A Microwave Imaging Procedure for Lung Lesion Detection: Preliminary Results on Multilayer Phantoms
Abstract
:1. Introduction
2. Experimental Configuration
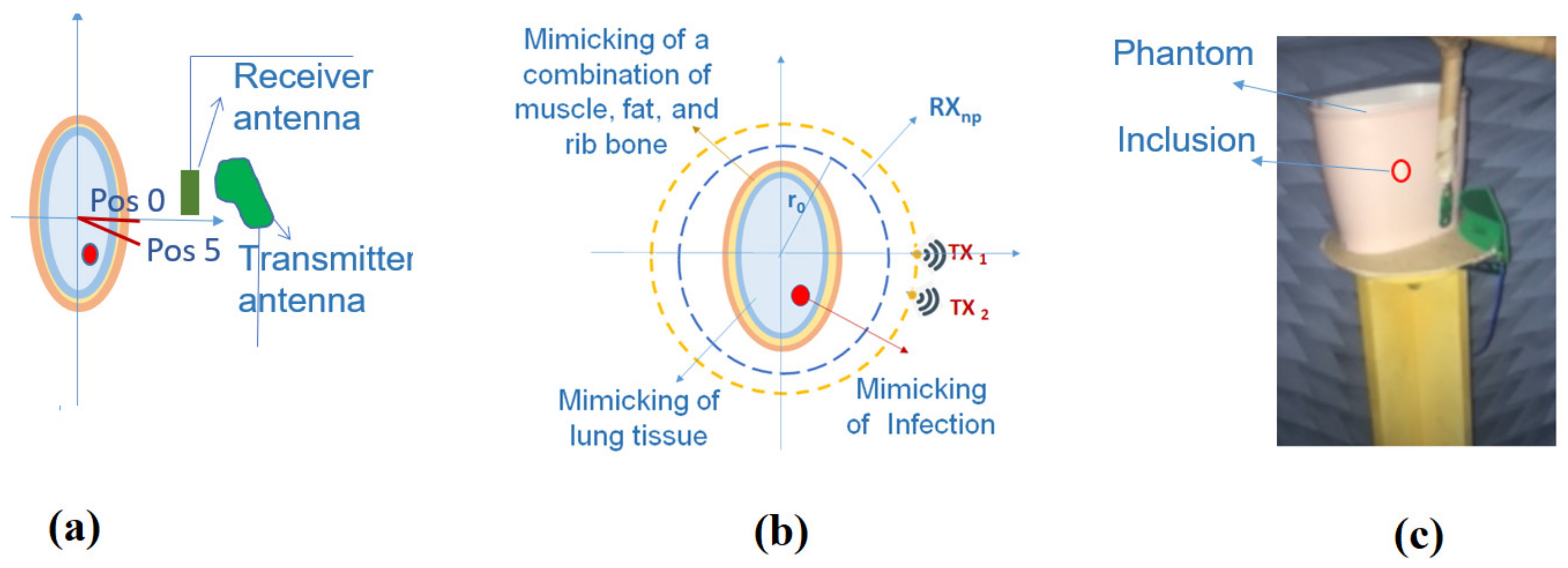
2.1. Phantom Fabrication
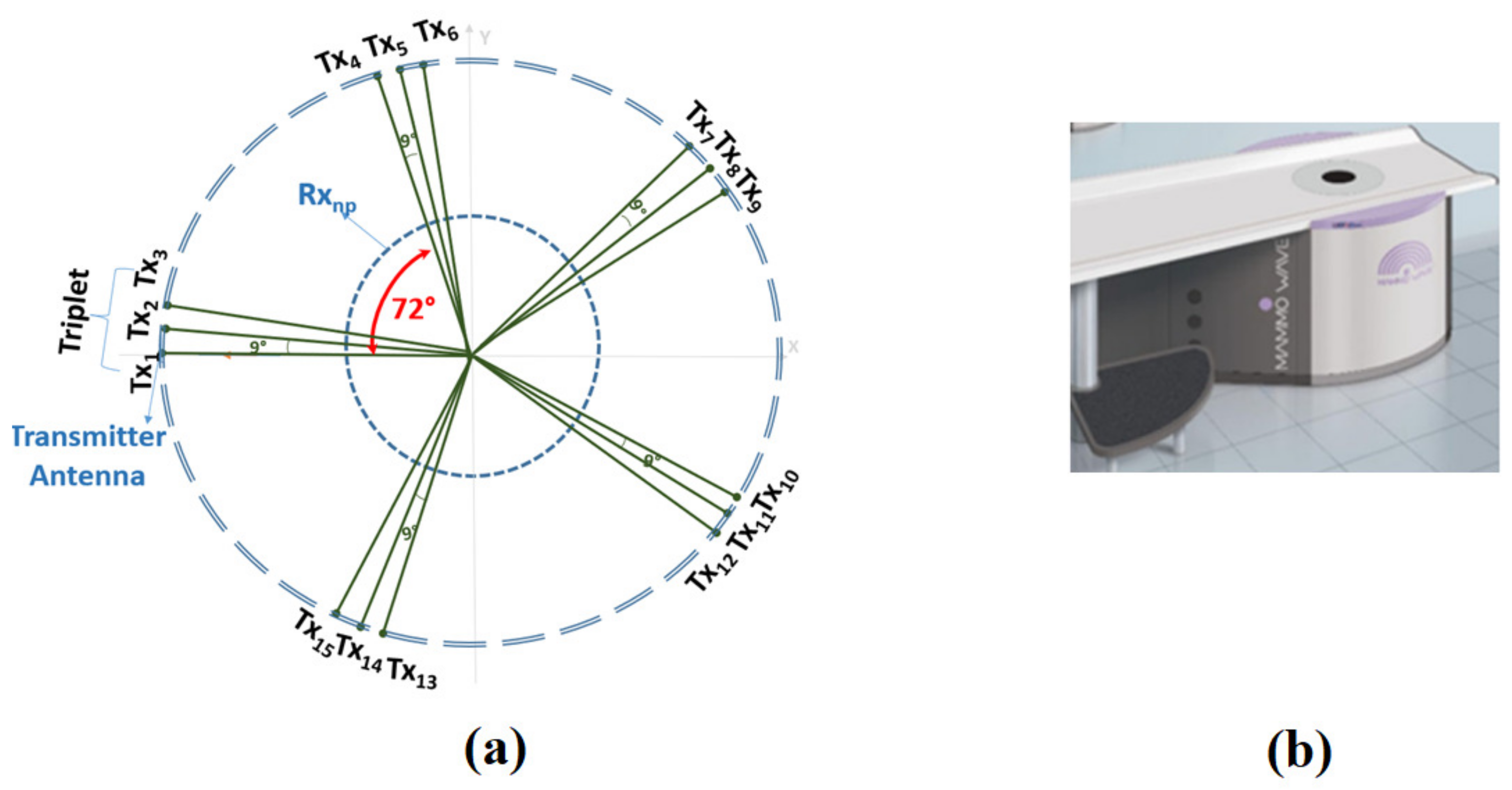
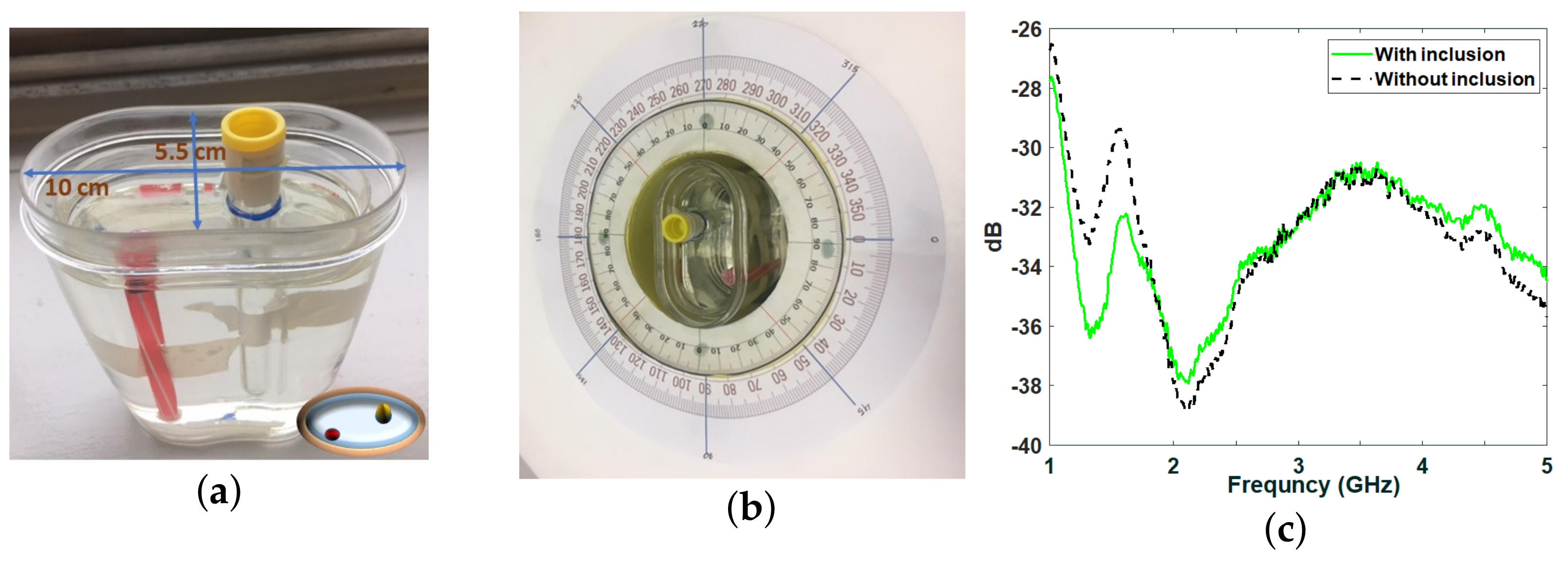
2.2. Measurements Inside the Anechoic Chamber
2.3. Microwave Imaging Device Description
2.4. Imaging Procedure Based on Huygens’ Principle
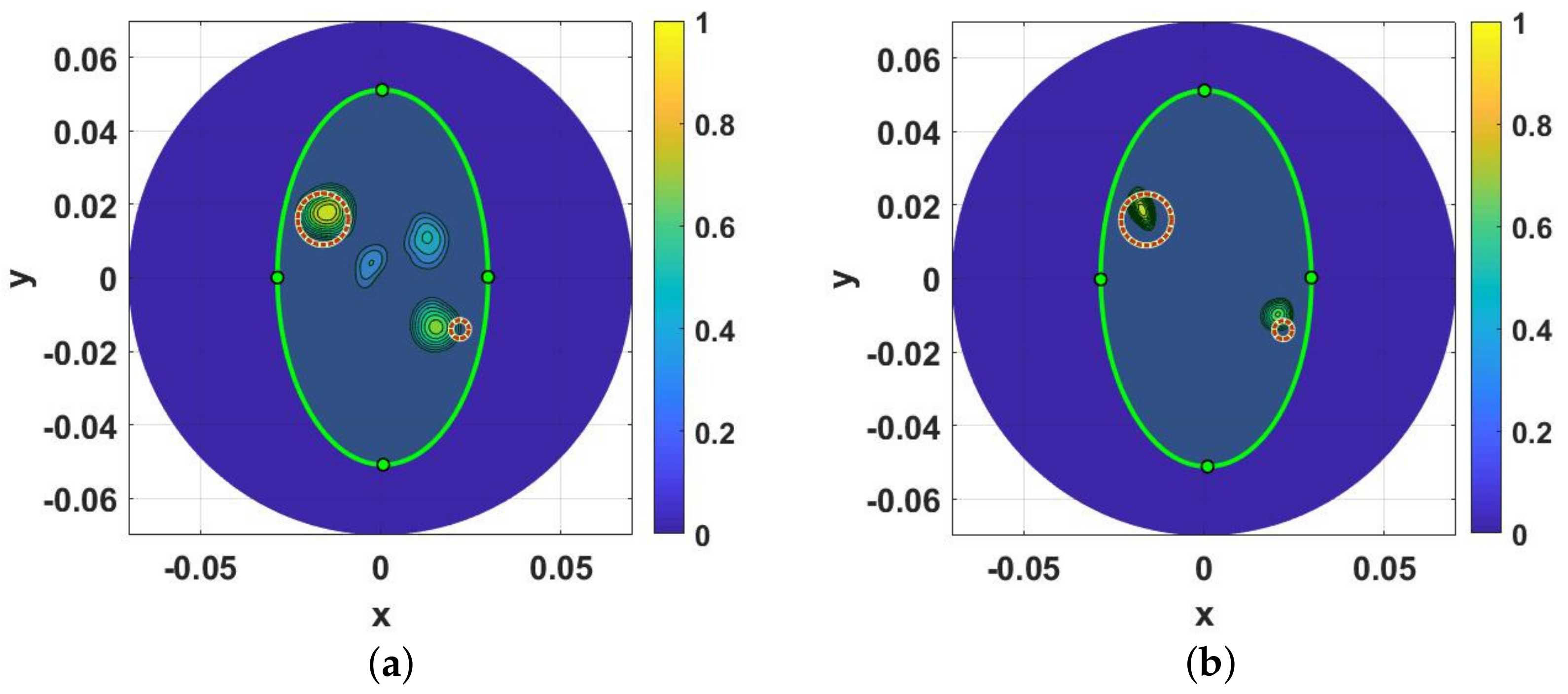
2.5. Image Quantification
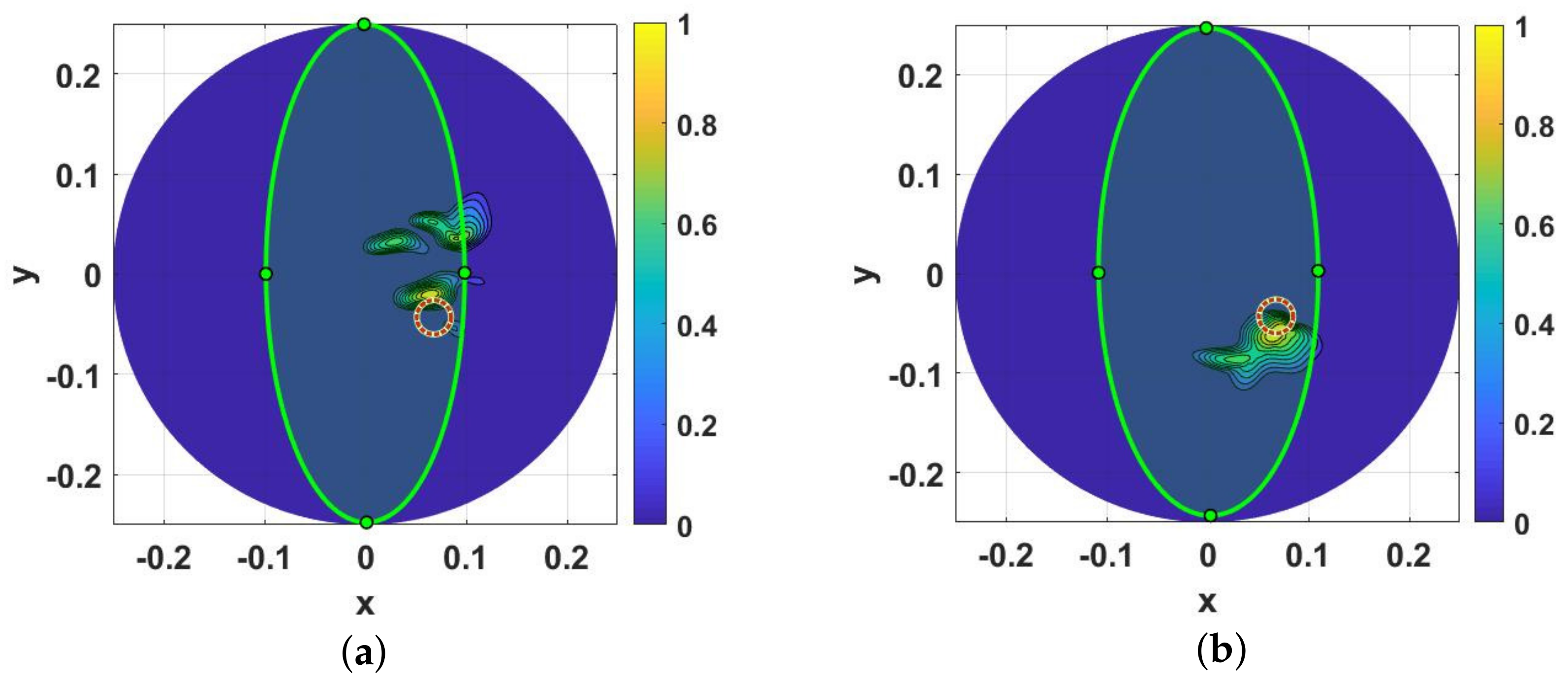
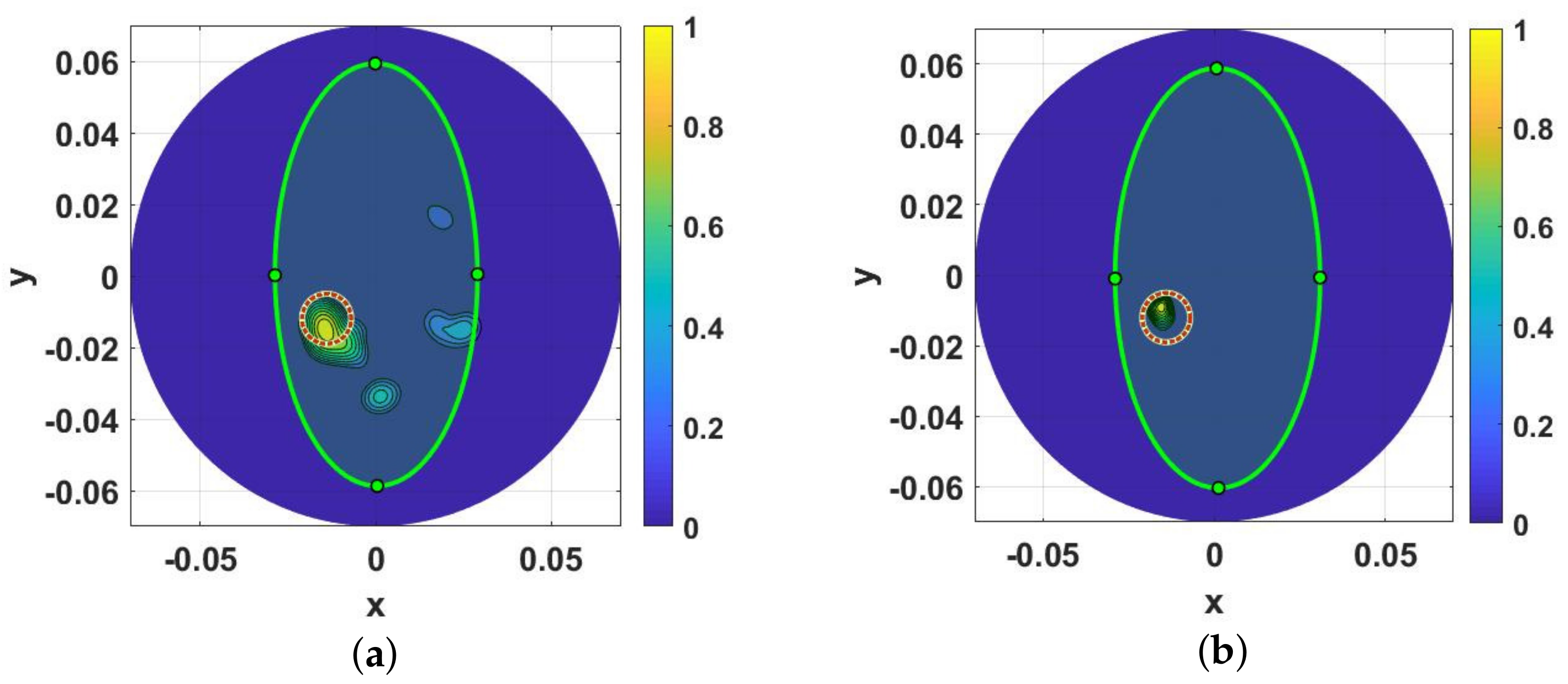
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalender, W.A. Computed tomography: Fundamentals. In System Technology, Image Quality, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Kim, K.; Choi, H. High-efficiency high-voltage class F amplifier for high-frequency wireless ultrasound systems. PLoS ONE 2021, 16, e0249034. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, M.; Randazzo, A. Microwave Imaging Methods and Applications; Artech House: Boston, MA, USA, 2018. [Google Scholar]
- Byrne, D. An Introduction to Microwave Imaging for Breast Cancer Detection; Biological and Medical Physics, Biomedical Engineering; Springer International Publishing: Basel, Switzerland, 2016; pp. 47–73. [Google Scholar]
- Khalesi, B.; Sohani, B.; Ghavami, N.; Ghavami, M.; Dudley, S.; Tiberi, G. A phantom investigation to quantify Huygens principle based microwave imaging for bone lesion detection. Electronics 2019, 8, 1505. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, B.; Sohani, B.; Ghavami, N.; Ghavami, M.; Dudley, S.; Tiber, G. Free-Space Operating Microwave Imaging Device for Bone Lesion Detection: A Phantom Investigation. IEEE Antennas Wirel. Propag. Lett. 2020, 19, 2393–2397. [Google Scholar] [CrossRef]
- Babarinde, O.J.; Jamlos, M.F.; Ramli, N.B. UWB microwave imaging of the lungs: A review. In Proceedings of the 2014 IEEE 2nd International Symposium on Telecommunication Technologies (ISTT), Langkawi, Malaysia, 24–26 November 2014; pp. 188–193. [Google Scholar]
- Zamani, A.; Rezaeieh, S.A.; Abbosh, A.M. Lung cancer detection using frequency-domain microwave imaging. Electron. Lett. 2015, 51, 740–741. [Google Scholar] [CrossRef]
- Rezaeieh, S.A.; Zamani, A.; Bialkowski, K.S.; Abbosh, A.M. Novel microwave torso scanner for thoracic fluid accumulation diagnosis and monitoring. Sci. Rep. 2017, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.N.; Isa, M.M.; Jamlos, F. Review article of microwave imaging techniques and dielectric properties for lung tumor detection. AIP Conf. Proc. 2020, 2203, 20012. [Google Scholar]
- Lin, X.; Gong, Z.; Ding, Y.; Chen, Y.; Sosa, P.A.V.; Sosa, M.J.V. Feasibility Study of Detection of Coronavirus Disease 2019 with Microwave Medical Imaging. In Proceedings of the 2021 15th European Conference on Antennas and Propagation (EuCAP), Dusseldorf, Germany, 22–26 March 2021; pp. 1–4. [Google Scholar]
- Alam, N.; Ahmed, A.; Oni, M.A.I.; Meen, T.A.; Rahman, M.A. UWB Microwave Imaging for Non-Invasive Anomaly Detection in Human Lung and Possible Application in COVID-19 Diagnosis: A Review. In Proceedings of the 2021 2nd International Conference on Robotics, Electrical and Signal Processing Techniques (ICREST), Dhaka, Bangladesh, 5–7 January 2021; pp. 772–776. [Google Scholar] [CrossRef]
- Khalesi, B.; Khalid, B.; Ghavami, N.; Ghavami, M.; Dudley, S.; Tiberi, G. Microwave Imaging for Lung COVID-19 Infection Detection through Huygens Principle. In Proceedings of the 2021 Photonics & Electromagnetics Research Symposium (PIERS 2021), Hangzhou, China, 21–25 November 2021. [Google Scholar]
- Ghavami, N.; Tiberi, G.; Edward, D.J.; Monorchio, A. UWB Microwave Imaging of Objects with Canonical Shape. IEEE Trans. Antennas Propag. 2012, 60, 231–239. [Google Scholar] [CrossRef]
- Ender, P. Huygens’ principle as universal model of propagation. Latin Amer. J. Phys. Educ. 2009, 3, 19–32. [Google Scholar]
- Vispa, A.; Sani, L.; Paoli, M.; Bigotti, A.; Raspa, G.; Ghavami, N.; Caschera, S.; Ghavami, M.; Duranti, M.; Tiberi, G. UWB Device for Breast Microwave Imaging: Phantom and Clinical Validations. Measurement 2019, 146, 582–589. [Google Scholar] [CrossRef]
- Camacho, L.M.; Tjuatja, S. FDTD simulation of microwave scattering from a lung tumor. In Proceedings of the IEEE Antennas and Propagation Society International Symposium, Washington, DC, USA, 3–8 July 2005; pp. 815–818. [Google Scholar]
- Hasgall, P.A.; Neufeld, E.; Gosselin, M.C.; Klingenböck, A.; Kuster, N.; Kuster, N.; Hasgall, P.; Gosselin, M. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues, Version 4.0; ScienceOpen: Berlin, Germany, 2018. [Google Scholar]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurich Med Tech. Available online: https://zmt.swiss/validation-hw/tsm/tle5c-24-2450/ (accessed on 26 October 2019).
- Meaney, P.M.; Fox, C.J.; Geimer, S.D.; Paulsen, K.D. Characterization of Glycerin: Water Mixtures: Implications for Use as a Coupling Medium in Microwave Tomography. IEEE Trans. Microw. Theory Tech. 2017, 65, 1471–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiberi, G.; Ghavami, N.; Edwards, D.J.; Monorchio, A. Ultrawideband microwave imaging of cylindrical objects with inclusions. IET Microw. Antennas Propag. 2011, 5, 1440–1446. [Google Scholar] [CrossRef]
- Fear, E.C.; Li, X.; Hagness, S.C.; Stuchly, M.A. Confocal microwave imaging for breast cancer detection: Localization of tumors in three dimensions. IEEE Trans. Biomed. Eng. 2002, 49, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Tissues | Relative Permittivity | Conductivity (S/m) |
|---|---|---|
| Fat | 5.33 | 0.08 |
| Muscle tissue | 53.3 | 1.45 |
| Rib bone tissue | 11.7 | 0.3 |
| Lung inflated | 20 | 0.1 |
| Lung lesion | 60 | 2.5 |
| Artifact Removal | Anechoic Chamber | MammoWave |
|---|---|---|
| Method | SCR (dB) | Device SCR (dB) |
| Rotation subtraction | 4.65 | 6 |
| Ideal artifact removal | 7 | 9.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalesi, B.; Khalid, B.; Ghavami, N.; Raspa, G.; Ghavami, M.; Dudley-McEvoy, S.; Tiberi, G. A Microwave Imaging Procedure for Lung Lesion Detection: Preliminary Results on Multilayer Phantoms. Electronics 2022, 11, 2105. https://doi.org/10.3390/electronics11132105
Khalesi B, Khalid B, Ghavami N, Raspa G, Ghavami M, Dudley-McEvoy S, Tiberi G. A Microwave Imaging Procedure for Lung Lesion Detection: Preliminary Results on Multilayer Phantoms. Electronics. 2022; 11(13):2105. https://doi.org/10.3390/electronics11132105
Chicago/Turabian StyleKhalesi, Banafsheh, Bilal Khalid, Navid Ghavami, Giovanni Raspa, Mohammad Ghavami, Sandra Dudley-McEvoy, and Gianluigi Tiberi. 2022. "A Microwave Imaging Procedure for Lung Lesion Detection: Preliminary Results on Multilayer Phantoms" Electronics 11, no. 13: 2105. https://doi.org/10.3390/electronics11132105
APA StyleKhalesi, B., Khalid, B., Ghavami, N., Raspa, G., Ghavami, M., Dudley-McEvoy, S., & Tiberi, G. (2022). A Microwave Imaging Procedure for Lung Lesion Detection: Preliminary Results on Multilayer Phantoms. Electronics, 11(13), 2105. https://doi.org/10.3390/electronics11132105










