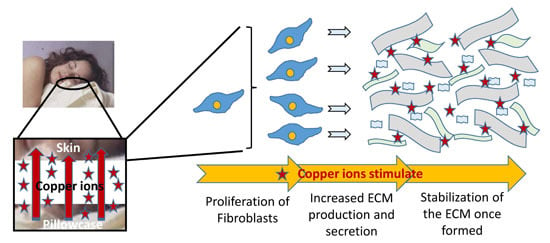
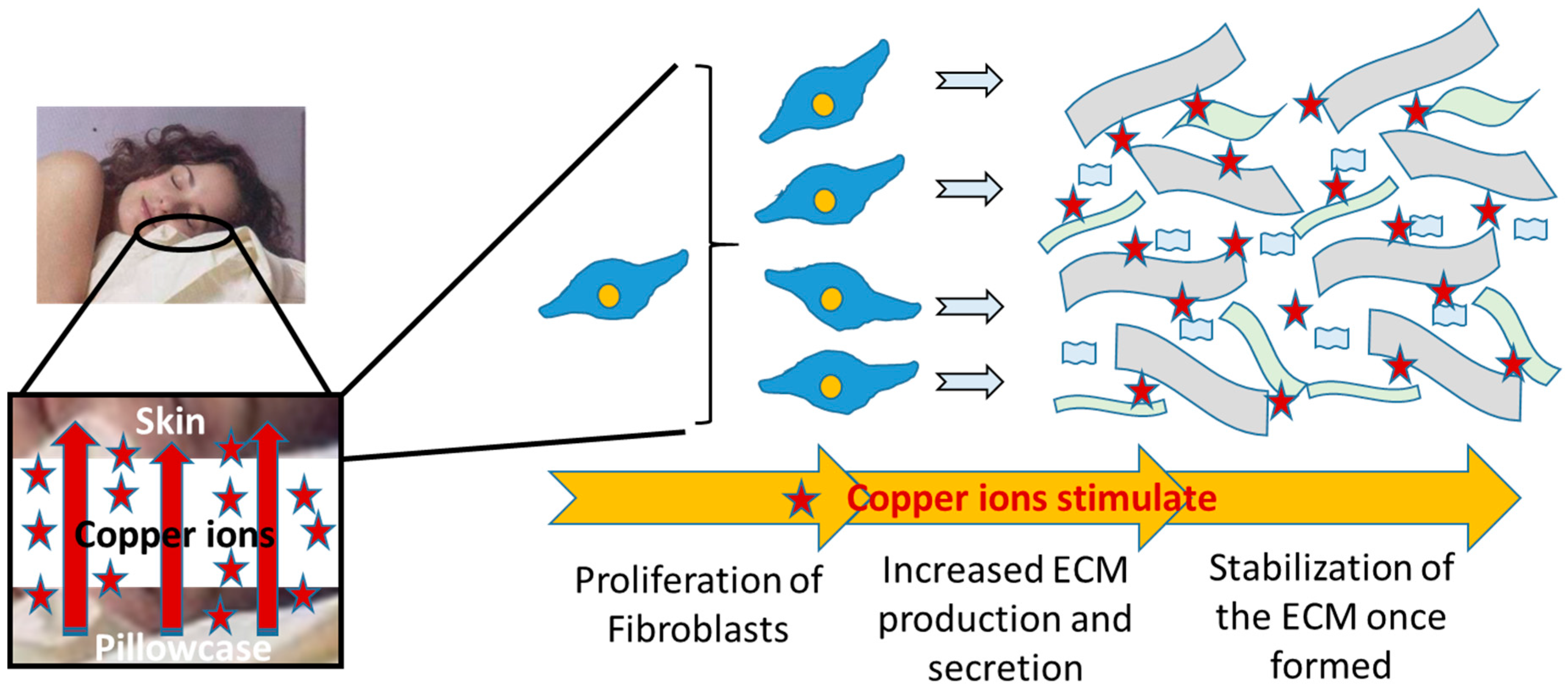
Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases
Abstract
:1. Introduction
2. Methods
2.1. Test Items
2.2. Test Procedures
2.3. Evaluation of Facial and Eye Skin Lifting
2.4. Measurement of Skin Brightness
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of Study Participants
3.2. Evaluation of Facial (Cheek) and Eye Skin Lifting
3.3. Evaluation of Skin Brightness
3.4. Adverse Reactions
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krieg, T.; Aumailley, M. The extracellular matrix of the dermis: Flexible structures with dynamic functions. Exp. Dermatol. 2011, 20, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.C.; Watson, R.E.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, S.; Chen, Y.; Lyga, J.; Wyborski, R.; Santhanam, U. Investigation of age-related decline of microfibril-associated glycoprotein-1 in human skin through immunohistochemistry study. Clin. Cosmet. Investig. Dermatol. 2013, 6, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Shao, Y.; He, T.; Qin, Z.; Perry, D.; Voorhees, J.J.; Quan, T. Reduction of fibroblast size/mechanical force down-regulates TGF-beta type II receptor: Implications for human skin aging. Aging Cell 2016, 15, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.M.; Wilhelm, K.P. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int. J. Cosmet. Sci. 2008, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Sherratt, M.J.; Griffiths, C.E.; Watson, R.E. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Luebberding, S.; Krueger, N.; Kerscher, M. Mechanical properties of human skinin vivo: A comparative evaluation in 300 men and women. Skin Res. Technol. 2014, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Eller, M.S.; Gilchrest, B.A. Fifty years of skin aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging differences in ethnic skin. J. Clin. Aesthet. Dermatol. 2016, 9, 31–38. [Google Scholar] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [PubMed]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-beta1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am. J. Clin. Nutr. 1998, 67, 996S–1002S. [Google Scholar] [PubMed]
- Szauter, K.M.; Cao, T.; Boyd, C.D.; Csiszar, K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol. Biol. 2005, 53, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D.; Rayton, J.K.; Balthrop, J.E.; DiSilvestro, R.A.; Garcia-de-Quevedo, M. Copper and the synthesis of elastin and collagen. Ciba Found. Symp. 1980, 79, 163–182. [Google Scholar] [PubMed]
- Lansdown, A.B. Metallothioneins: Potential therapeutic aids for wound healing in the skin. Wound Repair Regen. 2002, 10, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Royce, P.M.; Camakaris, J.; Danks, D.M. Reduced lysyl oxidase activity in skin fibroblasts from patients with Menkes’ syndrome. Biochem. J. 1980, 192, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Briden, A.; Hall, S.; Brown, R.A. Stabilisation of cables of fibronectin with micromolar concentrations of copper: In vitro cell substrate properties. Biomaterials 2004, 25, 803–812. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Ramamurthi, A. Copper nanoparticle cues for biomimetic cellular assembly of crosslinked elastin fibers. Acta Biomater. 2009, 5, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Gorter, R.W.; Butorac, M.; Cobian, E.P. Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am. J. Ther. 2004, 11, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Hostynek, J.J.; Dreher, F.; Maibach, H.I. Human stratum corneum penetration by copper: In vivo study after occlusive and semi-occlusive application of the metal as powder. Food Chem. Toxicol. 2006, 44, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Heilmittel, W. Wala Therapeutic Preparations (Handbook), 5th ed.; Weleda: Congers, NY, USA, 1994. [Google Scholar]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, J.; Mishal, J.; Magen, E.; Zatcoff, R.C.; Shemer-Avni, Y.; Borkow, G. Copper oxide impregnated textiles with potent biocidal activities. J. Ind. Text. 2006, 35, 323–335. [Google Scholar] [CrossRef]
- Borkow, G. Using copper to fight microorganisms. Curr. Chem. Biol. 2012, 6, 93–103. [Google Scholar] [CrossRef]
- Borkow, G. Safety of using copper oxide in medical devices and consumer products. Curr. Chem. Biol. 2012, 6, 86–92. [Google Scholar] [CrossRef]
- Weinberg, I.; Lazary, A.; Jefidoff, A.; Vatine, J.-J.; Borkow, G.; Ohana, N. Safety of using diapers containing copper oxide in chronic care elderly patients. Open Biol. J. 2013, 6, 54–59. [Google Scholar]
- Dykes, P. Increase in skin surface elasticity in normal volunteer subjects following the use of copper oxide impregnated socks. Skin Res. Technol. 2015, 21, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G. Protection of Soldiers’ feet by copper oxide impregnated socks. Adv. Mil. Technol. 2013, 8, 101–108. [Google Scholar]
- Borkow, G.; Zatcoff, R.C.; Gabbay, J. Reducing the risk of skin pathologies in diabetics by using copper impregnated socks. Med. Hypotheses 2009, 73, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Yoo, M.A.; Koh, J.S.; Borkow, G. Reduction of facial wrinkles depth by sleeping on copper oxide-containing pillowcases: A double blind, placebo controlled, parallel, randomized clinical study. J. Cosmet. Dermatol. 2012, 11, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J.; Lyakhovitsky, A.; Huszar, M. Improvement of facial skin characteristics using copper oxide containing pillowcases: A double-blind, placebo-controlled, parallel, randomized study. Int. J. Cosmet. Sci. 2009, 31, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Nishijima, T.; Fujimura, T.; Moriwaki, S.; Takema, Y. Development of a new evaluation method for cheek sagging using a Moire 3D analysis system. Skin Res. Technol. 2008, 14, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Pickart, L. The human tri-peptide GHK and tissue remodeling. J. Biomater. Sci. Polym. Ed. 2008, 19, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. An in vitro study on the role of metal catalyzed oxidation in glycation and crosslinking of collagen. Mol. Cell. Biochem. 1999, 194, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Hosoi, J.; Amano, S.; Tsuchiya, T. Sagging of the cheek is related to skin elasticity, fat mass and mimetic muscle function. Skin Res. Technol. 2009, 15, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Tenaud, I.; Sainte-Marie, I.; Jumbou, O.; Litoux, P.; Dreno, B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br. J. Dermatol. 1999, 140, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Saito, N.; Takemori, N.; Iizuka, S.; Suzuki, K.; Taniguchi, N.; Iizuka, H. Ultrastructural localization of superoxide dismutase in human skin. Acta Derm. Venereol. 1993, 73, 41–45. [Google Scholar] [PubMed]
- Borkow, G.; Mellibovsky, J.C. Resolution of skin maladies of the trapped Chilean miners: The unplanned underground copper-impregnated antifungal socks “trial”. Arch. Dermatol. 2012, 148, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Zatcoff, R.C.; Smith, M.S.; Borkow, G. Treatment of tinea pedis with socks containing copper-oxide impregnated fibers. Foot (Edinb.) 2008, 18, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hostynek, J.J.; Maibach, H.I. Copper hypersensitivity: Dermatologic aspects—An overview. Rev. Environ. Health 2003, 18, 153–183. [Google Scholar] [CrossRef] [PubMed]

 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.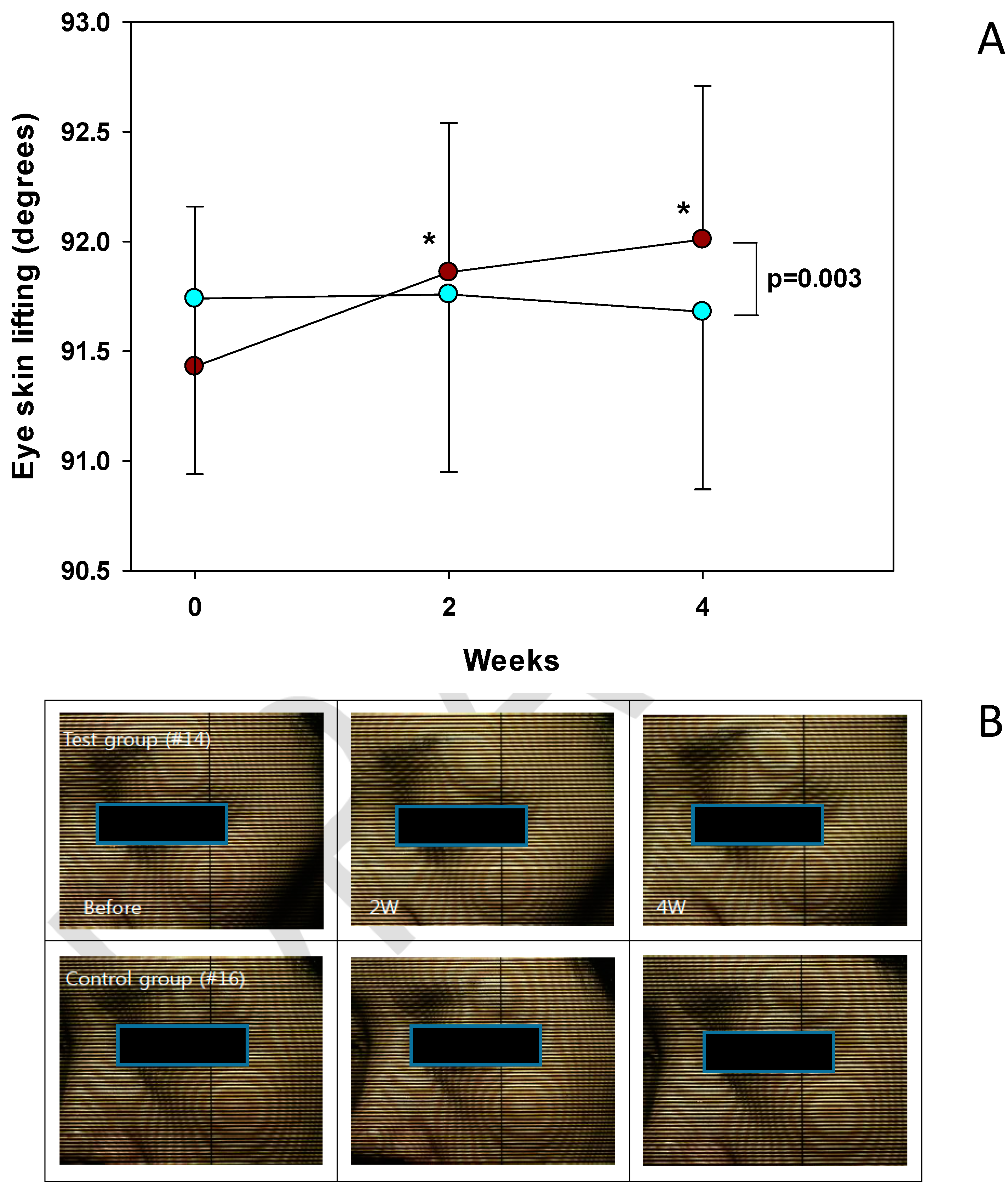
 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
 ) and Test (
) and Test (  ) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
) Groups (A) and representative pictures (B). * p < 0.05 vs. before treatment.
| Selection Criteria | Exclusion Criteria |
|---|---|
|
|
| Parameter | Grade | Control Group | Test Group | p Value | |||
|---|---|---|---|---|---|---|---|
| Frequency (n) | Percentage (%) | Frequency (n) | Percentage (%) | Chi-Square Test | Likelihood Ratio Test | ||
| Skin Type | Dry | 8 | 34.78 | 11 | 50 | 0.341 | 0.248 |
| Normal | 7 | 30.43 | 4 | 18.18 | |||
| Oily | 2 | 8.70 | 0 | 0.00 | |||
| Dry and oily | 6 | 26.09 | 7 | 31.82 | |||
| Problematic | 0 | 0.00 | 0 | 0.00 | |||
| Hydration | Sufficient | 0 | 0.00 | 0 | 0.00 | 0.436 | 0.436 |
| Normal | 11 | 47.83 | 8 | 36.36 | |||
| Deficient | 12 | 52.17 | 14 | 63.64 | |||
| Sebum | Glossy | 2 | 8.70 | 4 | 18.18 | 0.585 | 0.581 |
| Normal | 12 | 52.17 | 9 | 40.91 | |||
| Deficient | 9 | 39.13 | 9 | 40.91 | |||
| Surface | Smooth | 6 | 26.09 | 2 | 9.09 | 0.223 | 0.210 |
| Normal | 13 | 56.52 | 18 | 81.82 | |||
| Rough | 4 | 17.39 | 2 | 9.09 | |||
| Thickness | Thin | 8 | 34.78 | 10 | 45.45 | 0.178 | 0.169 |
| Normal | 14 | 60.87 | 10 | 45.45 | |||
| Thick | 1 | 4.35 | 2 | 9.09 | |||
| Time of UV exposure per day | <1 h | 5 | 21.74 | 7 | 31.82 | 0.510 | 0.498 |
| 1–3 h | 15 | 65.22 | 14 | 63.64 | |||
| >3 h | 3 | 13.04 | 1 | 4.55 | |||
| Time of sleeping per night | <5 h | 1 | 4.35 | 0 | 0.00 | 0.368 | 0.250 |
| 5–8 h | 22 | 95.65 | 21 | 95.45 | |||
| >8 h | 0 | 0.00 | 1 | 4.55 | |||
| Sleeping habits | Front side | 0 | 0.00 | 0 | 0.00 | 0.205 | 0.199 |
| Left side | 3 | 13.04 | 7 | 31.82 | |||
| Right side | 5 | 21.74 | 6 | 27.27 | |||
| Both sides | 15 | 65.22 | 9 | 40.91 | |||
| Irritability | Yes | 3 | 13.04 | 4 | 18.18 | 0.634 | 0.634 |
| No | 20 | 86.96 | 18 | 81.82 | |||
| Stinging | Yes | 1 | 4.35 | 0 | 0.00 | 0.323 | 0.243 |
| No | 22 | 95.65 | 22 | 100.00 | |||
| Smoking | Yes | 0 | 0.00 | 0 | 0.00 | 1.000 | 1.000 |
| No | 23 | 100.00 | 22 | 100.00 | |||
| Group | Week | Mean 1 | SD 2 | SEM 3 | p-Value 4 | Decrement (%) |
|---|---|---|---|---|---|---|
| Test Group | 0 | 37.79 | 5.09 | 1.09 | – | – |
| 2 | 37.52 | 5.35 | 1.14 | 0.280 | 0.70 ▼ | |
| 4 | 37.20 | 4.78 | 1.02 | 0.039 * | 1.56 ▼ | |
| Control Group | 0 | 37.95 | 4.64 | 0.97 | – | – |
| 2 | 38.17 | 4.62 | 0.96 | 0.063 | 0.59 ▲ | |
| 4 | 38.51 | 4.89 | 1.02 | 0.097 | 1.48 ▲ |
| Group | Week | Mean 1 | SD 2 | SEM 3 | p-Value 4 | Decrement (%) |
|---|---|---|---|---|---|---|
| Test Group | 0 | 91.43 | 3.04 | 0.73 | – | – |
| 2 | 91.86 | 3.19 | 0.68 | 0.011 * | 0.46 ▲ | |
| 4 | 92.01 | 3.29 | 0.70 | 0.001 * | 0.63 ▲ | |
| Control Group | 0 | 91.74 | 3.84 | 0.80 | – | – |
| 2 | 91.76 | 3.88 | 0.81 | 0.881 | 0.02 ▲ | |
| 4 | 91.68 | 3.89 | 0.81 | 0.505 | 0.07 ▼ |
| Group | Week | Mean 1 | SD 2 | SEM 3 | p-Value 4 | Decrement (%) |
|---|---|---|---|---|---|---|
| Test Group | 0 | 64.15 | 2.30 | 0.49 | – | – |
| 2 | 64.38 | 2.20 | 0.47 | 0.024 * | 0.37 ▲ | |
| 4 | 64.44 | 2.26 | 0.48 | 0.008 * | 0.45 ▲ | |
| Control Group | 0 | 64.01 | 1.95 | 0.41 | – | – |
| 2 | 63.91 | 1.94 | 0.40 | 0.108 | 0.15 ▼ | |
| 4 | 63.93 | 1.84 | 0.38 | 0.139 | 0.13 ▼ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkow, G.; Del Carmen Elías, A. Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases. Cosmetics 2016, 3, 24. https://doi.org/10.3390/cosmetics3030024
Borkow G, Del Carmen Elías A. Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases. Cosmetics. 2016; 3(3):24. https://doi.org/10.3390/cosmetics3030024
Chicago/Turabian StyleBorkow, Gadi, and Adriana Del Carmen Elías. 2016. "Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases" Cosmetics 3, no. 3: 24. https://doi.org/10.3390/cosmetics3030024
APA StyleBorkow, G., & Del Carmen Elías, A. (2016). Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases. Cosmetics, 3(3), 24. https://doi.org/10.3390/cosmetics3030024