1. Introduction
Knee injuries are certainly the most common cause of problems among individuals who practice sports, at both amateur and professional levels [
1,
2]. In general, any activity requiring repetitive stresses and high load for the knee joint can lead to knee injury. More sedentary people can be subject to knee injury as well due to the destabilization of the joint as a consequence of atrophied muscles [
3]. The diagnosis is generally made by an orthopedic specialist and after the cure (that sometimes may require surgery), the patient can resume his activity or begin a rehabilitation process by undergoing periodic checks consisting in physical examinations [
4] or in diagnostic imaging. As far as diagnostic imaging is concerned, depending on the public or private healthcare system organization, long waiting lists may be endured, and in any case, they are time consuming (a magnetic resonance investigation, for example, takes about half an hour plus the time required for the preparation and collection of the medical report, which could take up to a few days) and can be quite expensive [
5].
Electrical impedance spectroscopy (EIS) has been widely used in biomedical applications for biological tissues’ characterization [
6], for disease identification, such as diabetes [
7,
8,
9], for body composition analysis [
10], for cardiac functions monitoring [
11,
12], for muscles’ condition monitoring during sport activity [
13], in dentistry [
14] and in many other diagnostic fields.
Innovative methods that can result in simplified procedures for monitoring the health status of the knees would be more than welcome in the scientific and medical community and indeed, a few techniques to pursue this end (such as the analysis of the audio signals of degenerative arthritis [
15]) have been proposed.
A few systems based on bioimpedance measurements for determining the progress of osteoarthritis are reported in the scientific literature [
16,
17]. Acute injury characterized by edema can also be identified [
18,
19], and a wearable vector electrical bioimpedance system to assess knee joint health has been proposed [
20], employing an MP150 data acquisition system for data recording [
21].
In consideration of the widespread occurrence of knee injuries, in this paper, we propose a portable system that is easy and safe to use even by individuals who do not have specialized electronic or medical skills. Moreover, the system can be easily reproduced due to its simple structure, its cost effectiveness and the easy availability of all the required components.
To assess the functionality of the new system, it was tested on nine volunteers, with different characteristics of gender, age and activity. Our preliminary results show that a few biological parameters, like the presence of intracellular and extracellular liquids, which are closely related to the state of health of the knee, and the capacitance of the cellular membrane, which is related to the amount of lipids, can be estimated with reference to a simplified model. Different pathologies like osteoarthritis or cartilage damage can be identified as well as the presence of a dominant leg, that is a leg that is subjected to a significantly higher load with respect to the other because of health problems or as a result of asymmetries in posture habits or in sport practices. As part of the study reported in this paper, a new parameter has been investigated as an indicator for comparing the knee conditions between healthy subjects based on the ratio between bioelectrical parameters. Overall, the instrument we propose is suitable to be embedded in a portable system that is a fundamental requirement for many applications.
While we clearly recognize that the limited number of test subjects is insufficient for considering the system and the approach we propose as an established complementary medical device suitable for monitoring the knee health status of any possible individual, we regard our preliminary results as encouraging and worthy of further investigation in the future, including rigorous clinical trials under strict medical supervision.
The hardware and the software components of the system will be discussed in
Section 2 and
Section 3, respectively.
Section 4 will be devoted to a discussion of the methodology adopted for preliminary testing on volunteers, while
Section 5 is dedicated to the discussion of the results that have been obtained.
2. Design and Implementation of the Knee Impedance Measurement System
In the design of the knee impedance measurement system, we regarded portability, low hardware complexity and high versatility as the most important goals to be obtained. Portability is required in order to allow testing outside specialized medical facilities, such as sport centers, outdoors training centers or home environment, and low hardware complexity is required to enable other research groups, especially those interested in the medical application of bioimpedance measurements, to easily reproduce the hardware required for performing experimentation with the system. High versatility is required in order to allow for experimenting with different data acquisition and data elaboration approaches. High versatility can be easily obtained whenever Digital Signal Processing (DSP) approaches can be used for signal generation and data elaboration. In recent years, personal computer stereo sound systems capable of sampling frequencies up to 192 kHz and with a resolution of 24 bits for both inputs and outputs have become widespread and are available at quite affordable cost also as standalone USB-based peripherals. With the ability to process signals in a bandwidth extending above 50 kHz (up to about 80 kHz), any such USB audio board, when coupled with the computing power of any mid-range notebook, can represent a rather standard and easily available building block for signal generation, signal acquisition and signal elaboration. Moreover, standard and easily available software libraries can be used for software development that can be made independent of the specific audio board hardware. With these considerations in mind, we developed our system around the USB board Behringer U-PHORIA UMC202HD (192 kHz, 24 bit [
22]) and the PortAudio library [
23] available in the public domain. Any other audio board with equivalent characteristics can be used. Moreover, if a sound system with the same characteristics is already integrated in the notebook, the external USB audio board is not required. By taking advantage of the audio board and of the computing power of the notebook, the front-end electronics required for the measurement of the bioimpedance can be greatly simplified and can indeed be reduced to the circuit in
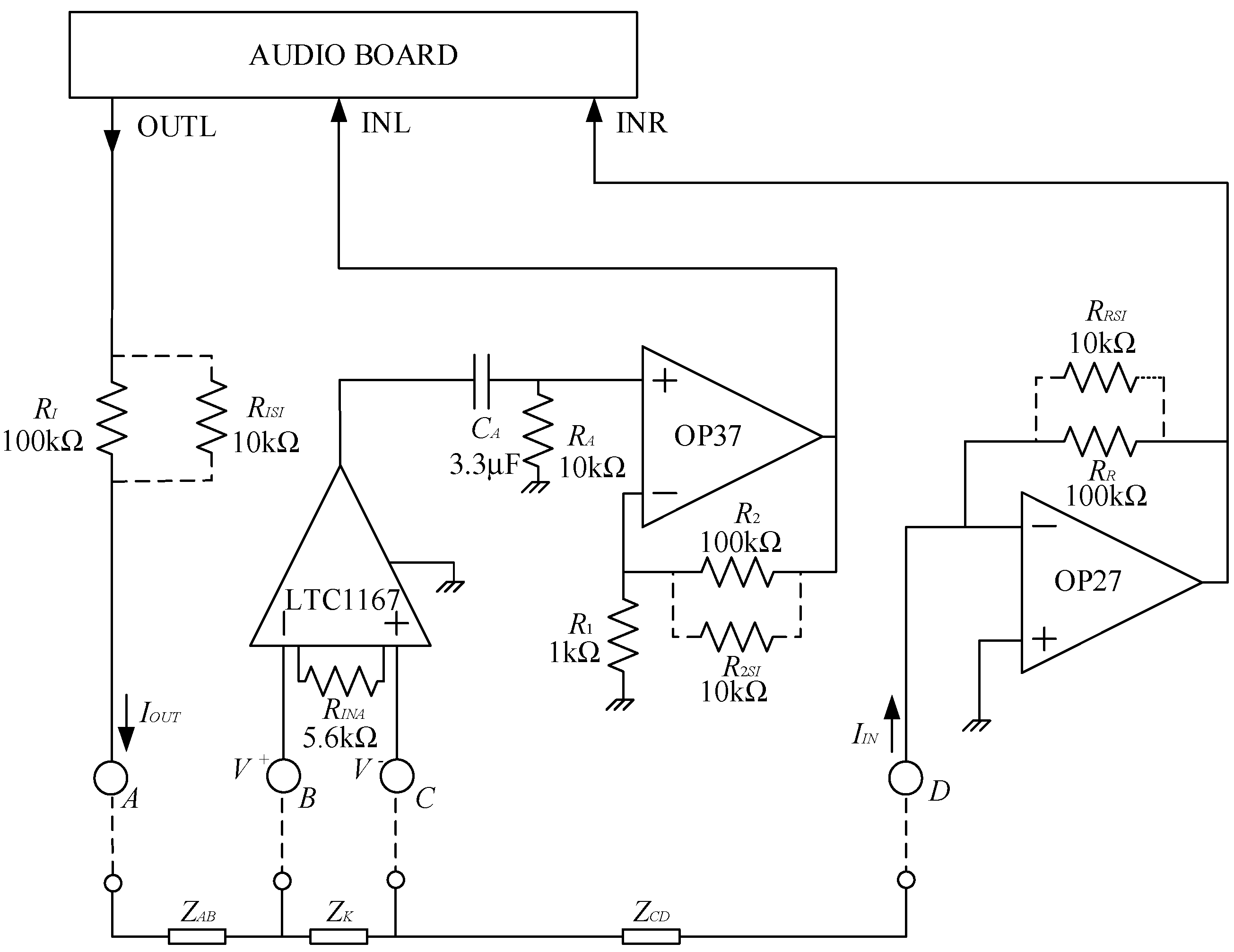
Figure 1.
The audio board is used for both generating the test signal (OUTL) and measuring the current and the voltage drop across the test region. The circuit is supplied by two 9 V batteries. Using a notebook, besides portability, allows to disconnect the system from the mains during operation (the USB audio board is powered by the notebook, as well), thus ensuring the electrical safety of the test subjects. Moreover, since the maximum DC voltages and currents to which the test subject is exposed are low, insulation and other safeties are not needed for the operation of the prototype according to the standard IEC 60601-1.
With reference to the circuit in
Figure 1,
ZK is the impedance of the knee, and
ZAB and
ZCD are the parasitic impedances between electrodes A and B and C and D, respectively. The resistances with the dashed lines (
RxSI) can be inserted (snapped in, hence the subscript
SI) or removed from the circuit in order to change gains and ranges, as it will be discussed in the following. In the prototype used in this work, these changes must be made manually, and this fact allows to employ an extremely simple electronic board. Manual switches can be used, of course, or programmability can be added if required. It must be noted, however, that changing the resistances in the field is seldom required, since, as it will be discussed in the following, the expected range of possible values for
ZK can be easily managed with a fixed setting. Indeed, all testing on actual subjects reported in the following sections have been performed in a fixed configuration with all the
RxSI (with
RxSI = 10 kΩ) inserted.
The resistance,
RI, in
Figure 1 is used to limit the maximum current sent toward the patient. When performing bioimpedance measurements, it is preferable to use current signals rather than voltages because the safety of applying electrical signals in vivo is limited by the magnitude of applied current, and therefore a better control is possible. Assuming
RISI removed from the circuit, with a maximum value of the voltage at the output OUTL of 1 Vrms, the maximum current toward the patient is limited to 10 μA rms (
RI = 100 kΩ). A resistance (
RISI) can be snapped in to increase the level of the current. Typically, we employed
RISI of 10 kΩ that, in parallel with
RI, allows for a maximum current of about 110 μA, that is still well within the safe range for the patient [
24]. As we have noted before, the resistance
RISI, if present, is installed during initial system configuration and it is never accessible to the operator when performing measurements on a patient. Note that the maximum current can only be obtained if the impedance between nodes A and D (|
ZAD| = |
ZAB +
ZK +
ZCD|) is negligible with respect to
RI (or to
RI in parallel to
RISI). This is indeed the case in impedance measurements on a knee, where |
ZAD| is typically in the order of 100 Ω or below, as we shall discuss in the following. The current exiting node A reaches node D (virtual ground) at the input of a trans-resistance amplifier (OP 27 and
RR or
RR//
RRSI) with a trans-resistance gain
GR = −
RR (or
GR = −
RR//
RRSI). The trans-resistance amplifier is used to send a voltage proportional to the actual current through the circuit to the right input INR of the audio board. Clearly, for aligning the output dynamic of the output OUTL with the input dynamic of INR (1 V rms in both cases), we have
RI =
RR, and, if snap-in resistances are used, they also must match, that is
RISI =
RRSI. In order to measure the impedance
ZK between nodes B and C, the voltage between these nodes must be detected and amplified. The values of |
ZK| typically range from few tens of ohms up to about 100 Ω. When working with a maximum current of 10 μA, a gain of about 1000 is required to take advantage of the full dynamic range of the input INL of the audio board (1 V rms), while when operating with
RISI = 10 kΩ in place, a gain of 100 is sufficient. To reach a large bandwidth, so that accurate measurements above 50 kHz can be made, we divided the voltage-amplifying chain into two stages. The first stage is a large bandwidth, JFET input instrumentation amplifier LTC1167, that provides for an amplification of 10 of the differential voltage across the impedance
ZK with a −3 dB bandwidth of 800 kHz. The second stage is a voltage amplifier based on an OP37 operational amplifier. In its default configuration (
R2SI not present), the voltage gain of the second stage is 101 with a −3 dB bandwidth in excess of 600 kHz. When the 10 kΩ snap-in resistance (
R2SI) is in place, the gain reduces to 10.1 with a −3 dB bandwidth in excess of 5 MHz. The AC coupling stage
RA,
CA is used to reject the DC component at the output of the instrumentation amplifier. With
RA = 10 kΩ and
CA = 3.3 μF, the low corner frequency is about 5 Hz, well below the frequency range in which bioimpedance measurements are usually carried out (above a few kHz [
25]).
When performing impedance measurements with the approach outlined in
Figure 1, the impedance at any given frequency is obtained by evaluating the ratio between the phasors at the outputs of the trans-resistance and voltage amplifiers in
Figure 1 (INL and INR). Because of the division, as long as the left and right acquisition channels of the audio board are well-matched (as it is expected to be the case), the detailed amplitude and phase responses of the audio board channels do not introduce errors in the estimation of the impedance. On the other hand, amplitude and phase deviation from the ideal gain of the voltage and trans-resistance amplification chains in
Figure 1 can result in significant errors. As an example, let us assume that we are configuring the circuit with all
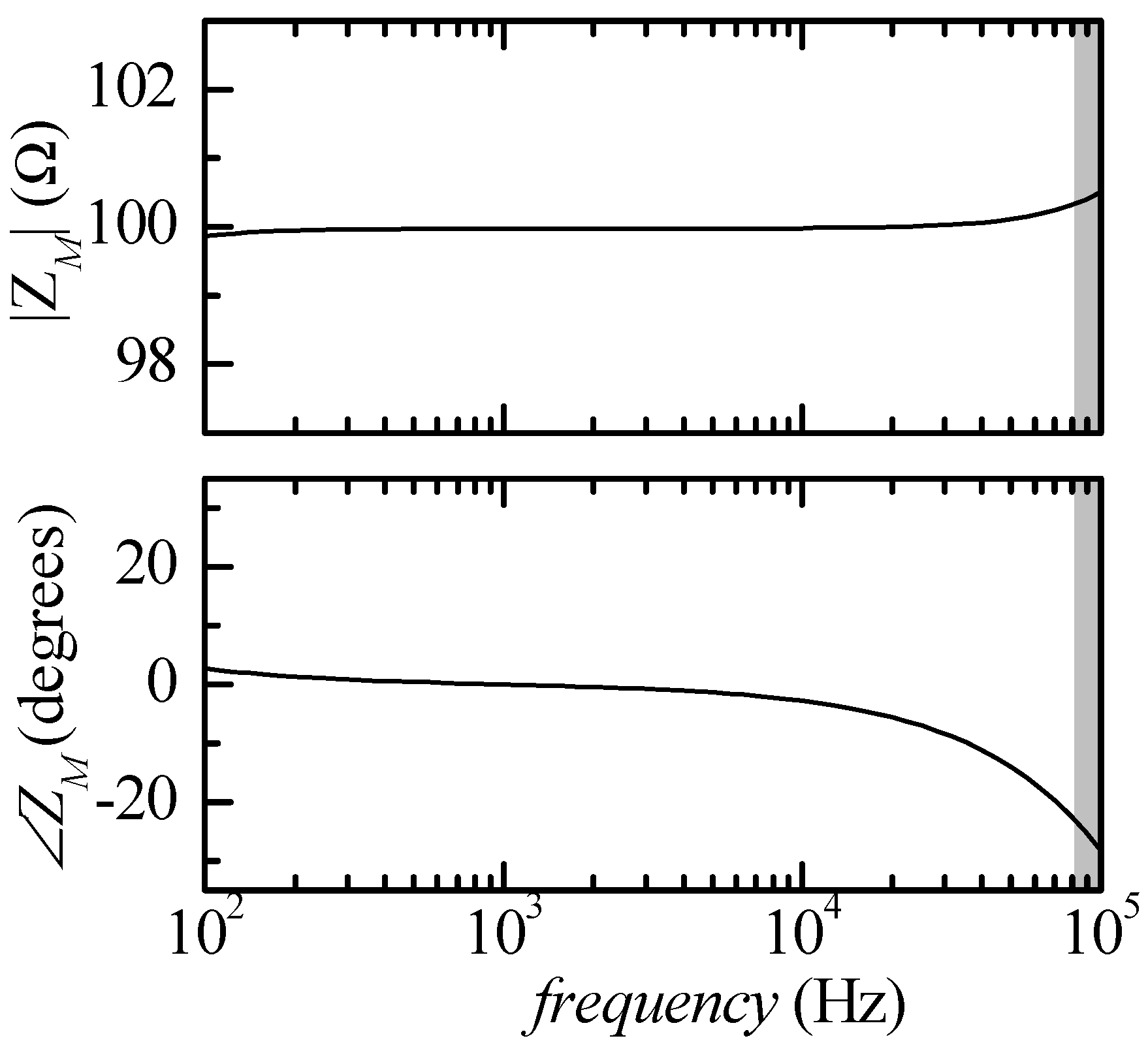
RXSI removed from the circuit (maximum test current about 10 μA rms). We can resort to SPICE simulation to verify what would be the result of measurements in the particular simple case of
ZK =
RK = 100 Ω,
ZAB =
RAB = 10 Ω and
ZCD =
RCD = 10 Ω. The resulting estimated impedance (
ZM) according to such a simulation is reported in
Figure 2, where the grey area indicates the frequency region outside the measuring capability of the audio board being used (
f > 80 kHz).
The deviation with respect to the ideal response |
ZM| = 100 Ω and ∠Z
M= 0 at low frequencies (
f < 500 Hz) is due to the presence of the high pass filter
CARA in
Figure 1. The effect of the high pass filter could be reduced by decreasing the cut-in frequency (by increasing
CA) but, since, as we will discuss presently, we need a calibration procedure for correcting the error at high frequencies, this is not really necessary. Moreover, bioimpedance measurements are rarely performed below a few kHz.
As far as the deviation at higher frequencies is concerned, whereas the error in the modulus of the impedance is small (less than 1%), the error in the phase is much more significant and can be linked to the phase deviation that occurs below the bandwidth limit of the amplifiers in
Figure 1. It must be noted that whereas there could be simple ways to extend the bandwidth of the trans-resistance amplifier and of the second-stage voltage amplifier in the circuit in
Figure 1, this is not the case as far as the instrumentation amplifier is concerned. As far as we have been able to ascertain, there are not many alternatives to the LT1167 in terms of overall bandwidth and CMMR at the highest frequencies of interest.
Starting from the (simulated) result of the measurement reported in
Figure 2, we can however define a complex correction function
HC(
f), defined as follows:
where
ZK is the actual true value of the impedance and
ZM is the impedance as obtained from the measurement with the circuit in
Figure 1. Suppose now we can ensure that the correction function
HC is essentially the same regardless of the actual
ZK (in a reasonable and useful range): if this is the case, we can obtain
HC(
f) from an actual measurement on an actual test impedance (a known calibration impedance
ZC) and then obtain the actual value of any impedance by multiplying the measured value
ZM at any frequency by
HC, as suggested by Equation (1).
In order to verify that this is indeed the case for the specific application we are interested in, we need to mention that the result of bioimpedance measurement on a knee will be interpreted with reference to the equivalent model in
Figure 3 [
16], where the resistance
Rext and
Rint, whose physical meaning will be discussed in the following sections, are expected to range between 30 and 100 Ω and the capacitance
C in the range between 10 and 15 nF.
We therefore performed Monte Carlo SPICE simulations by repeating more than 10,000 spice runs with
ZK in the form of
Figure 3 with random and uncorrelated values, within the expected ranges, for
Rin,
Rext and
C for each run. The correction function
HC was evaluated for each set of values in order to check how much it changed depending on the actual
ZK being tested. In a first simulation set-up, we performed the test in the same conditions in which
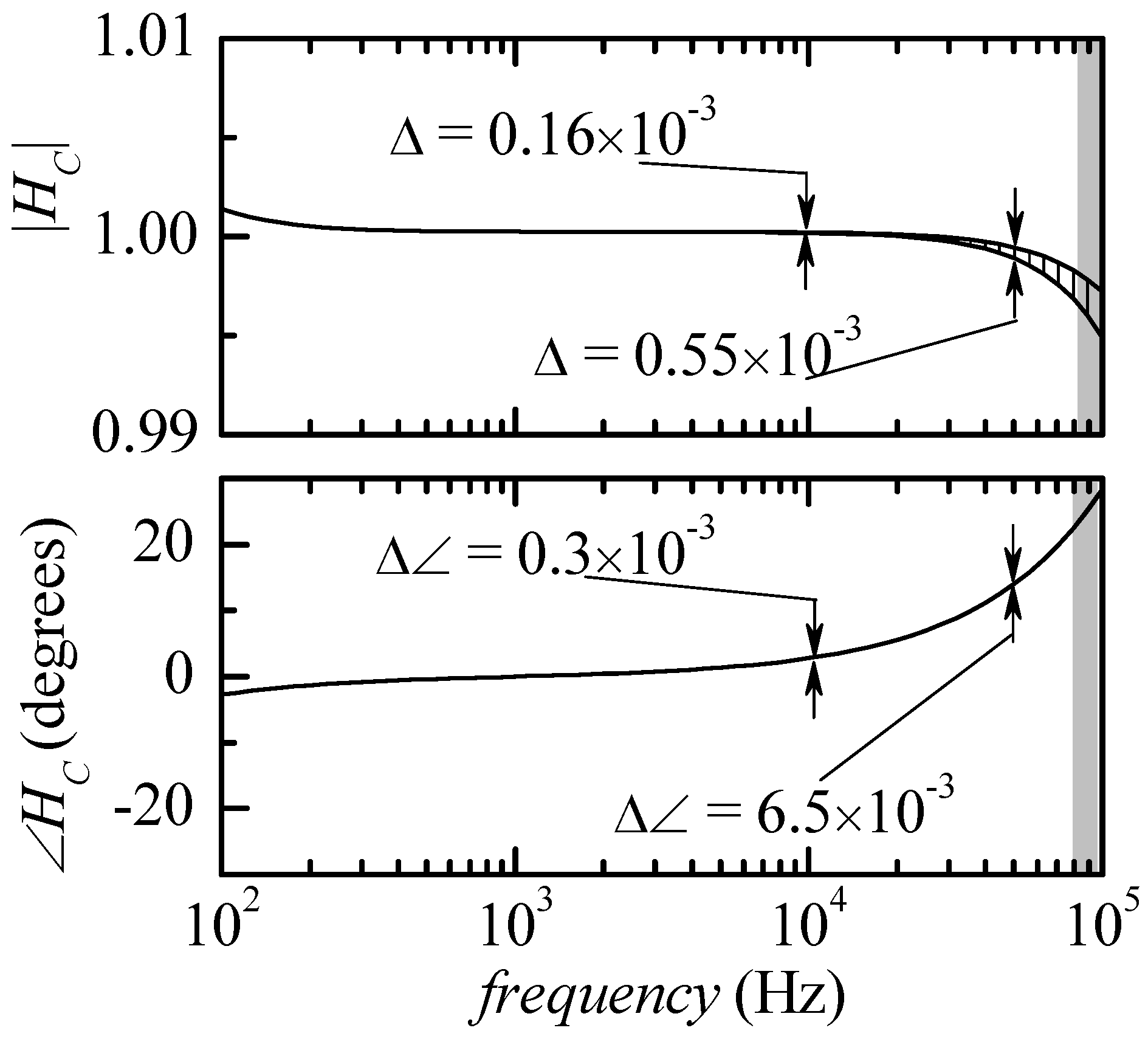
Figure 2 was obtained, that is with all snap-in resistances removed from the circuit. The results are summarized in
Figure 4. All curves representing the modulus and the phase of the correction functions lie inside two limiting curves: the deviation is however so small that the limiting curves can be distinguished, with the scale used for the figure, only in the case of the modulus and only for frequencies above about 20 kHz. It can be noticed that in the entire frequency range that can be explored (outside the gray areas), the deviation in the modulus of the correction function is expected to be less than 0.2% and, more importantly, the deviation in the phase is expected to be less than 0.03 degrees. This means that any calibration curve obtained from a calibrated impedance (in the possible range for
ZK) can be used to obtain a calibration function
HC that can be used for all actual measurements with small errors in the estimation of the modulus (less than 0.2%) and an almost negligible error in the phase.
We have investigated in some detail the origin of the spread in the results for
HC and the reason why such spread increases for increasing frequency. It is clear that for
HC to change as a function of
ZK, either the gain of the voltage amplification chain (from
VBC to the input INL in
Figure 1) or the gain of the current amplification chain (from
IIN to the input INR in
Figure 1), or both, should depend on the value of
ZK. We clearly expect the gain of these two chains to depend on the frequency, but the reason why we observe a dependence, at the same frequency, on the value of
ZK and the reason why this change increases with frequency (with
ZK changing in the same predefined range) are not obvious. There is certainly no reason, as can also be easily verified through simulation, for the gain of current amplification chain to depend on
ZK or in any circuit component to the left of node
D in
Figure 1. Similarly, if the inverting input of the instrumentation amplifier was actually grounded, there would be no reason for the gain of the voltage amplification chain to depend on
ZK. The fact is, however, that the non-inverting input of the instrumentation amplifier is not grounded, and this is indeed the reason why we may obtain a gain that changes with the value of the impedance. In order to understand why, let us assume, for the sake of simplicity, that the impedance
ZCD be completely negligible. In this case, we observe that node C is “virtually” grounded through the input of the trans-resistance stage. More in detail, the impedance from node C toward ground (with
ZCD = 0) is the input impedance of the trans-resistance stage. Whereas in most applications we may assume this impedance to be negligible, in our particular case in which |
ZK| is at most 100 Ω, this is not true in the entire frequency range in which we are interested. In order to estimate such an impedance in our operating conditions, we can safely assume the OP27 to be described by a single pole frequency response with a DC gain
AV0 and a pole frequency
fop. Since we typically operate at frequencies,
f, above 100 Hz (hence
f >>
fop) and well below the gain bandwidth product of the operational amplifier, we can estimate the modulus of the input impedance
ZIT of the transresistance stage to be:
Note that with
RR = 100 kΩ and typical values for
AV0 and
fOP (
AV0 × fOP = 8 MHz for the OP27), at the frequency of 10 kHz we have |
ZIT|≈125 Ω, that is an input impedance larger than the typical
ZK on which we want to perform measurements. The result of this situation is that the common mode voltage at the input of the instrumentation amplifier can be larger than the differential voltage across
ZK. More in general, at low frequencies, |
ZIT| is small, hence the common mode voltage at the input of the LT1167 is small compared to the differential voltage, and this fact, combined with the high value of the CMRR (105 dB for
f < 100 Hz), results in the almost complete rejection of the contribution of the common mode input voltage to the output voltage at node
INL in
Figure 1. As the frequency increases, however, the common mode input voltage for the same differential voltage across
ZK increases because |
ZIT| increases (by 20 dB/decade), and, at the same time, the CMRR decreases (−20 dB/decade above 100 Hz). These two effects both cause a larger and larger contribution of the common mode input voltage to the output voltage at
INL as the frequency increases. The contribution of the common mode input voltage to the output voltage introduces an error, and we can reasonably expect this error to change as
ZK changes and to be larger and larger as the frequency increases. This fact is indeed nicely confirmed by the result of the Monte Carlo simulations reported in
Figure 5, where the maximum deviation (∆) in the modulus of the correction function
HC is reported vs. frequency in the very same conditions used to obtain
Figure 5 (empty squares) and in the case in which 10 kΩ snap-in resistances are inserted in parallel to
RI,
R2 and
RR. We can clearly see that the maximum deviation (∆) increases at a rate of about 40 dB/decade, which can be directly correlated to the increase by 20 dB/decade of the input impedance of the trans-resistance stage and the decrease of the CMRR of the instrumentation amplifier by −20 dB/decade, as discussed above. As a further evidence of the correctness of our interpretation, it can be noticed that when the snap-in resistances are inserted, this causes a reduction by a factor of about 10 in the input impedance of the trans-resistance stage, and this, according to the mechanism discussed above, causes in turn a decrease of the maximum deviation (∆) by the same factor at all frequencies, as can be seen in
Figure 5. Since there is not much that can be done about the CMRR of the instrumentation amplifier, it is clear that the only way to further reduce the maximum deviation would be to design a trans-resistance amplifier with a much smaller equivalent input impedance. On the other hand, especially when the snap-in resistances are present, the maximum deviation is sufficiently small and there is no need to increase the complexity of the circuit. With the snap-in resistances in place, the maximum deviation in the modulus of
HC at 50 kHz is about 0.05 × 10
−3 and, at the same frequency, the maximum deviation in the phase can be estimated to be below 10
−3 degrees.
With these results and given that whenever possible we will employ the circuit in
Figure 1 with all snap-in resistance inserted (larger test current and, hence, larger signal and large signal-to-noise ratio at the input of the audio board), we can conclude that, provided that a calibration measurement is performed on a calibrated impedance, the accuracy that can be obtained with the simple circuit we propose is more than adequate for its application in bioimpedance measurements.
The calibrated impedance does not really need to be in the form of
Figure 3, as long as its modulus is within the expected range for the actual measurement. Indeed, we typically employ a single high-accuracy (0.1%) 100 Ω resistor for
ZK with
ZAB =
ZCD = 10 Ω. Using a single resistor greatly simplifies performing the calibration measurement while remaining effective. To demonstrate this fact, we first extracted the calibration function
HC from actual measurements on the 100 Ω resistor, and then used
HC for correcting the actual measurements on a test impedance in the form of
Figure 3, with
Rext = 100 Ω,
C = 10 nF,
Rin = 100 Ω. The results of such procedure are summarized in
Figure 6. The measurements obtained without correction (black dots) are shown together with the measurement modified using the correction function
HC obtained from a previous measurement on the calibration impedance (empty squares) and with the calculated values for the modulus and the phase of the impedance being tested (continuous black line). As it can be noticed, after correction, the measured values are essentially coincident with the expected ones.
3. Knee Impedance Measurement System: Software
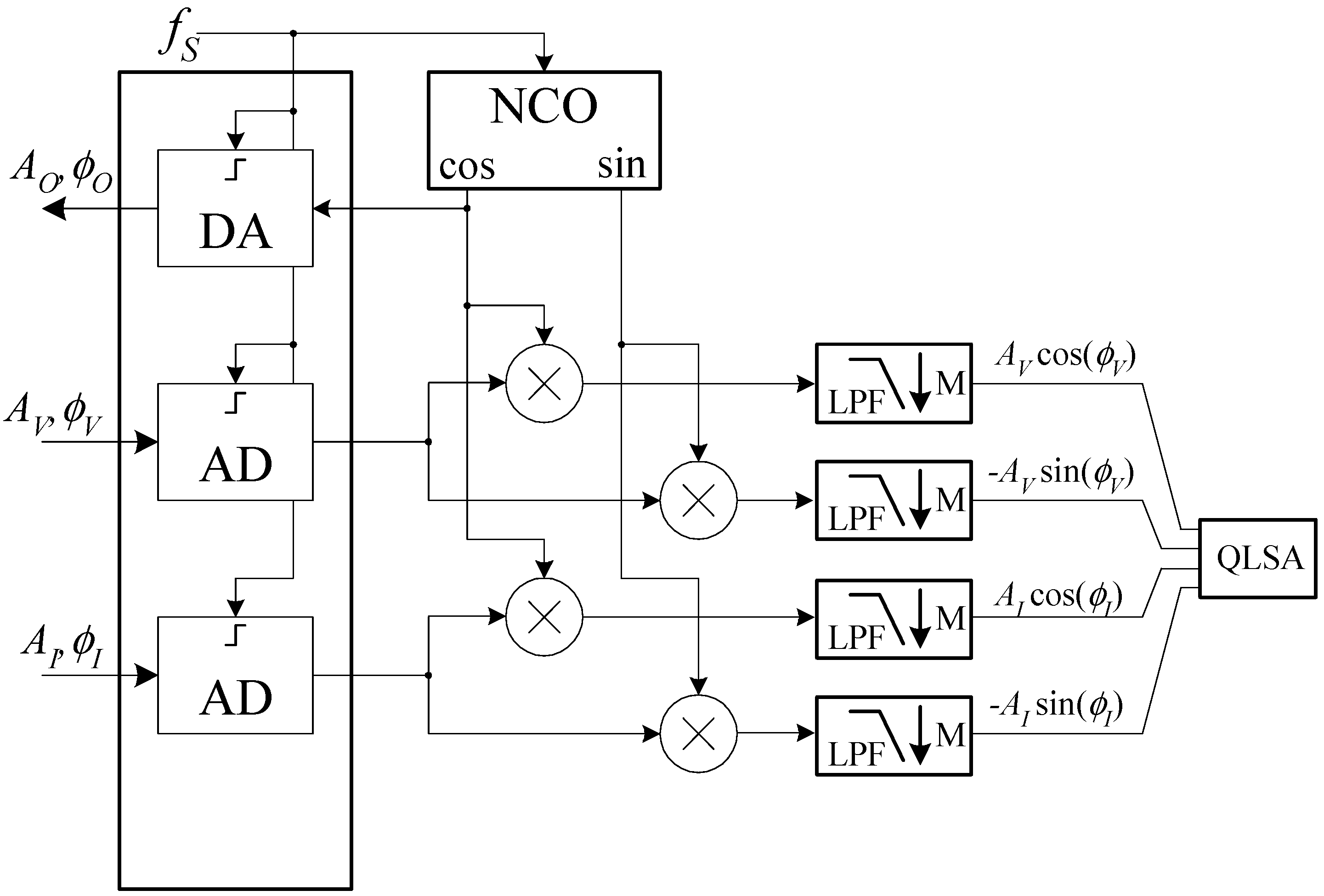
The software must allow to perform impedance measurements vs. frequency, but, in view of the possibility of expanding the application of the system we propose, should also allow to monitor the fluctuations of the impedance vs. time at fixed frequencies. While this last feature has not been implemented yet, the software architecture was designed to enable this functionality in future work. A schematic diagram of the operation of the Digital Signal Processing (DSP) section of the system software is reported in
Figure 7.
The NCO in
Figure 7 is a Numerically Controlled Oscillator working at the audio board sampling frequency. The NCO is based on a 30 bits phase accumulator resulting in a frequency resolution ∆
f well below 1 mHz (∆
f = 179 μHz). The NCO produces two sinusoidal signals that we will assume to be zero-phase cosine and sine functions with an amplitude of 1 V. The cosine signal generated by the NCO is sent to the audio board, resulting in a voltage signal
VO(
t) given by:
As a result of the stimulus
VO, we obtain a sinusoidal current
ik (
t) through the impedance chain
ZAB,
ZK and
ZCD that, in turn, results in a sinusoidal voltage drop
vk(
t) across the impedance of interest
ZK. The board in
Figure 1 amplifies both signals to produce the voltages
VV and
VI at the left and right input of the audio board:
where we have numerically multiplied
GR by −1 so that we obtain the same phase for the voltage and the current in the case of a pure resistance for
ZK and we have assumed, for the sake of simplicity, real gains independent of the frequency. We have, however, explicitly noted the fact that both the amplitude and the phase of the signals can depend on the time as a result of physiological causes, such as the cardiac activity. Both amplitude and phase changes are expected to be small and occurring over a time much longer than the signal period. After analog to digital conversion, each of the input signals is sent to a coherent demodulator implemented in software, so that their amplitude and phase can be extracted. The cut-off frequency of the fourth-order IIR low pass filters (LPF in
Figure 7) is set in such a way that, besides eliminating the high-frequency components at the output of the multipliers, the numerical signal can be decimated by a factor
M = 16, thus reducing the data rate from 192 down to 12 kHz. This sampling frequency is largely sufficient to retain the information due to the possible modulation in the amplitudes and phases of the detected signals. At the outputs of the decimation stages, the in-phase and in-quadrature components can be further low pass filtered in order to reduce noise before the estimation of the impedance. Depending on whether or not we want to retain information on the amplitude and phase fluctuations due to physiological activities, we may have to employ different cut-off frequencies to distinguish phenomena occurring in different frequency bands. In order to simplify such a process and to maintain high flexibility in the data elaboration process, we resorted to the QLSA public domain library [
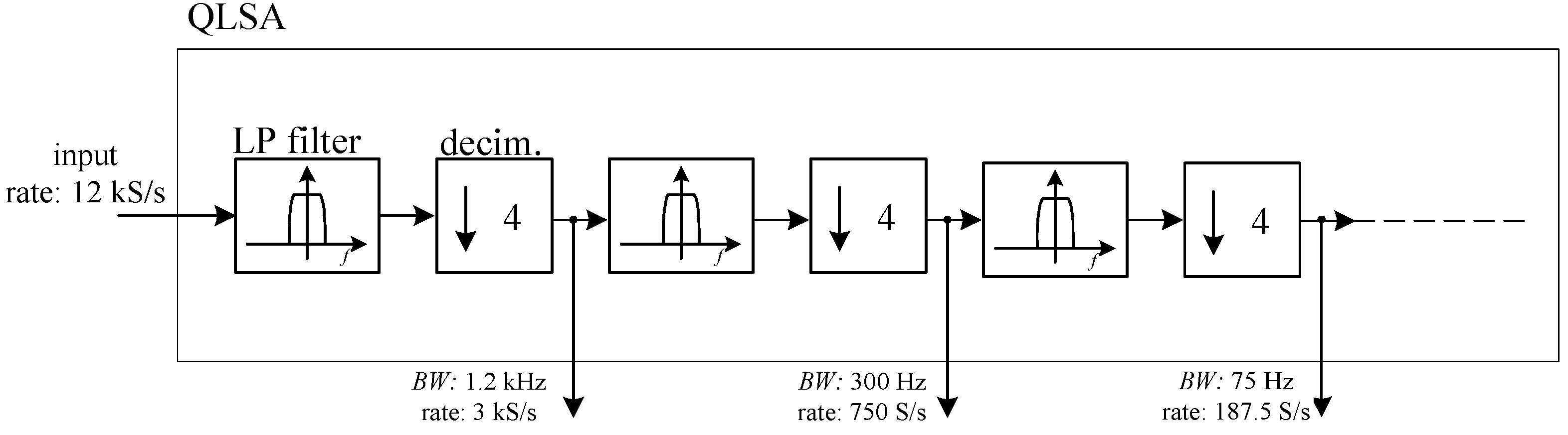
26]. While QLSA is mainly intended for multi-channel spectral analysis, as part of its inner working operation, it performs multi-stage low pass filtering and decimation on the incoming signal stream. More precisely, for each input signal sequence, QLSA is used to perform the filtering operations schematically shown in
Figure 8.
The LP filters in
Figure 7 are low pass FIR filters with a flat response from 0 to 0.1 times the frequency
fR corresponding to the input sampling rate and with an attenuation larger than 70 dB above
fR/4 [
26]. In this way, we can obtain several low pass filtered outputs with geometrically decreasing bandwidth limits (by a factor of 4 at each step) and correspondingly lower sampling rate, thus lowering the load for analysis algorithms at lower sampling rates.
As it is discussed in Reference [
26], adding more filtering and decimation stages after the first two or three has a negligible computational cost, and for this reason, we set the number of stages to 8, although it is rare that one may be interested to the lowest bandwidth obtained in this way (about 70 mHz).
Using any one of the output stages at the output of QLSA, it is trivial to obtain an estimate of the modulus and the impedance of ZK at any given time from the knowledge of the quantities AVcos(ΦV), AVsin(ΦV), AIcos(ΦI), AIsin(ΦI) and from the knowledge of the voltage and trans-resistance gains GV and GR.
In impedance vs. frequency measurements, as used in this work, estimates of the impedance at each frequency are averaged over time in order to reduce the error due to noise (average time is typically one to two seconds).
The software required for the operation of the system is freely available to any researcher willing to experiment with the bioimpedance measurement approach we propose.
4. System Testing
Nine volunteers with different ages (in the range 17–83 years old) and different knee history and conditions participated to the test of the proposed system, after having been thoroughly informed about the measurement procedures and after having signed an informed consent. As we stated in
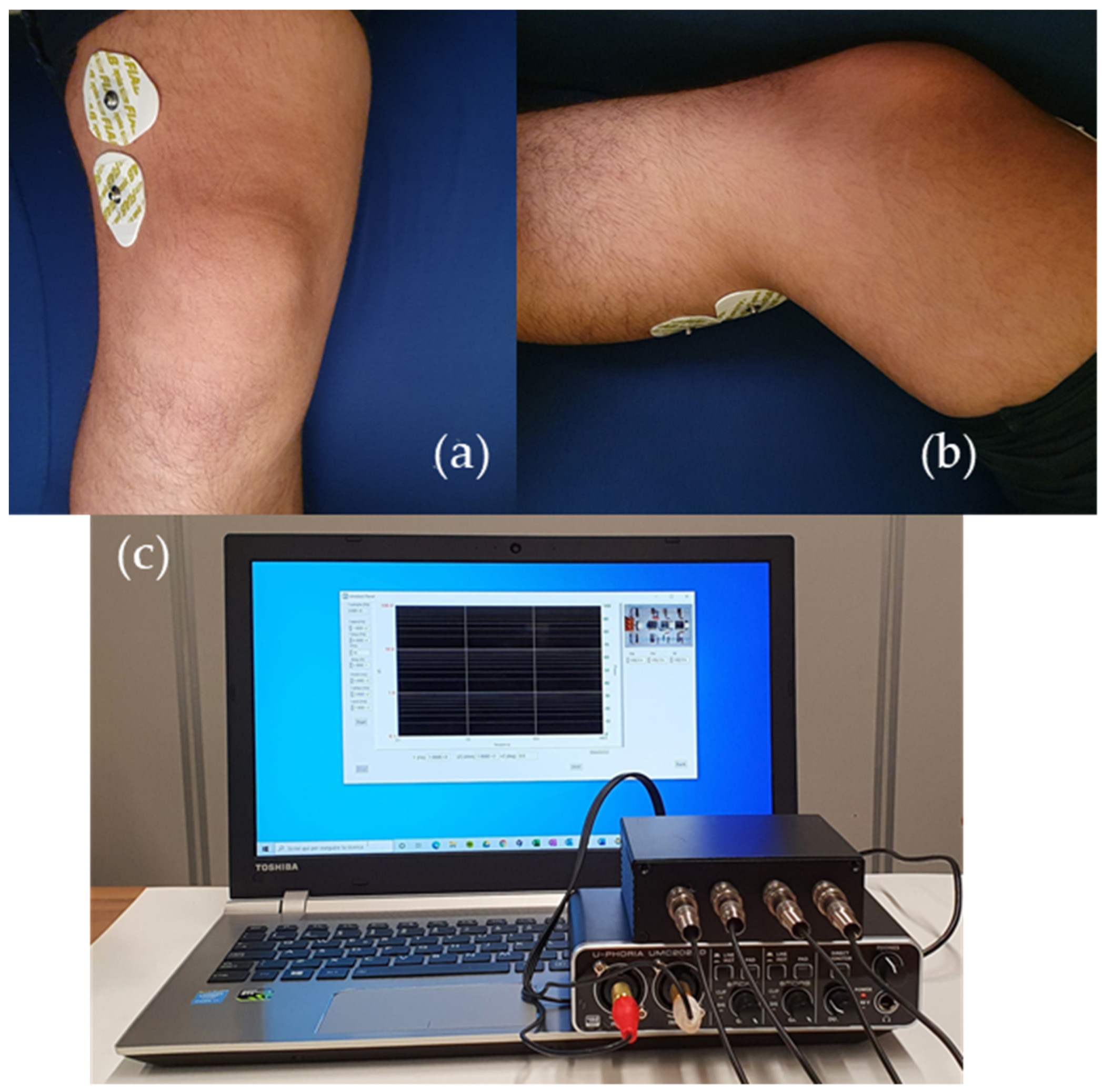
Section 2, the system is used without any connection to the mains, DC voltages and currents are very low and, therefore, insulation and other safeties are not needed for the operation of the prototype, according to the standard IEC 60601-1. Prior to actual measurements, the test impedance used for calibration can be connected to the system in order to check that it is in good working order. Measurements were performed employing standard Ag/AgCl electrodes, disposed to maximize the path of the current within the knee region and to minimize the influence of possible unwanted variables, such as muscle mass, for example. While a few electrode arrangements were tested, the solution employed to obtain the data discussed in this work is the one shown in
Figure 9, together with a picture of the measurement system. The upper and lower (on the back) electrodes are dedicated to the injection and detection of the current and correspond to electrodes A and D in
Figure 1, whereas the electrodes for measuring the voltage across the region of interest are the ones in the middle (electrodes B and C in
Figure 1). This positioning allows to exclude the contribution by the quadriceps and the calf muscles. Moreover, the four-electrodes configuration allows to remove parasitic effects due to cables, electrodes resistances and skin-electrode impedances that could change with skin hydration state and sweat [
27]. To ensure consistency among different test sessions for each single individual, the position of the electrodes with respect to the center of the patella, determined with the help of a measuring tape, was recorded. In this way, we were able to perform consistent measurements on both knees of the same patient, even in different trials, in reproducible conditions. High consistency in the positioning of the electrodes from one individual to another is not necessary, since the interpretation of the measurement result does not rely on the comparison of data obtained from different patients.
No significant differences have been noticed between the execution of the measurements with the subject seated (with the leg extended) or standing. However, since even small involuntary movement can cause fluctuations in the measurements, it was determined to have the patient sitting with the leg extended as this was deemed to be the most stable and most easily reproducible configuration for the leg of the individual during the time required for completing the measurement.
Notice that, on the other hand, significant changes are observed if the leg is bent instead of being extended, possibly because it is difficult to exactly reproduce the same posture in successive trials.
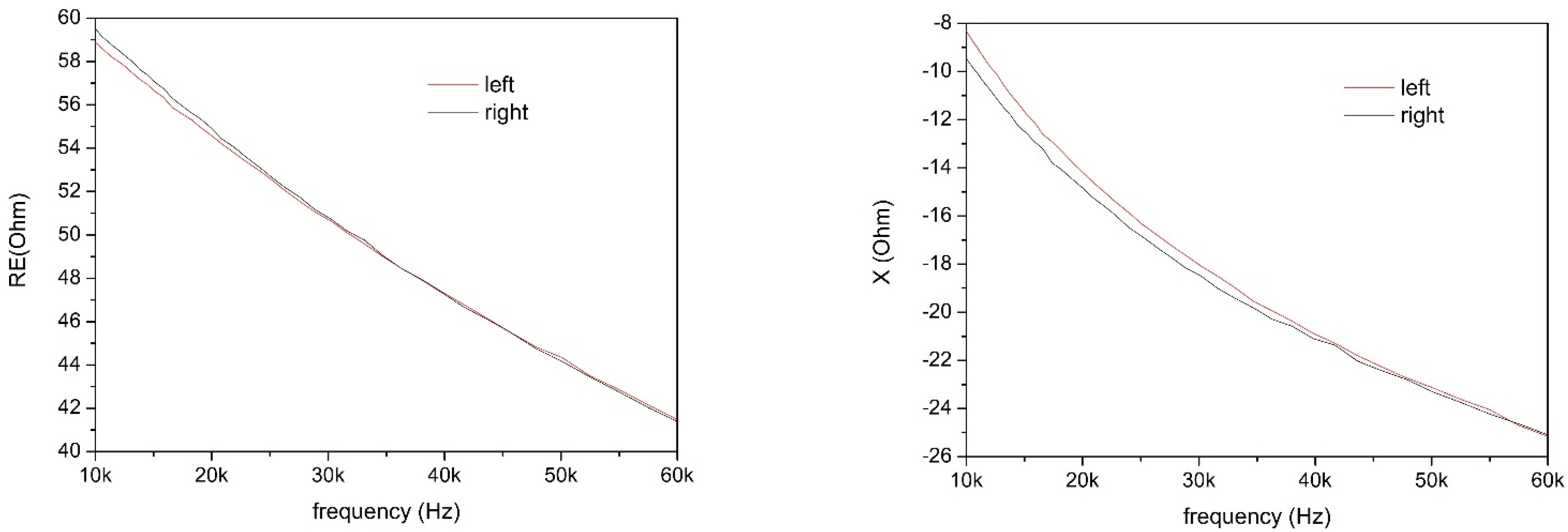
Measurements were performed in the frequency range 10–60 kHz, because this range allows both to reduce losses due to the skin–electrode interface and to employ lower current amplitudes, thus allowing to use the system in better safety conditions [
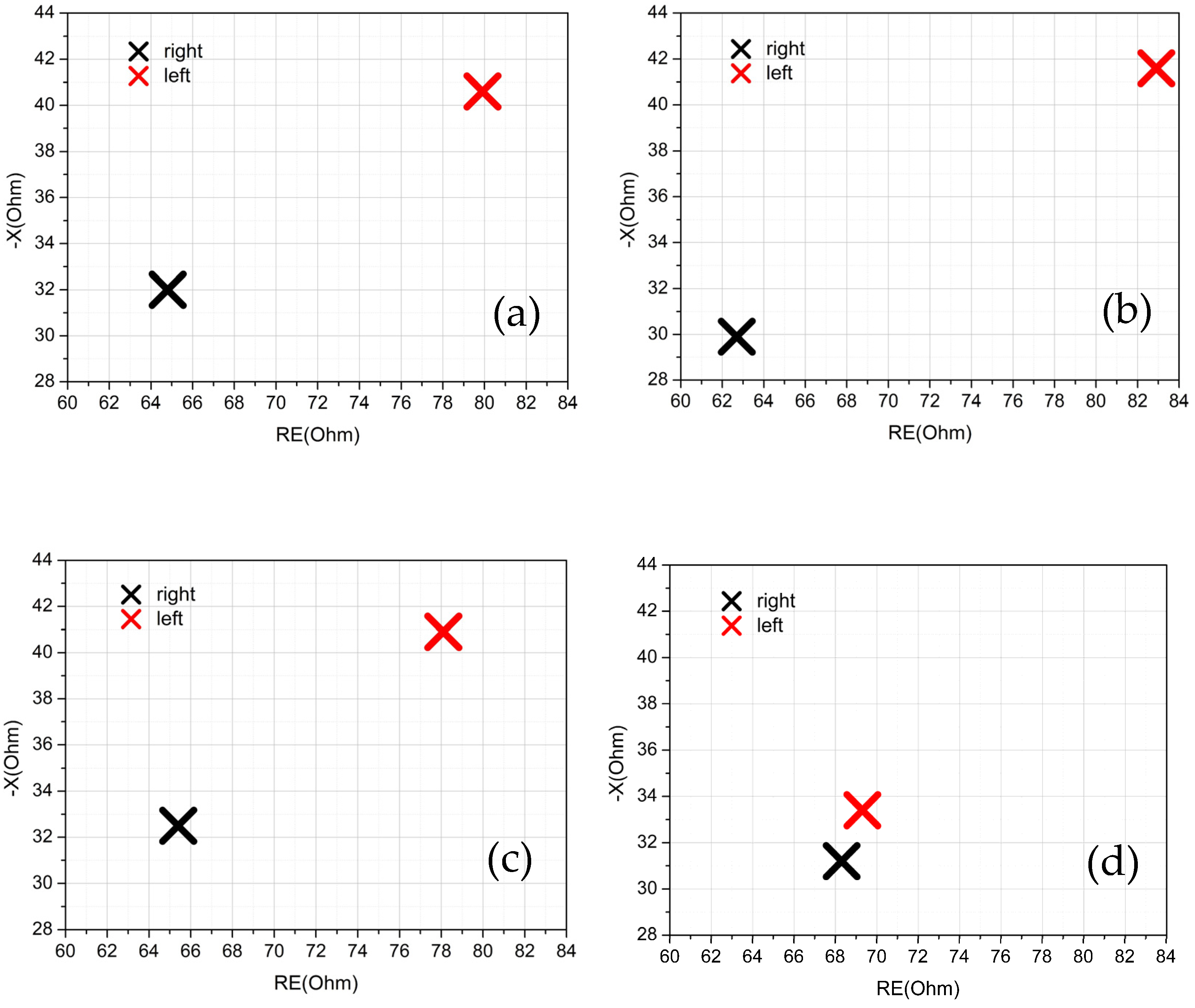
25]. A few bioimpedance parameters were obtained and analyzed through direct measurements and through the study of an equivalent circuit model. In particular, intracellular and extracellular resistances and the real and imaginary parts of the equivalent impedance have been correlated, together with the anamnesis of the volunteers, with the state of health of the knee of each individual.
The results of the measurements are consistent with the knee being modeled as an
RC network as in
Figure 3, where
Rext models the extracellular liquids,
Rin the intracellular liquids and
C the capacitance of the cellular membrane [
16,
28]. The ratio
Rext/
Rin was found to be an important indicator for identifying osteoarthritis or cartilage damage [
16,
17]. Moreover, the ratio of the intracellular resistances of the two knees allows to identify the dominant leg (if any). In addition to this term, the real part (
RE) and the imaginary part (
X) of the impedance were also discriminating factors for determining the health status of the knee, and their value at 50 kHz [
29] was used. Parameters
Rext,
Rin and
C (not used for the study) where extracted by fitting the experimental data (using OriginPro 8 by OriginLab Corporation) against the real (RE) and imaginary (X) parts of the impedance in
Figure 3: