In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulations
2.2. Human Skin Explants
2.3. Immunostaining of Human Skin Explants
2.4. Tyrosinase Activity Assay
2.5. RT-qPCR Analysis
2.6. Human Volunteers and Clinical Study Design
2.7. Dermatological Assessments and Clinical Scoring
2.8. Skin Lightness Measurement
2.9. Pore Diameter Measurements
2.10. Desquamation Assessment
2.11. Skin Roughness on the Periocular Area
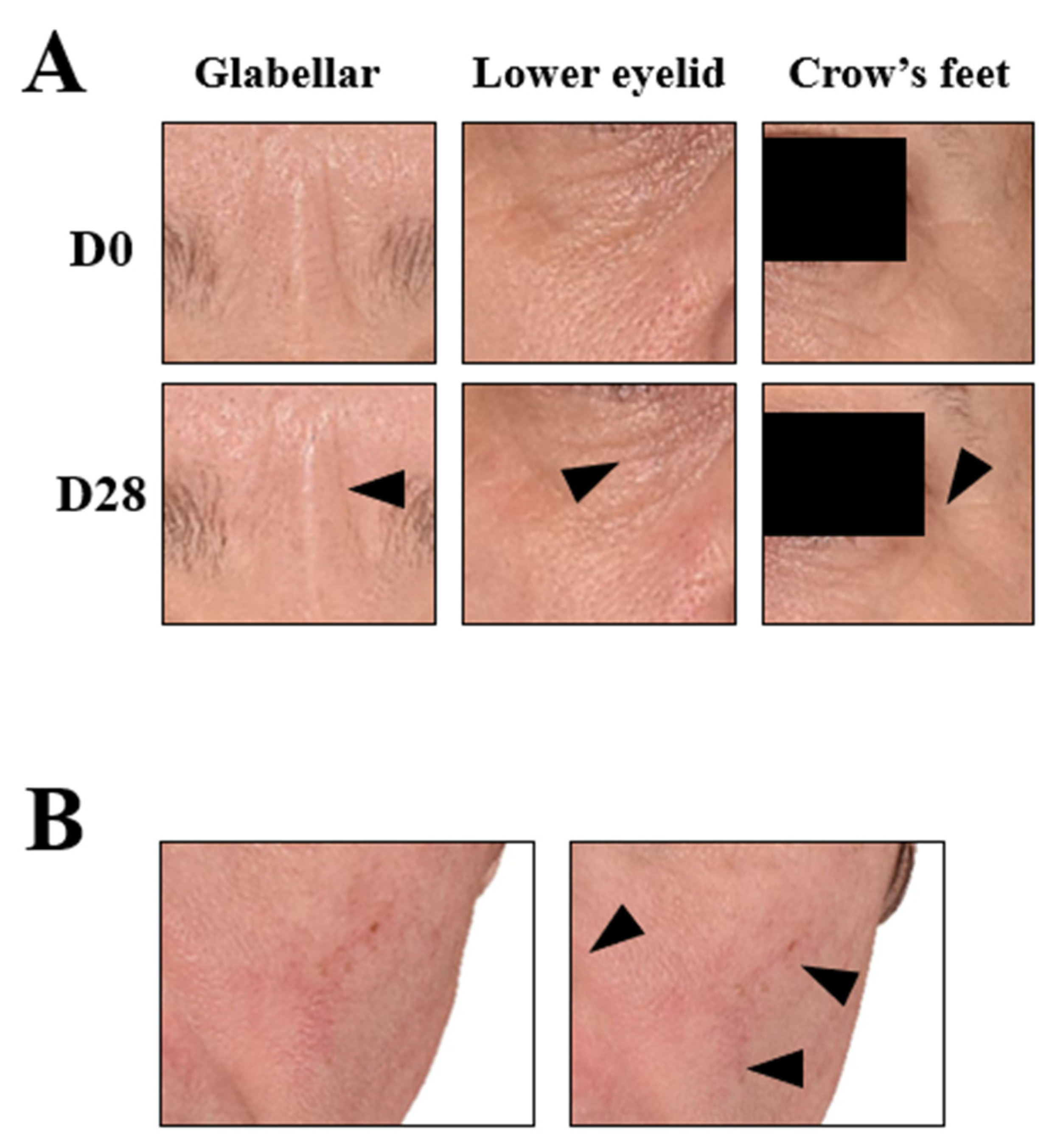
2.12. 2D and 3D Photographs
2.13. Statistical Analysis
3. Results
3.1. Ex Vivo Inflammation Assessment
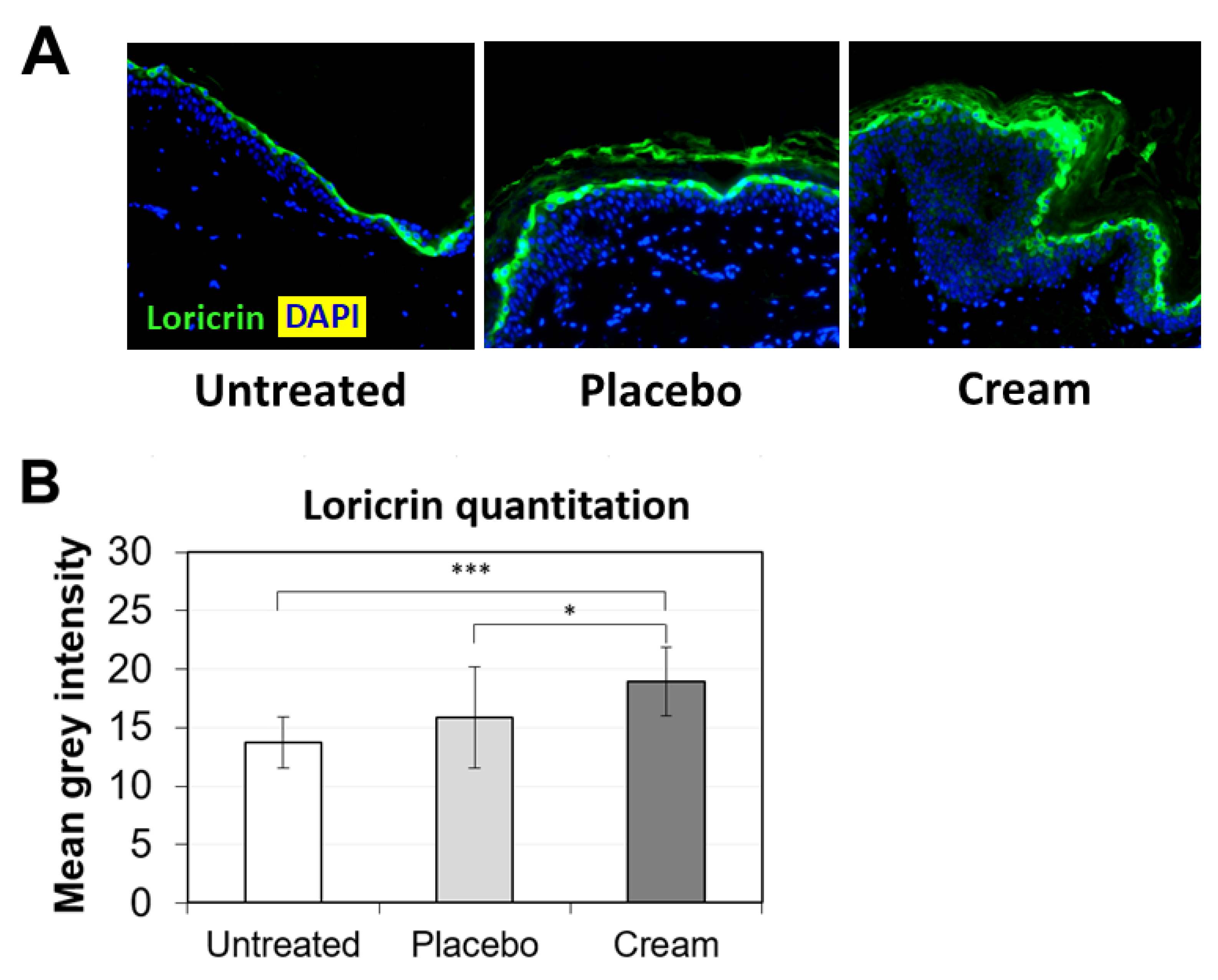
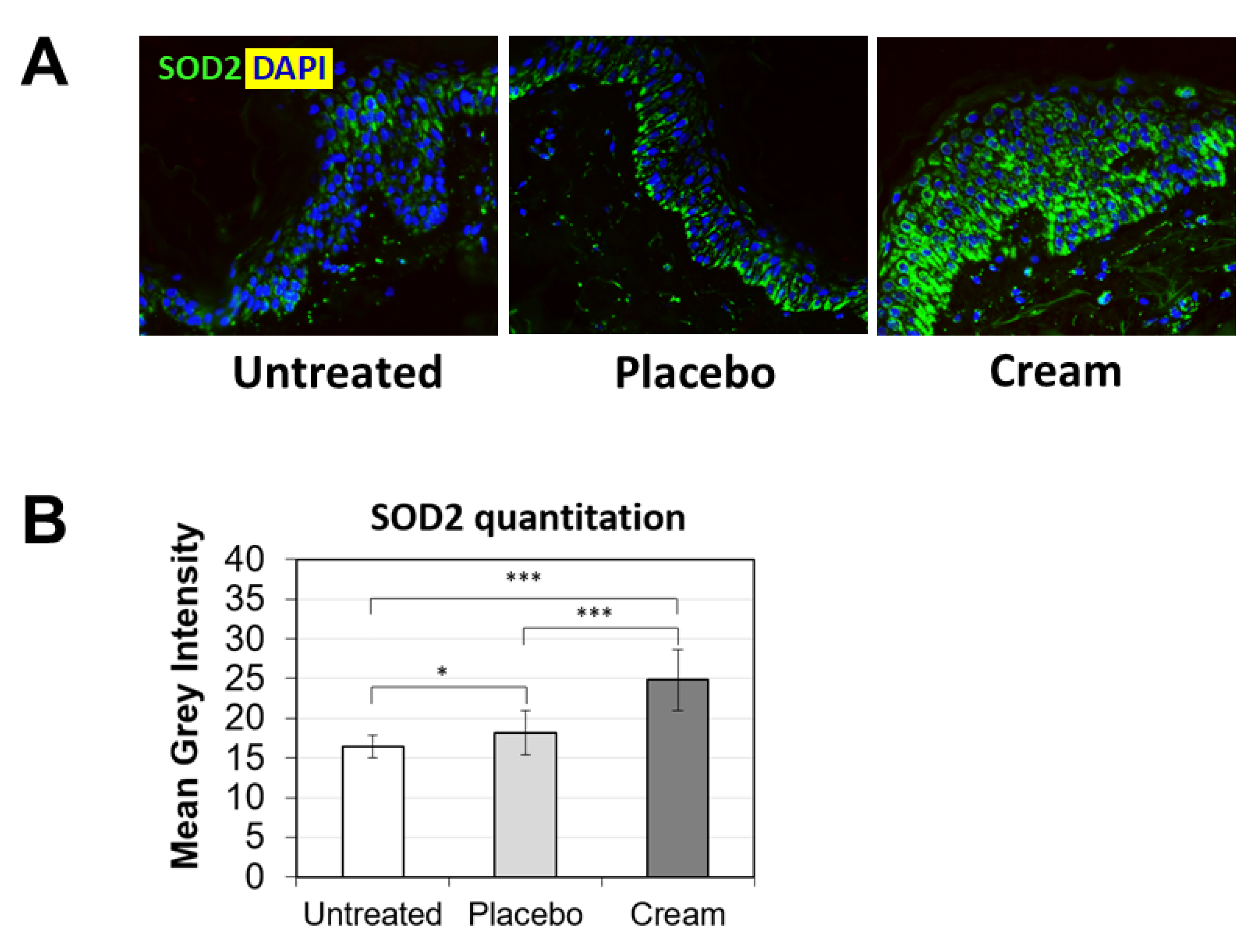
3.2. Increased Loricrin and SOD2 Expression for Improved Epidermal Function
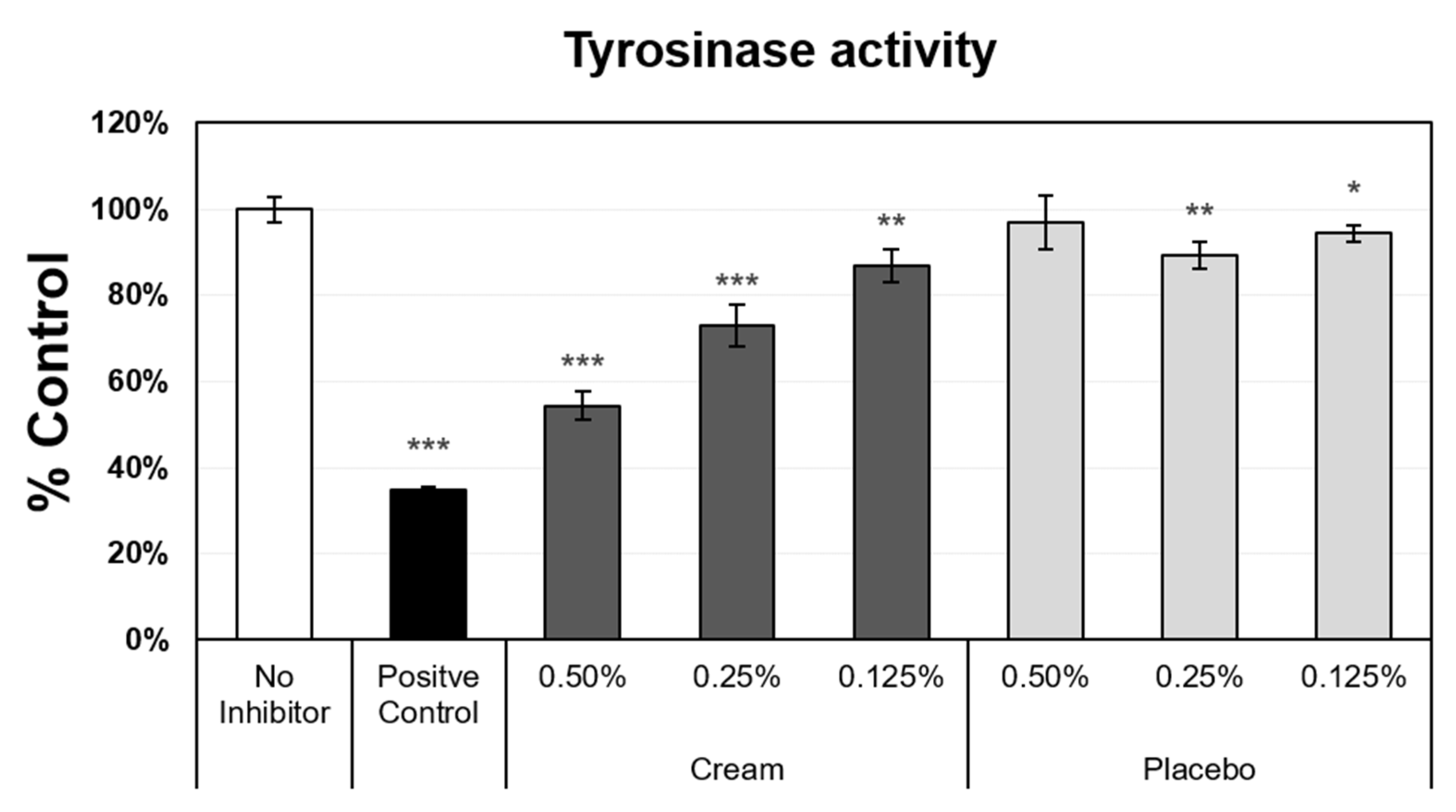
3.3. Tyrosinase Activity Inhibition
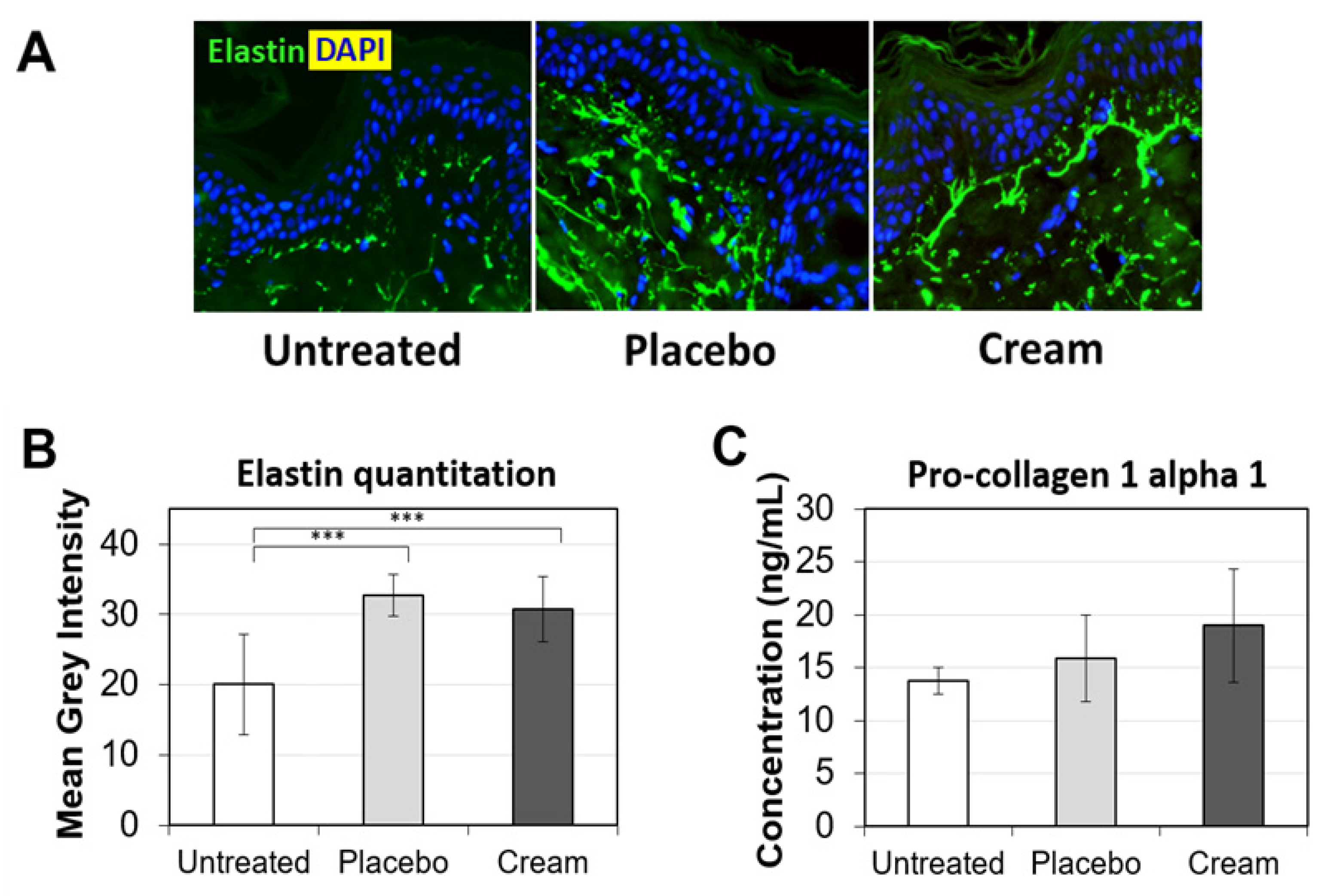
3.4. Impact on Collagen and Elastin Expression
3.5. Clinical Study and Demographic Analysis
3.6. Improved Dermatological Assessments
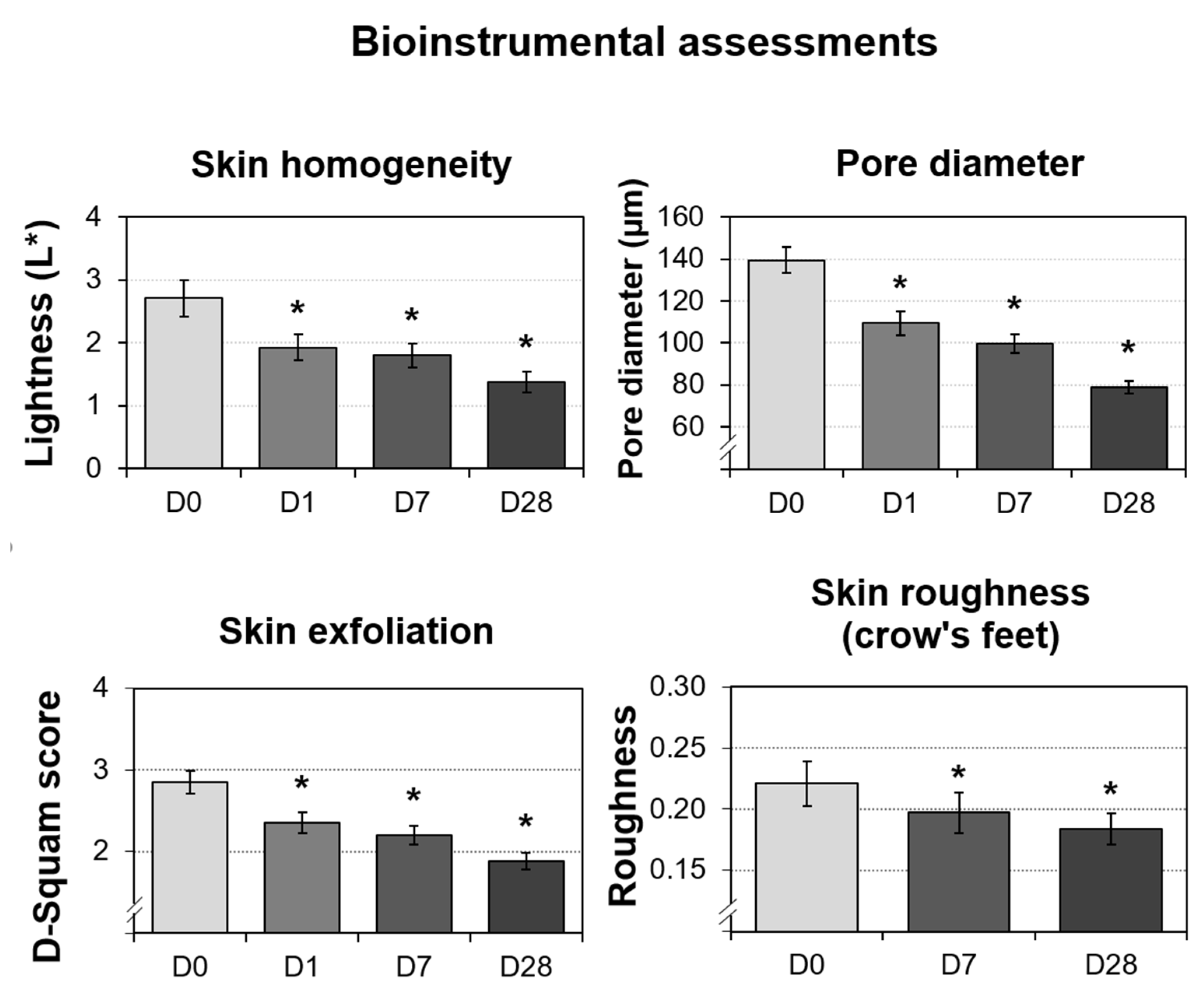
3.7. Significant Improvements in the Bioinstrumental Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Cream (Test Product) Ingredient List
Appendix A.2. Placebo (Negative Control) Ingredient List
References
- Rajanala, S.; Vashi, N.A. Cleopatra and Sour Milk-The Ancient Practice of Chemical Peeling. JAMA Dermatol. 2017, 153, 1006. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, T.; Lanoue, J.; Rahman, Z. A Practical Approach to Chemical Peels: A Review of Fundamentals and Step-by-step Algorithmic Protocol for Treatment. J. Clin. Aesthet. Dermatol. 2018, 11, 21–28. [Google Scholar] [PubMed]
- Rendon, M.I.; Berson, D.S.; Cohen, J.L.; Roberts, W.E.; Starker, I.; Wang, B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J. Clin. Aesthet. Dermatol. 2010, 3, 32–43. [Google Scholar] [PubMed]
- O’Connor, A.A.; Lowe, P.M.; Shumack, S.; Lim, A.C. Chemical peels: A review of current practice. Australas. J. Dermatol. 2018, 59, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Sharma, K.; Maksimovic, S.; Fan, A.; Adams-Woodford, A.; Mao, J. Professional-Grade TCA-Lactic Acid Chemical Peel: Elucidating Mode of Action to Treat Photoaging and Hyperpigmentation. Front. Med. 2021, 8, 617068. [Google Scholar] [CrossRef]
- Chiriac, A.; Brzezinski, P. Topical malic acid in combination with citric acid: An option to treat recalcitrant warts. Dermatol. Ther. 2015, 28, 336–338. [Google Scholar] [CrossRef]
- Abels, C.; Kaszuba, A.; Michalak, I.; Werdier, D.; Knie, U.; Kaszuba, A. A 10% glycolic acid containing oil-in-water emulsion improves mild acne: A randomized double-blind placebo-controlled trial. J. Cosmet. Dermatol. 2011, 10, 202–209. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Underhill, C.B.; Lakkakorpi, J.; Ditre, C.M.; Uitto, J.; Yu, R.J.; Scott, E.V. Citric acid increases viable epidermal thickness and glycosaminoglycan content of sun-damaged skin. Dermatol. Surg. 1997, 23, 689–694. [Google Scholar] [CrossRef]
- Smith, W.P. Epidermal and dermal effects of topical lactic acid. J. Am. Acad. Dermatol. 1996, 35, 388–391. [Google Scholar] [CrossRef]
- Denda, S.; Denda, M.; Inoue, K.; Hibino, T. Glycolic acid induces keratinocyte proliferation in a skin equivalent model via TRPV1 activation. J. Dermatol. Sci. 2010, 57, 108–113. [Google Scholar] [CrossRef]
- Narda, M.; Trullas, C.; Brown, A.; Piquero-Casals, J.; Granger, C.; Fabbrocini, G. Glycolic acid adjusted to pH 4 stimulates collagen production and epidermal renewal without affecting levels of proinflammatory TNF-alpha in human skin explants. J. Cosmet. Dermatol. 2021, 20, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Rendl, M.; Mayer, C.; Weninger, W.; Tschachler, E. Topically applied lactic acid increases spontaneous secretion of vascular endothelial growth factor by human reconstructed epidermis. Br. J. Dermatol. 2001, 145, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Farage, M.; Maibach, H. Sensitive skin: An overview. Int. J. Cosmet. Sci. 2013, 35, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, L. Efficacy of a combined chemical peel and topical salicylic acid-based gel combination in the treatment of active acne. J. Cosmet. Dermatol. 2021, 20 (Suppl. S2), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Nofal, E.; Nofal, A.; Gharib, K.; Nasr, M.; Abdelshafy, A.; Elsaid, E. Combination chemical peels are more effective than single chemical peel in treatment of mild-to-moderate acne vulgaris: A split face comparative clinical trial. J. Cosmet. Dermatol. 2018, 17, 802–810. [Google Scholar] [CrossRef]
- Sharad, J. Glycolic acid peel therapy—A current review. Clin. Cosmet. Investig. Dermatol. 2013, 6, 281–288. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szygula, R.; Dziendziora-Urbinska, I.; Taradaj, J. A comparison of the effectiveness of azelaic and pyruvic acid peels in the treatment of female adult acne: A randomized controlled trial. Sci. Rep. 2020, 10, 12612. [Google Scholar] [CrossRef]
- Bonneville, M.; Saint-Mezard, P.; Benetiere, J.; Hennino, A.; Pernet, I.; Denis, A.; Nicolas, J.F. Laminaria ochroleuca extract reduces skin inflammation. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 1124–1125. [Google Scholar] [CrossRef]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12 (Suppl. S2), 43–50. [Google Scholar] [CrossRef]
- Bazin, R.; Doublet, E. Skin Aging Atlas: Caucasian Type; MED’COM: Paris, France, 2007. [Google Scholar]
- Robin, S.; Fanian, F.; Courderot-Masuyer, C.; Tordjman, M.; Braccini, F.; Boisnic, S.; Philippon, V.; Vincent, A.G.; Salomon, C.; Manfait, M.; et al. Efficacy of a Biorevitalizing-Filler Solution on All Skin Aspects: 10 Years Approach through <i>in Vitro</i> Studies and Clinical Trials. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 18–37. [Google Scholar] [CrossRef]
- Petit, L.; Zugaj, D.; Bettoli, V.; Dreno, B.; Kang, S.; Tan, J.; Torres, V.; Layton, A.M.; Martel, P. Validation of 3D skin imaging for objective repeatable quantification of severity of atrophic acne scarring. Skin Res. Technol. 2018, 24, 542–550. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Skoczynska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and lipofuscin as hallmarks of skin aging. Postepy Dermatol. I Alergol. 2017, 34, 97–103. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Catoi, C.; Drugan, T. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef]
- Farage, M.A. The Prevalence of Sensitive Skin. Front. Med. 2019, 6, 98. [Google Scholar] [CrossRef]

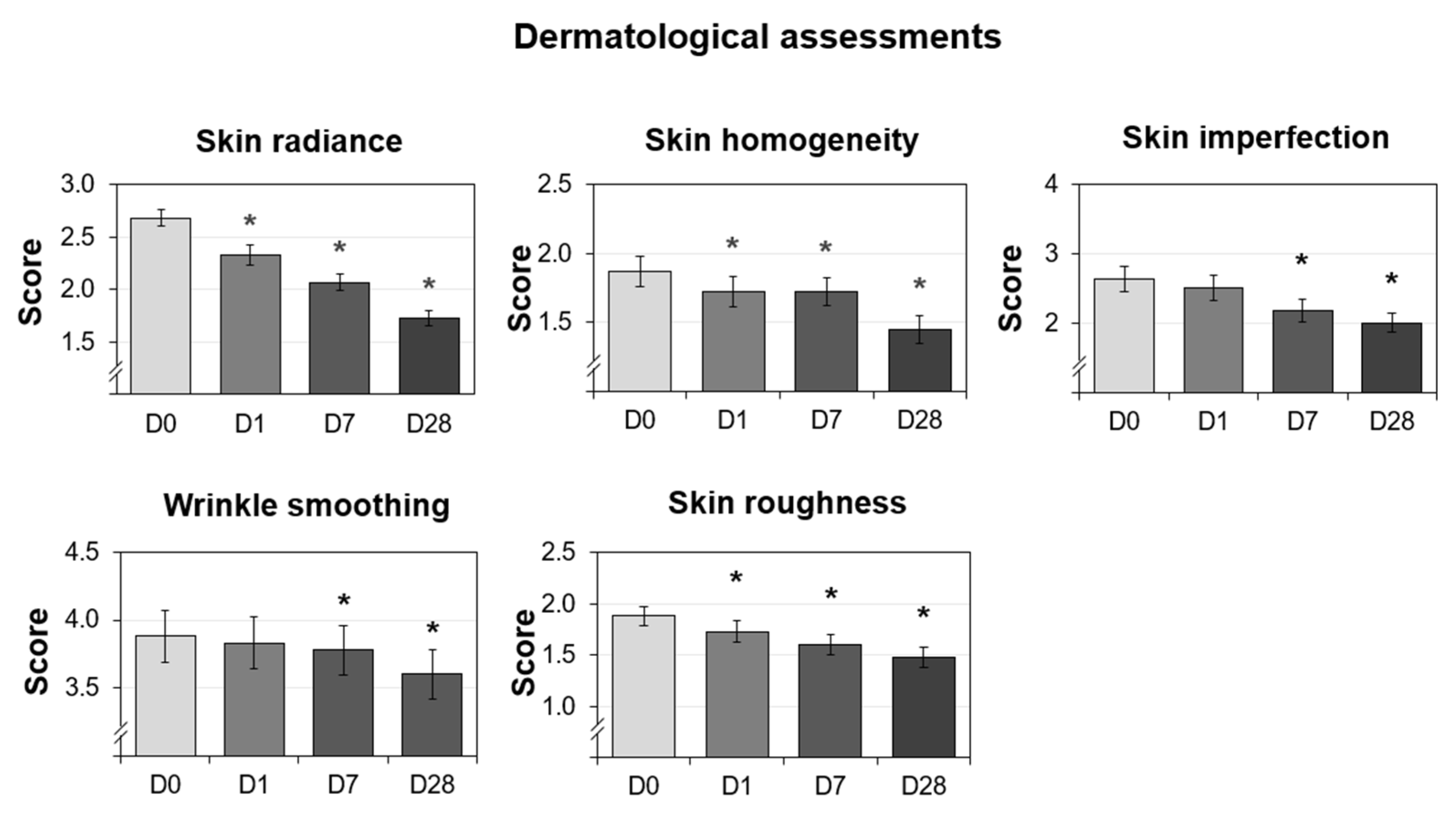

| Radiance score |
|---|
| 0 Very bright complexion 1 Luminous complexion 2 Medium radiance complexion 3 Dull complexion and lacking radiance 4 Very dull complexion |
| Homogeneity score |
| 0 Homogeneous skin 1 Slight inhomogeneity of the complexion 2 Moderate skin tone inhomogeneity 3 Very inhomogeneous complexion with the presence of pigmented spots |
| Imperfections—pore dilatation score |
| 0 Absence of pore dilation 1 Slight dilation of the pores 2 Moderate pore dilation 3 Important pore dilation 4 Very important dilatation of the pores |
| Imperfections—pimple score |
| 0 No button 1 One to four buttons 2 Five to nine buttons 3 Ten to nineteen buttons 4 Twenty or more buttons |
| Overall skin imperfections score |
| Score (0–9) = (pore dilatation score) + (pimple score) |
| Wrinkle smoothing score |
| 0 Young face without visible wrinkles 9 Very marked face with many deep wrinkles |
| Skin roughness score |
| 0 Smooth skin without irregularities 1 Slightly rough skin 2 Moderately rough skin 3 Skin with important irregularities |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namkoong, J.; Goswami, S.; Tartar, O.; Diaz, I.; Wu, J. In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin. Cosmetics 2022, 9, 138. https://doi.org/10.3390/cosmetics9060138
Namkoong J, Goswami S, Tartar O, Diaz I, Wu J. In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin. Cosmetics. 2022; 9(6):138. https://doi.org/10.3390/cosmetics9060138
Chicago/Turabian StyleNamkoong, Jin, Sayantani Goswami, Océane Tartar, Isabel Diaz, and Joanna Wu. 2022. "In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin" Cosmetics 9, no. 6: 138. https://doi.org/10.3390/cosmetics9060138
APA StyleNamkoong, J., Goswami, S., Tartar, O., Diaz, I., & Wu, J. (2022). In-Vitro Efficacy Investigation and an Open-Label, Single-Arm Clinical Study of a Gentle Micropeeling Cream for Sensitive and Non-Sensitive Skin. Cosmetics, 9(6), 138. https://doi.org/10.3390/cosmetics9060138






