Anti-Skin-Aging Activity of a Standardized Extract from Panax ginseng Leaves In Vitro and In Human Volunteer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Cell Culture
2.2. Chemicals and Reagent
2.3. Preparation of the Extract
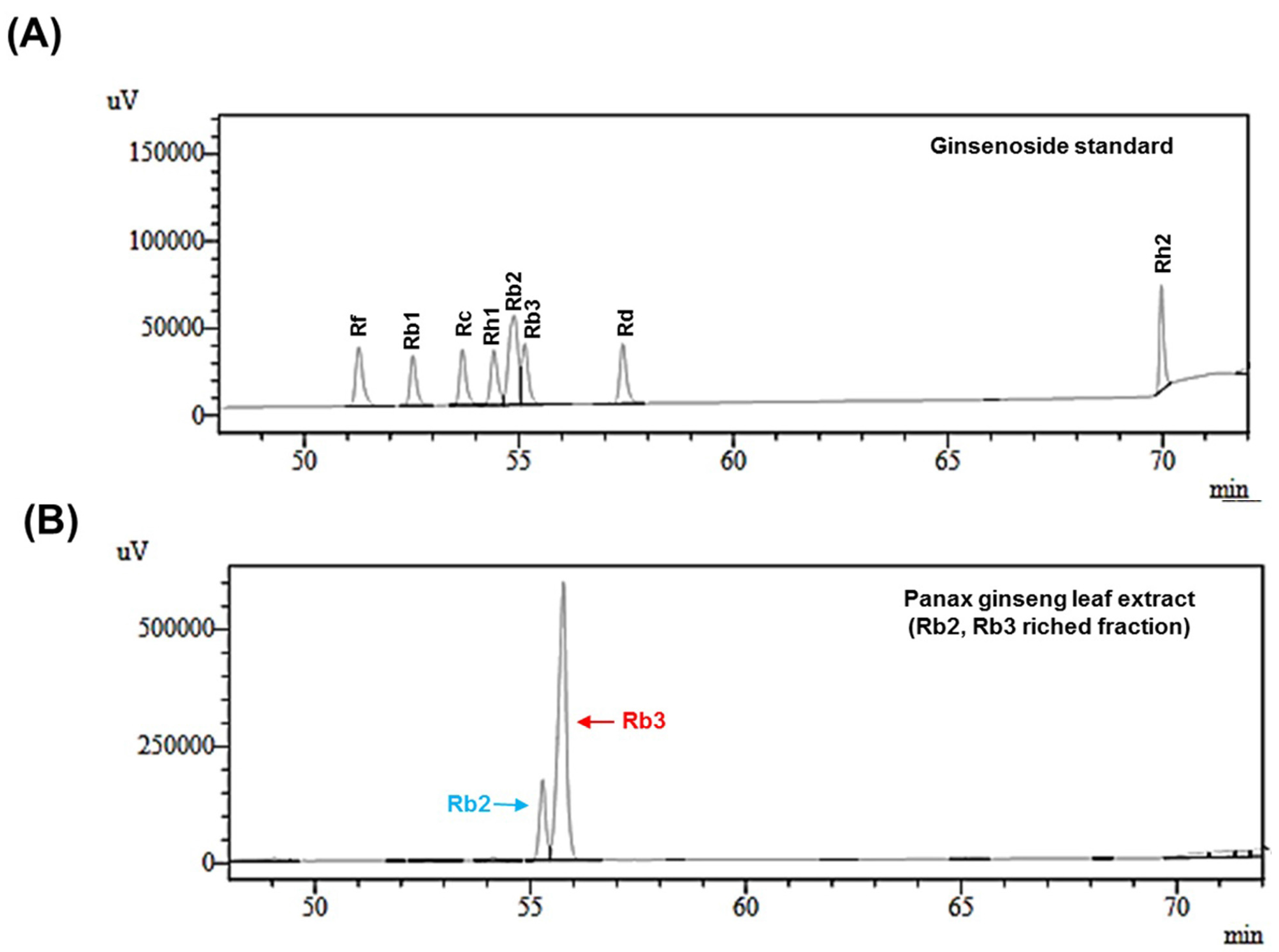
2.4. HPLC Analysis of Ginsenoside
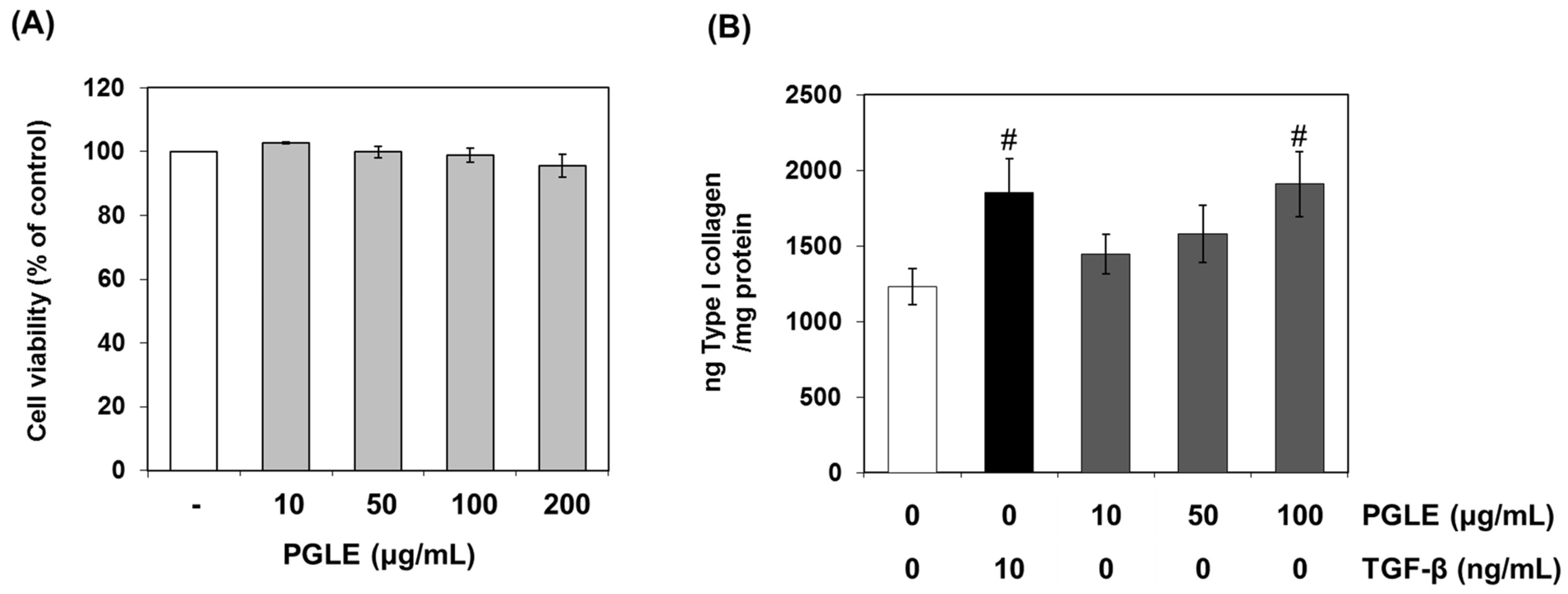
2.5. Cell Viability Assay
2.6. Quantitative Detection of Type I Collagen
2.7. Study Design
2.8. Human Volunteers
2.9. Preparation of Lotion Containing 0.05% PGLE
2.10. General Conditions during Measurement
2.11. Skin Replicas and Image Analysis
2.12. Human Skin Primary Irritation Test
2.13. Statistical Analysis
3. Results
3.1. Ginsenosides Analysis of Panax ginseng Leaf Extract
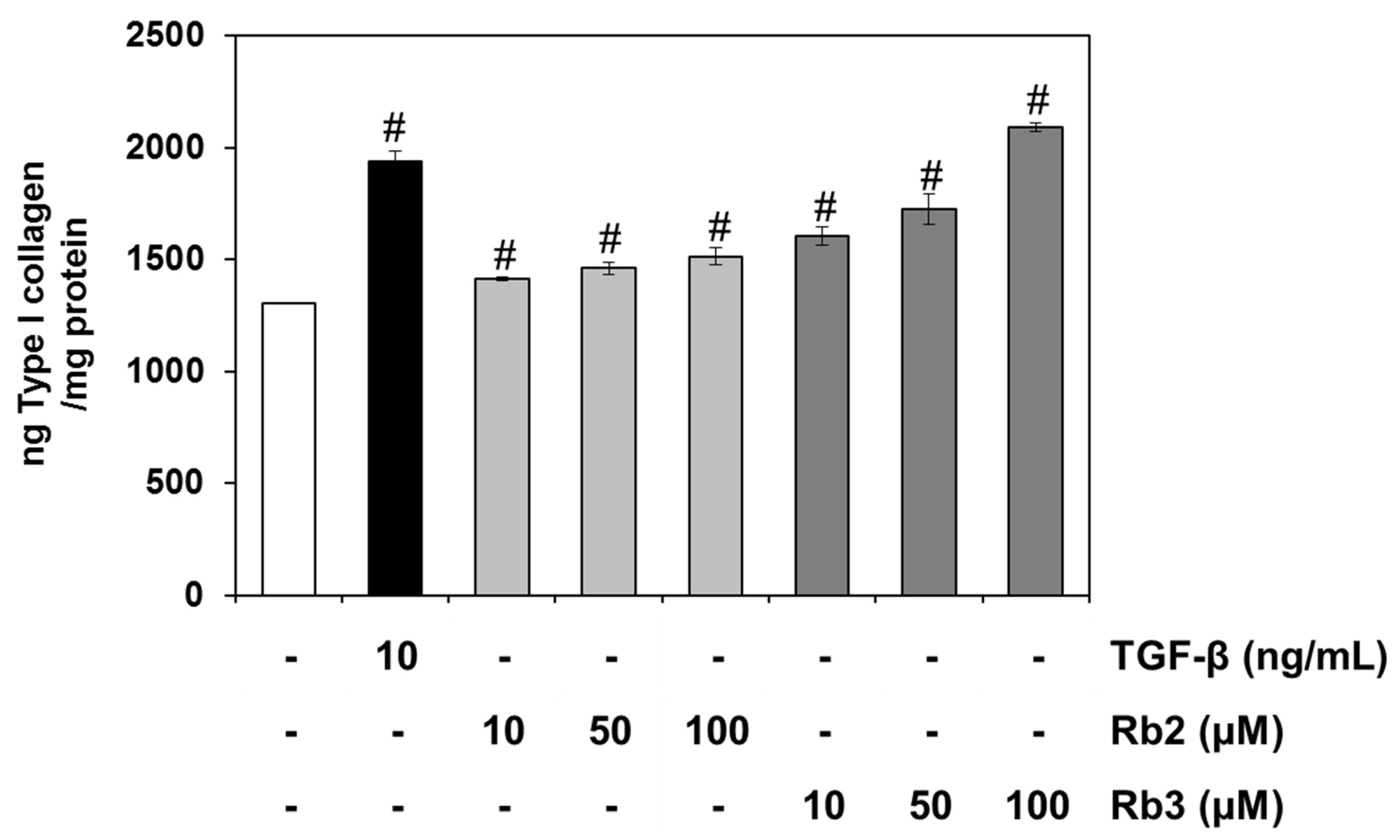
3.2. Effect of PGLE and its Two Main Ginsenosides, Rb2 and Rb3, on Type I Collagen Synthesis
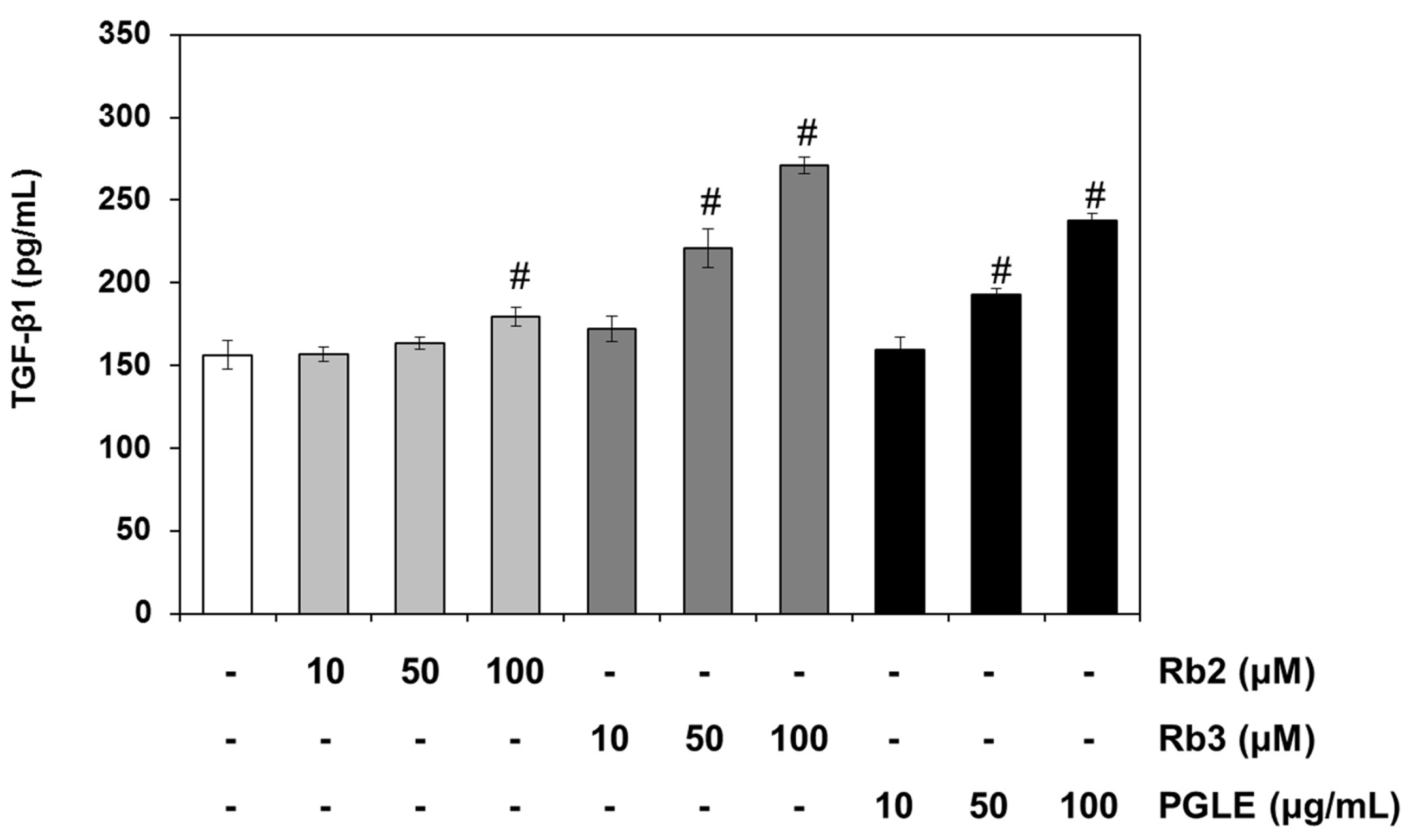
3.3. Effect of PGLE and its Two Main Ginsenosides, Rb2 and Rb3, on TGF-β Pathway
3.4. Human Skin Primary Irritation Test of PGLE
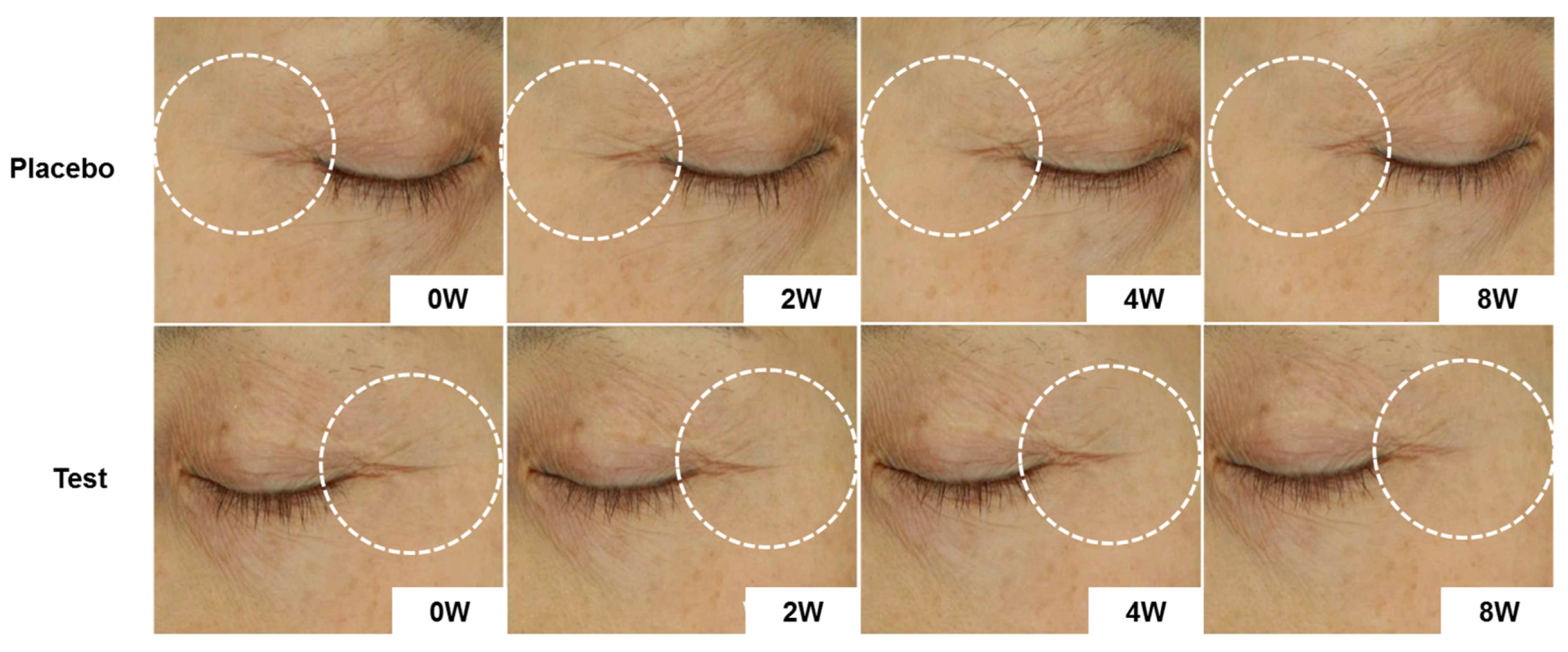
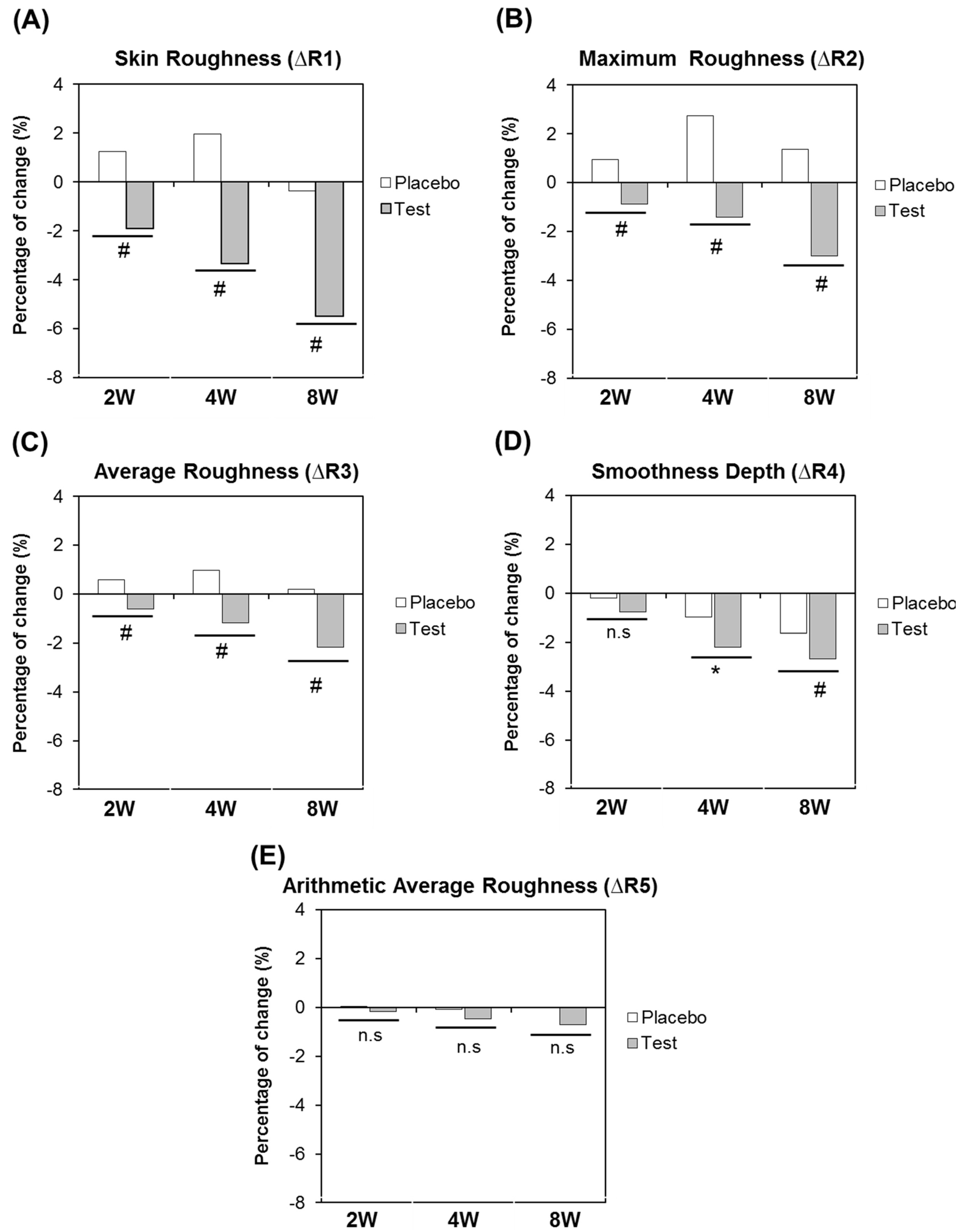
3.5. Clinical Trial on Healthy Volunteers
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Kim, J.; Park, M.; So, J.; Ham, Y.; Jung, K.; Hyun, C.G.; et al. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J. Ethnopharmacol. 2007, 109, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.T.; Li, J.; Zang, H.O.; Ding, J. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 664–668. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.Z.; Kim, Y.K.; Uddin, M.R.; Bae, H.; Kim, H.H.; Park, S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012, 9, 679–683. [Google Scholar]
- Zhang, Y.C.; Li, G.; Jiang, C.; Yang, B.; Yang, H.J.; Xu, H.Y.; Huang, L.Q. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules 2014, 19, 17381–17399. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jeon, J.N.; Jang, M.G.; Oh, J.Y.; Kwon, W.S.; Jung, S.K.; Yang, D.C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J. Ginseng Res. 2014, 38, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ragheb, K.; Lawler, G.; Sturgis, J.; Rajwa, B.; Melendez, J.A.; Robinson, J.P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003, 278, 8516–8525. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, K.; Lagarde, J.M.; Gall, Y.; Roseeuw, D.; Rogiers, V. Microrelief of the skin using a light transmission method. Arch. Dermatol. Res. 2000, 292, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Grove, G.L.; Grove, M.J.; Leyden, J.J.; Lufrano, L.; Schwab, B.; Perry, B.H.; Thorne, E.G. Skin replica analysis of photodamaged skin after therapy with tretinoin emollient cream. J. Am. Acad. Dermatol. 1991, 25, 231–237. [Google Scholar] [CrossRef]
- Frosch, P.J.; Kligman, A.M. The soap chamber test. A new method for assessing the irritancy of soaps. J. Am. Acad. Dermatol. 1979, 1, 35–41. [Google Scholar] [CrossRef]
- Anita, S.C.; Stephen, D.G.; G N McEwen, J. CTFA Safety Testing Guideline; Cosmetic Toiletry and Fragrance Association: Washington, DC, USA, 1981; p. 20005. [Google Scholar]
- Statview 5. A statistics Application Released for Apple Macintosh Computers; Abacus Concepts: Piscataway, NJ, USA, 2009.
- Kim, Y.G.; Sumiyoshi, M.; Sakanaka, M.; Kimura, Y. Effects of ginseng saponins isolated from red ginseng on ultraviolet B-induced skin aging in hairless mice. Eur. J. Pharmacol. 2009, 602, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Woo, H.J.; Lee, I.S.; Lee, J.W.; Cho, Y.C.; Lee, I.N.; Chae, H.J. Optimization of enzymatic pretreatment for the production of fermented ginseng using leaves, stem and roots of ginseng. J. Ginseng Res. 2010, 34, 68–75. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, J.J. Production of ginseng and its bioactive components in plant cell culture: Current technological and applied aspects. J. Biotechnol. 1999, 68, 89–99. [Google Scholar] [CrossRef]
- Keum, Y.S.; Park, K.K.; Lee, J.M.; Chun, K.S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surh, Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000, 150, 41–48. [Google Scholar] [CrossRef]
- Chang, L.K.; Whitaker, D.C. The impact of herbal medicines on dermatologic surgery. Dermatol. Surg. 2001, 27, 759–763. [Google Scholar] [PubMed]
- Choi, S. Epidermis proliferative effect of the Panax ginseng ginsenoside Rb2. Arch. Pharm. Res. 2002, 25, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Mehendale, S.R.; Wang, A.; Han, A.H.; Wu, J.A.; Osinski, J.; Yuan, C.S. American ginseng leaf: Ginsenoside analysis and hypoglycemic activity. Pharmacol. Res. 2004, 49, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C; Chen, C.R.; Chang, C.J. Carbon dioxide extraction of ginseng root hair oil and ginsenosides. Food Chem. 2001, 72, 505–509. [Google Scholar] [CrossRef]
- Li, T.S.C.; Mazza, G.; Cottrell, A.C.; Gao, L. Ginsenosides in roots and leaves of American ginseng. J. Agric. Food Chem. 1996, 44, 717–720. [Google Scholar] [CrossRef]
- El-Domyati, M.; El-Ammawi, T.S.; Medhat, W.; Moawad, O.; Mahoney, M.G.; Uitto, J. Expression of transforming growth factor-β after different non-invasive facial rejuvenation modalities. Int. J. Dermatol. 2015, 54, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.; Akutsu, N.; Ogura, Y.; Nishiyama, T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br. J. Dermatol. 2004, 151, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Krüger, M.; Quondamatteo, F.; Knittel, T.; Saile, B.; Ramadori, G. Transforming growth factor-beta1 stimulates the synthesis of basement membrane proteins laminin, collagen type IV and entactin in rat liver sinusoidal endothelial cells. J. Hepatol. 1999, 31, 692–702. [Google Scholar] [CrossRef]
- Vindevoghel, L.; Kon, A.; Lechleider, R.J.; Uitto, J.; Roberts, A.B.; Mauviel, A. Smad-dependent transcriptional activation of human type VII collagen gene (COL7A1) promoter by transforming growth factor-beta. J. Biol. Chem. 1998, 273, 13053–13057. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J. Investig. Dermatol. 2002, 119, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Phelps, R.; Goldberg, D.J. Clinical, histologic, and ultrastructural changes after use of human growth factor and cytokine skin cream for the treatment of skin rejuvenation. J. Cosmet. Laser Ther. 2008, 10, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Rao, J.; Pabby, A.; Goldman, M.P. Improvement in the appearance of wrinkles with topical transforming growth factor β1 and l-ascorbic acid. Dermatol. Surg. 2006, 32, 618–625. [Google Scholar] [CrossRef] [PubMed]
| Phase | INCI Name | %W/W Placebo | %W/W Test |
|---|---|---|---|
| A | Deionized Water | To 100 | To 100 |
| Glycerin | 3.00 | 3.00 | |
| B | Shea Butter | 3.00 | 3.00 |
| Cetearyl Alcohol | 2.00 | 2.00 | |
| Beeswax | 2.00 | 2.00 | |
| Cetearyl Olivate (and) Sorbitan Olivate | 2.00 | 2.00 | |
| Mineral Oil | 6.00 | 6.00 | |
| C | Panax ginseng leaf extract | 0.05 | |
| D | 1,2-Hexandiol | 2.00 | 2.00 |
| Ethylhexylglycerin | 0.10 | 0.10 | |
| Fragrance | Q.S | Q.S |
| Type of Ginsenosides | Content (%) |
|---|---|
| Rb1 | 0.68 |
| Rb2 | 12.04 |
| Rb3 | 55.72 |
| Rc | 0.84 |
| Rd | 0.10 |
| Rf | 0.26 |
| Rh2 | 0.038 |
| Total content of ginsenosides | 69.68 |
| No | Test Material | 48 h | 72 h | Reaction Grade (b) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | 1+ | 2+ | 3+ | 4+ | ± | 1+ | 2+ | 3+ | 4+ | 48 h | 72 h | Mean | ||
| 1 | Squalene | - (a) | - | - | - | - | - | - | - | - | - | 0 | 0 | 0 |
| 2 | PGLE (0.5%) | - | - | - | - | - | - | - | - | - | - | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Lee, J.-A.; Son, D.; Park, D.; Jung, E. Anti-Skin-Aging Activity of a Standardized Extract from Panax ginseng Leaves In Vitro and In Human Volunteer. Cosmetics 2017, 4, 18. https://doi.org/10.3390/cosmetics4020018
Shin S, Lee J-A, Son D, Park D, Jung E. Anti-Skin-Aging Activity of a Standardized Extract from Panax ginseng Leaves In Vitro and In Human Volunteer. Cosmetics. 2017; 4(2):18. https://doi.org/10.3390/cosmetics4020018
Chicago/Turabian StyleShin, Seoungwoo, Jung-A Lee, Dahee Son, Deokhoon Park, and Eunsun Jung. 2017. "Anti-Skin-Aging Activity of a Standardized Extract from Panax ginseng Leaves In Vitro and In Human Volunteer" Cosmetics 4, no. 2: 18. https://doi.org/10.3390/cosmetics4020018
APA StyleShin, S., Lee, J.-A., Son, D., Park, D., & Jung, E. (2017). Anti-Skin-Aging Activity of a Standardized Extract from Panax ginseng Leaves In Vitro and In Human Volunteer. Cosmetics, 4(2), 18. https://doi.org/10.3390/cosmetics4020018






