Abstract
Artificial intelligence (AI) is revolutionizing plastic surgery through its remarkable advancements in various domains such as image analysis, robotic assistance, predictive analytics, and augmented reality. Predictive analytics, powered by AI, harnesses patient data to predict surgical outcomes, minimize risks, and tailor treatment plans, thereby optimizing patient care and safety. Augmented reality and virtual reality technology are also reshaping the cosmetic surgery landscape, providing immersive experiences for preoperative imaging, intraoperative guidance, and advanced skills through simulation. Looking ahead, the future of AI in plastic surgery holds great promise, including personalized medicine, bioprinting of tissues and organs, and continuous learning through iterative improvement algorithms based on real-world surgical experience. However, amid these transformational advances, ethical considerations and regulatory frameworks must evolve to ensure the responsible deployment of AI, protect patient privacy, minimize errors and algorithmic deviation, and uphold standards of fairness and transparency. Our study aims to explore the role of AI in the field of plastic surgery with the potential for the future in mind. In summary, AI is considered a beacon of innovation in plastic surgery, enhancing surgical precision, enhancing patient outcomes, and heralding a future where interventions rely on personalized technology that will redefine the boundaries of aesthetic and regenerative medicine.
1. Introduction
Artificial intelligence (AI) is rapidly gaining prominence in modern healthcare, significantly altering various medical practice fields, including diagnosis, treatment, and broader applications [1]. Using advanced machine learning algorithms and deep learning to analyze extensive datasets, including patient electronic health records (EHRs) [2,3], medical imaging [4,5], genomic sequences [6,7], and real-time physiological data streams (electrocardiograms, electroencephalograms, and electromyograms) [8,9,10]. In diagnostic tasks, AI demonstrates exceptional proficiency in interpreting complex medical images, aiding radiologists and pathologists in the early detection and categorization such as cancers, vascular diseases, neurological disorders, and other diseases [11,12,13,14]. Moreover, AI facilitates personalized medicine by integrating patient-specific clinical and genetic data to customize treatment strategies, predict treatment responses, and optimize therapeutic outcomes, thereby revolutionizing traditional approaches to healthcare [15,16,17,18,19]. Additionally, AI technologies streamline healthcare operations by automating administrative tasks, optimizing resource allocation, and improving patient care coordination. Furthermore, AI-driven telemedicine platforms and remote monitoring tools empower patients and healthcare providers by providing real-time access to medical expertise, enabling remote consultations, continuous patient monitoring, and proactive disease management, which are particularly beneficial in remote or underserved areas [20,21].
Nowadays, physicians need to make accurate and rapid decisions based on clinical examinations, test results, history, and demographics. These decisions are impacted by various influences, such as cognitive biases, personal traits, emotions, and external factors, which can introduce variability, uncertainty, and errors into the therapeutic decision-making process [22]. AI emerges as a crucial tool in overcoming these human limitations by aiding in medical decision-making and reasoning. Simultaneously, the proliferation of informatization has led to a significant increase in both pre- and postoperative patient data, including patient-generated health data and information from wearable sensors, collectively referred to as “big data”, as shown in Figure 1. In the medical field, machine learning (ML) includes a variety of statistical techniques and methods that are particularly adept at detecting and combining subtle influences to predict a specific outcome effectively [23]. In recent years, medical image analysis has gravitated toward deep learning (DL), showcasing significant advancements across various medical imaging tasks, especially in radiology and pathology [24,25]. DL employs data-driven techniques, autonomously crafting and organizing image features based on their predictive capacity rather than relying on human-derived knowledge [26]. As the volume of digital data expands, relying solely on manual analysis becomes inadequate. AI support in medical decision-making has improved the accuracy, efficiency, and effectiveness of healthcare delivery, finally leading to better patient outcomes [27,28]. Therefore, leveraging AI becomes indispensable for effectively managing and utilizing this vast dataset to solve complex clinical challenges.
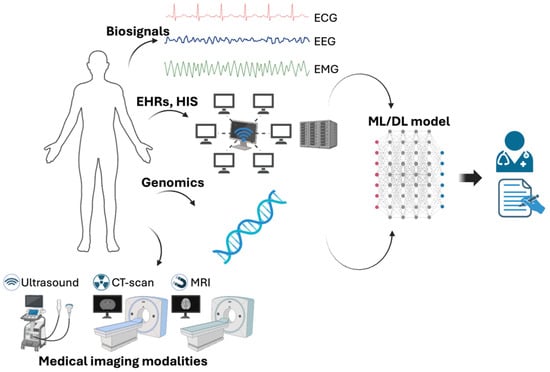
Figure 1.
Biomedical information and machine learning/deep learning to support physicians make decision. Created with BioRender.com.
Plastic surgery has seen remarkable progress over time, experiencing a notable shift with the introduction of cutting-edge AI. Previous studies reported the role of AI in several applications, such as breast surgery, wound recovery, hand surgery, flap monitoring, and aesthetics [29,30,31,32]. In cosmetic surgery, AI-powered simulations utilize advanced algorithms to generate visual representations of potential outcomes for surgical procedures [33,34,35]. AI simulations play a pivotal role during pre-operative consultations, facilitating communication between patients and surgeons. These simulations enable surgeons to intricately outline the proposed treatment plan and address any queries or anxieties the patient might harbor. Moreover, patients can offer feedback on these simulations, ensuring that their expectations align with the feasible outcomes of the surgery. Such simulations are instrumental in establishing realistic expectations for both parties. By visually portraying the anticipated results, patients attain a clearer comprehension of the potential alterations to their appearance, along with any associated limitations or risks. This proactive approach mitigates unrealistic expectations and ensures that patients are comprehensively informed prior to undergoing surgery.
The main branches of AI are machine learning, deep learning, and natural language processing, which represent tools that plastic surgeons can employ to enhance their surgical procedures. The peer-reviewed scientific literature and ongoing scientific research were searched using relevant terms in the following databases: MEDLINE and EMBASE (Ovid), the Cochrane Central Register of Controlled Trials, and the Cumulative Index of Nursing and Allied Health Literature. All levels of evidence were considered. Only articles published in English were included. The review provides an overview of the fundamental technical components and concepts underlying AI techniques and explores current developments, applications, and future perspectives of AI in plastic surgery. It emphasizes the importance of plastic surgeons promptly embracing AI technology to drive the discipline’s development.
2. Concept of AI
The scope of AI encompasses various technologies and applications dedicated to developing intelligent machines capable of tasks traditionally associated with human intelligence. In this part, our focus is specifically on the AI aspects relevant to the field of plastic surgery.
2.1. Machine Learning
Machine learning (ML) can be divided into several types based on the learning approach and the availability of labeled data: supervised learning, unsupervised learning, semi-supervised learning, and transfer learning.
In supervised learning, the algorithm learns from labeled data, where each example is matched with a corresponding label or outcome [36]. The algorithm aims to establish a mapping from inputs to outputs, enabling accurate prediction of outcomes for new, unseen inputs. Unsupervised learning, conversely, entails training algorithms on unlabeled data to unveil underlying patterns or structures within the dataset [37]. Without explicit labels, the algorithm must discern relationships or clusters within the data. Semi-supervised learning amalgamates aspects of both supervised and unsupervised learning [38] (Figure 2). The algorithm is trained on a blend of labeled and unlabeled data. Finally, transfer learning involves harnessing knowledge acquired from training on one task to enhance performance on a related task [39]. Rather than initiating the learning process anew, transfer learning enables models to transfer learned features or representations from one domain to another.
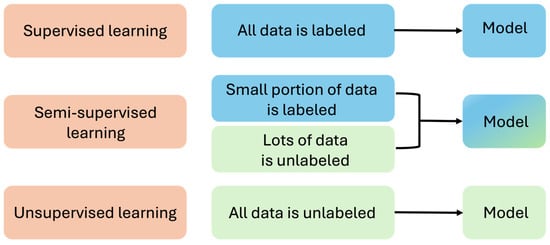
Figure 2.
Supervised learning, unsupervised learning, and semi-supervised learning.
2.2. Deep Learning
Deep learning is a branch of machine learning that uses neural networks composed of multiple layers to understand hierarchical data representations [40]. These networks, called deep neural networks, contain interconnected nodes, or neurons, across various layers, allowing them to automatically distinguish features from raw data [41]. Deep learning algorithms proficiently extract features at different levels of abstraction, starting with rudimentary features in the early layers and progressing to more complex features in subsequent layers [42]. The hierarchical nature of this approach empowers deep learning models to effectively identify intricate patterns and correlations within data, making them highly adept at tasks such as image and speech recognition, natural language processing, and medical diagnosis.
2.3. Natural Language Processing
Natural language processing (NLP) uses computational methods to understand, interpret, and produce human language. It merges linguistics, computer science, and artificial intelligence to enable computers to interact with and grasp human language meaningfully. NLP algorithms can analyze text, translate languages, discern sentiment, summarize text, and generate human-like responses. Speech processing involves the study and creation of algorithms and software programs designed to analyze human language presented in speech form, typically audio data. Key applications of speech processing include speech recognition and synthesis. While speech recognition involves converting spoken language into text, speech synthesis, conversely, transforms text into spoken language. Its applications span across diverse areas, including virtual assistants, chatbots, search engines, sentiment analysis, language translation, and healthcare.
3. Current Developments in AI for Plastic Surgery
AI revolutionises plastic surgery by enhancing predictive analytics, enabling precise 3D imaging and simulations, and supporting planning and consultant pre-operative. Personalized treatment plans are crafted using AI algorithms that analyze individual patient data, while automated diagnostics aid in identifying conditions requiring surgical intervention (Figure 3). AI also improves surgical techniques by analyzing past data and monitoring post-surgical recovery. Additionally, AI-driven research leverages large datasets to innovate and refine surgical methods, improving patient care and outcomes.
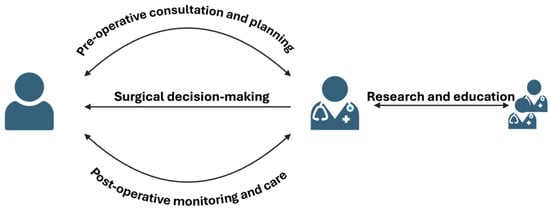
Figure 3.
Schematic diagram of the application of AI in plastic surgery.
3.1. Using AI in Pre-Operative Consultation and Planning
Patients seeking plastic surgery treatment often have numerous questions that cannot always be fully addressed during the initial consultation. Currently, the common method to address these frequently asked questions in plastic surgery outside of the clinic involves compiling lists of commonly asked questions along with their corresponding answers. These resources are typically made available on websites or in informational booklets. In online patient consultations, artificial intelligent virtual assistants (AIVA) equipped with NLP technology comprehend the meaning behind human speech and respond through dialogue [43]. A study employed AIVA to engage patients in discussions about typical plastic surgery subjects. The plastic surgery AIVA achieved an overall answer accuracy of 92.3%, and 83.3% of participants found the answers to be accurate. The adoption and implementation of this technology are anticipated to relieve plastic surgeons, reducing the need for initial medical consultations.
The AI model can generate visual simulations of potential outcomes based on the patient’s body measurements, desired breast size, and implant type. This allows patients to see realistic representations of how different options may look on their bodies, aiding in decision-making and managing expectations. Hammond et al. [44] used Crisalix Virtual Aesthetics 3D, a cloud-based software tailored for 3D breast modeling, with a virtual tool to estimate the 3D breast volume, offering patients a preview of their potential postoperative results. The findings suggest that this approach can effectively facilitate communication with patients considering breast augmentation. Among the 40 patients in the study cohort, the virtual reality tool received commendable ratings for its usefulness (62%), accuracy (78%), and importance (88%) in supporting patients in choosing their desired implant size.
In hand surgery, AI aids surgeons in carefully planning and precisely executing complex procedures [30]. Machine learning algorithms can be utilized for risk stratification, identifying patients who may not experience significant improvement in patient-reported outcome measures before undergoing surgery. Subsequently, these algorithms can suggest alternative non-operative treatments or allocate enhanced postoperative monitoring and more frequent support, including face-to-face training sessions.
3.2. Using AI in Surgical Decision-Making and Performance
AI serves as a valuable resource during surgeries, providing reference information to aid in decision-making processes [45]. Hoogendam et al. [46] created a predictive model for clinically significant symptom improvement six months post-carpal tunnel release. A gradient boosting model used five patient-reported predictors to train on 1589 patients who underwent a mini-open carpal tunnel. Their model reached an area under the receiver operating characteristic curve (AUC-ROC) of 0.723. It is accessible online and could aid in shared decision-making for patients contemplating a carpal tunnel release procedure. Loos et al. [47] developed a model offering the potential for clinicians to anticipate functional enhancement in patients undergoing surgery for thumb carpometacarpal osteoarthritis. This model could significantly aid in clinical decision-making. Regarding functional outcomes, the gradient boosting machine demonstrated a commendable AUC-ROC of 0.74 and exhibited satisfactory calibration in the test dataset.
The AI robotic surgery system serves as a navigation tool for surgeons during robot-assisted surgeries. Utilizing supervised automated procedures, it can identify anatomical structures and streamline navigation during the procedure, thus assisting in surgical decision-making for operating on those structures [48]. This robotic surgical assistant technology is utilized in procedures like cleft lip and palate repair surgery, employing deep learning techniques to enhance surgical outcomes through a reduction in technical hurdles [49]. In the foreseeable future, the emergence of automated robotic surgical systems incorporating AI technology is poised to deliver precise surgical interventions, enhance surgical procedure efficiency, and mitigate complications. By reducing complications and shortening hospital stays, these advancements are anticipated to yield significant patient advantages and reduce the financial burden of treatment costs.
Phillips et al. [50] developed an algorithm capable of identifying malignant tumors from dermoscopic images of specific lesions with an accuracy equivalent to that of experts. The algorithm analyzed 1550 images of suspicious and benign skin lesions. When compared to histopathological diagnoses, the algorithm achieved an AUC-ROC of 91.8%. The study emphasizes the potential of deep learning (DL) in supporting clinical physicians in preventing secondary skin cancer and serving as a valuable diagnostic and decision-making tool.
A review highlighted the usefulness of intelligent robotic systems in supporting surgical procedures [51]. However, the authors noted that despite their superior dexterity, cognitive robotics often lack contextual understanding of the surgical environment and struggle to adapt to complex workflows. They propose that integrating procedural analysis and semantic knowledge through deep learning methods could enhance the potential of AI-supported surgery [52].
3.3. Using AI in Simulating Surgical Outcomes
Using AI in plastic surgery includes predictive models that utilize the pattern recognition abilities of ML/DL to help surgeons make pre-operative decisions. Since cosmetic surgery plans are typically customized to the unique aesthetic preferences of both the patient and the surgeon, AI can create simulated images to offer surgical guidelines and enhance the planning process. Patients commonly express their aesthetic desires and envisioned outcomes before undergoing surgery.
BreastGAN is an AI-equipped tool trained on breast images to simulate breast augmentation outcomes [53]. The breast images of bilateral breasts of 926 individuals were used to train neural network algorithms and test on 309 images. This study indicates that Generative Adversarial Networks (GANs) can be utilized to perform image translation tasks, such as transforming before images into after images, particularly when working with clinical images. In the field of rhinoplasty, an AI model can generate simulated images of cosmetic rhinoplasty surgeries that align with the aesthetic standards of plastic surgeons [54]. The resemblance between these simulated images and actual rhinoplasty outcomes reaches 92%. Technologies like these provide patients with AI-based predictions, assisting them in making informed decisions. This, in turn, contributes to lowering reoperation rates and enhancing postoperative satisfaction. Participants evaluated sequentially presented simulated rhinoplasty images, generated randomly by both plastic surgeons and an AI model. They used a seven-point Likert scale to indicate their agreement level with the simulated images, ranging from one for completely disagree to seven for completely agree. An AI model can replicate the aesthetic standards of plastic surgeons to create computer-generated rhinoplasty images. This capability allows patients to gain a realistic view of potential rhinoplasty outcomes before scheduling direct consultation appointments.
The prior study presented a swarm intelligence-based artificial neural network aimed at predicting the successful outcome of a nerve graft, providing valuable insights into its potential success in particular conditions [55]. The artificial neural network was trained by researchers using more than 30 variables extracted from experimental data derived from nerve graft studies conducted on rats. These variables covered various categories, including the biomaterials utilized for the wall and filling, extracellular matrix proteins, growth factors, scaffold type, and surface characteristics. Through the implementation of a swarm intelligence-based artificial neural network, the success of regenerating any nerve grafts was estimated with an accuracy of approximately 92.59%.
Patients tend to be more inclined to undergo surgery when they have access to accurate information about the expected postoperative outcome. This information empowers them to make informed decisions about their healthcare and increases their confidence in the procedure and its potential benefits. On the other hand, it is important to note that certain AI technologies can have detrimental effects on medical communication. For instance, the widespread adoption of photo editing technology has enhanced the credibility of video presentations in medical advertising. However, advanced AI techniques such as deep learning and computational modeling, collectively referred to as “deepfakes,” have the capability to manipulate people’s faces, facial expressions, and body movements in videos [56]. In a concerning example, deepfakes were employed to modify a patient’s postoperative video outcome, leading to an exaggerated depiction of the effects of plastic surgery.
3.4. Using AI in Postoperative Monitoring and Care
AI can be used to analyze large amounts of patient data, such as biosignals, test results, and patient-reported symptoms, allowing for early detection of complications or errors that deviate from the expected recovery trajectory. Furthermore, AI-powered predictive analytics can identify patients at high risk for post-surgical complications, allowing healthcare providers to intervene proactively and personalize appropriate care plans. AI algorithms can also assist in optimizing medication administration and pain control, ensuring patients receive the most effective and timely interventions [57].
Aasvang et al. [58] demonstrated AI-based alert continuous vital sign monitoring, leading to a reduction of 10–30% in-hospital risk of severe postoperative complications. AI-assisted continuous vital sign monitoring detects complications earlier, thereby potentially reducing staff workload and the occurrence of severe complications.
Chairat et al. [59] created an AI-supported wound assessment tool designed to assist physicians in monitoring wound healing progress and making treatment adjustments as necessary. Even when integrated into remote monitoring devices, AI algorithms retain the ability to analyze wound images, identify early-stage complications, and enable timely interventions to enhance postoperative care. Furthermore, AI-based predictive models can assess patient outcomes and refine treatment algorithms progressively, fostering a culture of continuous improvement and evidence-based practice.
In addition, machine learning has surpassed traditional statistical methods in predicting surgical site infections in immunosuppressed burn patients [60]. By constructing nonlinear models that integrate various data sources, such as diagnoses, treatments, and laboratory values, machine learning achieves superior performance in this predictive task. They employed a matched cohort of patients undergoing gastrointestinal surgery to construct a predictive model for postoperative surgical site infections.
AI holds promise for aiding patients during the post-operative phase by enabling them to play a more proactive role in their recovery. This technology can support patients in tracking their progress with physiotherapy and can schedule reminders to promote participation and adherence to their treatment regimen.
3.5. Using AI in Research and Education
AI tools aid in education and training initiatives for healthcare professionals. Virtual reality simulations and AI-powered educational platforms enable trainees to rehearse surgical procedures in an immersive virtual setting, thereby improving their proficiency and confidence prior to actual surgeries. Furthermore, AI-driven personalized learning systems can customize educational materials to suit the unique requirements and preferences of each learner, resulting in enhanced learning outcomes.
Integrating virtual reality (VR) with authentic data-driven simulations will be crucial for educating future burn care professionals [61]. The urgency of burn injuries creates a high-pressure environment where effective management requires a skilled, communicative, and coordinated team. In burn emergency cases, many unfavorable outcomes stem from non-technical skills such as communication, leadership, and teamwork. Therefore, leveraging comprehensive data on serious adverse events has the potential to significantly enhance the development of non-technical skills.
Augmented reality (AR) integrates computer-generated images with real-world surroundings, enhancing the visual experience. Proximie, a secure cloud-based augmented reality (AR) platform, facilitates live collaboration between a local (operating) surgeon and a remote (assisting) surgeon by offering a comprehensive view of the surgical field [62]. From a bird’s-eye view, both surgeons communicate through a two-way audio stream, leveraging integrated augmented reality (AR) features to enhance clarity. For example, the remote surgeon can overlay their hands onto the virtual surgical field using a webcam or highlight critical structures using a range of annotation and drawing tools.
4. Future Perspectives and Challenges
4.1. Potential Applications of AI in Plastic Surgery beyond Current Capabilities
AI promises to advance the practice of cosmetic surgery, enabling personalized, precise, and innovative approaches to patient care. By harnessing the potential of AI technology, plastic surgeons can enhance surgical outcomes, improve patient satisfaction, and drive transformative changes in the field.
Although current advancements in AI seem rapid, many different functions in plastic surgery have not yet been explored, leaving most applications only in the preclinical stage. Artificial intelligence has made strides in imaging diagnostics, but in the future, it may accurately detect anatomical landmarks in images to assist surgeons in performing precise surgical interventions. It can also use specific patient data to develop personalized treatment plans [63]. By considering factors such as patient preferences, anatomical variations, and previous surgical outcomes, AI can support the adjustment of procedures tailored to each individual [64]. Furthermore, it can optimize the patient’s recovery process and outcomes by monitoring progress, analyzing wound healing processes, and providing personalized recovery protocols for optimal restoration.
A study [65] found that the majority of participants had limited or no prior experience with AI. While some perceive AI as capable of improving precision and imaging in surgery, opinions vary regarding its impact on surgical duration, patient recovery, and satisfaction. Participants’ sentiments differed. Concerns have been raised regarding patient privacy, data security, costs, and obtaining informed consent for AI-assisted procedures. The study also identifies valuable sources of AI training data and emphasizes the importance of establishing standards and ensuring transparency in AI applications. Participants anticipate an expanding role for AI in reconstructive and cosmetic surgery, suggesting the integration of AI training into residency programs to address administrative challenges and core disease complications. Despite acknowledging the potential benefits of AI, participants expressed confidence in the enduring significance of human expertise in surgery. There is also an interest in further research to explore AI’s potential in surgical practice.
Advancements in technology are reshaping the landscape of medical education, potentially reducing the emphasis on memorization and retention of vast amounts of information. Instead, there’s a shift towards cultivating other essential skills. The evolving system is expected to prioritize competencies such as communication, emotional intelligence, and IT proficiency. To adapt to these changes, medical students and residents should have opportunities to acquire AI-related skills. This can be integrated into electives, leadership or business tracks, or post-graduate research programs.
4.2. Challenges and Limitations in AI Implementation
The widespread application of AI across diverse patient demographics may face limitations if there is a scarcity of data on certain groups, often due to explicit or implicit exclusion from clinical trials and patient registries. Additionally, accurate data labeling is paramount, as mislabeled variables can result in inaccurate outputs. Concerns arise regarding AI’s automated data interpretation, given its opaque nature. Human oversight is crucial to comprehending how AI identifies patterns in data, as algorithms do not provide explanations for their interpretations. Effective integration of AI in clinical settings will require extensive collaboration among clinicians, engineers, and hospital system support staff.
Ethical concerns emerge with the use of AI, particularly in cosmetic surgery, where algorithms may be utilized to “objectively” evaluate beauty. However, the development of such algorithms entails translating subjective attributes into objective parameters, posing significant ethical questions regarding the generalization of cultural norms.
Moreover, the scarcity of clinical research on AI raises safety concerns in AI-assisted surgery. While recent reviews have underscored the potential of intelligent robotic systems in aiding surgical procedures, it has been noted that, despite their remarkable dexterity, these robots lack understanding of the surgical context and struggle to adapt to intricate workflows. Integrating surgical procedure analysis and semantic knowledge through deep learning methods offers promise for enhancing the potential of AI-assisted surgery.AI cannot entirely substitute for doctors in diagnosing and making decisions. Currently, AI is often utilized with limitations, and the results provided by algorithms are merely correlations. Physicians must assess whether these results are applicable to individual clinical cases and circumstances. The accuracy of outcomes needs to be further analyzed through more studies in the future. Additionally, AI worsens existing issues like overdiagnosis, excessive detection, and overtreatment [66]. Surgeons must thoroughly comprehend both the advantages and disadvantages of AI to encourage its use and advancement in the field of medicine.
In 2024, the European Union introduced the AI Act, a comprehensive piece of legislation overseeing AI usage [67]. Similarly, in various states across the United States, ongoing deliberations revolve around the necessity of AI regulation [68]. This underscores the importance of implementing strong safeguards to protect sensitive patient data when adopting AI technologies.
4.3. Opportunities for Collaboration between AI Researchers, Plastic Surgeons, and Regulatory Bodies
AI researchers and plastic surgeons can collaborate to develop customized algorithms tackling specific challenges in plastic surgery, such as surgical planning, outcome prediction, and complication detection. Plastic surgeons can assist AI researchers by granting access to clinical data and aiding in the annotation of datasets for AI model training. Regulatory bodies play a vital role in ensuring privacy and compliance with ethical guidelines during data collection and sharing. Together, collaborative efforts can focus on validating AI-driven tools and technologies’ accuracy, reliability, and safety in plastic surgery. Regulatory bodies offer guidance on study design and evaluation criteria. Their oversight ensures AI-driven plastic surgery technologies adhere to regulatory standards and safety requirements. This collaboration streamlines the regulatory approval process, fostering the safe adoption of AI in plastic surgery. Ultimately, this cooperation fosters innovation, safeguards patient health, and encourages responsible AI technology integration in plastic surgery.
5. Conclusions
In conclusion, the potential of AI in plastic surgery is clear, marking an exciting new era. AI-driven technologies offer unparalleled opportunities in all phases of plastic surgery, including pre-operative planning, surgical procedures, and post-operative care, promising significant improvement in patient outcomes. However, seamless integration of AI into clinical practice requires a strong regulatory framework to protect patient safety and maintain ethical standards. It is necessary to promote AI research, education, and awareness within the aesthetic community, along with open dialogue, to ensure AI’s ethical and responsible incorporation in plastic surgery and achieve optimal results. Although AI should never replace a surgeon’s expertise and judgment, it should be considered a valuable tool to supplement surgical decision-making and enhance patient care.
Author Contributions
Conceptualization, T.V.D.; methodology T.V.D.; validation, V.P.T.V. and T.N.K.H.; writing—original draft preparation, T.V.D.; writing—review and editing, T.V.D., V.P.T.V. and T.N.K.H.; formal analysis, T.V.D. and V.P.T.V.; visualization, V.P.T.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Landi, I.; Glicksberg, B.S.; Lee, H.-C.; Cherng, S.; Landi, G.; Danieletto, M.; Dudley, J.T.; Furlanello, C.; Miotto, R. Deep representation learning of electronic health records to unlock patient stratification at scale. NPJ Digit. Med. 2020, 3, 96. [Google Scholar] [CrossRef] [PubMed]
- Hobensack, M.; Song, J.; Scharp, D.; Bowles, K.H.; Topaz, M. Machine learning applied to electronic health record data in home healthcare: A scoping review. Int. J. Med. Inf. 2023, 170, 104978. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.N.K.; Vy, V.P.T.; Tri, N.M.; Hoang, L.N.; Tuan, L.V.; Ho, Q.T.; Le, N.Q.K.; Kang, J.H. Automatic Detection of Meniscus Tears Using Backbone Convolutional Neural Networks on Knee MRI. J. Magn. Reson. Imaging 2023, 57, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Lassau, N.; Ammari, S.; Chouzenoux, E.; Gortais, H.; Herent, P.; Devilder, M.; Soliman, S.; Meyrignac, O.; Talabard, M.-P.; Lamarque, J.-P.; et al. Integrating deep learning CT-scan model, biological and clinical variables to predict severity of COVID-19 patients. Nat. Commun. 2021, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Yordanov, B.; Gaunt, A.; Wang, M.X.; Dai, P.; Chen, Y.-J.; Zhang, K.; Fang, J.Z.; Dalchau, N.; Li, J.; et al. A deep learning model for predicting next-generation sequencing depth from DNA sequence. Nat. Commun. 2021, 12, 4387. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, J. Machine Learning Methods for Exploring Sequence Determinants of 3D Genome Organization. J. Mol. Biol. 2022, 434, 167666. [Google Scholar] [CrossRef] [PubMed]
- Mincholé, A.; Camps, J.; Lyon, A.; Rodríguez, B. Machine learning in the electrocardiogram. J. Electrocardiol. 2019, 57, S61–S64. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Massey, S.L.; Kirschen, M.P.; Yuan, I.; Padiyath, A.; Simpao, A.F.; Tsui, F.R. Electroencephalogram-based machine learning models to predict neurologic outcome after cardiac arrest: A systematic review. Resuscitation 2024, 194, 110049. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Agostini, V.; Knaflitz, M.; Fioretti, S.; Di Nardo, F. Machine-Learning-Based Prediction of Gait Events from EMG in Cerebral Palsy Children. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 819–830. [Google Scholar] [CrossRef]
- Vy, V.P.T.; Yao, M.M.-S.; Khanh Le, N.Q.; Chan, W.P. Machine Learning Algorithm for Distinguishing Ductal Carcinoma In Situ from Invasive Breast Cancer. Cancers 2022, 14, 2437. [Google Scholar] [CrossRef]
- Abdollahi, J.; Mehrpour, O. Using Machine Learning Algorithms for Coronary Artery Disease (CAD) Prediction Prediction of Coronary Artery Disease (CAD) Using Machine Learning Algorithms. In Proceedings of the 2024 10th International Conference on Artificial Intelligence and Robotics (QICAR), Qazvin, Iran, 29 February 2024; pp. 164–172. [Google Scholar]
- Jeong, J.; Wang, L.; Ji, B.; Lei, Y.; Ali, A.; Liu, T.; Curran, W.J.; Mao, H.; Yang, X. Machine-learning based classification of glioblastoma using delta-radiomic features derived from dynamic susceptibility contrast enhanced magnetic resonance images. Quant. Imaging Med. Surg. 2019, 9, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, H.; Ming, Y.; Liu, Q.; Huang, C.; Xu, J.; Zhang, J.; Li, Y. Predicting myometrial invasion in endometrial cancer based on whole-uterine magnetic resonance radiomics. J. Cancer Res. Ther. 2020, 16, 1648–1655. [Google Scholar] [PubMed]
- Fujihara, K.; Sone, H. Machine Learning Approach to Drug Treatment Strategy for Diabetes Care. Diabetes Metab. J. 2023, 47, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, X.; Pan, W. Deep reinforcement learning for personalized treatment recommendation. Stat. Med. 2022, 41, 4034–4056. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yu, H.; Ke, J.; Ding, P.; Yi, Y.; Jiang, X.; Duan, X.; Tang, J.; Chang, D.T.; Wu, X.; et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat. Commun. 2021, 12, 1851. [Google Scholar] [CrossRef]
- Rafique, R.; Islam, S.M.R.; Kazi, J.U. Machine learning in the prediction of cancer therapy. Comput. Struct. Biotechnol. J. 2021, 19, 4003–4017. [Google Scholar] [CrossRef] [PubMed]
- Squarcina, L.; Villa, F.M.; Nobile, M.; Grisan, E.; Brambilla, P. Deep learning for the prediction of treatment response in depression. J. Affect. Disord. 2021, 281, 618–622. [Google Scholar] [CrossRef]
- Sharma, S.; Rawal, R.; Shah, D. Addressing the challenges of AI-based telemedicine: Best practices and lessons learned. J. Educ. Health Promot. 2023, 12, 338. [Google Scholar] [CrossRef]
- Bhaskar, S.; Bradley, S.; Sakhamuri, S.; Moguilner, S.; Chattu, V.K.; Pandya, S.; Schroeder, S.; Ray, D.; Banach, M. Designing futuristic telemedicine using artificial intelligence and robotics in the COVID-19 era. Front. Public. Health 2020, 8, 556789. [Google Scholar] [CrossRef]
- Beldhuis, I.E.; Marapin, R.S.; Jiang, Y.Y.; Simões de Souza, N.F.; Georgiou, A.; Kaufmann, T.; Castela Forte, J.; van der Horst, I.C.C. Cognitive biases, environmental, patient and personal factors associated with critical care decision making: A scoping review. J. Crit. Care 2021, 64, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Kooi, T.; Litjens, G.; van Ginneken, B.; Gubern-Mérida, A.; Sánchez, C.I.; Mann, R.; den Heeten, A.; Karssemeijer, N. Large scale deep learning for computer aided detection of mammographic lesions. Med. Image Anal. 2017, 35, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Cicero, M.; Bilbily, A.; Colak, E.; Dowdell, T.; Gray, B.; Perampaladas, K.; Barfett, J. Training and Validating a Deep Convolutional Neural Network for Computer-Aided Detection and Classification of Abnormalities on Frontal Chest Radiographs. Investig. Radiol. 2017, 52, 281–287. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Lysaght, T.; Lim, H.Y.; Xafis, V.; Ngiam, K.Y. AI-Assisted Decision-making in Healthcare: The Application of an Ethics Framework for Big Data in Health and Research. Asian Bioeth. Rev. 2019, 11, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, G.; Arbelaez Ossa, L.; Shaw, D.M.; Elger, B.S. Artificial intelligence and the doctor–patient relationship expanding the paradigm of shared decision making. Bioethics 2023, 37, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.W.; Tsai, T.Y.; Hsieh, Y.H.; Hsu, C.C.; Chen, S.H.; Lee, C.H.; Lin, Y.T.; Kao, H.K.; Lin, C.H. Reliability of Postoperative Free Flap Monitoring with a Novel Prediction Model Based on Supervised Machine Learning. Plast. Reconstr. Surg. 2023, 152, 943e–952e. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Farnebo, S.; Horwitz, M.D. Insights and trends review: Artificial intelligence in hand surgery. J. Hand Surg. Eur. Vol. 2023, 48, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.R. Art, Artificial Intelligence, and Aesthetics in Plastic Surgery. Plast. Reconstr. Surg. 2021, 148, 529e–530e. [Google Scholar] [CrossRef]
- Hassan, A.M.; Biaggi-Ondina, A.; Asaad, M.; Morris, N.; Liu, J.; Selber, J.C.; Butler, C.E. Artificial Intelligence Modeling to Predict Periprosthetic Infection and Explantation following Implant-Based Reconstruction. Plast. Reconstr. Surg. 2023, 152, 929–938. [Google Scholar] [CrossRef]
- Turner, A.E.; Abu-Ghname, A.; Davis, M.J.; Ali, K.; Winocour, S. Role of simulation and artificial intelligence in plastic surgery training. Plast. Reconstr. Surg. 2020, 146, 390e–391e. [Google Scholar] [CrossRef]
- Qin, F.; Gu, J. Artificial intelligence in plastic surgery: Current developments and future perspectives. Plast. Aesthetic Res. 2023, 10, 3. [Google Scholar] [CrossRef]
- TerKonda, S.P.; TerKonda, A.A.; Sacks, J.M.; Kinney, B.M.; Gurtner, G.C.; Nachbar, J.M.; Reddy, S.K.; Jeffers, L.L. Artificial Intelligence: Singularity Approaches. Plast. Reconstr. Surg. 2024, 153, 10–1097. [Google Scholar] [CrossRef]
- Nasteski, V. An overview of the supervised machine learning methods. HORIZONSB 2017, 4, 51–62. [Google Scholar] [CrossRef]
- Alloghani, M.; Al-Jumeily, D.; Mustafina, J.; Hussain, A.J.; Aljaaf, A.J. A Systematic Review on Supervised and Unsupervised Machine Learning Algorithms for Data Science. In Supervised and Unsupervised Learning for Data Science; Unsupervised Semi-Supervised Learn; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–21. [Google Scholar]
- Zhu, X.; Goldberg, A.B. Overview of Semi-Supervised Learning. In Introduction to Semi-Supervised Learning; Springer International Publishing: Cham, Switzerland, 2009; pp. 9–19. [Google Scholar]
- Wang, J.; Chen, Y. Overview of Transfer Learning Algorithms. In Introduction to Transfer Learning: Algorithms and Practice; Wang, J., Chen, Y., Eds.; Springer Nature: Singapore, 2023; pp. 53–66. [Google Scholar]
- Sarker, I.H. Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef]
- Kotu, V.; Deshpande, B. Chapter 10—Deep Learning. In Data Science, 2nd ed.; Kotu, V., Deshpande, B., Eds.; Morgan Kaufmann: Burlington, MA, USA, 2019; pp. 307–342. [Google Scholar]
- Chen, Y.; Li, L.; Li, W.; Guo, Q.; Du, Z.; Xu, Z. Chapter 3—Deep learning. In AI Computing Systems; Chen, Y., Li, L., Li, W., Guo, Q., Du, Z., Xu, Z., Eds.; Morgan Kaufmann: Burlington, MA, USA, 2024; pp. 53–121. [Google Scholar]
- Boczar, D.; Sisti, A.; Oliver, J.D.; Helmi, H.; Restrepo, D.J.; Huayllani, M.T.; Spaulding, A.C.; Carter, R.; Rinker, B.D.; Forte, A.J. Artificial Intelligent Virtual Assistant for Plastic Surgery Patient’s Frequently Asked Questions: A Pilot Study. Ann. Plast. Surg. 2020, 84, e16–e21. [Google Scholar] [CrossRef]
- Hammond, D.C.; Kim, K.; Bageris, M.H.; Chaudhry, A. Use of three-dimensional imaging to assess the effectiveness of volume as a critical variable in breast implant selection. Plast. Reconstr. Surg. 2022, 149, 70–79. [Google Scholar] [CrossRef]
- Kooi, K.; Martinez, E.T.; Freundt, L.; Oflazoglu, K.; Ritt, M.J.P.F.; Eberlin, K.R.; Selles, R.W.; Clemens, M.W.; Rakhorst, H.A. From Data to Decisions: How AI Is Revolutionizing Clinical Prediction Models in Plastic Surgery. Plast. Reconstr. Surg. 2021, 10–1097. [Google Scholar] [CrossRef]
- Hoogendam, L.; Bakx, J.A.C.; Souer, J.S.; Slijper, H.P.; Andrinopoulou, E.-R.; Selles RWobotHWSG. Predicting Clinically Relevant Patient-Reported Symptom Improvement After Carpal Tunnel Release: A Machine Learning Approach. Neurosurgery 2022, 90, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Loos, N.L.; Hoogendam, L.; Souer, J.S.; Slijper, H.P.; Andrinopoulou, E.-R.; Coppieters, M.W.; Selles, R.W.; Group tH-WS. Machine Learning Can be Used to Predict Function but Not Pain after Surgery for Thumb Carpometacarpal Osteoarthritis. Clin. Orthop. Relat. Res. 2022, 480, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Shademan, A.; Decker, R.S.; Opfermann, J.D.; Leonard, S.; Krieger, A.; Kim, P.C. Supervised autonomous robotic soft tissue surgery. Sci. Transl. Med. 2016, 8, 337ra364. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Mei, H.; Ma, H.; Chen, Z.; Li, Y. CLPNet: Cleft Lip and Palate Surgery Support with Deep Learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 3666–3672. [Google Scholar] [PubMed]
- Phillips, M.; Marsden, H.; Jaffe, W.; Matin, R.N.; Wali, G.N.; Greenhalgh, J.; McGrath, E.; James, R.; Ladoyanni, E.; Bewley, A.; et al. Assessment of Accuracy of an Artificial Intelligence Algorithm to Detect Melanoma in Images of Skin Lesions. JAMA Netw. Open 2019, 2, e1913436. [Google Scholar] [CrossRef]
- Bodenstedt, S.; Wagner, M.; Müller-Stich, B.P.; Weitz, J.; Speidel, S. Artificial Intelligence-Assisted Surgery: Potential and Challenges. Visc. Med. 2020, 36, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Nadri, H.; Lotfnezhad Afshar, H.; Timpka, T. A Systematic Review of the Technology Acceptance Model in Health Informatics. Appl. Clin. Inf. 2018, 9, 604–634. [Google Scholar] [CrossRef]
- Chartier, C.; Watt, A.; Lin, O.; Chandawarkar, A.; Lee, J.; Hall-Findlay, E. BreastGAN: Artificial Intelligence-Enabled Breast Augmentation Simulation. Aesthet. Surg. J. Open Forum 2022, 4, ojab052. [Google Scholar] [CrossRef]
- Chinski, H.; Lerch, R.; Tournour, D.; Chinski, L.; Caruso, D. An Artificial Intelligence Tool for Image Simulation in Rhinoplasty. Facial Plast. Surg. 2022, 38, 201–206. [Google Scholar] [CrossRef]
- Conforth, M.; Meng, Y.; Valmikinathan, C.; Yu, X. Nerve graft selection for peripheral nerve regeneration using neural networks trained by a hybrid ACO/PSO method. In Proceedings of the 2009 IEEE Symposium on Computational Intelligence in Bioinformatics and Computational Biology, Nashville, TN, USA, 30 March–2 April 2009; pp. 208–214. [Google Scholar]
- Crystal, D.T.; Cuccolo, N.G.; Ibrahim, A.M.S.; Furnas, H.; Lin, S.J. Photographic and Video Deepfakes Have Arrived: How Machine Learning May Influence Plastic Surgery. Plast. Reconstr. Surg. 2020, 145, 1079–1086. [Google Scholar] [CrossRef]
- Chartier, C.; Gfrerer, L.; Knoedler, L.; Austen, W.G., Jr. Artificial Intelligence–Enabled Evaluation of Pain Sketches to Predict Outcomes in Headache Surgery. Plast. Reconstr. Surg. 2023, 151, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Aasvang, E.K.; Meyhoff, C.S. The future of postoperative vital sign monitoring in general wards: Improving patient safety through continuous artificial intelligence-enabled alert formation and reduction. Curr. Opin. Anesthesiol. 2023, 36, 683–690. [Google Scholar] [CrossRef]
- Chairat, S.; Chaichulee, S.; Dissaneewate, T.; Wangkulangkul, P.; Kongpanichakul, L. AI-Assisted Assessment of Wound Tissue with Automatic Color and Measurement Calibration on Images Taken with a Smartphone. Healthcare 2023, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Soguero-Ruiz, C.; Fei, W.M.; Jenssen, R.; Augestad, K.M.; Álvarez, J.L.; Jiménez, I.M.; Lindsetmo, R.O.; Skrøvseth, S.O. Data-driven Temporal Prediction of Surgical Site Infection. AMIA Annu. Symp. Proc. 2015, 2015, 1164–1173. [Google Scholar] [PubMed]
- Sadideen, H.; Goutos, I.; Kneebone, R. Burns education: The emerging role of simulation for training healthcare professionals. Burns 2017, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, M.J.; Luck, J.; Billingsley, M.L.; Heyes, R.; Smith, O.J.; Mosahebi, A.; Khoussa, A.; Abu-Sittah, G.; Hachach-Haram, N. Demonstration of the Effectiveness of Augmented Reality Telesurgery in Complex Hand Reconstruction in Gaza. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1708. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Artificial intelligence and personalized medicine. In Precision Medicine in Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 265–283. [Google Scholar]
- Mir, M.A. Artificial Intelligence Revolutionizing Plastic Surgery Scientific Publications. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Farid, Y.; Fernando Botero Gutierrez, L.; Ortiz, S.; Gallego, S.; Zambrano, J.C.; Morrelli, H.U.; Patron, A. Artificial Intelligence in Plastic Surgery: Insights from Plastic Surgeons, Education Integration, ChatGPT’s Survey Predictions, and the Path Forward. Plast. Reconstr. Surg. Glob. Open 2024, 12, e5515. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Artificial Intelligence Is Still Far from Truly Revolutionizing Plastic Surgery. Plast. Reconstr. Surg. 2020, 146, 390e. [Google Scholar] [CrossRef]
- Parliament, E. Artificial Intelligence Act; European Parliament: Strasbourg, France, 2024. [Google Scholar]
- Farid, Y. A call for guidelines and regulatory body in adopting artificial intelligence for plastic surgeons. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).