Abstract
Lithium-ion capacitors (LICs) have gained significant attention due to the combination on the advantages of electric double-layer capacitors (EDLCs) and lithium-ion batteries (LIBs). Herein, the LIC pouch cell was fabricated by an activated carbon (AC) cathode and a Li4Ti5O12 (LTO) anode. Two organic electrolytes (1 mol L−1 LiBF4/acetonitrile (AN) and 1 mol L−1 LiPF6/ ethylene carbonate (EC) + ethyl methyl carbonate (EMC) + dimethyl carbonate (DMC)) were chosen and the gas swelling behavior was studied. Compared with the ester-based LIC, the AN-based LIC displays higher energy density of 13.31 Wh kg−1 at 11.4 W kg−1 and even provides a value of 9.1 Wh kg−1 at 1075 W kg−1. Because of the lower DC Resistance of 0.761 mΩ, the maximum power density of the AN-based LIC reaches 12.5 kW kg−1. The AN-based LIC delivers good stability with an energy retention of 88.3% after 900 cycles. It is discovered that the swelling behavior of AN-based LICs is more serious and the major component is H2. The difference of swelling behavior among the LICs, lithium nickel cobalt manganese oxide (NCM)/LTO LIB and AC/AC EDLC is proposed to be caused by the AC electrode and the interfacial reaction of LTO.
1. Introduction
A lithium-ion capacitor (LIC) [1,2,3,4,5,6] is an energy storage device which combines the advantages of a lithium-ion battery (LIB) and an electric double-layer capacitor (EDLC). The most prominent feature is the higher energy density compared with the EDLC, and the power density far exceeds batteries. Amatucci et al. [1] were among the first to fabricate a prototype LIC with a LTO anode and an AC cathode. The LIC displayed the working voltage of 3 V and a packaged energy density of 20 Wh kg−1, which corresponded to a two to three times increase compared with conventional EDLCs at the time. LTO has gained lots of attention since then and has become one of the research highlights for LICs [2,3,4,5,6,7,8,9,10,11].
The LTO anode exhibits excellent reversibility due to its zero-strain characteristics in the charge and discharge process. In addition, LTO demonstrates excellent safety and cyclic performance, making it a potential anode material for high power applications [12,13,14,15,16]. Unfortunately, the LTO anode is not considered the most preferable choice for large-scale applications by the power LIB industries, such as power devices for vehicles or energy storage devices in smart grids, mainly due to low capacities, low energy densities and limited low-temperature performances, especially the gas swelling problem during usage and storage [17,18,19]. The gas swelling, coupled with the consumption of electrolytes, worsens the electrical contact between anode and cathode, which impedes the transmission of ions and electrons. As a result, the impedance and polarization increase, and the capacity of the cell decays rapidly. The mechanism of gas swelling is still under debate [20,21,22,23] and there are only a few studies on the mechanisms of gas swelling of LTO anode [2,17,18,24,25]. Xiong et al. [24] found that LiMn2O4/Li4Ti5O12 cell showed the most severe gas swelling behavior at 100% SOC. Belharouak et al. [17] studied the relationship between the gas swelling and temperature and proposed that elevated temperature would cause capacity and power loss with severe gas swelling. Moreover, they analyzed the gas components and found that the main components include H2, CO2, CO, CH4, C2H6, C2H4, C3H8 and C3H6. Wu et al. [18,26,27] assembled LIB with the LiNi1/3Co1/3Mn1/3O2 (NCM) cathode and the LTO anode, demonstrating that the type and the ratio of electrolyte solvents would affect the gas components, in which PC+DMC (1:1) exhibited the best behavior to suppress the cell swelling. However, most studies focused on the gas swelling of the LTO anode in LIB. Since no gas swelling study on the LIC, it is necessary to explore the research on the field and study the effect of electrolytes on electrochemical behavior and gas swelling in LIC to improve the performance and suppress the gas swelling behavior of LTO cells.
Herein, we assembled LIC with the AC cathode and the LTO anode, using 1 mol L−1 LiBF4/acetonitrile (AN) and 1 mol L−1 LiPF6/ethylene carbonate + ethyl methyl carbonate + dimethyl carbonate (EC+EMC+DMC) as electrolyte, respectively, according to our previous work [28,29]. The electrochemical performances and gas swelling behavior were systematically investigated. It is demonstrated that AN-based LIC possesses higher energy density of 38.1 Wh kg−1 (based on the mass of active materials), higher power density of 12.5 kW kg−1, excellent energy retention of 91.9% at a rate of 10 C, and stable cycle characteristics. The major swelling gas of LICs is H2, the volume fraction of which is higher in AN-based LICs, resulting in more serious gas swelling behavior. The swelling difference among AC/LTO LICs, NCM/LTO LIB and AC/AC EDLC was discussed to confirm that both impacts of AC electrode and interfacial reaction of LTO cause the swelling behavior coordinatively.
2. Materials and Methods
2.1. Electrode Preparation and LIC Assemble
Commercial active materials were utilized as received. The cathode was prepared by coating the mixture of activated carbon (AC, YP50, Kuraray, Tokyo, Japan), carbon black (Super P, Timcal, Bodio, Swizerland), sodium carboxymethyl cellulose (CMC, BVH8, Ashland, Covington, America) and styrene butadiene rubber (SBR, BM-451B, ZEON, Tokyo, Japan) dissolving in deionized water by a mass ratio 100:5:1:4 on Al foil. The active mass loading of the cathode is 110 g/m2 and the size of the cathode is 85 × 207 mm. The anode was prepared by coating the mixture of LTO (LTO-2S, BTR, Shenzhen, China), SP and polyvinylidene fluoride (PVDF, Solef6020, Solvay, Brussels, Belgium) dissolving in N-methyl pyrrolidone (NMP, BYN Chemical, Shandong, China) by a mass ratio of 90:5:5 on Cu foil. The active mass loading of the anode is 100 g/m2 and the size of the anode is 89 × 210 mm. After drying and rolling for as-coated electrodes, the punch LICs were fabricated with a stacked sandwich structure of cathode, separator and anode. A total of 1M LiBF4 in acetonitrile (AN), with the ionic conductivity of 18.4 mS cm−1, and 1 M LiPF6 in the mixture solvent of ethylene carbonate (EC), ethyl methyl carbonate (EMC) and dimethyl carbonate (DMC) (volume ratio, 1:1:1, BASF, Ludwigshafen, Germany) with the ionic conductivity of 9.7 mS cm−1 were chosen as electrolytes, respectively, which were labeled as 1# electrolyte and 2# electrolyte. The preparation of NCM (TLD5506, Xinxiang Tianli Lithium Energy, Henan, China)/LTO LIB and AC/AC EDLC was similar to that of LICs. All of the as-prepared LICs (AC/LTO), LIBs (NCM/LTO) and EDLCs (AC/AC) were assembled in the size of 304 × 109 × 8 mm, as shown in Figure 1.
Figure 1.
As-prepared LIC sample.
2.2. Characterization Techniques
Powder X-ray diffraction (XRD, Bruker D8/Germany) using Cu Kα radiation was employed to identify the properties of the crystal. The XRD test was performed by step mode with a fixed time of 3 s and a step size of 0.02°. Surface morphologies of the electrode samples were observed with a scanning electron microscope (SEM, Hitachi SU1510/Tokyo, Japan). Gas chromatography and mass spectrometry (GC-MS, Agilent 7890B-5977B/ Palo Alto, CA, USA) were conducted to analyze the gas compositions. The galvanostatic charge–discharge (GCD) measurements, rate capability and long-time cycle stability were conducted by using the battery test system (Arbin BT-2000/TX, America). To calculate the swelling ratio, the volumes of LICs, LIBs and EDLCs were measured utilizing the water draining method.
2.3. Electrochemical Measurements
The as-prepared LICs were tested by GCD test within the voltage range 1.5~2.7 V at 1.2 A (1 C), conducted by the 50% SOC power test at for 10 s, tested by the rate capability test at 1.2 A (1 C), 6 A (5 C), 12 A (10 C), 24 A (20 C), 36 A (30 C), 60 A (50 C), 120 A (100 C), and conducted by long-time cycle test within the voltage range 1.5~2.7 V at 6 A (5 C).
3. Results and Discussion
3.1. Electrochemical Performance of LIC
Figure 2a shows the charging–discharging curves of LICs with two electrolytes; all the curves exhibit triangular symmetry, which conforms to the capacitance characteristics, and the AN-based LIC shows larger capacitance. Figure 2b displays the long-time cycle curves of two types of LICs, which obtain their energy retention by 88.3% for AN-based LIC and 84.1% for ester-based LIC after 900 cycles. During the whole cycle life, the AN-based LIC exhibits stable performance with higher discharge energy. The higher energy of the AN-based electrolyte is attributed to high ionic conductivity (18.4 mS cm−1).
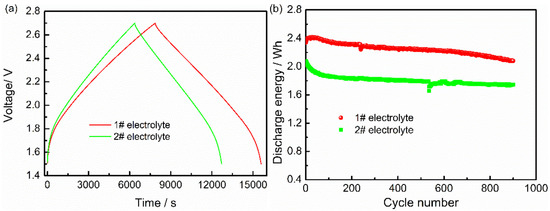
Figure 2.
The (a) charging/discharging curves and (b) cycle life curves of AC/LTO LIC-based different electrolytes.
Table 1 shows the comparison of two AC/LTO LIC-based electrolytes; the performances demonstrate that the AN-based LIC displays a smaller alternating current internal resistance (Rac) and direct current internal resistance (Rdc), coupled with higher discharge energy at 1 C rate and higher charging/discharging power density at 50% SOC, compared with the ester-based LIC.

Table 1.
The performance of AC/LTO LIC-based different electrolytes.
To evaluate the rate capability of LICs, the discharge energy is plotted as a function of C rate in Figure 3a. The energy retention ratio is normalized with respect to the C rate of the same electrode at the slowest rate of 1 C in this research. It can be seen that the AN-based LIC features excellent rate capability and processes the higher energy retention at any C rate compared with ester-based LIC. In addition, due to the insufficient charging at high C rate, the capacity decay is kinetic in nature. Hence, the AN-based LIC maintains up to 91.9% energy retention at a rate of 10 C and 57.2% energy retention at a rate of 100 C. Figure 3b shows the rate capability of LICs after charging and discharging for 900 cycles, it can be seen that the AN-based LIC maintains up to 74.8% energy retention at a rate of 10 C and 40% energy retention at a rate of 100 C, which rate capability is still superior to that of ester-based LIC.
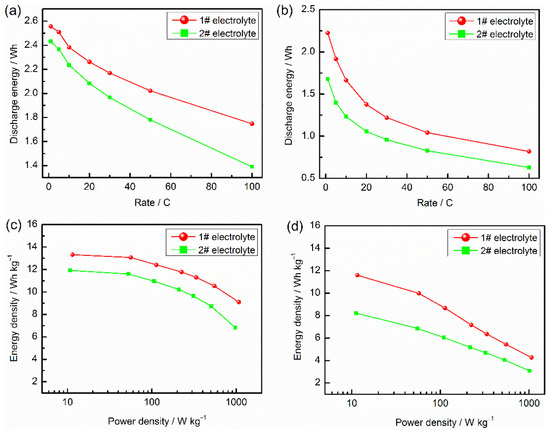
Figure 3.
Rate capability of AC/LTO LICs based different electrolytes: (a) before cycle; (b) after 900 cycles; Ragone plots of AC/LTO LICs based different electrolytes: (c) before cycle; (d) after 900 cycles.
The Ragone plots of specific energy vs. specific power for LIC employing two electrolytes is illustrated in Figure 3c. The LIC with AN electrolyte exhibits higher energy density of 13.31 Wh kg−1 at 11.4 W kg−1 and even provides a value of 9.1 Wh kg−1 at 1075 W kg−1. Moreover, the energy density of the AN-based LIC is 38.1 Wh kg−1 based on the mass of active materials. Figure 3d shows the Ragone plots of LICs after charging and discharging for 900 cycles. The LIC with AN electrolyte exhibits energy density of 11.59 Wh kg−1 at 11.62 W kg−1 and even provides a value of 4.27 Wh kg−1 at 1068.73 W kg−1. Compared with the LICs before cycle, the rate capability, power density and energy density of LTO are falling obviously, which is mainly attributed to the gas swelling behavior. The electrochemical performance is comparable with previously reported similar works ([6,30,31,32,33], Table S1).
The maximum power density of LIC can obtain when the terminal voltage reaches half of the rated voltage, during the process of instantaneous power output, which can be calculated by Equation (1). The maximum power density of the AN-based LIC is calculated at 12.5 kW kg−1, whereas that of the ester-based LIC is 8.38 kW kg−1 accordingly.
where Pmax (W kg−1) denotes the maximum power density of LIC, UR (V) denotes the rated voltage of LIC, R (Ω) denotes the direct current internal resistance of LIC, m (kg) denotes the weight of LIC, and I (A) denotes the instantaneous current of LIC when the terminal voltage reaches half of the rated voltage.
3.2. Gas Swelling Behavior of LIC
To investigate the influence of electrolyte on swelling behavior, the LICs before and after charging and discharging for 900 cycles (before cycle, after cycle), rested at 25 °C (RT, 2.7 V) and 55 °C (HT, 2.7 V) for 60 days were studied, respectively, and the volume expansibility is showed in Figure 4. It can be seen that the volume expansibility of all LICs at any state is over 300%, and are over 400% at HT significantly, indicating that elevated temperature would cause severe gas swelling even in the rest state. Moreover, the volume expansibility of LICs after 900 cycles is between the above two states and are about 350%. It should be noted that all LICs using an AN electrolyte expand more seriously; it is worth further analyzing the components of the swelling gas.
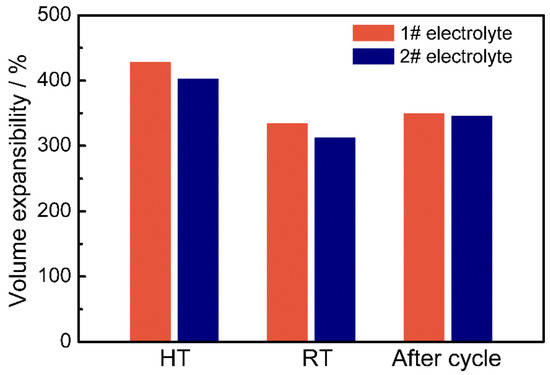
Figure 4.
The volume expansibility of AC/LTO LIC at different states (HT: high temperature, RT: room temperature).
Figure 5 shows the relative amounts of gases collected from the LICs with two electrolytes at three different states, and the volume fraction of the gases are listed in Table 2. It can be claimed that gases such as H2, CO2, N2, O2, CO and CxHy alkanes were determined by GC (raw data can be seen in Figures S1–S14). The most abundant gas collected in all LICs is H2, and the volume fractions of them are all over 70%, indicating that H2 generation of all LICs fundamentally causes the gas swelling behavior. The possible gas evolution mechanism can be suggested by the moisture in the cell and the intrinsic reaction of the LTO. The moisture in the graphite electrode could be eliminated during formation since the reduction of water peaked at 1.2 V vs. Li/Li+. However, a large part of the absorbed moisture in the LTO electrode remains intact after formation because its working potential is above 1.3 V vs. Li/Li+ [26]. The remained moisture would chemically react with the electrolytes, which could catalyze the solvents decomposing to various gas species. In terms of the intrinsic reaction of the LTO, a spontaneous charge transfer from Ti3+ to Ti4+ and release of an electron would further influence the oxidation/reduction in the organic molecules on the surface of the LTO electrode and result the decomposition of the electrolyte [34]. In this connection, the LTO electrodes before and after the cycle were characterized by XRD, as shown in Figure 6. The crystalline phases are still dominated by spinel LTO whether in AN-based or in ester-based electrolytes, indicating that charging/discharging and gas swelling do not lead to the structure collapse of LTO. It should be pointed out that the volume fraction of H2 of all AN-based LICs is higher than that of ester-based LICs, which is the root cause of the more severe expansion of AN-based LICs. In addition to H2, the generated gases of AN-based LICs mainly includes N2 and CO, resulting from the hydrolysis, hydrohalogenation, dimerisation, cyclisation and isomerisation reactions of AN solvent [35]. The gas of N2 can be generated by the oxidation of AN, and H2 can be produced by the reduction of AN [36].
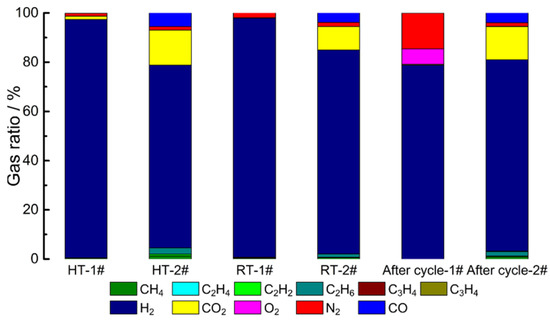
Figure 5.
The gas composition of AC/LTO LIC at different states.

Table 2.
The volume fraction of various gas for AC/LTO LIC.
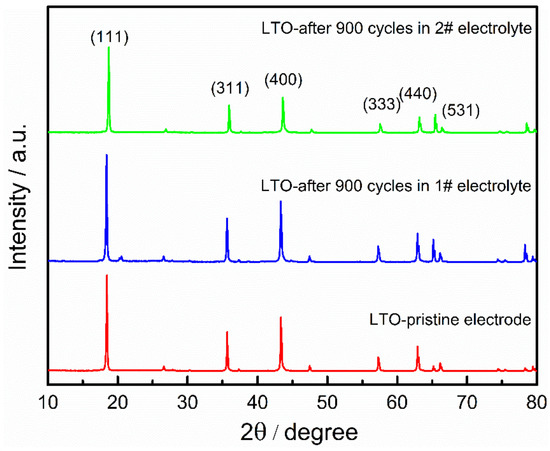
Figure 6.
XRD pattern of LTO electrode before and after 900 cycles.
As for the ester-based LICs, the gases generated primarily include CO2 and CO besides H2, deriving from the decarboxylation, decarbonylation and dehydrogenation reactions of the electrolyte [37]. Given the production of PF5, a strong Lewis acid and one of the decomposition products of LiPF6 [17], has been considered as the major source of initiating the gassing reactions and should be responsible for the generation of CO2, CO and CxHy alkanes. The gas of CO and CO2 can be produced by the decomposition routes for EC [17].
As mentioned above, the prime difference for gas swelling behavior of LICs is the impact of the electrolyte, which causes the more severe gas evolution of AN-based LICs. To further explore the influence of the electrolyte, the two electrolytes were employed at LIB (NCM/LTO) and EDLC (AC/AC). The gas swelling behavior of them was analyzed, including the volume expansibility after RT rest, as shown in Figure 7. It can be seen that the gas swelling behavior indeed exists in all kinds of cells; however, the volume expansibility of LIB was the least whether affected by any electrolyte significantly. The volume expansibility of EDLC cells is larger than that of LIBs, but smaller than that of LICs. Figure 8 and Figure 9 show the gas composition of LIB (HT), and of EDLC (HT and after cycle). It can be found that the major gas component of AN-based LIB is N2, which mainly comes from the hydrolysis, hydrohalogenation, dimerisation, cyclisation and isomerisation reactions of AN solvent [35]. The major gas conponent of ester-based LIB is CO2; the possible mechanism is that the terminated Ti4+ ions of LTO coordinate with the unshared electron pairs of O2− ions of carbonyl groups in the electrolyte [38]. The introduction of N2 is inevitable, similar to the reported research [17,34]. It should be noted that there are no H2 in both AN-based and ester-based LIBs. In stark contrast, the main components of the swelling gas in EDLCs are H2, N2, CO and CO2, similarly to LICs. The major gas components in AN-based EDLC include H2 and N2, which comes from the remaining moisture in the AC electrode and the reactions of the AN solvent. The major gas component in ester-based EDLC include H2 and CO2, which comes from the remaining moisture in the AC electrode and results in the decomposition of H2O. The electron from the decomposition may coordinate with the unshared electron pairs of O2− ions of ester-based electrolytes and then cause the formation of CO2. It could be concluded that the AC electrode, an absorber of moisture in the air with high specific surface area [39], is one of the root causes for gas swelling behavior of LICs. Considering the volume expansibility of EDLCs is smaller than LICs, it could be inferred that the intrinsic reaction of the LTO catalyzed the decomposition of the electrolyte coupled with the irreversible reactions on the surface of LTO electrode, resulting the more severe gas swelling behavior of LICs.
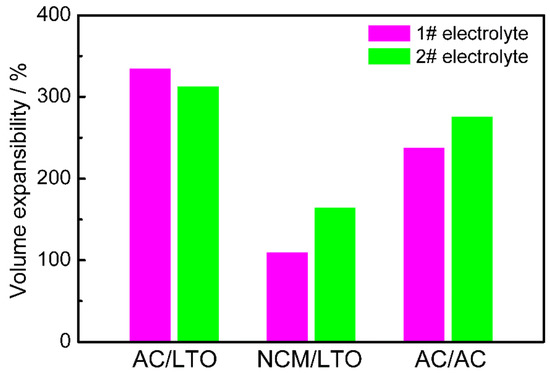
Figure 7.
The volume expansibility of AC/LTO, NCM/LTO and AC/AC devices after storage at room temperature.
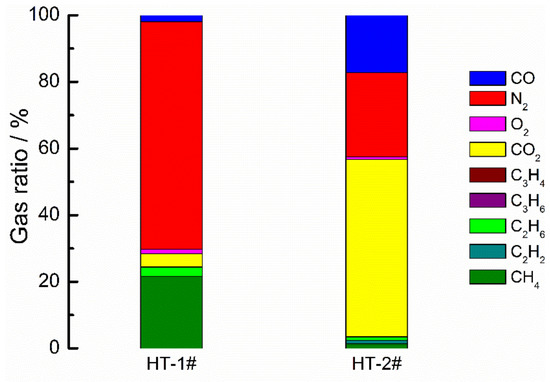
Figure 8.
The gas composition of NCM/LTO LIB at different states (HT: high temperature).
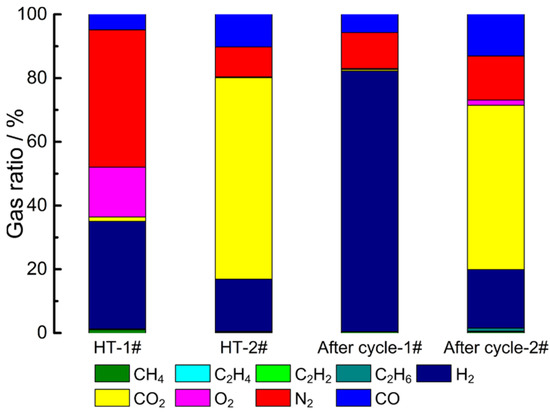
Figure 9.
The gas composition of AC/AC EDLC at different states (HT: high temperature).
4. Conclusions
In summary, we have fabricated LIC pouch cells applying AC as a cathode, and LTO as an anode and 1 mol L−1 LiBF4/AN and 1 mol L−1 LiPF6/EC+EMC+DMC as an electrolyte, respectively. Owing to the higher ionic conductivity of the AN-based electrolyte, the corresponding LIC displays a higher energy density of 38.1 Wh kg−1 (based on the mass of active materials), the maximum power density of 12.5 kW kg−1, the lower DC Resistance of 0.761 mΩ, and better rate capability of 91.9% energy retention at a rate of 10 C and 57.2% energy retention at a rate of 100 C. Furthermore, the AN-based LIC retains 88.3% of its original capacity after 900 cycles with a good stability. Gas analysis shows that the generated gas is mainly H2, which is the root cause of the volume expansion. The higher volume fraction of H2 in AN-based LICs leads to more serious gas swelling behavior. Further lucubrating the gas evolution of LIBs and EDLCs, both impacts of the AC electrode and the intrinsic reaction of the LTO could be proposed to catalyze the decomposition of the electrolyte and the irreversible reactions on the surface of the LTO, resulting in the more severe gas swelling behavior of LICs. More research on suppressing gassing formation is necessary and urgent; the surface coating on the LTO can avoid direct contact between the LTO and the electrolyte, and additives in electrolytes may promote the formation of stable films on LTO, moisture control on active materials, electrolyte solute/solvent selection, LTO surface modification and anode potential control, which are all feasible strategies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/electronics10212623/s1, Figure S1: GC raw data (inorganic part) of AN-based LIC after rest at 55 °C for 60 days, Figure S2: GC raw data (organic part) of AN-based LIC after rest at 55 °C for 60 days, Figure S3: GC raw data (inorganic part) of ester-based LIC after rest at 55 °C for 60 days, Figure S4: GC raw data (organic part) of ester-based LIC after rest at 55 °C for 60 days, Figure S5: GC raw data (inorganic part) of AN-based LIC after rest at 25 °C for 60 days, Figure S6: GC raw data (organic part) of AN-based LIC after rest at 25 °C for 60 days, Figure S7: GC raw data (inorganic part) of ester-based LIC after rest at 25 °C for 60 days, Figure S8: GC raw data (organic part) of ester-based LIC after rest at 25 °C for 60 days, Figure S9: GC raw data (inorganic part) of AN-based LIC after 900 cycles, Figure S10: GC raw data (organic part) of AN-based LIC after 900 cycles, Figure S11: GC raw data (inorganic part) of ester-based LIC after 900 cycles, Figure S12: GC raw data (organic part) of ester-based LIC after 900 cycles, Figure S13: calibration curves (inorganic part) of GC test, Figure S14: calibration curves (organic part) of GC test, Table S1: The comparison on electrochemical performance among this study and obtained results.
Author Contributions
Conceptualization, Z.A. and J.X.; methodology, Z.A. and X.X.; validation and formal analysis, L.F. and C.Y.; investigation and resources, Z.A.; data curation, X.X.; writing—original draft preparation, Z.A. and L.F.; writing—review and editing, C.Y.; visualization, supervision and project administration, Z.A.; funding acquisition, J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science and Technology Commission of Shanghai Municipality (19DZ1203102), Science and Technology Commission of Shanghai Municipality (19DZ2292400), Shanghai Sailing Program (21YF1411900).
Acknowledgments
The authors thank Shanghai Aowei Technology Development Co., Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, M.; Liu, W.; Zhu, Y.; Xu, Y. Electrochemical performance of pre-lithiated graphite as negative electrode in lithium-ion capacitors. Russ. J. Electrochem. 2014, 50, 1050–1057. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Menocal, S.; Amatucci, G. A comparative study of Li-ion battery, supercapacitor and nonaqueous asymmetric hybrid devices for automotive applications. J. Power Sources 2003, 115, 171–178. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Laforgue, A.; Simon, P. Li4Ti5O12/poly(methyl)thiophene asymmetric hybrid electrochemical device. J. Power Sources 2004, 125, 95–102. [Google Scholar] [CrossRef]
- Du Pasquier, A.; Plitz, I.; Gural, J.; Badway, F.; Amatucci, G.G. Power-ion battery: Bridging the gap between Li-ion and supercapacitor chemistries. J. Power Sources 2004, 136, 160–170. [Google Scholar] [CrossRef]
- Ajjan, F.N.; Khan, Z.; Riera-Galindo, S.; Lienemann, S.; Vagin, M.; Petsagkourakis, I.; Gabrielsson, R.; Braun, S.; Fahlman, M.; Inganäs, O.; et al. Doped Conjugated Polymer Enclosing a Redox Polymer: Wiring Polyquinones with Poly(3,4-Ethylenedioxythiophene). Adv. Energy Sustain. Res. 2020, 1, 2000027. [Google Scholar] [CrossRef]
- Raj, H.; Saxena, S.; Sil, A. Li4Ti5O12/AC Hybrid Supercapacitor combining High Power of Supercapacitor and High Energy of Li-ion battery. In Proceedings of the 9th International Conference of Materials Processing and Characterization (ICMPC), Gokaraju Rangaraju Inst Engn & Technol, Hyderabad, India, 8–10 March 2019. [Google Scholar]
- Choi, H.S.; Im, J.H.; Kim, T.; Park, J.H.; Park, C.R. Advanced energy storage device: A hybrid BatCap system consisting of battery–supercapacitor hybrid electrodes based on Li4Ti5O12–activated-carbon hybrid nanotubes. J. Mater. Chem. 2012, 22, 16986–16993. [Google Scholar] [CrossRef]
- Cericola, D.; Novák, P.; Wokaun, A.; Kötz, R. Hybridization of electrochemical capacitors and rechargeable batteries: An experimental analysis of the different possible approaches utilizing activated carbon, Li4Ti5O12 and LiMn2O4. J. Power Sources 2011, 196, 10305–10313. [Google Scholar] [CrossRef]
- Naoi, K. ‘Nanohybrid Capacitor’: The Next Generation Electrochemical Capacitors. Fuel Cells 2010, 10, 825–833. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.; Naoi, W. Second generation ‘nanohybrid supercapacitor’: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Jain, A.; Aravindan, V.; Jayaraman, S.; Kumar, P.S.; Balasubramanian, R.; Ramakrishna, S.; Madhavi, S.; Srinivasan, M.P. Activated carbons derived from coconut shells as high energy density cathode material for Li-ion capacitors. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ferg, E.; Gummow, R.J.; Kock, A.d. Spinel Anodes for Lithium-Ion Batteries. J. Electrochem. Soc. 1994, 141, 147–150. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero-strain insertion material of Li [Li1/3Ti5/3] O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
- Aldon, L.; Kubiak, P.; Womes, M.; Jumas, J.C.; Olivier-Fourcade, J.; Tirado, J.L.; Corredor, J.I.; Vicente, C.P. Chemical and electrochemical Li-insertion into the Li4Ti5O12 spinel. Chem. Mater. 2004, 16, 5721–5725. [Google Scholar] [CrossRef]
- Park, K.S.; Benayad, A.; Kang, D.J.; Doo, S.G. Nitridation-Driven Conductive Li4Ti5O12 for Lithium Ion Batteries. J. Am. Chem. Soc. 2008, 130, 14930. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, A.; Wibowo, A.; Asri, L.A.T.W.; Yin, S.; Purwasasmita, B.S. Improved ionic conductivity of porous Li4Ti5O12 synthe-sized by sol-gel method using eggshell membrane as soft template. Mater. Res. Express 2019, 6, 7. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Tan, T.; Yumoto, H.; Ota, N.; Amine, K. Performance Degradation and Gassing of Li4Ti5O12/LiMn2O4 Lithium-Ion Cells. J. Electrochem. Soc. 2012, 159, A1165–A1170. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Zhang, Y.; Wang, C.Y.; Wang, D.Y. Investigation on Li4Ti5O12 batteries developed for hybrid electric vehicle. J. Appl. Electrochem. 2012, 42, 989–995. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.; Zheng, S. Challenges of Spinel Li4Ti5O12 for Lithium-Ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Li, M.; Liang, Z.; Zheng, W. Progress of research on gassing prevention in lithium-ion batteries using an anode based on lithium titanate. Dongfang Electr. Rev. 2016, 30, 1–4. [Google Scholar]
- Xu, S.; Gao, Y.; Duan, Q.; Zhao, C. Research on rate performance and gas production of lithium titanate-based lithium ion battery using different electrolytes. Hans J. Chem. Eng. Technol. 2018, 8, 367–372. [Google Scholar] [CrossRef]
- Gao, J.; Gong, B.; Wang, G.; Dai, Y.; Fan, W. Research progress of gassing behavior of Li4Ti5O12 batteries. Chin. J. Power Sources 2015, 39, 2010–2013. [Google Scholar]
- Xiong, Y.; Xu, S.; Jing, L.; Du, P.; Gao, J. The study of gas swelling in the LiMn2O4/Li4Ti5O12 cell. In Proceedings of the 15th International Meeting on Lithium Batteries-IMLB, Montreal, QC, Canada, 27 June–2 July 2010; The Electrochemical Society: Pennington, NJ, USA, 2010. Abstract: MA2010-03 767. [Google Scholar]
- Ding, Z.J.; Zhao, L.; Suo, L.M.; Jiao, Y.; Meng, S.; Hu, Y.S.; Wang, Z.X.; Chen, L.Q. Towards understanding the effects of carbon and nitrogen-doped carbon coating on the electrochemical performance of Li4Ti5O12 in lithium ion batteries: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 15127–15133. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, L.; He, X.Q.; Xiao, R.J.; Gu, L.; Hu, Y.S.; Li, H.; Wang, Z.X.; Duan, X.F.; Chen, L.Q.; et al. Lithium Storage in Li4Ti5O12 Spinel: The Full Static Picture from Electron Microscopy. Adv. Mater. 2012, 24, 3233–3238. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.Y.; Xu, J.M.; Ning, F.; Wang, D.Y. Investigation on gas generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Umirov, N.; Yamada, Y.; Munakata, H.; Kim, S.S.; Kanamura, K. Analysis of intrinsic properties of Li4Ti5O12 using single-particle technique. J. Electroanal. Chem. 2019, 855, 113514. [Google Scholar] [CrossRef]
- An, Z.X.; Xia, H.H.; Xu, J.Q.; Li, H. Behavior of hybrid supercapacitor using pre-lithiated lithium titanate as anode. Electron. Compon. Mater. 2017, 36, 19–24. [Google Scholar]
- An, Z.X.; Fang, W.Y.; Xu, J.Q.; Zhang, J.J. Comparative analysis of electrochemical performances and capacity degrading behaviors in lithium ion capacitors based on different anodic materials. Ionics 2019, 25, 3277–3285. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.Y.; Cho, M.Y.; Kim, H.; Hong, J.; Jung, S.K.; Roh, K.C.; Kang, K. High-Performance Hybrid Supercapacitor Based on Graphene-Wrapped Li4Ti5O12 and Activated Carbon. Chem. Electro. Chem. 2014, 1, 125–130. [Google Scholar]
- Jiang, C.H.; Zhao, J.; Wu, H.Q.; Zou, Z.M.; Huang, R.Z. Li4Ti5O12/activated-carbon hybrid anodes prepared by in situ copolymerization and post-CO2 activation for high power Li-ion capacitors. J. Power Sources 2018, 401, 135–141. [Google Scholar] [CrossRef]
- Ye, L.; Liang, Q.H.; Lei, Y.; Yu, X.L.; Han, C.P.; Shen, W.C.; Huang, Z.H.; Kang, F.Y.; Yang, Q.H. A high performance Li-ion capacitor constructed with Li4Ti5O12/C hybrid and porous graphene macroform. J. Power Sources 2015, 282, 174–178. [Google Scholar] [CrossRef]
- Satish, R.; Aravindan, V.; Ling, W.C.; Woei, N.K.; Madhavi, S. Macroporous carbon from human hair: A journey towards the fabrication of high energy Li-ion capacitors. Electrochim. Acta 2015, 182, 474–481. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.H.; Wang, Q.; Zhang, J.; Xia, B.J.; Min, G.Q. Gas swelling behaviour at different stages in Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 pouch cells. J. Power Sources 2017, 369, 103–110. [Google Scholar] [CrossRef]
- Kurzweil, P.; Chwistek, M. Electrochemical stability of organic electrolytes in supercapacitors: Spectroscopy and gas analysis of decomposition products. J. Power Sources 2008, 176, 555–567. [Google Scholar] [CrossRef]
- Lindley, B.M.; Appel, A.M.; Krogh-Jespersen, K.; Mayer, J.M.; Miller, A.J.M. Evaluating the Thermodynamics of Electrocatalytic N-2 Reduction in Acetonitrile. ACS Energy Lett. 2016, 1, 698–704. [Google Scholar] [CrossRef]
- Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 1999, 81, 715–719. [Google Scholar] [CrossRef]
- He, Y.B.; Li, B.H.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.D.; Zhang, B.A.; Yang, Q.H.; et al. Gassing in Li4Ti5O12-based batteries and its remedy. In Proceedings of the E-MRS 2012 Spring Meeting, Strasbourg, France, 14–18 May 2012. [Google Scholar]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).