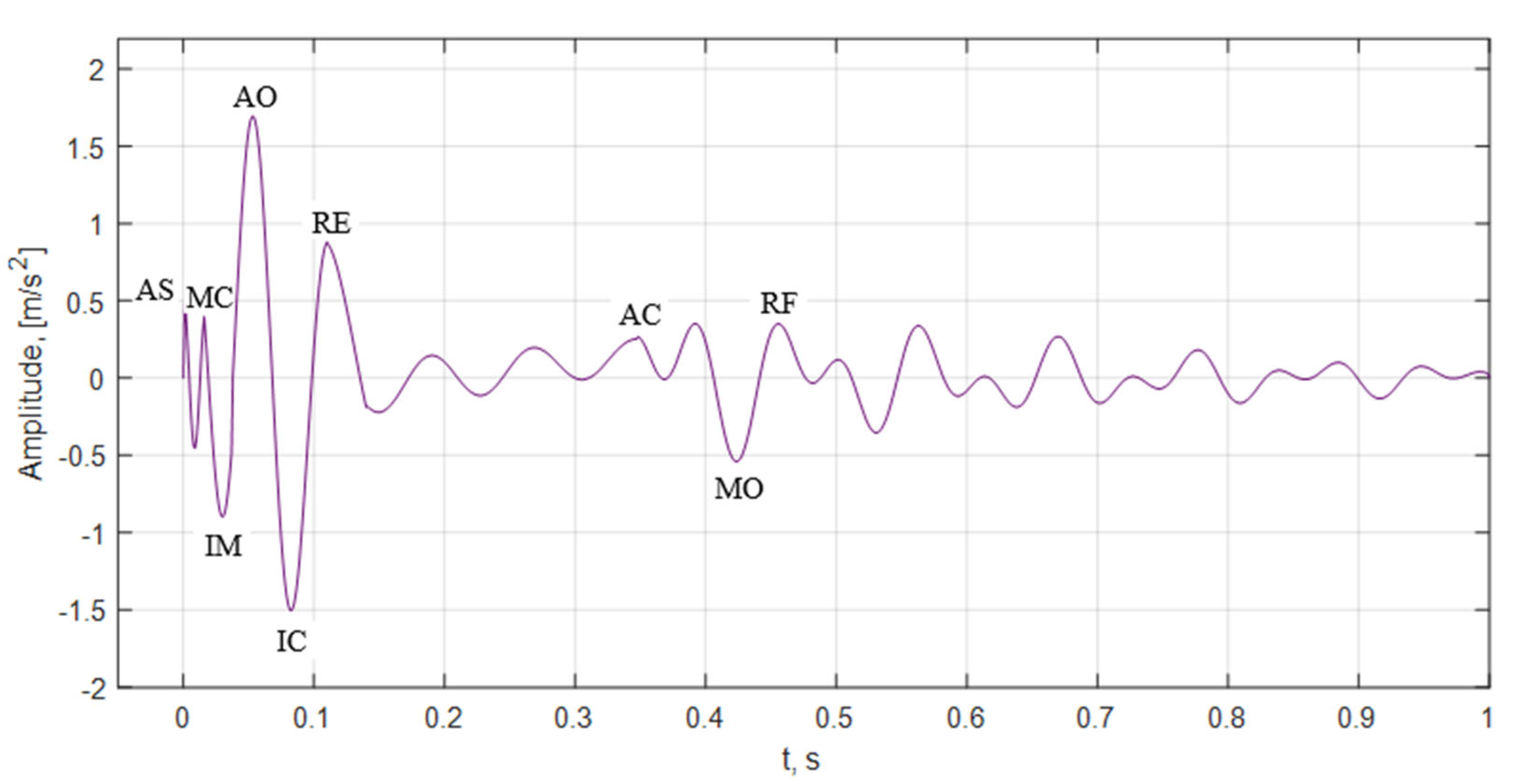

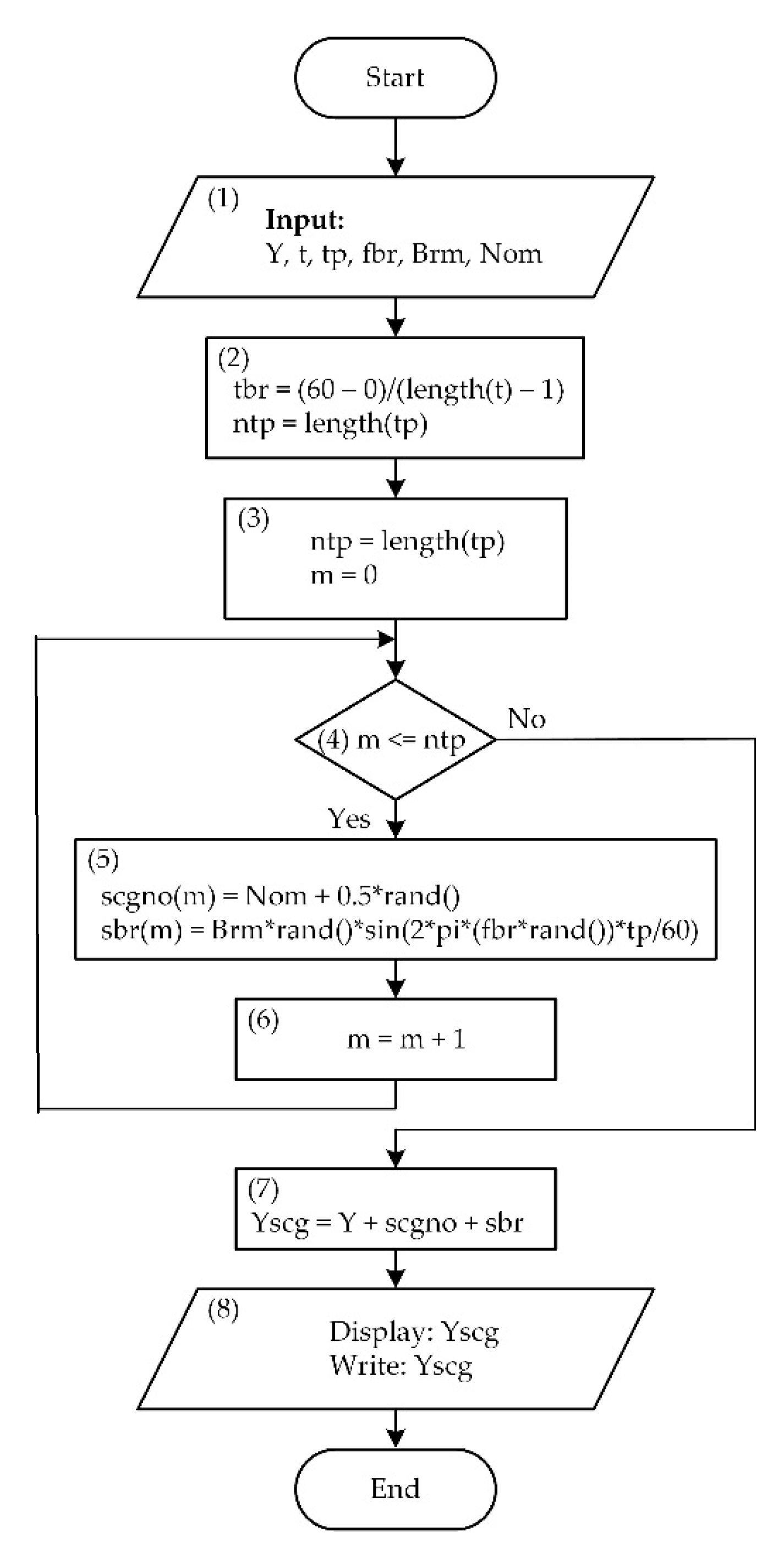
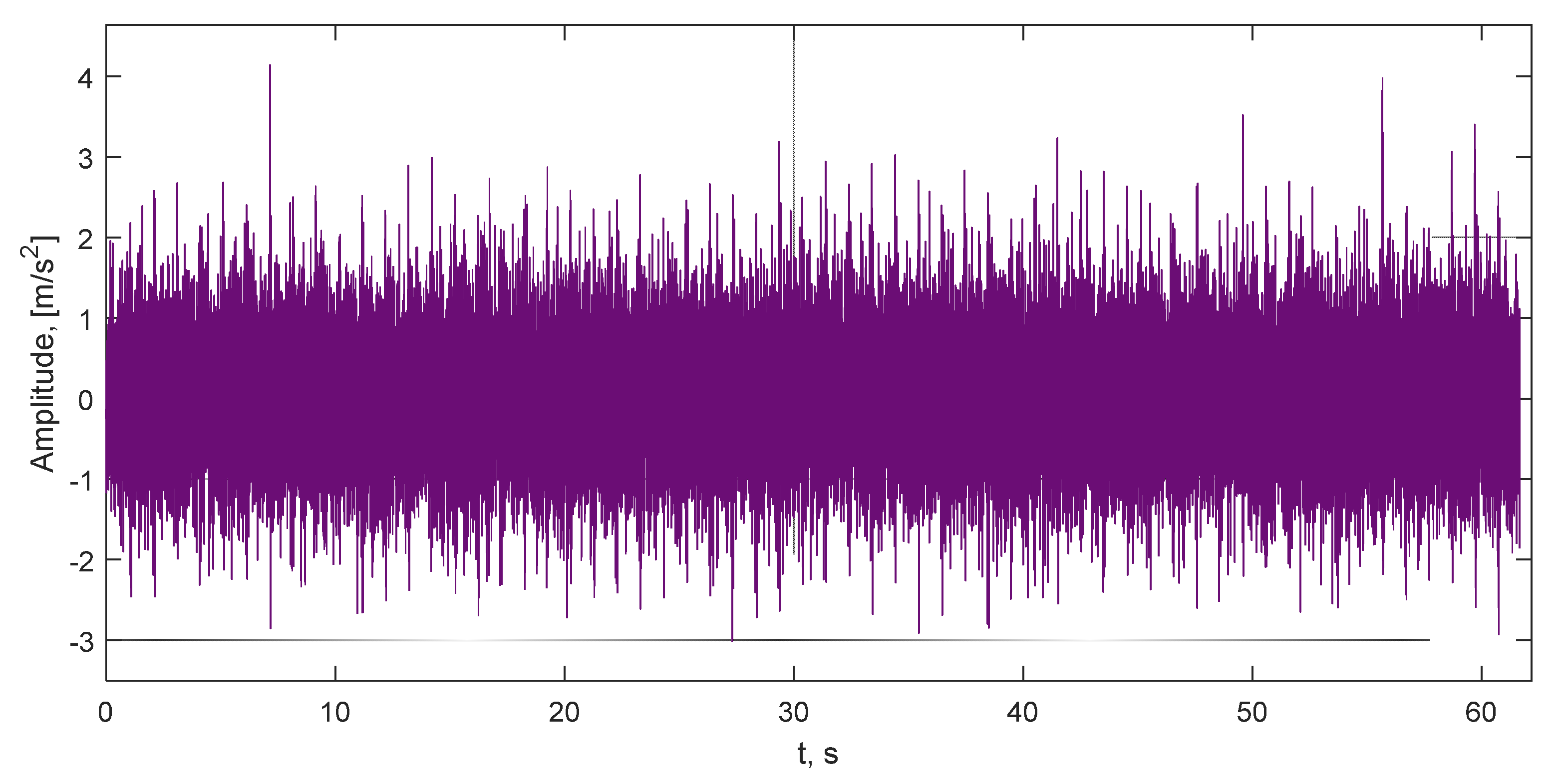
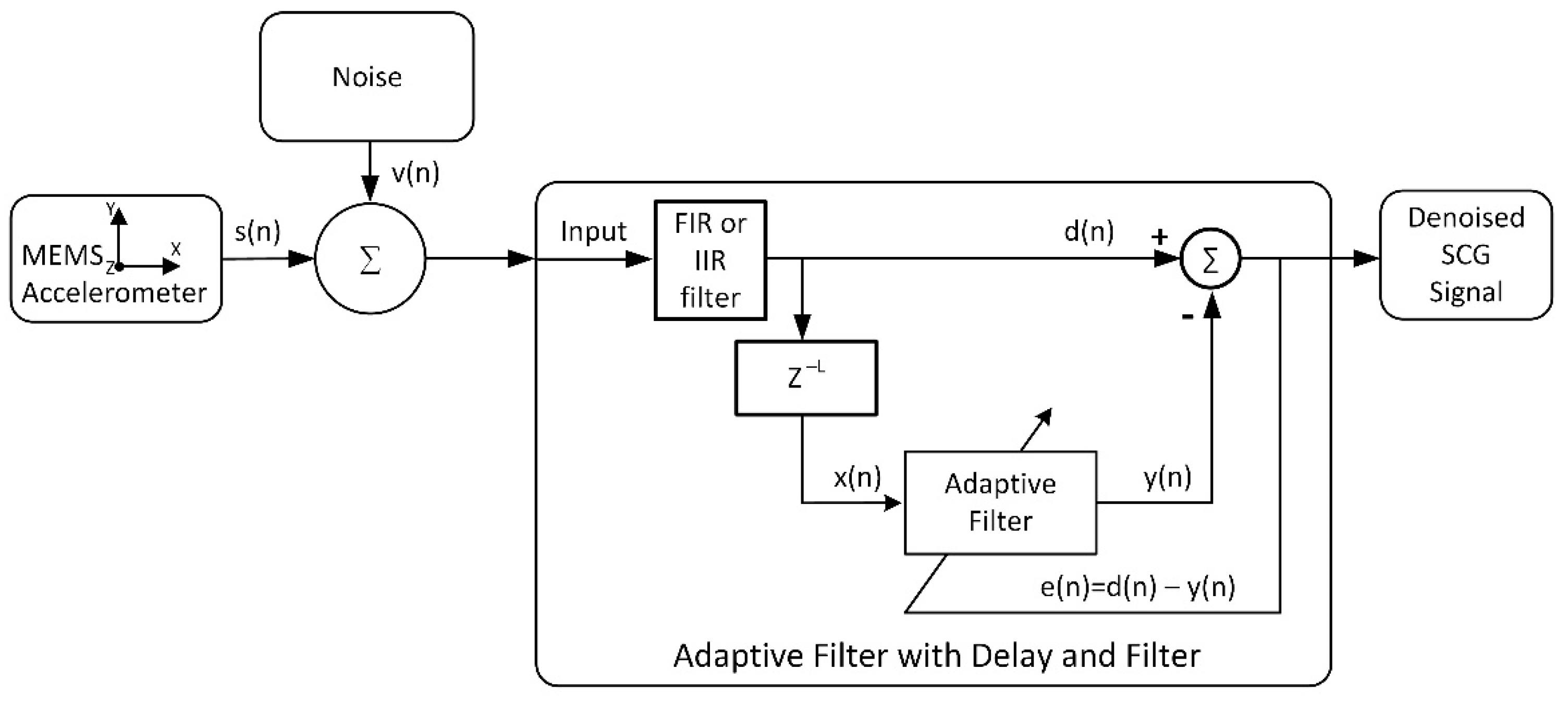
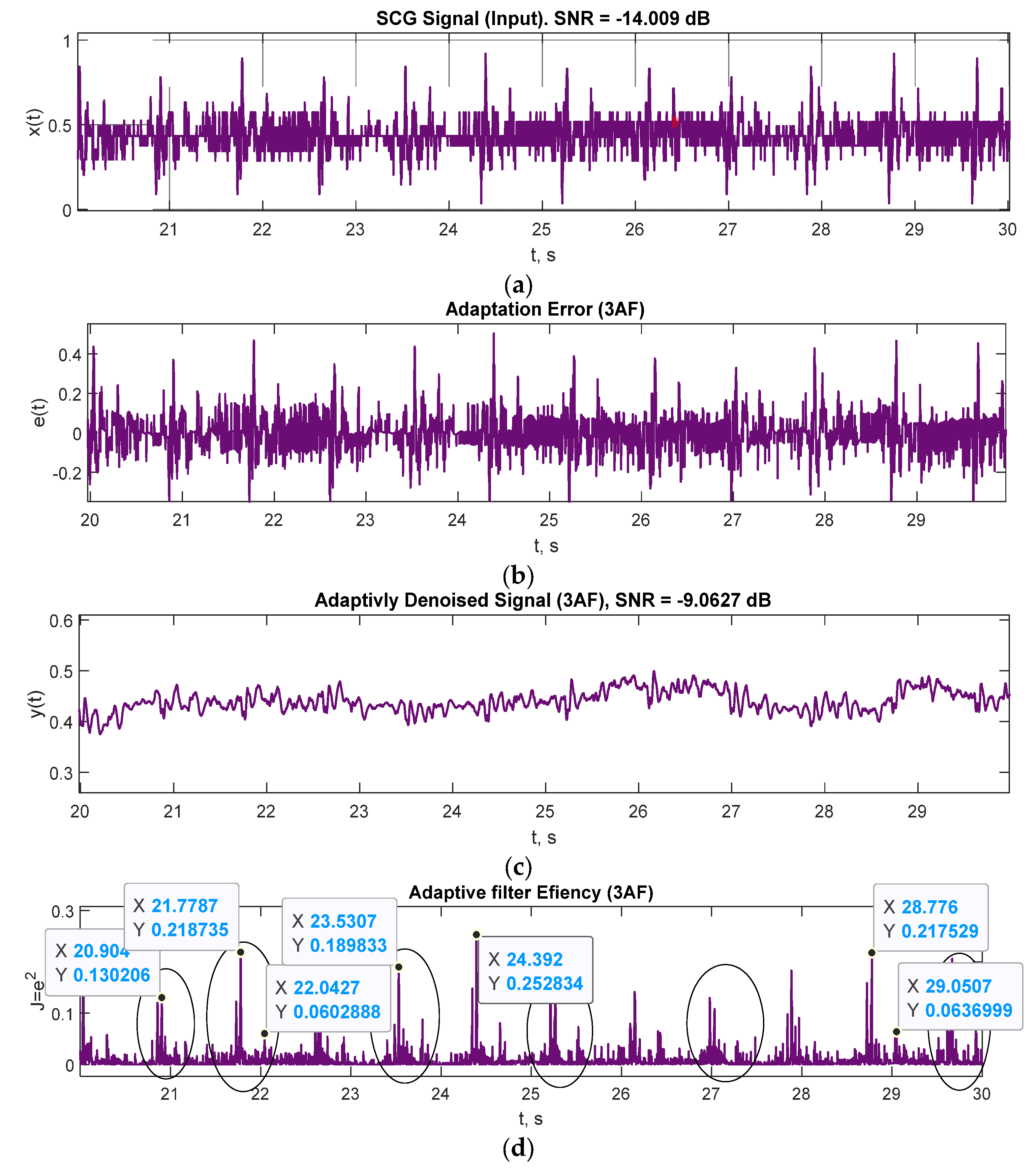

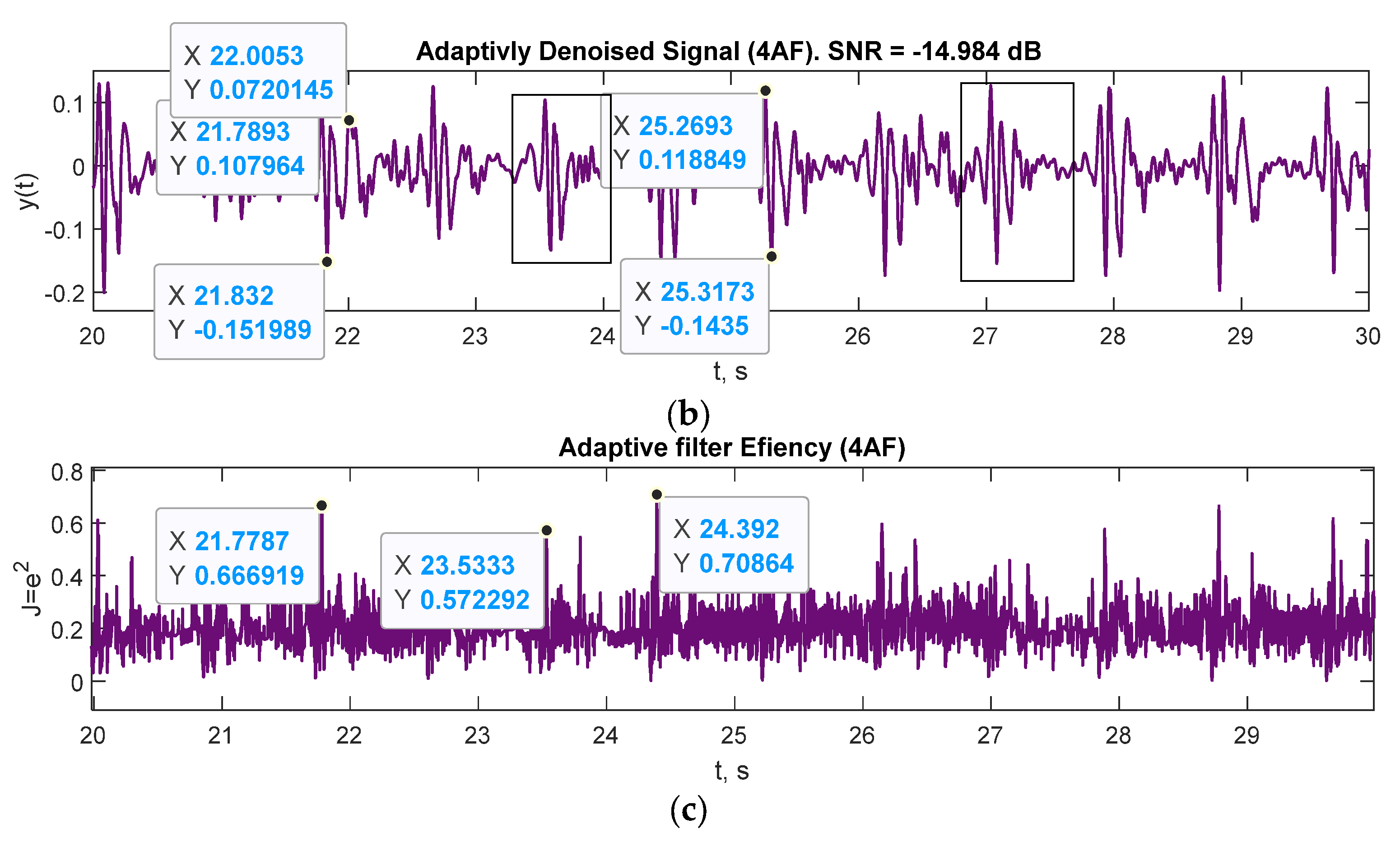
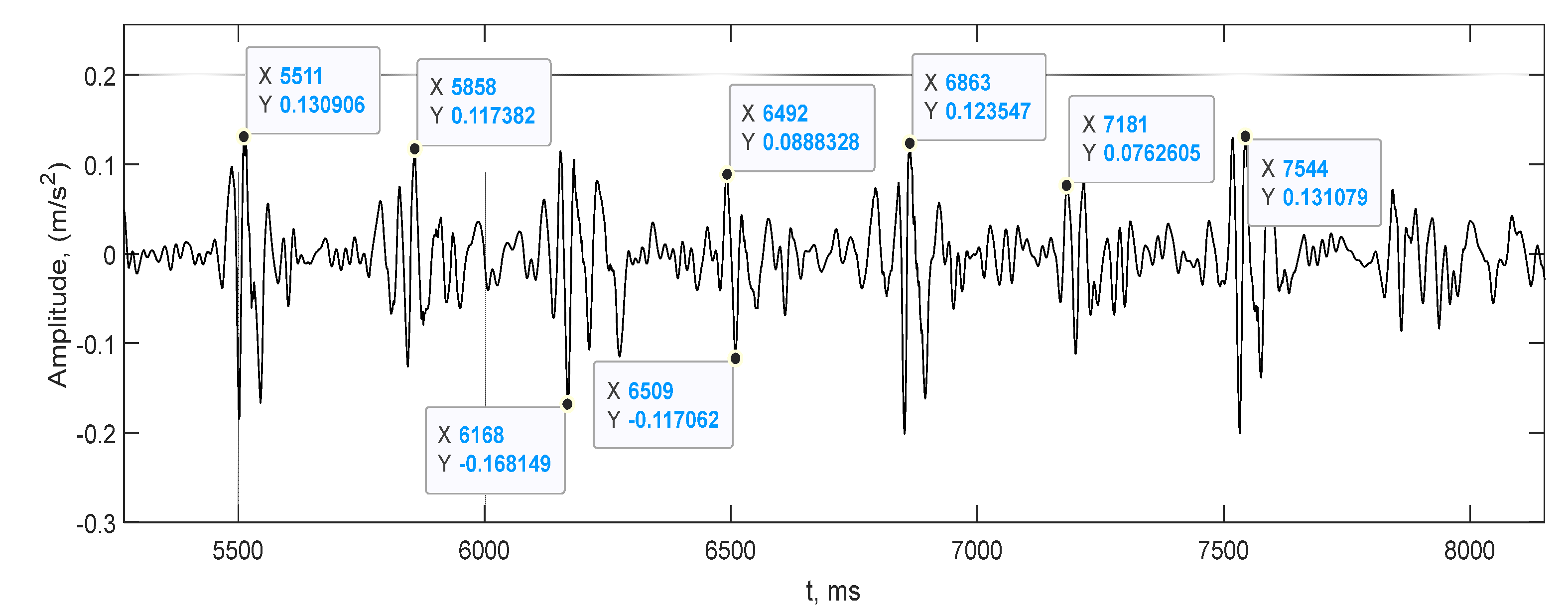
| Mark | Description | Points from | Points to | Duration, ms |
|---|---|---|---|---|
| TMC,AO | Duration from MC to AO | 16 (±14) | 59 (±11) | 43 (±14) |
| TAO,AC | Duration from AO to AC | 59 (±11) | 348 (±37) | 332 (±37) |
| TMC,MO | Duration from MC to MO | 16 (±14) | 437 (±37) | 378 (±37) |
| TAC,MO | Duration from AC to MO | 348 (±37) | 437 (±37) | 89 (±37) |
| TRBE | Duration of Systole | −111 (±24) | ||
| TRBF | Duration of Diastole | 505 (±41) |
| Mark | Acceleration Amplitude Value, m/s2 | Description |
|---|---|---|
| ascg1 | 0.5 | Arteries systole |
| ascg2 | 0.3 | Mitral valve closing |
| ascg3 | −0.9 | Isovolumetric moment |
| ascg4 | 1.8 | Aorta valve opening |
| ascg5 | −1.65 | Isovolumic contraction |
| ascg6 | 1.2 | Rapid systole ejection |
| ascg7 | −0.2 | Rapid filling |
| ascg8 | 0.25 | Aorta valve closing |
| ascg9 | −0.55 | Mitral valve opening |
| Mark | Fiducial Points Time Values, ms | Description |
|---|---|---|
| tas | 1 | Arteries systole |
| tmc | 16 | Mitral valve closing |
| tim | 21.5 | Isovolumetric moment |
| tao | 59 | Aorta valve opening |
| tic | 26 | Isovolumic contraction |
| trbe | 111 | Rapid systole ejection |
| trbee | 141 | Rapid filling |
| tac | 348 | Aorta valve closing |
| tmo | 437 | Mitral valve opening |
| Filter Order | 1 Adaptive Filter | 2 Adaptive Filter |
|---|---|---|
| Filter Order | FIR (100)/892 | IIR (5)/892 |
| mu AF step | 2.3105 × 10−4 | 1.9370 × 10−4 |
| Heart Rate, beats/min | 67 | 69 |
| RMS, m/s2 | 0.2145 | 0.2635 |
| SNR, dB | −6.3288 | −6.7995 |
| RMSE, m/s2 | 0.1447 | 0.3401 |
| Peaks number | 63 | 67 |
| Peaks Interval Mean, ms | 887.032 | 858.313 |
| Peaks Interval STD | 446.838 | 237.043 |
| Adaptation duration, s | 1.237476 | 1.185759 |
| Adaptation time, s | 1.200769 | 1.193749 |
| Filter Order | 1 Adaptive Filter | 2 Adaptive Filter |
|---|---|---|
| Filter Order | FIR (100)/892 | IIR (5)/892 |
| mu AF step | 3.5503 × 10−4 | 5.1336 × 10−4 |
| Heart Rate, beats/min | 107 | 98 |
| RMS, m/s2 | 0.5503 | 0.0523 |
| SNR, dB | −5.1047 | −7.8809 |
| RMSE, m/s2 | 0.0717 | 0.0945 |
| Peaks number | 106 | 97 |
| Peaks Interval Mean, ms | 556.406 | 611.619 |
| Peaks Interval STD | 137.600 | 171.220 |
| Peaks Variation | 18,933.805 | 29,316.363 |
| Adaptation time, s | 1.200769 | 1.193749 |
| Filter Order | 3 Adaptive Filter | 4 Adaptive Filter |
|---|---|---|
| Filter Order | 200 | 50 |
| mu AF step | 2.1226 × 10−3 | 1.4774 × 10−1 |
| Heart Rate, beats/min | 95 | 88 |
| RMS, m/s2 | 0.4373 | 0.0478 |
| SNR, dB | −9.0627 | −14.9807 |
| RMSE, m/s2 | 0.0503 | 0.0984 |
| Peaks number | 61 | 57 |
| Peaks Interval Mean, ms | 623.885 | 664.281 |
| Peaks Interval STD | 229.843 | 132.981 |
| Peaks Variation | 52,827.737 | 17,683.920 |
| Processing time, s | 1.091161 | 1.110726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uskovas, G.; Valinevicius, A.; Zilys, M.; Navikas, D.; Frivaldsky, M.; Prauzek, M.; Konecny, J.; Andriukaitis, D. A Novel Seismocardiogram Mathematical Model for Simplified Adjustment of Adaptive Filter. Electronics 2022, 11, 2444. https://doi.org/10.3390/electronics11152444
Uskovas G, Valinevicius A, Zilys M, Navikas D, Frivaldsky M, Prauzek M, Konecny J, Andriukaitis D. A Novel Seismocardiogram Mathematical Model for Simplified Adjustment of Adaptive Filter. Electronics. 2022; 11(15):2444. https://doi.org/10.3390/electronics11152444
Chicago/Turabian StyleUskovas, Gediminas, Algimantas Valinevicius, Mindaugas Zilys, Dangirutis Navikas, Michal Frivaldsky, Michal Prauzek, Jaromir Konecny, and Darius Andriukaitis. 2022. "A Novel Seismocardiogram Mathematical Model for Simplified Adjustment of Adaptive Filter" Electronics 11, no. 15: 2444. https://doi.org/10.3390/electronics11152444
APA StyleUskovas, G., Valinevicius, A., Zilys, M., Navikas, D., Frivaldsky, M., Prauzek, M., Konecny, J., & Andriukaitis, D. (2022). A Novel Seismocardiogram Mathematical Model for Simplified Adjustment of Adaptive Filter. Electronics, 11(15), 2444. https://doi.org/10.3390/electronics11152444










