Motor Unit Discharges from Multi-Kernel Deconvolution of Single Channel Surface Electromyogram
Abstract
:1. Introduction
- A single kernel is unlikely to be sufficient to represent a general EMG, including MUAPs corresponding to different conduction velocities (CV). Indeed, a widespread delay distribution is expected to be used to recover a MUAP with a larger support than the kernel (corresponding to a MU with a low muscle fibre CV), whereas, there will be problems in rebuilding MUAPs shorter than the kernel.
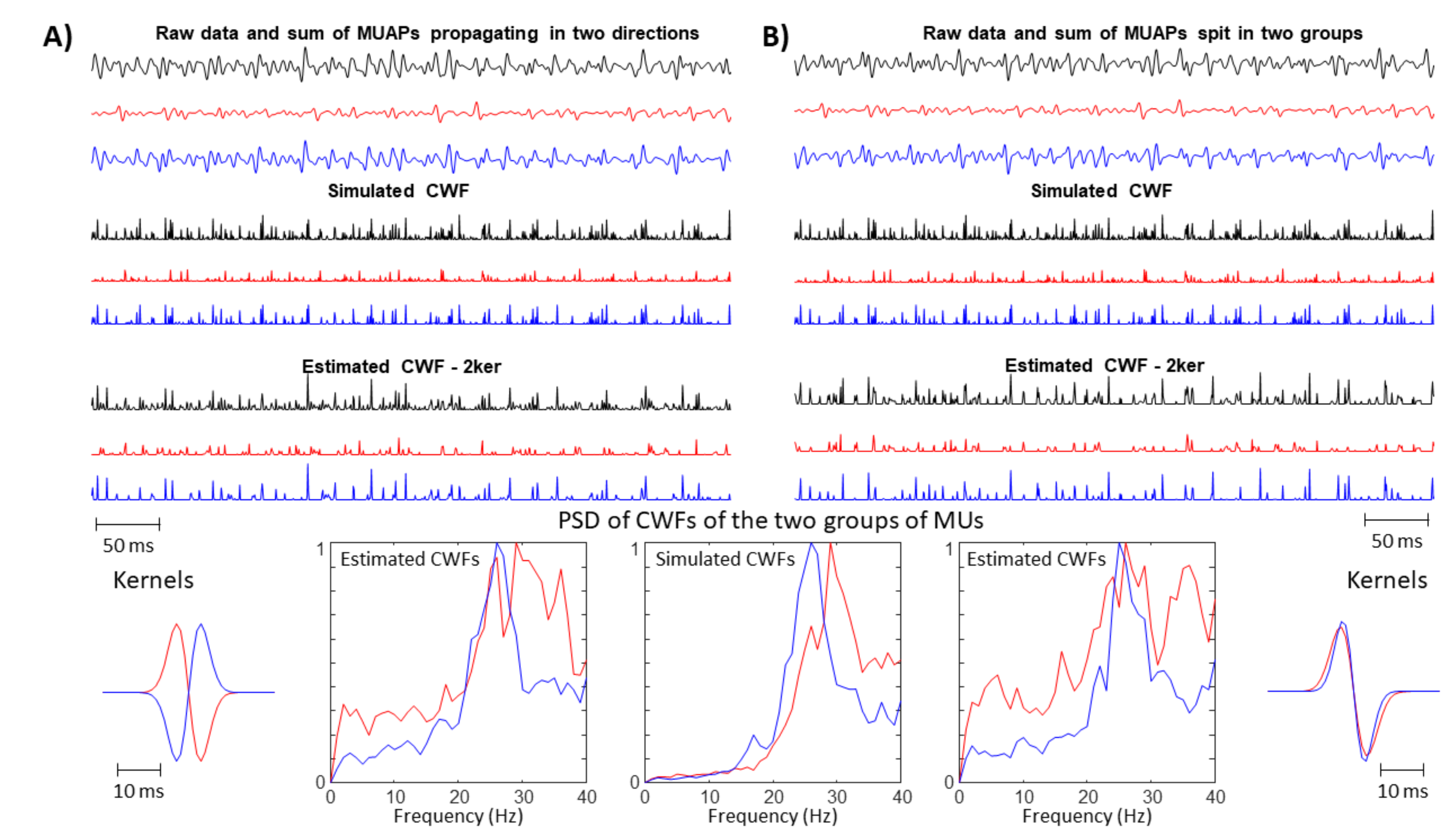
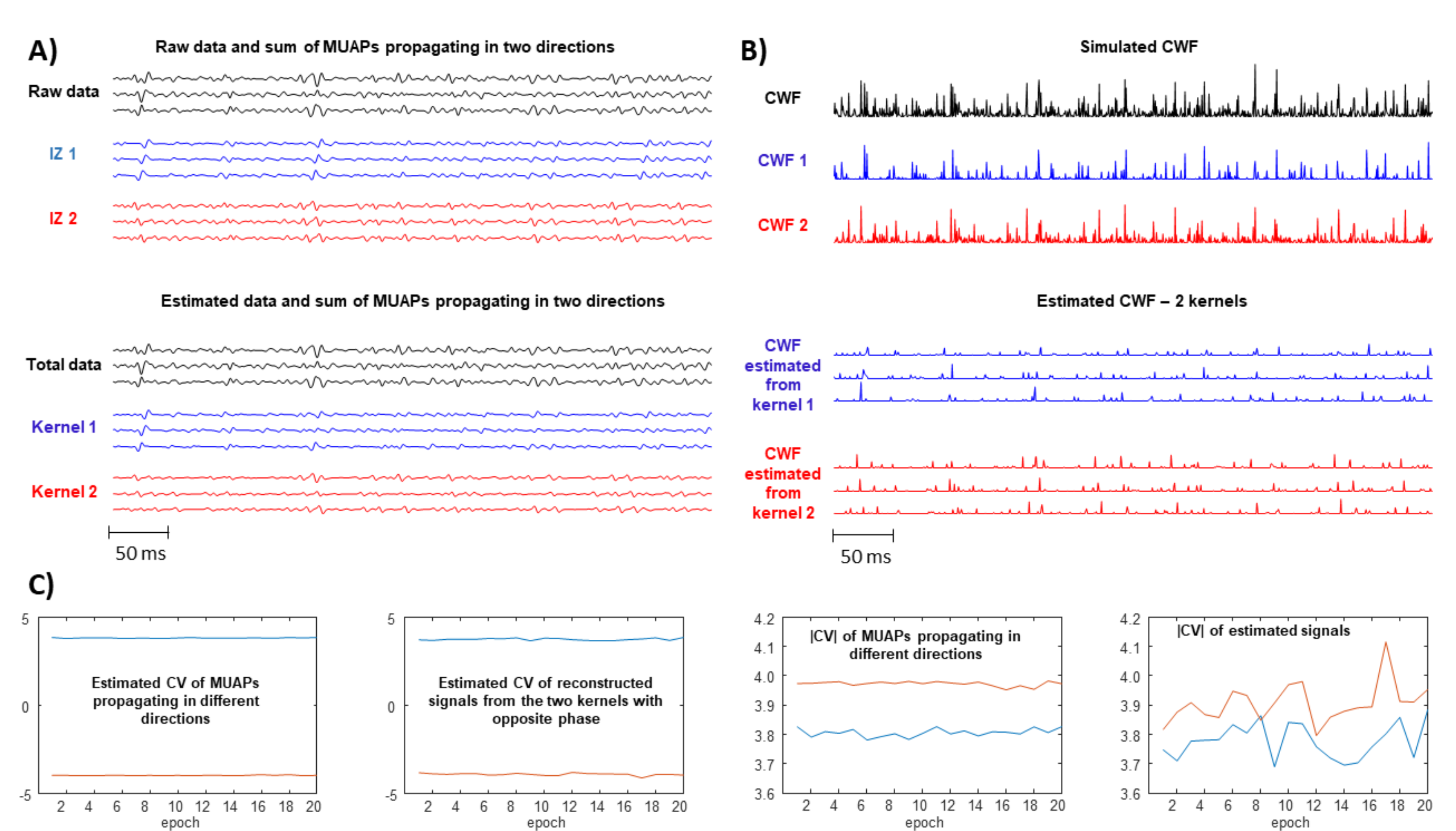
- Problems are expected if there are more innervation zones (IZs) and MUAPs are propagating in different directions under the detection point so that the single SD channel records waves with opposite phases.
- In ideal conditions, the deconvolution process would recover exactly the original data by convoluting the estimated cumulative firings with the selected kernel. As coherence is unaffected by filtering, it would be the same if applied to the original or the processed data. Thus, a generalization is needed to make the method applicable to important fields, such as intra- or inter-muscular coherence, overcoming the limitations of using the raw EMG.
2. Methods
2.1. Signal Processing
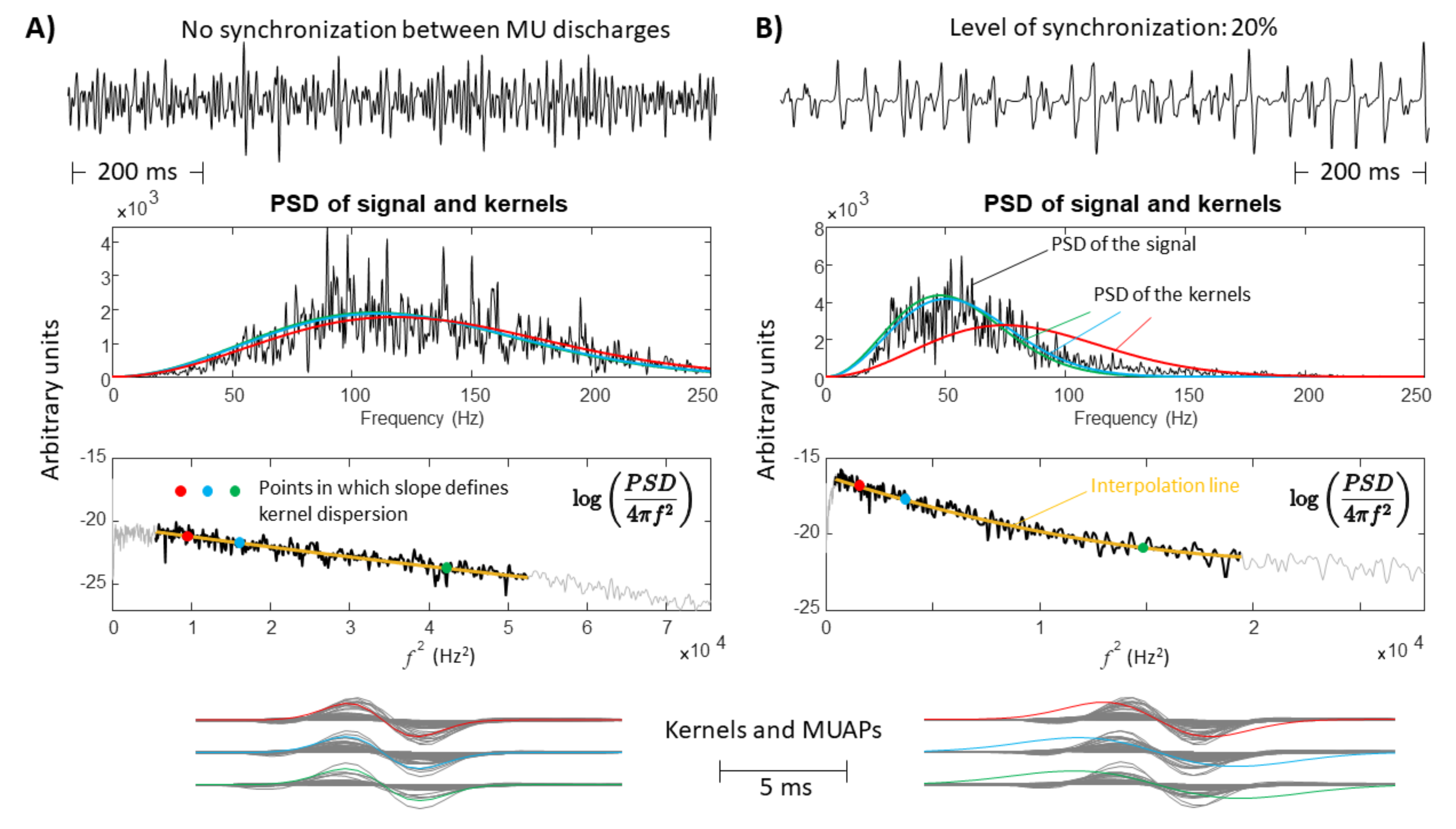
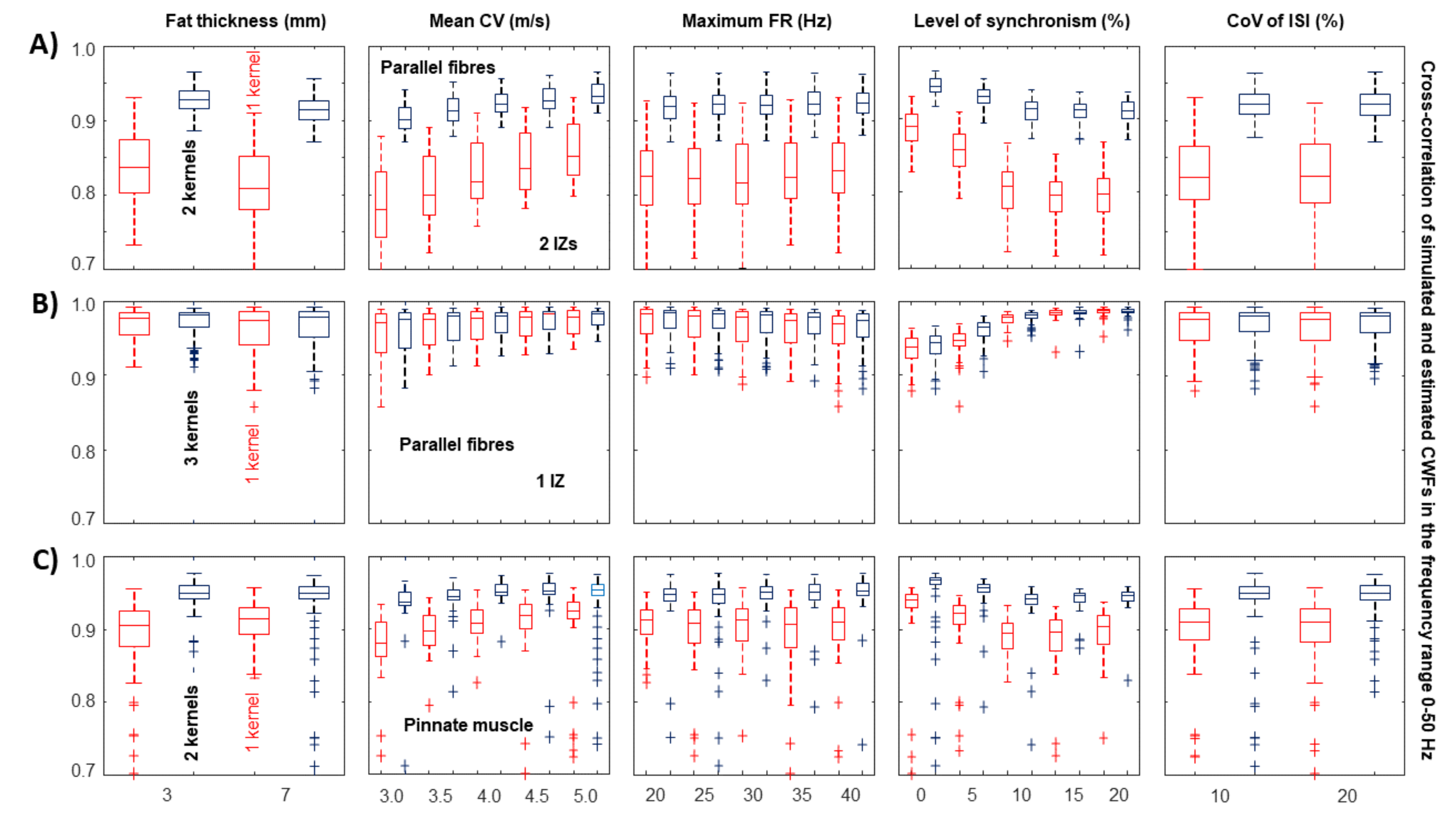
- A large spread of IZs was assumed, so that MUAPs could propagate under the electrodes in two opposite directions. This happens in many different conditions, e.g., in sphincter muscles [25], in the case of fibre pinnation or, in general, when the distribution of IZs is not perpendicular to the fibre direction [26]. As a consequence, waveforms with opposite phases are recorded by the considered SD channel. In such a case, two kernels were considered, with the same PSD resembling that of the original data but with opposite phase. Specifically, the PSD of the first derivative of a Gaussian function is -4.6cm0cmwhere F indicates the Fourier transform. In order to estimate the variance , the following 1D curve can be studiedIt is clear that can be estimated by the slope of this curve divided by . This procedure was applied to the PSD of the EMG, which is more complicated than the above expression, as different waveforms are summed, none of them are exactly obtained as a derivative of a Gaussian function, and noise is present. Thus, the PSD of the EMG was considered in a frequency range in which most of the power is found, i.e., in (), where is the median frequency and the standard deviation of the PSD (preliminary tests showed that this range provided stable results). Curve (5) was approximated by a straight line within this range and its slope was used to estimate . As detailed below, two different simulators were used to test this condition: a model with parallel fibres [27] and two different IZs and a simulator of pinnate muscle with fibres inclined with respect to the skin surface [28,29].
- A single direction of propagation was assumed, such as when electrodes are placed beyond the last IZ over a muscle with parallel fibre architecture. As MUAPs are generated by MUs with different CVs, the PSD of the EMG sometimes provides a curve (5) that is not well approximated by a straight line. The curve was then fit by a parabola, and its slopes in the 15th, 50th, and 85th percentile of the frequency range mentioned above were used to estimate the variances of three kernels. Those kernels ideally reflect MUAP prototypes of MUs with small, medium, and large values of CV. This way, the proposed method for the selection of the kernels adapts to the signal. Eventually, the method can come back to the single kernel case in the limit in which the curve (5) is linear, so that the three kernels are identical.
2.2. Test Data
2.3. Assessment of Performance
3. Results
4. Discussion
5. Conclusions and Further Work
Funding
Conflicts of Interest
Abbreviations
| CoV | Coefficient of Variation |
| CV | Conduction Velocity |
| CWF | Cumulative Weighted Firings |
| EMG | ElectroMyoGram |
| FR | Firing Rate |
| ISI | Inter-Spike Interval |
| MVC | Maximal Voluntary Contraction |
| SD | Single Differential |
References
- Mesin, L.; Dardanello, D.; Rainoldi, A.; Boccia, G. Motor unit firing rates and synchronisation affect the fractal dimension of simulated surface electromyogram during isometric/isotonic contraction of vastus lateralis muscle. Med. Eng. Phys. 2016, 38, 1530–1533. [Google Scholar] [CrossRef] [Green Version]
- Herda, T.J.; Siedlik, J.A.; Trevino, M.A.; Cooper, M.A.; Weir, J.P. Motor unit control strategies of endurance- versus resistance-trained individuals. Muscle Nerve 2015, 52, 832–843. [Google Scholar] [CrossRef]
- Contessa, P.; Adam, A.; De Luca, C.J. Motor unit control and force fluctuation during fatigue. J. Appl. Physiol. 2009, 107, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basmajian, J.; De Luca, C.J. Muscles Alive: Their Function Revealed by Electromyography, 5th ed.; Williams and Wilkins: Baltimore, MD, USA, 1985. [Google Scholar]
- Hu, X.; Suresh, A.K.; Rymer, W.Z.; Suresh, N.L. Altered motor unit discharge patterns in paretic muscles of stroke survivors assessed using surface electromyography. J. Neural Eng. 2016, 13, 046025. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Erim, Z. Common drive of motor units in regulation of muscle force. Trends Neurosci. 1994, 17, 299–305. [Google Scholar] [CrossRef]
- De Luca, C.J.; Erim, Z. Common drive in motor units of a synergistic muscle pair. J. Neurophysiol. 2002, 87, 2200–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.N.; Kilner, J.M.; Pinches, E.M.; Lemon, R.N. The role of synchrony and oscillations in the motor output. Exp. Brain Res. 1999, 128, 109–117. [Google Scholar] [CrossRef]
- Conway, B.A.; Halliday, D.M.; Farmer, S.F.; Shahani, U.; Maas, P.; Weir, A.I.; Rosenberg, J.R. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J. Physiol. 1995, 489 Pt 3, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, G.; Chen, M.; Chen, X.; Chen, X.; Zhou, P. Muscle Force Estimation Based on Neural Drive Information From Individual Motor Units. IEEE Trans. Neural Syst. Rehab. Eng. 2020, 28, 3148–3157. [Google Scholar] [CrossRef]
- Vecchio, A.; Negro, F.; Holobar, A.; Casolo, A.; Folland, J.P.; Felici, F.; Farina, D. You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J. Physiol. 2019, 597, 2445–2456. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Hu, X. Finger Joint Angle Estimation Based on Motoneuron Discharge Activities. IEEE J. Biomed. Health Inform. 2020, 24, 760–767. [Google Scholar] [CrossRef]
- Chen, C.; Yu, Y.; Sheng, X.; Farina, D.; Zhu, X. Simultaneous and proportional control of wrist and hand movements by decoding motor unit discharges in real time. J. Neural Eng. 2021, 18, 056010. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, A.; Schomaker, L.R. Motor unit firing rate during static contraction indicated by the surface EMG power spectrum. IEEE Trans. Biomed. Eng. 1983, 30, 601–609. [Google Scholar] [CrossRef]
- Myers, L.J.; Lowery, M.; O’Malley, M.; Vaughan, C.L.; Heneghan, C.; St Clair Gibson, A.; Harley, Y.X.; Sreenivasan, R. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J. Neurosci. Methods 2003, 124, 157–165. [Google Scholar] [CrossRef]
- Neto, O.P.; Christou, E.A. Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J. Neurophysiol. 2010, 103, 1093–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, C.J.; Adam, A.; Wotiz, R.; Gilmore, L.D.; Nawab, S.H. Decomposition of surface EMG signals. J. Neurophysiol. 2006, 96, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Holobar, A.; Zazula, D. Multichannel blind source separation using convolution kernel compensation. IEEE Trans. Sig. Proc. 2007, 55, 4487–4496. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The extraction of neural strategies from the surface EMG: An update. J. Appl. Physiol. 2014, 117, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Mesin, L. Separation of interference surface electromyogram into propagating and non-propagating components. Biomed. Signal Process. Control 2019, 52, 238–247. [Google Scholar] [CrossRef]
- Mesin, L. Non-propagating components of surface electromyogram reflect motor unit firing rates. IEEE Access 2019, 7, 106155–106161. [Google Scholar] [CrossRef]
- Mesin, L. Single channel surface electromyogram deconvolution to explore motor unit discharges. Med. Biol. Eng. Comput. 2019, 57, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Bourges, M.; Naik, G.R.; Mesin, L. Single channel surface electromyogram deconvolution is a useful pre-processing for myoelectric control. IEEE Trans. Biomed. Eng. 2021. submitted. [Google Scholar]
- Boyd, S.; Vandenberghe, L. Convex Optimization; Cambridge University Press: Los Angeles, CA, USA, 2004. [Google Scholar]
- Cescon, C.; Mesin, L.; Nowakowski, M.; Merletti, R. Geometry assessment of anal sphincter muscle based on monopolar multichannel surface EMG signals. J. Electromyogr. Kinesiol. 2011, 21, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Damiano, L.; Farina, D. Estimation of average muscle fiber conduction velocity from simulated surface EMG in pinnate muscles. J. Neurosci. Methods 2007, 160, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Farina, D.; Mesin, L.; Martina, S.; Merletti, R. A surface EMG generation model with multilayer cylindrical description of the volume conductor. IEEE Trans. Biomed. Eng. 2004, 51, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Mesin, L.; Farina, D. Simulation of surface EMG signals generated by muscle tissues with inhomogeneity due to fiber pinnation. IEEE Trans. Biomed. Eng. 2004, 51, 1521–1529. [Google Scholar] [CrossRef]
- Mesin, L.; Merletti, R.; Vieira, T.M. Insights gained into the interpretation of surface electromyograms from the gastrocnemius muscles: A simulation study. J. Biomech. 2011, 44, 1096–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrus, C.S. Iterative Reweighted Least Squares. OpenStax CNX. Available online: http://cnx.org/contents/92b90377-2b34-49e4-b26f-7fe572db78a1@12 (accessed on 24 December 2012).
- Mesin, L.; Cocito, D. A new method for the estimation of motor nerve conduction block. Clin. Neurophysiol. 2007, 118, 730–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craven, D.; McGinley, B.; Kilmartin, L.; Glavin, M.; Jones, E. Compressed sensing for bioelectric signals: A review. IEEE J. Biomed. Health Inform. 2015, 19, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Contessa, P.; De Luca, C.J. Neural control of muscle force: Indications from a simulation model. J. Neurophysiol. 2013, 109, 1548–1570. [Google Scholar] [CrossRef] [Green Version]
- Meekins, G.D.; So, Y.; Quan, D. American Association of Neuromuscular & Electrodiagnostic Medicine evidenced-based review: Use of surface electromyography in the diagnosis and study of neuromuscular disorders. Muscle Nerve 2008, 38, 1219–1224. [Google Scholar]
- Perry, J.; Easterday, C.S.; Antonelli, D.J. Surface versus intramuscular electrodes for electromyography of superficial and deep muscles. Phys. Ther. 1981, 61, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Crosstalk in surface electromyogram: Literature review and some insights. Phys. Eng. Sci. Med. 2020, 43, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Volume conductor models in surface electromyography: Computational techniques. Comput. Biol. Med. 2013, 43, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Volume conductor models in surface electromyography: Applications to signal interpretation and algorithm test. Comput. Biol. Med. 2013, 43, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.G.; Farina, D.; Maluf, K.S.; Merletti, R.; Enoka, R.M. Influence of amplitude cancellation on the simulated surface electromyogram. J. Appl. Physiol. 2005, 98, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Boccia, G.; Zoppirolli, C.; Bortolan, L.; Schena, F.; Pellegrini, B. Shared and task-specific muscle synergies of Nordic walking and conventional walking. Scand. J. Med. Sci. Sports 2018, 28, 905–918. [Google Scholar] [CrossRef] [Green Version]
- De Stefano, A.; Burridge, J.H.; Yule, V.T.; Allen, R. Effect of gait cycle selection on EMG analysis during walking in adults and children with gait pathology. Gait Posture 2004, 20, 92–101. [Google Scholar] [CrossRef]
- Reinvee, M.; Vaas, P.; Ereline, J.; Paasuke, M. Applicability of Affordable sEMG in Ergonomics Practice. Procedia Manuf. 2015, 3, 4260–4265. [Google Scholar] [CrossRef] [Green Version]
- Uslu, S.; Nuzket, T.; Uysal, H. Modified motor unit number index (MUNIX) algorithm for assessing excitability of alpha motor neuron in spasticity. Clin. Neurophysiol. Pract. 2018, 3, 127–133. [Google Scholar] [CrossRef]
- Javaid, H.A.; Tiwana, M.I.; Alsanad, A.; Iqbal, J.; Riaz, M.T.; Ahmad, S.; Almisned, F.A. Classification of Hand Movements Using MYO Armband on an Embedded Platform. Electronics 2021, 10, 1322. [Google Scholar] [CrossRef]
- Boonstra, T.W.; Breakspear, M. Neural mechanisms of intermuscular coherence: Implications for the rectification of surface electromyography. J. Neurophysiol. 2012, 107, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Simulation of surface EMG signals for a multilayer volume conductor with a superficial bone or blood vessel. IEEE Trans. Biomed. Eng. 2008, 55, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Farina, D. A model for surface EMG generation in volume conductors with spherical inhomogeneities. IEEE Trans. Biomed. Eng. 2005, 52, 1984–1993. [Google Scholar] [CrossRef]
- Mesin, L.; Farina, D. An analytical model for surface EMG generation in volume conductors with smooth conductivity variations. IEEE Trans. Biomed. Eng. 2006, 53, 773–779. [Google Scholar] [CrossRef]
- Mesin, L. Simulation of surface EMG signals for a multilayer volume conductor with triangular model of the muscle tissue. IEEE Trans. Biomed. Eng. 2006, 53, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Joubert, M.; Hanekom, T.; Merletti, R.; Farina, D. A finite element model for describing the effect of muscle shortening on surface EMG. IEEE Trans. Biomed. Eng. 2006, 53, 593–600. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L. Motor Unit Discharges from Multi-Kernel Deconvolution of Single Channel Surface Electromyogram. Electronics 2021, 10, 2022. https://doi.org/10.3390/electronics10162022
Mesin L. Motor Unit Discharges from Multi-Kernel Deconvolution of Single Channel Surface Electromyogram. Electronics. 2021; 10(16):2022. https://doi.org/10.3390/electronics10162022
Chicago/Turabian StyleMesin, Luca. 2021. "Motor Unit Discharges from Multi-Kernel Deconvolution of Single Channel Surface Electromyogram" Electronics 10, no. 16: 2022. https://doi.org/10.3390/electronics10162022
APA StyleMesin, L. (2021). Motor Unit Discharges from Multi-Kernel Deconvolution of Single Channel Surface Electromyogram. Electronics, 10(16), 2022. https://doi.org/10.3390/electronics10162022





