Abstract
Rutin, a flavonoid found in various plants, has gained attention for its potential applications in the cosmetic industry due to its antioxidant, anti-inflammatory, and potential photoprotective properties. Our review explored the use of rutin (rutoside, 3-O-rutinoside-quercetin) in cosmetics/dermocosmetics, focusing on its physicochemical properties and stability, cutaneous permeability, and efficacy in sunscreen systems, involving in vitro tests and the current state of clinical trials. Rutin’s ability to scavenge free radicals, prevent peroxidation, and reduce vascular fragility makes this flavonoid a promising ingredient for photoprotection. Studies have shown that rutin can enhance the sun protection factor (SPF) of sunscreen formulations, especially when combined with organic UV filters. The encapsulation of rutin in nanostructures has also been investigated to improve its applicability. Overall, rutin shows potential as a safe and effective ingredient in cosmetics/dermocosmetics, offering protection against the harmful effects of UV radiation and oxidative stress on the skin, as well as being a feasible strategy for developing environmentally friendly multifunctional sunscreens.
1. Introduction
Several bioactive substances are obtained from plant origin, finding applications in feeding, cosmetic, and medicinal products. Among the most widely used classes are flavonoids, comprising more than 8000 identified substances, and being broadly recognized for their properties, including antioxidant, anti-inflammatory, and anti-cancer, among several others. One of the most well-recognized subgroups of the flavonoid class is the flavonols (quercetin, mercetin, rutin etc.) [1,2,3,4,5]. Rutin (rutoside, 3-O-rutinoside-quercetin), also known as vitamin P, is a yellow/green and odorless pigment that develops in the form of needle crystals [6,7,8]. This compound has a free radical scavenging capacity and can also prevent peroxidation promoted by metal ions, as it is a metal chelator [9,10]. Moreover, it exhibits broad bioactivity, such as reducing vascular fragility, reducing hypertension, and possessing anti-inflammatory and antioxidant activities [7,11,12,13]. Such benefits to the vascular system, particularly the cutaneous superficial microcirculation, help to improve the efficacy of sunscreens.
Rutin was detected in 1842 in Ruta graveolens (common rue or herb-of-grace) and later isolated from Caparis spinosa (caper bush). It was classified as rutinic acid, since it is soluble in alkaline media, practically insoluble in water (0.13 g/L), and slightly soluble in alcohols (methanol 55.0 g/L; ethanol 5.5 g/L). Table 1 presents some of the physicochemical properties of rutin. Its molecular structure (C27H30O16) was elucidated in 1896 (Figure 1), with the discovery of the link between the sugars (glucose and rhamnose) in the quercetin molecule [1,6,14,15].

Table 1.
Physicochemical characteristics of rutin.
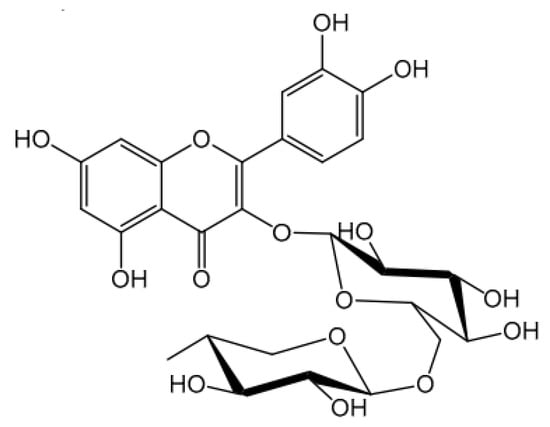
Figure 1.
Chemical structure of rutin [16].
The molecular structure of rutin shows aromatic rings and structures capable of resonance. Thus, rutin can be an ally in photoprotection, being able to interact with ultraviolet (UV) radiation, with the potential to improve the efficacy of sunscreen products [20,21,22]; however, according to our research group’s experience, rutin’s mechanism of action in the efficacy enhancement of sunscreen systems could be attributed to other biological functionalities, like antioxidant and anti-inflammatory properties. Furthermore, it is known that much of the skin damage caused by UV radiation is related to free radicals formed during UV exposure [23,24,25,26,27,28]. Therefore, considering the antioxidant capacity of rutin, it is believed that it can confer additional protection from oxidative damage to the skin caused by sun exposure, justifying its application in multifunctional photoprotective formulations [29,30,31,32]. It is still worth highlighting that this bioactive compound is biocompatible, and thus possesses a suitable profile to be used in cosmetic formulations [23,33,34,35,36]. In this narrative review, we provide a comprehensive description of the scientific reports exploring the potential use of rutin in the development of photoprotective formulations, discussing its physicochemical and functional properties, such as its antioxidant, anti-inflammatory, and photoprotective effects. We also examine the physicochemical stability of cosmetic preparations containing rutin and its cutaneous permeability, highlighting its relevance to cosmetology. Additionally, we discuss the efficacy of rutin in sunscreen systems, both in vitro and in vivo, and also explore the potential use of nanotechnology.
2. Physicochemical Stability of Cosmetic Preparations Containing Rutin
Rutin presents limited liability and solubility, making its inclusion into formulations challenging (Figure 2). However, due to its compatibility with different excipients/starting materials used in the preparation of formulations, the possibility of synergies with other compounds, and its potential for distinct biological activities, rutin continues to be the target of studies on use in topical applications. Rutin has been successfully incorporated into semi-solid systems for cosmetic applications after being previously dissolved in an alkaline (the pH value of the product can be adjusted to 5–7 by the end of the formulation production) or hydroalcoholic media, by using heat (aqueous medium) or tensoactives such as sodium lauryl sulfate [17,37,38,39,40,41,42,43,44].
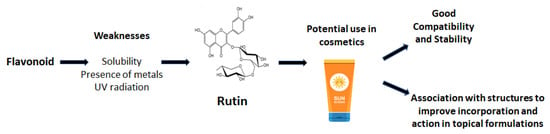
Figure 2.
Weaknesses and strengths of using rutin in topical formulations.
The development of cosmetic formulations requires the rigorous selection of starting materials, careful technological processes, and the validation of the analytical methodology used to determine the active substance(s), ensuring the quality (physical, physicochemical, chemical, microbiological, and toxicological aspects), safety, efficacy, acceptance, and adherence of the final product by the consumer [45,46,47].
Several extrinsic and intrinsic factors influence the stability of formulations:
- (i)
- Extrinsic factors—external conditions to which cosmetic products are exposed, such as temperature, light, oxygen, humidity, packaging materials, microorganisms, and movement, among others;
- (ii)
- Intrinsic factors—related to the nature of the formulation and the interactions among the components, leading mainly to physical and chemical incompatibilities [48,49].
Stability studies of cosmetic products aim to provide information indicating the relative stability of a product under various exposure conditions until the end of its shelf life. These studies should guide the development procedures, including the selection of formulation components, presentation form, alternative packaging materials, and confirmation of the estimated shelf life [48,49,50].
Preliminary Stability Evaluation (PSE) involves numerous formulations and tests applying extreme conditions of temperature, gravity, and humidity to select substances with the best physicochemical stability. Typically, preparations undergo centrifugation and thermal stress tests. PSE allows the formulator to choose which formulas, among those subjected to several tests from the development stage, are apparently stable [48,49,50]. Velasco and collaborators developed 14 oil-in-water (O/A) emulsions, containing 5.0% w/w of the commercial extract of Trichilia catigua Adr. Juss (and) Ptychopetalum olacoides Bentham, as a source of rutin. PSE facilitated the identification of signs of instability in certain samples, such as phase separation, color alteration, and creaming [51]. Formulations showing modifications after the test should be rejected, or possible modifications for improved stability should be investigated. Those showing better performance are subjected to Accelerated Stability Testing.
The Accelerated Stability Test acts as a guide in predicting the stability of a product under harsh storage conditions in a shortened timeframe. Samples are stored under extreme temperature and light conditions such as the following: 22.0 ± 0.5 °C (room temperature); 5.0 ± 0.5 °C; temperature cycles ranging from −10 ± 0.5 °C to +45 ± 0.5 °C; −10 ± 0.5 °C; exposure to indirect or direct sunlight; in an oven under 37.0 ± 0.5 °C to 50.0 ± 0.5 °C. They are carefully evaluated after the tests, and the best-performing samples are selected for sequential testing in the Normal Stability Test [49]. The evaluated parameters include appearance, color, odor, pH value, and apparent viscosity, indicating possible physical and physicochemical changes.
Our research group has previously determined the stability profiles of two emulsified systems containing 5.0% w/w of a commercial extract, standardized in total flavonoids quantified as rutin, through the Normal Stability Test [52]. The systems differed in the absence or presence of 2.0% w/w of soy lecithin as an additional tensoactive. The total analysis period was 90 days, with tests on the 3rd, 7th, 15th, 30th, 60th, and 90th days. The storage conditions were: 40.0 ± 0.5 °C; exposure to indirect and direct sunlight at room temperature (24.0 ± 2.0 °C); and 5.0 ± 0.5 °C. The evaluated parameters were the organoleptic characteristics, pH value, apparent viscosity (cP), and total flavonoid content, expressed as rutin equivalents, remaining in the samples (µg/mL) [52]. The quantification of rutin was performed through UV spectrophotometry at 361.0 nm [53,54,55]. Under the storage conditions at 24.0 ± 2.0 °C and 5.0 ± 0.5 °C (refrigerator), the samples showed reduced degradation of rutin content compared to the levels determined after their preparation. The sample without soy lecithin exhibited a reduction in the bioactive compound of 3.36% in the room temperature condition and 0.68% in the refrigerator after 90 days of storage. Although there was a variation in the pH value in the refrigerator condition, the concentration of total flavonoids was considered satisfactory, indicating that a pH value change in the range of −13.8% to −5.2% did not increase rutin degradation. For the sample with soy lecithin, at the end of the 90-day analysis, the percentage reductions were 6.38% and 1.31% for the room temperature and refrigerator conditions, respectively. In the oven condition (40.0 ± 0.5 °C), after 90 days, rutin deteriorated to a greater extent due to the temperature effect, indicating the chemical degradation of the active substance for both samples. There were reductions of 34.16 and 35.12% in the rutin concentration in the systems without and with lecithin, respectively [51]. Considering that the evaluation of the stability of emulsified systems is usually conducted empirically, the protocol used in this work offers advantages by systematically establishing experimental assays and objective criteria for the acceptance/rejection of samples in a short period [52,53,54,55,56].
Overall, flavonoids are sensitive to the presence of metals, UV radiation, temperature, and hydrolysis, which sensitivity is accelerated directly and proportionally to the temperature increase [57,58,59]. Bilia and collaborators evaluated the stability of flavonoids, as a dry extract with or without the addition of a mixture of ascorbic acid and citric acid, under storage conditions at room temperature and in an oven for periods of 90 and 45 days [60]. The researchers found the flavonoids to be stable, and observed that the presence of the antioxidants did not affect the stability of the dry extract. Thus, it can be suggested that the absence of a vehicle or cosmetic form containing water contributes to the stability and content of flavonoids. This highlights the importance of a careful selection of pharmaceutical adjuvants (starting materials) to favor the chemical stability of rutin. Therefore, another possible explanation for the high kinetics of rutin deterioration in the presence or absence of lecithin could be the inadequate concentration of, for example, the chelating/sequestering agent sodium heptanoate at 0.1% w/w [51]. Banov and coworkers evaluated the stability of gels and emulsions containing Ginkgo biloba L. extract, standardized in total flavonoid content, and the chelating/sequestering agent EDTA Na2. The results show the suitability of this agent in maintaining the quantified levels of total flavonoids according to the Normal Stability Test, at a concentration of 0.1% w/w and under oven conditions (40.0 ± 0.5 °C) [61].
Additionally, Nishikawa and coworkers developed hydroalcoholic gel systems (10.0% w/w cereal alcohol) based on polyvinyl alcohol with 0.05% w/w rutin [62]. The authors aimed to obtain a suitable vehicle containing the bioactive compound for facial application as a peel-off mask. The samples differed by the absence or presence of EDTA Na2 at 0.1% w/w. The stability study was conducted for 45 days, and the samples were stored at 22.0 ± 2.0 °C, 5.0 ± 0.5 °C, and 40.0 ± 0.5 °C. The quantitative content of rutin was determined by first-order derivative spectrophotometry at 410.0 nm [53,63]. The authors found that the presence of the chelating agent, at the employed concentration, improved the stability of rutin in the peel-off mask under room- (22.0 ± 2.0 °C) and low-temperature storage conditions. In the sample without EDTA Na2, the bioactive compound degraded under all storage conditions. It was also observed that the pH values of both samples tended to decrease when stored at 40.0 ± 0.5 °C. However, as also noted by Velasco and collaborators, this did not interfere with the chemical stability of rutin [51]. Thus, Nishikawa and coworkers (2007) concluded that the chelating agent increased the stability of the bioactive compound in hydroalcoholic gel systems when stored at room and low temperatures [62]. Under the storage conditions of 40.0 ± 0.5 °C, the presence of EDTA Na2 did not inhibit rutin degradation, contrary to the findings of Banov and coworkers as mentioned above [61], reinforcing the interpretation that higher water contents in the vehicle intended to carry rutin necessitate additional care in the selection and concentration of pharmaceutical adjuvants.
3. Cutaneous Permeability of Rutin: Relevance to Cosmetology and Photoprotection
Bioactives must reach the site of action at a sufficient concentration for their effectiveness. Therefore, rutin, when formulated in different cosmetic forms, should mainly located on the outer layers of the skin, its target tissue, to satisfactorily exert its protective effects [34,64]. Experiments on the release of active ingredients provide data on the behavior of the active component in semi-solids, supporting the characterization and comparison of formulations, the evaluation of production quality, and batch-to-batch uniformity. Therefore, it is essential to compare the performance of products under development with those available on the market [64]. In vitro penetration studies offer advantages such as cost-effectiveness, rapid results acquisition, experiment condition control, and the ability to assess a greater number of assays and replicas, among others. The ideal situation would be to use human skin as a model; however, the limited availability of this type of material, the need to submit the experiment to an Ethics Committee, and the difficulties and costs associated with storage and viability restrict its use [64]. As alternatives to human skin, researchers use experimental animal skin, synthetic membranes, and three-dimensional cultures, such as reconstructed epidermis. There is an interest in using shed snake skin as a substitute model for human skin, and researchers have evaluated its applicability in permeation studies, finding favorable responses [64].
Baby and coworkers developed emulsions containing 5.0% w/w rutin, differing in the presence of urea, isopropanol, and/or propylene glycol at 2.5 or 5.0% w/w (concentrations and combinations defined according to a two-level experimental factorial design) [64,65]. The authors evaluated the release profile and, subsequently, the skin penetration of the bioactive compound in vertical diffusion cells for 6 h, using cellulose acetate membrane and a biomembrane model (shed skin from Crotalus durissus snake). The rutin content in the receptor compartment was quantified by spectrophotometry at 410.0 nm [64]. Rutin dissolution was found to be a limiting factor in the diffusion process (zero-order model) [65]. Nonetheless, higher amounts of released and accumulated rutin over time were found with propylene glycol addition (5.0% w/w). Since the presence of propylene glycol showed a tendency to be more suitable in favoring the release of rutin from the investigated sample, this formulation was selected for further studies with shed skin from Crotalus durissus, as a model biomembrane. Even though the spectrophotometric method used in this investigation had a limit of quantification value equal to 0.308 μg/mL, no penetration of rutin through the model’s biomembrane was observed over 6 h. The authors attributed this behavior to the reduced logP of rutin (−0.69), since similar results were reported in other permeation studies of flavonoids using human and pig skin [18]. Valenta, Nowack, and Bernkop-Schnürch observed the penetration of rutin through rat skin and suggested that it interacted with the membrane model, in agreement with the results obtained in shed snake skin [63].
In a study of the penetration of quercetin and its 3-O-acyl esters through abdominal human skin, the authors proposed the duration of the experiment as an extremely relevant factor [66]. In their opinion, the diffusion assay should be conducted for at least 22 h, as only after long time intervals was the identification and quantification of quercetin in the receptor fluid of the diffusion cells possible. To verify whether the experimental duration in this research was appropriate, Baby and collaborators conducted a diffusion assay for 52 h [64]. Despite not being able to detect rutin in the receptor compartment after this interval, they were successful in quantifying the rutin retained in the model membrane, finding 0.931 + 0.039 μg/mg after just 6 h, thus confirming that rutin is significantly retained in shed snake skin.
The association of rutin with nanostructures has been used to aid the penetration of this active ingredient into deeper layers of the skin, and to thus improve the action of this compound in the deeper layers of the epidermis [8,67,68,69]. In vitro studies using nanostructures such as nanocrystal, glycerosomal, and microencapsulated nanostructured lipid carriers showed enhanced permeation of the active ingredient, demonstrating the potential of these nanocarriers for use in bioactive delivery [70,71,72,73]. The encapsulation into ethosomes was also found to improve rutin penetration ex vivo, which was attributed to the characteristics of the nanostructure, since these malleable vesicles can facilitate the passage of the active ingredient to deeper skin layers [23]. These studies indicate that this strategy should be considered in the development of photoprotective formulations. A compilation of reports from the literature on different nanostructures used to enhance rutin skin penetration/permeation is presented in Table 2.

Table 2.
Nanostructures that promote enhanced skin permeation of rutin.
Despite the technologies used to improve rutin penetration/permeation across the epidermis and dermis with different levels of success, this is still a challenging task that must be investigated in parallel with the establishment of safety and efficacy in the new strategies, which is pertinent to the function of the product/system and its target site.
4. Rutin and Sunscreen Systems: In Vitro and In Vivo Efficacy Assessment
As previously discussed, rutin exhibits poor penetration/permeation through the skin, being retained within the stratum corneum and, therefore, being appropriate for topical formulations [64,80,81,82]. The observed superficial action profile of rutin, related to its aglycone counterpart, aligns with the desired characteristics of sunscreens, which ideally should remain predominantly at the application site’s surface [66,70,72,73,83]. Other factors, combined with the stability of this bioactive compound in topical vehicles, support the incorporation of rutin into dermocosmetics designed for skin protection against UVA and UVB radiations (Figure 3) [12,64,80]. The structural resemblance of flavonoids with organic UV filters and the antioxidant properties inherent to polyphenolic compounds indicate the potential to prevent photooxidative stress in the skin. Additionally, the absorption spectra in the UV radiation region position this compound as a promising candidate for providing complementary or adjuvant photoprotective activity [4,14,84,85,86,87].
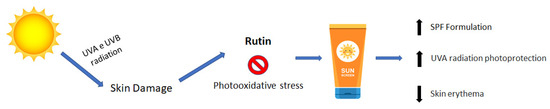
Figure 3.
Putative mechanism of action of rutin in sunscreen formulations.
Velasco and coworkers showed, based on reflectance spectrophotometry with an integrating sphere, that the inclusion of rutin in photoprotective formulations, isolated or combined with octyl p-methoxycinnamate (UVB filter) and benzophenone-3 (UVA filter) in varying proportions, enhanced the in vitro the sun protection factor (SPF) and protected against UVA radiation [14]. Notably, 0.1% w/w rutin increased the estimated SPF value from 7.34 ± 0.24 to 9.97 ± 0.18, in the presence of octyl p-methoxycinnamate at 3.5% w/w and benzophenone-3 at 1.0% w/w, representing an increase of 2.63 units. Interestingly, the combination of rutin at 0.1% w/w with filters at a maximum concentration (octyl p-methoxycinnamate at 7.0% w/w and benzophenone-3 at 2.0% w/w) did not result in a significant increase in the SPF value. The combination of rutin with organic UV filters, at various concentrations, resulted in a reduction in the critical wavelength (λC) and the UVA/UVB ratio. The photoprotective interaction between rutin and the UV filters was found to be concentration-dependent. Based on these results, it is plausible to suggest that an interaction occurred between the active ingredients, leading to a decrease in UVA protection that rutin has the potential to induce [14].
UVA radiation directly impacts the dermal compartment of the skin, and it is well-established that this radiation contributes significantly to skin photoaging [84,88,89,90,91,92]. The primary cumulative effects of UVA radiation (320–400 nm) include the generation of reactive oxygen species and alterations in tumor suppressor genes, such as p53. UVA radiation is further categorized into UVA II (320–340 nm) and UVA I (340–400 nm). It is known that UVA I radiation induces damage to dermal fibroblasts, leading to the induction of cytokines, matrix metalloproteinases, and mutations in mtDNA [93,94,95,96]. In a complementary study, Baby and coworkers expanded upon the research to determine the in vitro anti-UVA I efficacy of the previously described photoprotectors [97]. The anti-UVA I efficacy was assessed through diffuse reflectance spectrophotometry with an integrating sphere, followed by mathematical treatment [98,99]. The outcomes indicate that formulations containing octyl p-methoxycinnamate at 3.5% w/w and benzophenone-3 at 1.0% w/w, and formulations with octyl p-methoxycinnamate at 7.0% w/w and benzophenone-3 at 2.0% w/w (representing a twofold increase in filter concentration), did not provide a directly proportional escalation in anti-UVA I protection. This observation persisted despite a substantial increase in SPF from 7.34 ± 0.24 to 14.63 ± 2.05 [14], a finding also supported by other studies that established that the SPF value is directly dependent on the concentration of filters utilized [99,100]. Contrastingly, Baby and coworkers found that increasing the proportion of UV filters did not inherently result in a proportional enhancement in protection against UVA radiation [97]. Samples with rutin at 0.1% w/w combined with filters at intermediate concentrations yielded a modest increase in anti-UVA I protection, a phenomenon not replicated in samples with the doubled proportion of octyl p-methoxycinnamate and benzophenone-3. The authors attributed this behavior to the electronic stabilization–destabilization mechanism of UV filter molecules (resonant structures), influenced by the presence of rutin. However, given that the concentration of UV filters in the aforementioned samples was at least ten times higher than that of rutin, it can be proposed that the maximal proportions of octyl p-methoxycinnamate and benzophenone-3 may have hindered the potential adjuvant photoprotective effect of rutin. In terms of the estimated anti-UVA efficacy, benzophenone-3 made a limited contribution to the augmentation of its UV radiation-absorbing effects with the respective increase in concentration in the systems proposed by Velasco and coworkers, and by Baby and coworkers [14,97].
Considering in vivo studies, the use of rutin was evaluated by incorporating this bioactive into a photoprotective formulation containing butyl methoxydibenzoylmethane and octyl dimethyl PABA, resulting in an increase in the SPF of the formulation by around 70% compared to the formulation without the compound (in vivo SPF 12.4 with rutin and SPF 7.3 without rutin). This fact can be attributed mainly to the anti-inflammatory activity of rutin, which results in a reduction in erythema and consequently increases the photoprotective action [34]. Polyphenolic compounds present a diverse array of biological properties, encompassing antiallergic, anti-inflammatory, hepatoprotective, vasoactive, antithrombotic, antioxidant, free radical scavenging, antitumor, antibacterial, and antiprotozoal actions, among others [23,34,101,102]. Considering the presence of polyphenols, studies were conducted to investigate the photoprotective properties of various plants, including Aloe, Helichrysum, Chamomile, Hamamelis, Cinnamomum, Camellia, Rosa, Ginkgo, and Polypodium, among others [103]. In a clinical study on the dorsal skin of rats, Aronia melanocarpa (black chokeberry), whose extract composition is rich in rutin and chlorogenic acid, significantly influenced the reduction in the severe effects of UVB radiation, such as erythema, excoriation, and scarring, after topical treatment for 7 days. Furthermore, it was clear that the extract reduced the thickening of the epidermis, as well as the degradation of fibroblasts and collagen, in addition to helping to reduce the inhibition of collagen production [104].
In this context, Velasco and coworkers developed sunscreens containing rutin and extracts of Passiflora incarnata L. and Plantago lanceolata associated or not with chemical (octyl p-methoxycinnamate and benzophenone-3) and physical filters (TiO2) [105]. The extracts were chosen as potential bioactive compounds for sunscreens due to their phytochemical compositions, which also included polyphenolic compounds [106,107,108,109]. The authors standardized the concentrations of the mentioned extracts in the samples based on their total flavonoid contents, expressed in rutin, which has been previously quantified [51,52,53]. The concentrations of P. incarnata L. and P. lanceolata extracts in the photoprotective formulations were 1.68 and 2.78% w/w, respectively, corresponding to 0.1% w/w of rutin. Chemical filters were incorporated at different concentrations [14,97], while TiO2 was used at 1.0% or 2.0% w/w. The formulated samples exhibited a range of in vitro SPF values, spanning from 0.972 ± 0.004 (control, without active compounds) to 28.064 ± 2.429 (P. lanceolata extract at 2.78% w/w, octyl p-methoxycinnamate at 7.0% w/w, benzophenone-3 at 2.0% w/w, and TiO2 at 2.0% w/w). The best SPF values were observed in samples containing rutin (27.574 ± 2.055) or P. lanceolata extract (28.064 ± 2.429) combined with filters at their maximum concentrations, which values were significantly higher than the SPF of the control (24.256 ± 3.276), clearly demonstrating the activity of rutin or the P. lanceolata extract. Unexpectedly, the inclusion of P. incarnata L. extract led to a significant reduction in the SPF value when associated with octyl p-methoxycinnamate at 7.0% w/w, benzophenone-3 at 2.0% w/w, and TiO2 at 2.0% w/w, resulting in a decrease of approximately four SPF units. A similar trend was observed for the combination of P. lanceolata extract with octyl p-methoxycinnamate at 3.5% w/w, benzophenone-3 at 1.0% w/w, and TiO2 at 1.0% w/w, causing a reduction in anti-UVB efficacy by approximately seven SPF units. The authors justified this observed phenomenon by considering factors such as vehicle composition, the presence of inorganic filters, the quantitative composition of organic filters, and the phytochemical compositions of P. incarnata L. and P. lanceolata extracts [105].
Organic filters represent active compounds widely employed in diverse cosmetic formulations, particularly in sunscreens. These filters mitigate the impacts of UV radiation on the skin by absorbing radiation through the promotion of electrons from the lowest-energy molecular orbital (highest occupied molecular orbital—HOMO) to a higher energy value (lowest unoccupied molecular orbital—LUMO). Considering this mechanism, it is conceivable that the phytochemical compositions of extracts, with the presence of flavonoids as electronegative polyphenolic compounds, might have facilitated the stabilization of the filter system, augmenting the energy gap between HOMO and LUMO. As energy is inversely proportional to wavelength (nm), this could result in a shift in the maximum wavelength to values below 290 nm, thereby leading to a decrease in SPF. Conversely, an opposing effect could have favored anti-UVA protection, potentially pushing the maximum wavelength beyond 320 nm, justifying improvements in the in vitro anti-UVA efficacy of bioactive photoprotectors [33,105].
From a practical standpoint, when UV filters were combined with bioactive compounds in a complex medium like an emulsion, a distinct photochemical profile emerged, deviating from that identified for isolated bioactive compounds. Consistent with the findings of Velasco and coworkers, Dondi and coworkers also discovered interactions in the associations between bioactive compounds and UV filters (such as octyl p-methoxycinnamate and avobenzone), and noted that these interactions were concentration- and media-dependent [105,110].
Considering the limited solubility of rutin in topical formulations and the possibility of negative interactions with the organic filters, the encapsulation of rutin can offer an interesting alternative to improve its benefits in photoprotective samples, such as by enhancing photostability, active compound incorporation, safety, and efficacy. Also, when using rutin, the amounts of chemical filters could be reduced, which is an important approach to preventing adverse effects, toxicity, and also ecological impacts [70,111,112,113,114,115,116].
The encapsulation of rutin into gelatin nanostructures (R-NC) was evaluated by Oliveira and collaborators, which demonstrated in vitro the photoprotective efficacy of this nanostructure by creating a possible adjuvant UV filter with higher wavelength ranges in the UVB and UVA regions compared to free rutin. The effect was attributed to the interaction of R-NC with the skin surface, resulting in a protective film capable of reflecting and sequestering UV radiation [111]. Also, the formulation containing R-NC and the filters ethylhexyl dimethyl PABA, ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane resulted in a 48% increase in the SPF determined in vitro, attributed to a synergistic effect among the components of the formulation. However, these results were not corroborated when R-NC was evaluated in vivo, since the nanostructures failed to enhance the SPF [36].
5. Conclusions
Among the flavonoids, the multifaceted properties of rutin (antioxidant and anti-inflammatory, for instance) make it a promising candidate for inclusion into cosmetics/dermocosmetics, particularly in photoprotective products, combined with its biocompatibility (safety), relative stability, and low cost. However, the successful incorporation of rutin into formulations requires the careful consideration of its interactions with other components. In vitro and in vivo studies have demonstrated rutin’s ability to enhance the sun protection factor (SPF) of sunscreen systems, mainly by acting in synergy with some organic UV filters. Nonetheless, the efficacy of rutin in sunscreens can be influenced by its concentration, formulation composition, and interactions with other UV-active ingredients. The encapsulation/association of rutin in nanostructures has been explored as a strategy to improve its photoprotective properties, although further research is needed to optimize its efficacy in vivo. Overall, rutin shows potential as a bioactive compound for use in photoprotective formulations toward environmental-friendly sunscreens, i.e., with the maintenance or improvement of efficacy, while keeping or reducing the concentration of organic UV filters and also providing multiple benefits for the cutaneous tissue.
Author Contributions
Conceptualization, A.R.B., M.V.R.V., M.B.A., T.M.C. and W.A.G.J.; methodology, A.R.B., M.B.A., F.V.L.S.P. and W.A.G.J.; formal analysis, A.R.B., M.V.R.V., C.R., C.d.O.R.-Y., M.B.A., F.V.L.S.P. and T.M.C.; research, A.R.B. and M.B.A.; resources, A.R.B., M.M.G.B.d.A., C.R., C.d.O.R.-Y., M.B.A. and W.A.G.J.; writing—original draft preparation, A.R.B., W.A.G.J. and M.B.A.; writing—review and editing, C.R., F.V.L.S.P., M.V.R.V., C.d.O.R.-Y. and A.R.B.; visualization, C.R., C.d.O.R.-Y. and A.R.B.; supervision, A.R.B.; project administration, A.R.B.; acquisition financing, M.M.G.B.d.A., C.R., C.d.O.R.-Y. and A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnolόgico (CNPq, Process 303862/2022-0); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Process 2024/01920-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES, Finance Code 001); and FCT (Foundation for Science and Technology), I.P. through national funds under DOI 10.54499/UIDP/04567/2020, DOI 10.54499/UIDB/04567/2020 and DOI 10.54499/EXPL/BTM-MAT/0112/2021 projects attributed to CBIOS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
W.A.G.J. is thankful for the scholarship PIBIC-CNPq. M.M.G.B.d.A. is grateful to the Provost of Inclusion and Belonging (Pró-Reitoria de Inclusão e Pertencimento, PRIP) and Provost of Research and Innovation (Pró-Reitoria de Pesquisa e Inovação, PRPI), University of São Paulo, for the post-doctoral fellowship (001/2023). A.R.B. is thankful to the CNPq, for the Research Productivity Scholarship, and to FAPESP.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goyal, J.; Verma, P.K. An Overview of Biosynthetic Pathway and Therapeutic Potential of Rutin. Mini-Rev. Med. Chem. 2023, 23, 1451–1460. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as Promising Natural Compounds in the Prevention and Treatment of Selected Skin Diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Garcia-Baeza, A.; Vidal-Limon, H.R.; Balderas-Renteria, I.; Ramírez-Cabrera, M.A.; Ramirez-Estrada, K. Plant Secondary Metabolites against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, 11, 220. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef] [PubMed]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–117. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic Benefits of Rutin and Its Nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Kola, A.; Dudek, D.; Valensin, D. Metal Complexation Mechanisms of Polyphenols Associated to Alzheimer’s Disease. Curr. Med. Chem. 2021, 28, 7278–7294. [Google Scholar] [CrossRef]
- Noon, J.; Mills, T.B.; Norton, I.T. The Use of Natural Antioxidants to Combat Lipid Oxidation in O/W Emulsions. J. Food Eng. 2020, 281, 110006. [Google Scholar] [CrossRef]
- De Araújo, J.I.R.; de Oliveira, J.H.P.; da Silva, J.W.V.; da Silva, D.T.C.; de Soares, M.F.L.R.; Soares-Sobrinho, J.L. Métodos Analíticos para Avaliação da Estabilidade de Rutina e Análise da Formação de Seus Produtos de Degradação: Uma Revisão. Res. Soc. Dev. 2022, 11, e399111234657. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and in Vitro Toxicological Profile Using 3d Reconstructed Human Epidermis Tissue and 2d Cells. Antioxidants 2021, 10, 85. [Google Scholar] [CrossRef]
- Sathiya Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus Sinensis and Conventional Antibiotic Gentamicin on Pseudomonas Aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Balogh, T.S.; Pedriali, C.A.; Sarruf, F.D.; Pinto, C.A.S.O.; Kaneko, T.M.; Baby, A.R. Rutin Association with Ethylhexyl Methoxycinnamate and Benzophenone-3: In Vitro Evaluation of the Photoprotection Effectiveness by Reflectance Spectrophotometry. Lat. Am. J. Pharm. 2008, 27, 23–27. [Google Scholar]
- Rashidinejad, A.; Jameson, G.B.; Singh, H. The Effect of PH and Sodium Caseinate on the Aqueous Solubility, Stability, and Crystallinity of Rutin towards Concentrated Colloidally Stable Particles for the Incorporation into Functional Foods. Molecules 2022, 27, 534. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, Y.K.; Lin, C.F.; Wang, P.W.; Chen, E.L.; Fang, J.Y. Elucidating the Skin Delivery of Aglycone and Glycoside Flavonoids: How the Structures Affect Cutaneous Absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef]
- Lin, C.F.; Leu, Y.L.; Al-Suwayeh, S.A.; Ku, M.C.; Hwang, T.L.; Fang, J.Y. Anti-Inflammatory Activity and Percutaneous Absorption of Quercetin and Its Polymethoxylated Compound and Glycosides: The Relationships to Chemical Structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic Sunscreens for Photoprotection. Green Chem. 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Karunakaran, T.; Hamdan, M.R. Exploring the Potential Use of Hylocereus Polyrhizus Peels as a Source of Cosmeceutical Sunscreen Agent for Its Antioxidant and Photoprotective Properties. Evid.-Based Complement. Altern. Med. 2020, 2020, 7520736. [Google Scholar] [CrossRef]
- Franco, J.G.; Cefali, L.C.; Ataide, J.A.; Santini, A.; Souto, E.B.; Mazzola, P.G. Effect of Nanoencapsulation of Blueberry (Vaccinium myrtillus): A Green Source of Flavonoids with Antioxidant and Photoprotective Properties. Sustain. Chem. Pharm. 2021, 23, 100515. [Google Scholar] [CrossRef]
- Cândido, T.M.; De Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. Aaps Pharmscitech 2018, 19, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Chen, X.; Jin, C.; Chai, F.; Tian, M. Selective Enrichment of Rutin in Sunscreen by Boronate Affinity Molecularly Imprinted Polymer Prior to Determination by High Performance Liquid Chromatography. Biochem. Eng. J. 2023, 191, 108811. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.P.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of Flavonoids and Cinnamates, the Main Photoprotectors with Natural Origin. Adv. Pharmacol. Sci. 2018, 2018, 5341487. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, B.; Soman, A.; Johnson, J.; Narayanan, P.S.; John, A.P. Plants and phytoconstituents having sunscreen activity. World J. Curr. Med. Pharm. Res. 2020, 2, 14–20. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa Oleifera Leaf Extracts as Multifunctional Ingredients for “Natural and Organic” Sunscreens and Photoprotective Preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; de Siqueira Martins, S.; Barbosa, G.L.F.; Fonseca, M.J.V.; Rochette, P.J.; Moulin, V.J.; de Freitas, L.A.P. Photoprotective Effect of Solid Lipid Nanoparticles of Rutin against UVB Radiation Damage on Skin Biopsies and Tissue-Engineered Skin. J. Microencapsul. 2022, 39, 668–679. [Google Scholar] [CrossRef]
- Roquete Amparo, T.; Cherem Peixoto da Silva, A.; Brandão Seibert, J.; dos Santos da Silva, D.; Martins Rebello dos Santos, V.; Melo de Abreu Vieira, P.; Célio Brandão, G.; Henrique Bianco de Souza, G.; Aloise Maneira Corrêa Santos, B. In Vitro and In Silico Investigation of the Photoprotective and Antioxidant Potential of Protium Spruceanum Leaves and Its Main Flavonoids. J. Photochem. Photobiol. A Chem. 2022, 431, 114037. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Gonçalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In Vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. Aaps Pharmscitech 2019, 20, 9. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) Var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef]
- Fonseca, M.; Rehman, M.; Soares, R.; Fonte, P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules 2023, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF Enhancement Provided by Rutin in a Multifunctional Sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Tabolacci, E.; Tringali, G.; Nobile, V.; Duca, S.; Pizzoferrato, M.; Bottoni, P.; Clementi, M.E. Rutin Protects Fibroblasts from UVA Radiation through Stimulation of Nrf2 Pathway. Antioxidants 2023, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Efficacy Evaluation of Gelatin-Based Nanoparticles Associated with UV Filters. Colloids Surf. B Biointerfaces 2016, 140, 531–537. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, C.; Zoghbi, A.; Huang, J.; Xia, Q. Oil-in-Oil-in-Water Pre-Double Emulsions Stabilized by Nonionic Surfactants and Silica Particles: A New Approach for Topical Application of Rutin. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 399–407. [Google Scholar] [CrossRef]
- Berlier, G.; Gastaldi, L.; Sapino, S.; Miletto, I.; Bottinelli, E.; Chirio, D.; Ugazio, E. MCM-41 as a Useful Vector for Rutin Topical Formulations: Synthesis, Characterization and Testing. Int. J. Pharm. 2013, 457, 177–186. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, Synthesis and Biological Activity of a Novel Rutin Analogue with Improved Lipid Soluble Properties. Bioorg. Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Soni, H.; Singhai, A.K. Formulation and Development ff Hydrogel Based System for Effective Delivery Of Rutin. Int. J. App. Pharm. 2013, 5, 5–13. [Google Scholar]
- Mishra, S.; Ranjan Mishra, S.; Soni, H. Hydrogel Containing Rutin in Wound Healing. EAS J. Pharm. Pharmacol. 2021, 3, 161–167. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Cristiano, M.C.; Fresta, M.; Cosco, D. Rutin-Loaded Poloxamer 407-Based Hydrogels for In Situ Administration: Stability Profiles and Rheological Properties. Nanomaterials 2020, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sri Venkateswarlu, B.; Balasubramani, R.; Chandira, R.M.; Pethappachetty, P. Topical Application Using Non-Ionic Surfactant for Formulation and Evaluation of Flavonoid Proniosomes. Asian J. Biol. Life Sci. 2022, 11, 397–403. [Google Scholar] [CrossRef]
- Gandhi, S.V.; Nilgar, N.M.; Bhalekar, M.R. Formulation and evaluation of phytoconstituents cream for the treatment of varicose veins. World J. Pharm. Res. SJIF Impact Factor 2018, 7, 733. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Hansson, A.C.; Johansson, H.; Lemkine, G.F.; Thénot, J.P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (Nams). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological Analytical Techniques for Cosmetics Quality Control and Process Monitoring. Processes 2021, 9, 1982. [Google Scholar] [CrossRef]
- De Argollo, G.; Camila, M.; Hiraishi, F.; Ivo, P.; Macedo, D.S.; Aparecida, C.; De Oliveira, S.; João, P.; Catarina, G. HPLC-TBARS-EVSC (High-Performance Liquid Thiobarbituric Acid Reactive Substances—Ex Vivo Stratum Corneum) Protocol: Selection of the Subjects and Approach to Present the Results. Int. J. Cosmet. Sci. 2023, 45, 647–654. [Google Scholar] [CrossRef]
- Shanbhag, S.; Nayak, A.; Narayan, R.; Nayak, U.Y. Anti-Aging and Sunscreens: Paradigm Shift in Cosmetics. Adv. Pharm. Bull. 2019, 9, 348–359. [Google Scholar] [CrossRef]
- ANVISA. Guia de Controle de Qualidade de Produtos Cosméticos: Uma Abordagem Sobre Os Ensaios Físicos e Químicos; Agência Nacional de Vigilância Sanitária: Brasilia, Brazil, 2008; ISBN 9788588233348. [Google Scholar]
- Badruddoza, A.Z.M.; Thean Yeoh, J.C.S.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J. Pharm. Sci. 2023, 112, 1772–1793. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Maciel, C.R.M.; Sarruf, F.D.; Pinto, C.A.S.O.; Consiglieri, V.O.; Kaneko, T.M.; Baby, A.R. Desenvolvimento e Teste Preliminar da Estabilidade de Formulaç Ões Cosméticas Acrescidas de Extrato Comercial de Trichilia catigua Adr. Juss (e) Ptychopetalum olacoides Bentham. Rev. Cienc. Farm. Basica Apl. 2008, 29, 179–194. [Google Scholar]
- Baby, A.R.; Migliato, K.F.; Maciel, C.P.M.; Zague, V.; Aparecida Sales de Oliveira Pinto, C.; Salgado, H.R.N.; Kaneko, T.M.; Velasco, M.V.R. Accelerated Chemical Stability Data of O/W Fluid Emulsions Containing the Extract of Trichilia Catigua Adr. Juss (and) Ptychopetalum olacoides Bentham. Braz. J. Pharm. Sci. 2007, 43, 405–412. [Google Scholar] [CrossRef][Green Version]
- Maciel, C.P.M.; Kaneko, T.M. UV Spectrophotometric Determination of Bioflavonoids from a Semisolid Pharmaceutical Dosage Form Containing. J. AOAC Int. 2006, 89, 1532–1537. [Google Scholar] [CrossRef]
- Passos, M.L.; Saraiva, M.L.M. Detection in UV-Visible Spectrophotometry: Detectors, Detection Systems, and Detection Strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Haque, S.M. Optimized Box–Behnken Experimental Design Based Response Surface Methodology and Youden’s Robustness Test to Develop and Validate Methods to Determine Nateglinide Using Kinetic Spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120712. [Google Scholar] [CrossRef]
- Civan, F.; Alarcon, L.J.; Campbell, S.E. Laboratory Confirmation of New Emulsion Stability Model. J. Pet. Sci. Eng. 2004, 43, 25–34. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The Photostability of Flavanones, Flavonols and Flavones and Evolution of Their Antioxidant Activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Morgenni, F.; Mazzi, G.; Vincieri, F.F. Evaluation of Chemical Stability of St. John’s Wort Commercial Extract and Some Preparations. Int. J. Pharm. 2001, 213, 199–208. [Google Scholar] [CrossRef]
- Banov, D.; Baby, A.R.; Del Bosco, L.M.; Kaneko, T.M.; Velasco, M.V.R. Caracterização do Extrato Seco de Ginkgo biloba L. em Formulações de Uso Tópico. Acta Farm. Bonaer. 2006, 25, 219–224. [Google Scholar]
- Nishikawa, D.O.; Zague, V.; Pinto, C.A.S.O.; Vieira, R.P.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Avaliação Da Estabilidade de Máscaras Faciais Peel-off Contendo Rutina. Rev. Ciências Farm. Básica Apl. 2007, 28, 227–232. [Google Scholar]
- Valenta, C.; Nowack, E.; Bernkop-Schnürch, A. Deoxycholate-Hydrogels: Novel Drug Carrier Systems for Topical Use. Int. J. Pharm. 1999, 185, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Tavante-Júnior, C.R.; de Pinto, C.A.S.O.; Zague, V.; Arêas, E.P.G.; Kaneko, T.M.; Velasco, M.V.R. Stability and In Vitro Penetration Study of Rutin Incorporated in a Cosmetic Emulsion through an Alternative Model Biomembrane. Braz. J. Pharm. Sci. 2008, 44, 233–248. [Google Scholar] [CrossRef]
- Baby, A.R.; Haroutiounian-Filho, C.A.; Sarruf, F.D.; Pinto, C.A.S.D.O.; Kaneko, T.M.; Velasco, M.V.R. Influence of Urea, Isopropanol, and Propylene Glycol on Rutin In Vitro Release from Cosmetic Semisolid Systems Estimated by Factorial Design. Drug Dev. Ind Pharm. 2009, 35, 272–282. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Maniscalco, C.; Lambusta, D.; Nicolosi, G.; Ventura, C.A.; Puglisi, G. In Vitro Evaluation of Quercetin-3-O-Acyl Esters as Topical Prodrugs. Int. J. Pharm. 2007, 336, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Wadher, K.; Trivedi, S.; Rarokar, N.; Umekar, M. Development and Assessment of Rutin Loaded Transfersomes to Improve Ex Vivo Membrane Permeability and In Vitro Efficacy. Hybrid Adv. 2024, 5, 100144. [Google Scholar] [CrossRef]
- Hassan, A.S.; Soliman, G.M. Rutin Nanocrystals with Enhanced Anti-Inflammatory Activity: Preparation and Ex Vivo/In Vivo Evaluation in an Inflammatory Rat Model. Pharmaceutics 2022, 14, 2727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, R.; Huang, J.; Xia, Q. Development and Characterization of a New Non-Aqueous Self-Double-Emulsifying Drug Delivery System for Topical Application of Rutin. J. Drug Deliv. Sci. Technol. 2021, 61, 101243. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, C.; Wu, F.; Li, H.; Zhang, W.P.; Zhang, Q. Microencapsulated Nanostructured Lipid Carriers as Delivery System for Rutin. Mater. Technol. 2018, 33, 357–363. [Google Scholar] [CrossRef]
- Li, J.; Ni, W.; Aisha, M.; Zhang, J.; Sun, M. A Rutin Nanocrystal Gel as an Effective Dermal Delivery System for Enhanced Anti-Photoaging Application. Drug Dev. Ind Pharm. 2021, 47, 429–439. [Google Scholar] [CrossRef]
- Alam, M.S.; Sultana, N.; Rashid, M.A.; Alhamhoom, Y.; Ali, A.; Waheed, A.; Ansari, M.S.; Aqil, M.; Mujeeb, M. Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate. Gels 2023, 9, 752. [Google Scholar] [CrossRef]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin-Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (SmartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Alam, A.; Imran, M. Rutin-Loaded Transethosomal Gel for Topical Application: A Comprehensive Analysis of Skin Permeation and Antimicrobial Efficacy. ACS Omega 2024, 9, 27300–27311. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lee, H.J.; Gu, H.A. Enhanced Skin Delivery and Characterization of Rutin-Loaded Ethosomes. Korean J. Chem. Eng. 2014, 31, 485–489. [Google Scholar] [CrossRef]
- Pelikh, O.; Stahr, P.L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for Improved Dermal Drug Delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Lee, M.H.; Kim, S.J.; Yu, E.R. Preparation of Quercetin and Rutin-Loaded Ceramide Liposomes and Drug-Releasing Effect in Liposome-in-Hydrogel Complex System. Biochem. Biophys. Res. Commun. 2013, 435, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Kalita, B. Design and Evaluation of Phyto-Phospholipid Complexes (Phytosomes) of Rutin for Transdermal Application. J. Appl. Pharm. Sci. 2014, 4, 51–57. [Google Scholar] [CrossRef]
- Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; De Almeida, T.S.; Fonte, P. Ionic Liquid-Polymer Nanoparticle Hybrid Systems as New Tools to Deliver Poorly Soluble Drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, A.; Salabat, A. Microemulsion and Microemulsion Gel Formulation for Transdermal Delivery of Rutin: Optimization, In-Vitro/Ex-Vivo Evaluation and SPF Determination. J. Dispers. Sci. Technol. 2022, 43, 1848–1857. [Google Scholar] [CrossRef]
- Sionkowska, A.; Lewandowska, K.; Kurzawa, M. Chitosan-Based Films Containing Rutin for Potential Cosmetic Applications. Polymers 2023, 15, 3224. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Flavonoid Nanocrystals Produced by ARTcrystal®-Technology. Int. J. Pharm. 2015, 482, 27–37. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Borin, M.F.; Lopez, R.F.; Fonseca, M.J. In Vitro Evaluation of Quercetin Cutaneous Absorption from Topical Formulations and Its Functional Stability by Antioxidant Activity. Int. J. Pharm. 2007, 328, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S. Do the Polyphenolic Compounds from Natural Products Can Protect the Skin from Ultraviolet Rays? Results Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Anim. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Fatharani, R.M.; Fitrian, U.A.; Ishak, S.S.O.; Adlia, A. Sun Protection Factor and Tyrosinase Inhibitory Activity of Several Plant Secondary Metabolites. Curr. Res. J. Biol. Sci. 2023, 5, 315–319. [Google Scholar] [CrossRef]
- Pinzaru, I.; Chioibas, R.; Marcovici, I.; Coricovac, D.; Susan, R.; Predut, D.; Georgescu, D.; Dehelean, C. Rutin Exerts Cytotoxic and Senescence-Inducing Properties in Human Melanoma Cells. Toxics 2021, 9, 226. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; Da Peres, D.; Rugno, C.M.; Kojima, M.; De Oliveira Pinto, C.A.S.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional Photostability and Cutaneous Compatibility of Bioactive UVA Sun Care Products. J. Photochem. Photobiol. B 2015, 148, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Pasuch Gluzezak, A.J.; Dos Santos, J.L.; Maria-Engler, S.S.; Gaspar, L.R. Evaluation of the Photoprotective and Antioxidant Potential of an Avobenzone Derivative. Front. Physiol. 2024, 15, 1347414. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schalka, S.; Watson, R.E.B.; Wei, L.; Morita, A. Daily Photoprotection to Prevent Photoaging. Photodermatol. Photoimmunol. Photomed. 2021, 37, 482–489. [Google Scholar] [CrossRef]
- Flament, F.; Mercurio, D.G.; Catalan, E.; Bouhadanna, E.; Delaunay, C.; Miranda, D.F.; Passeron, T. Impact on Facial Skin Aging Signs of a 1-Year Standardized Photoprotection over a Classical Skin Care Routine in Skin Phototypes II–VI Individuals: A Prospective Randomized Trial. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2090–2097. [Google Scholar] [CrossRef]
- Lan, C.C.E.; Hung, Y.T.; Fang, A.H.; Ching-Shuang, W. Effects of Irradiance on UVA-Induced Skin Aging. J. Dermatol. Sci. 2019, 94, 220–228. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Yamauchi, R.; Tsuji, T.; Krutmann, J.; Morita, A. The Expression of Matrix Metalloproteinase-1 MRNA Induced by Ultraviolet A1 (340–400 Nm) Is Phototherapy Relevant to the Glutathione (GSH) Content in Skin Fibroblasts of Systemic Sclerosis. J. Dermatol. 2003, 30, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, E.; Goss, G.; Hiratsuka, T.; Sipilä, K.H.; Kirk, T.; Kober, K.I.; Lui, P.P.; Tsang, V.S.K.; Hawkshaw, N.J.; Pilkington, S.M.; et al. Role of Distinct Fibroblast Lineages and Immune Cells in Dermal Repair Following UV Radiation Induced Tissue Damage. Elife 2021, 10, e71052. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Domingues, P.; Skrzydlewska, E. Proteins Involved in the Antioxidant and Inflammatory Response in Rutin-Treated Human Skin Fibroblasts Exposed to UVA or UVB Irradiation. J. Dermatol. Sci. 2018, 90, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Baby, A.R.; Balogh, T.S.; Pedriali, C.A.; Kaneko, T.M.; Velasco, M.V.R. Uva I-Protection Effectiveness of Bioactive Compound and Organic Uv Filters: An In Vitro Assessment. Quim. Nova 2009, 32, 1321–1323. [Google Scholar] [CrossRef]
- Food and Drug Administration, HHS. Labeling and Effectiveness Testing; Sunscreen Drug Products for over-the-Conter Human Use. Final Rule. Fed. Regist. 2011, 76, 46. [Google Scholar]
- El-Boury, S.; Couteau, C.; Boulande, L.; Paparis, E.; Coiffard, L.J.M. Effect of the Combination of Organic and Inorganic Filters on the Sun Protection Factor (SPF) Determined by In Vitro Method. Int. J. Pharm. 2007, 340, 1–5. [Google Scholar] [CrossRef]
- Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L.J.M. Study of the Photostability of 18 Sunscreens in Creams by Measuring the SPF In Vitro. J. Pharm. Biomed. Anal. 2007, 44, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Nadia, M.A.; Zulkarnain, A.K.; Sulaiman, T.N.S. Determination of Photoprotective Capacity of Topical Gel Formulations Containing Bioactive Compound Rutin and Evaluation of Physicochemical Stability. Trop. J. Nat. Prod. Res. 2023, 7, 3923–3931. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T. Plant Polyphenols: Recent Advances in Epidemiological Research and Other Studies on Cancer Prevention. Stud. Nat. Prod. Chem. 2013, 39, 269–295. [Google Scholar] [CrossRef]
- Her, Y.; Lee, T.K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.C.; Ahn, J.H.; Park, J.H.; Lee, J.W.; Hong, J.; et al. Topical Application of Aronia Melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25, 4577. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.V.R.; Sarruf, F.D.; Salgado-Santos, I.M.N.; Haroutiounian-Filho, C.A.; Kaneko, T.M.; Baby, A.R. Broad Spectrum Bioactive Sunscreens. Int. J. Pharm. 2008, 363, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Monsalve-bustamante, Y.A.; Puertas-mejia, M.A.; Mejia-giraldo, J.C. Comparison of the Photoprotective Effect between Hydrolyzed and Aglycones Flavonoids as Sunscreen: A Systematic Review. J. Appl. Pharm. Sci. 2020, 10, 116–123. [Google Scholar] [CrossRef]
- Opriş, O.; Soran, M.L.; Lung, I.; Stegarescu, A.; Guţoiu, S.; Podea, R.; Podea, P. Optimization of Extraction Conditions of Polyphenols, Antioxidant Capacity and Sun Protection Factor from Prunus Spinosa Fruits. Application in Sunscreen Formulation. J. Iran. Chem. Soc. 2021, 18, 2625–2636. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef]
- Dondi, D.; Albini, A.; Serpone, N. Interactions between Different Solar UVB/UVA Filters Contained in Commercial Suncreams and Consequent Loss of UV Protection. Photochem. Photobiol. Sci. 2006, 5, 835–843. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.; Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; De Araújo, G.L.B.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Kamel, R.; Mostafa, D.M. Rutin Nanostructured Lipid Cosmeceutical Preparation with Sun Protective Potential. J. Photochem. Photobiol. B 2015, 153, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Kuthi, N.; Basar, N.; Chandren, S. Nanonutrition- and Nanoparticle-Based Ultraviolet Rays Protection of Skin. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 227–280. [Google Scholar] [CrossRef]
- Kumar, N.; Jose, J. Current Developments in the Nanomediated Delivery of Photoprotective Phytochemicals. Environ. Sci. Pollut. Res. 2020, 27, 38446–38471. [Google Scholar] [CrossRef] [PubMed]
- Gonçalo das Neves e Silva, E.; Ferreira Barbosa, G.L.; Alves Confessor, M.V.; Bacalhau de Sousa, W.J.; Lia Fook, M.V.; Siqueira-Júnior, J.P.; Pedro de Freitas, L.A.; Martins, R.M. Artemia Salina Leach in Photoprotection: A New Model to Evaluate the Potential of Nanoparticles for Topical Application. J. Drug Deliv. Sci. Technol. 2023, 90, 105164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).