1. Introduction
The skin is composed of three main layers: the epidermis, dermis, and subcutaneous tissue. Each of them has a specific structure and function [
1]. Skin hydration and barrier properties are primarily controlled by the condition of the most external part of the epidermis—the
stratum corneum. The elasticity and firmness of the skin depend mainly on its middle layer, the dermis. In adults, it is mainly composed of extracellular matrix (EMC), consisting of 70–90% type I collagen, elastin, proteoglycans, and hyaluronic acid [
2]. Young skin is composed of 80% type I collagen and about 15% type III collagen. Along with the aging process, the ability to replenish collagen naturally decreases by about 1–1.5% per year. When its levels begin to decline, the structure of the skin becomes increasingly brittle, leading to a weakening of its structural support [
3].
The
stratum corneum of the elderly has an overall reduced lipid content as well as a reduced water content, partly due to a decrease in cholesterol synthesis [
4]. In the skin of the elderly, vessel wall thinning has been reported to be less than half of the normal value in younger individuals, which contributes to vascular fragility. In contrast, loss of elastin contributes to vascular stiffness [
5]. Sensory and autonomic innervation of the epidermis and dermis is reduced, and the skin appendages are also affected.
Exercise makes it possible to halt involutionary changes, improve well-being, maintain a correct figure, and improve balance. Thanks to participation in group activities, older people or those with certain dysfunctions (such as obesity) are socially active and do not feel excluded and lonely [
6]. After the introduction of regular training, one can observe a reduction in blood pressure, increased ventricular compliance, improved inotropism, increased cardiac output, reduced arterial stiffness, positive effects on peripheral circulation disorders, improved respiratory ventilation and chest mobility, deepening of inspiration and lengthening of expiration, and a reduction in subjectively perceived dyspnea. In addition, regularly repeated physical activity increases the level of antibodies of the IgG and IgM classes, the production of anti-inflammatory cytokines, and the number of Th cells. When properly applied, exercise intervention is, in addition to diet therapy, the cornerstone of overweight and obesity treatment [
7]. The World Health Organization (WHO) clearly defines recommendations for physical activity that have health benefits for different age groups. It pays particular attention to the need to reduce sedentary lifestyles and replace them with any kind of movement, and it stresses that any kind of physical activity is better than none. Adults (18–64 years) and older adults (65 years and older) should undertake weekly moderate-intensity aerobic training of 150–300 min or high-intensity training of 75–150 min, and for a minimum of two days, introduce exercises that affect muscle strengthening [
8].
Physical activity has a major impact on the functioning of many systems and organs, including the skin. It performs two very important functions in the processes associated with exercise. Through physical activity, the condition of the skin is improved through hormonal changes in the body, sweating, increased blood flow, and regular muscle stimulation. However, the effects of activity on the skin’s condition have not been sufficiently studied, in no uncertain terms [
9,
10,
11].
Hypoxia is a condition characterized by a deficiency of tissue oxygenation relative to its demand. In the natural environment, it occurs in high-mountain conditions when the altitude exceeds 2000 m above sea level, and it can also be artificially simulated in environmental and climate chambers [
12,
13]. Biochemical changes in the human body under the influence of hypoxia, including acute hypoxia, depend on its intensity (rate and level of decrease in oxygen partial pressure). It causes deteriorative changes in the body, which are manifested by cognitive dysfunction, changes in body composition, and a decrease in body weight, as well as a decrease in motivation to act. More beneficial effects for the body are related to chronic hypoxia, which is based on slow and prolonged acclimatization of the body to the environment. This allows the induction of a group of genes responsible for maintaining the body’s homeostasis, glucose metabolism, or vascular tone, among other things.
Hypoxia can be applied in the prevention and treatment of obesity and many other diseases of civilization (type II diabetes, cardiovascular, respiratory, and neurological diseases), as well as in improving the quality of life of the elderly [
12]. It has been proven that physical activity conducted under hypoxic conditions has a stimulating effect on the regenerative processes of the nervous system, kidneys, and heart.
Hypoxic workouts are recommended not only for athletes but also for obese people [
14]. For the latter population, several beneficial effects are indicated. Exercise in a hypoxic environment simultaneously accelerates lipolysis, increases metabolism, reduces appetite (which still needs scientific confirmation), and reduces cholesterol levels. In addition, this form of physical activity strengthens blood vessels and dilates their diameter, lowers blood glucose levels, improves blood pressure, slows down the aging process, reduces free radicals, positively affects the immune system, increases lung capacity, reduces neuronal degeneration, and finds use in rehabilitation processes. Training under hypoxia affects the metabolic adaptation of skeletal muscle tissue. Increased hypertrophy of muscle fibers is observed as a result of the achievement of protein balance and a positive balance of satellite cells responsible for remodeling and growing muscle mass. Workouts performed under such conditions affect hematological changes in the body related to improved oxygen delivery to tissues and muscles. The number of mitochondria in cells and the buffering capacity of muscles and blood also increase. This improves the aerobic capacity of athletes, contributing to better performance in endurance and power sports [
15,
16].
In cosmetology and esthetic medicine, the phenomenon of hypoxia, as a state of temporary tissue hypoxia, is widely used through the subcutaneous application of carbon dioxide during a carboxytherapy treatment. Such action leads to local vasodilation, improving microcirculation, and increasing blood flow locally. The consequence is greater oxygenation of tissues. The treatment is used, among other things, to reduce cellulite, firm and smooth the skin, and locally reduce adipose tissue [
17].
It should be noted that, in addition to the positive effects of hypoxic training, pathophysiological hypoxia can also occur. In order to avoid undesirable effects, it is important to individually adjust the conditions of sports training. The following are considered to be symptoms of concern: inflammatory changes, immune reactions, vasoconstriction, psychological disorders (anxiety, depression, difficulty expressing emotions), and increased oxidative stress. Complications associated with training under hypoxic conditions can also become apparent on the skin in the form of cyanosis, coagulation disorders, or erythrocytosis [
16]. The paucity of research on this issue prompted us to try to indicate how a series of workouts under hypoxic conditions would affect basic skin characteristics.
The aim of this study was to evaluate the effect of four weeks of training performed under hypoxic conditions three times a week on hydration, the amount of TEWL, and skin elasticity status in overweight and obese women.
2. Materials and Methods
2.1. Study Protocol
Participants meeting the inclusion criteria for the project were selected from a group of female volunteers, and those with contraindications and other exclusion factors were excluded during medical qualification. Each qualified woman was subjected to a body composition analysis. The measurement took place before the start of the training series and again after 4 weeks. During those times, blood was drawn for analytical tests. Skin features on the jaw and hand were examined using probes before and after the first training session and before and after the last training session. The control group had the test done before and after the first 1-h training session, and then again 4 weeks later before and after the last 1-h training session. During the 4-week period, subjects in the study group performed interval exercise on a bicycle cycloergometer under hypoxia (2500 m above sea level), while subjects in the control group were not subjected to any training interventions. The study protocol is shown in
Figure 1.
2.2. Study Participants
Twenty-four women with obesity or overweight were enrolled in the study with the approval of the Bioethics Committee at the Regional Medical Board (No. 47/KBL/OIL/2022). During the project, 4 women dropped out (1 from the study group and 3 from the control group). In the end, the study group consisted of 11 subjects, and the control group consisted of 9.
Before entering the study, contraindications to exercise and the presence of glycemic disorders that would allow the diagnosis of diabetes were excluded. During the training series, the subjects were not allowed to change their diet or eating habits, take part in other physical activities, or have neurological or orthopedic health problems.
2.3. The Training Intervention
The study took place at the Laboratory of Physiological Basis of Adaptation, Central Scientific and Research Laboratory, AWF Krakow (
Figure 2). For 4 weeks, the subjects performed interval physical exercise on a bicycle cycloergometer under hypoxia (2500 m above sea level) 3× a week for 60 min, according to the scheme: interval 6 min/6 min—effort with 85% HRmax, active break with 70% HRmax, i.e., 12 min × 5 repetitions = 60 min.
In order to determine the level of physical fitness of the subjects and determine individual training loads, a graded test “to subjective refusal” was performed on a bicycle cycloergometer at an ambient temperature of 21 ± 0.5 °C and a relative humidity of 40 ± 3%. It was preceded by a 3-min warm-up on a bicycle cycloergometer at a pedaling rate (RPM) of 60 revolutions per minute at an intensity of 90 W, after which the load was increased by 20 W every 2 min. The effort lasted until the subjective feeling of not being able to maintain the desired pedaling rhythm, i.e., refusing to continue the effort at the specified pedaling resistance. During the effort, respiratory exchange rates were recorded in 30-s sequences, and heart rate (HR) was continuously recorded.
2.4. Body Composition Analysis
Each participant had her body height (BH) measured using a stadiometer (Seca, Hamburg, Germany), body weight determined using a Sartorius type F 105 scale (DZA, Berlin, Germany), and body composition estimated using the IOI 353 body composition analyzer (JAWON MEDICAL, Daejeon, Republic of Korea) with certification for scientific research (EC0197). The following components were indicated: TT (body fat content in kg); PBF (body fat percentage); SLM (soft tissue mass in kg); VFA (visceral fat area in cm2); and TBW (total water content in kg). The BMI (body mass index) was also calculated from the data obtained.
2.5. Blood Collection and Biochemical Tests
Blood collection was performed on an empty stomach by specialized medical personnel in accordance with current standards. Venous blood was collected at the elbow bend into Vacumed system (Italy) tubes. The following baseline morphology and serum lipid profiles were then determined: total cholesterol (TC); high-density lipoprotein cholesterol (HDL); non-HDL cholesterol; low-density lipoprotein cholesterol (LDL); and triglycerides (TG). The tests were performed at an external diagnostic laboratory.
2.6. Objective Assessment of Skin Characteristics
Tewametry is based on the measurement of TEWL (transepidermal water loss) expressed in grams of water per unit area (1 m2) of skin in one hour. The correct TEWL range varies for each type of tewameter and is individually specified by the device manufacturer. In the present project, the Tewameter® TM 300 (Courage + Khazaka electronic GmbH, Cologne, Germany), which belongs to the tewameters with an open measuring chamber, was used to evaluate the magnitude of TEWL. Measurement in each area was carried out until the reading stabilized (at least 30 s). The manufacturer of the device recommends the following interpretation of the results [g/(m2 h)]: 0–10: very good condition; 10–15: good condition; 15–25: normal condition; 25–30: deteriorated condition; above 30: critical condition.
An indentometer is used to assess the stiffness of the skin. The skin is deformed under the force generated by the spring on the pins coming out of the small recess of the probe. The device measures how the probe indentor displaces the skin. The depth of penetration is measured in millimeters (0–3 mm). The stiffer the skin, the shallower the depth of penetration [
18]. An indentomer IDM 800 probe (Courage + Khazaka electronic GmbH, Cologne) was used to assess skin elasticity. Measurements were taken at three locations on the hand and mandible, and the average was drawn.
The instrument used to assess epidermal hydration is the corneometer. The test involves measuring the capacitance and electrical resistance of the skin as well as the electrical conductivity in the epidermis. Applying the device to the skin for a second with a force of about 7.1 N/cm2 allows measurement at a depth of 20–30 μm of the epidermis. The lower the reading, the less hydrated the area of skin. The study was performed with a Corneometer® CM 825 (Courage + Khazaka electronic GmbH, Cologne). Measurements were taken at three non-overlapping points, and then the average value was calculated from the three results on a given area of skin.
The measurements were performed one person at a time in women from both groups before and after the first training and before and after the last training (for the control group at corresponding time points without training intervention). Readings were taken from the mandible and the hand. The tests were conducted under the conditions specified by the manufacturer: room temperature between 20 and 24 °C, humidity 50–65%. Before the measurement, the subject did not use emollients or detergents and did not wash their hands for 30 min before the test.
2.7. Surveys
The author’s survey questionnaire was anonymously filled out by female respondents after completing a series of training sessions. The form contained 16 questions concerning daily care, cosmetic products used, and subjective evaluation of the skin. The respondents were asked to rate selected skin features on a scale of 1 to 5, with 1 being the most negative and 5 being the most positive.
2.8. Statistical Analysis
Results are presented using basic descriptive statistics (mean; ± standard deviation, SD; minimum value, MIN; maximum value, MAX; median, MED). Survey results of the baseline characteristics of the selected groups were compared using the Chi2 test. The type of distribution was tested with the Shapiro-Wilk test. The elimination of results significantly different from the others was performed using the Grubs test. Results were compared using an ANOVA test and a Student’s t-test for dependent and independent samples. The presence of correlations between skin characteristics, somatic traits, and biochemical variables was examined using a Pearson’s test or a Spearman’s test, depending on the type of distribution of the variables in question. In each test, the result was considered statistically significant at p < 0.05. Analysis of the results was performed using Statistica 13 (SoftStat, Krakow, Poland) and Excel (Microsoft, Redmond, WA, USA) software.
3. Results
Table 1 shows a comparison of the baseline characteristics of the women assigned to these groups. Participants assigned to the study group and the control group at baseline were not significantly different.
The results of the questionnaires did not indicate differences between skin care habits or subjective assessments of skin quality among women assigned to the groups (
Table 2).
Among the respondents, 20% of the women declared that they smoke cigarettes (1 from GB and 4 from GK); this characteristic did not differentiate the groups (p = 0.177). It was indicated that 75% of the respondents confirmed drinking alcohol daily or less often, while 25% of the women do not drink alcoholic beverages at all. The Chi2 test indicates that there was no significant difference in the emergent groups (p = 0.795).
When it comes to subjective assessment of skin type, the most common answer is mixed and vascular skin (45%). This is followed by 20% of women stating that they have sensitive skin and 20% stating that they have oily skin. Only 10% of respondents do not know what kind of skin they have, and the fewest respondents (5%) recognize that they have normal skin. The distribution in the emerging groups was similar. The same was true for the subjective assessment of the severity of acne lesions and the occurrence of skin discoloration. As many as 65% of the women surveyed indicated that they had no or very little acne; 15% said their acne was minor or moderate; and only 5% said their acne was very severe. Almost half of the women (55%) indicated that they have no or very little hyperpigmentation; 10% of respondents have small hyperpigmentation; 30% have moderate hyperpigmentation; and only 5% complain of very severe hyperpigmentation. More than half of the women in both groups indicated a problem with excessive skin luminosity (all over the face or in the T-zone). The women also indicated factors causing skin irritation. The most common factors were low temperatures (65% of the women surveyed); the impact of emotions and stress (55%); hormonal changes (50%); high temperatures and wind (45%); sun and UV radiation (35%); and air conditioning (10%).
A subjective assessment of skin hydration and elasticity is shown in
Table 2. Subjective comparison to peers in terms of overall skin appearance and sweating intensity was also determined. No differences were indicated between GB and GK.
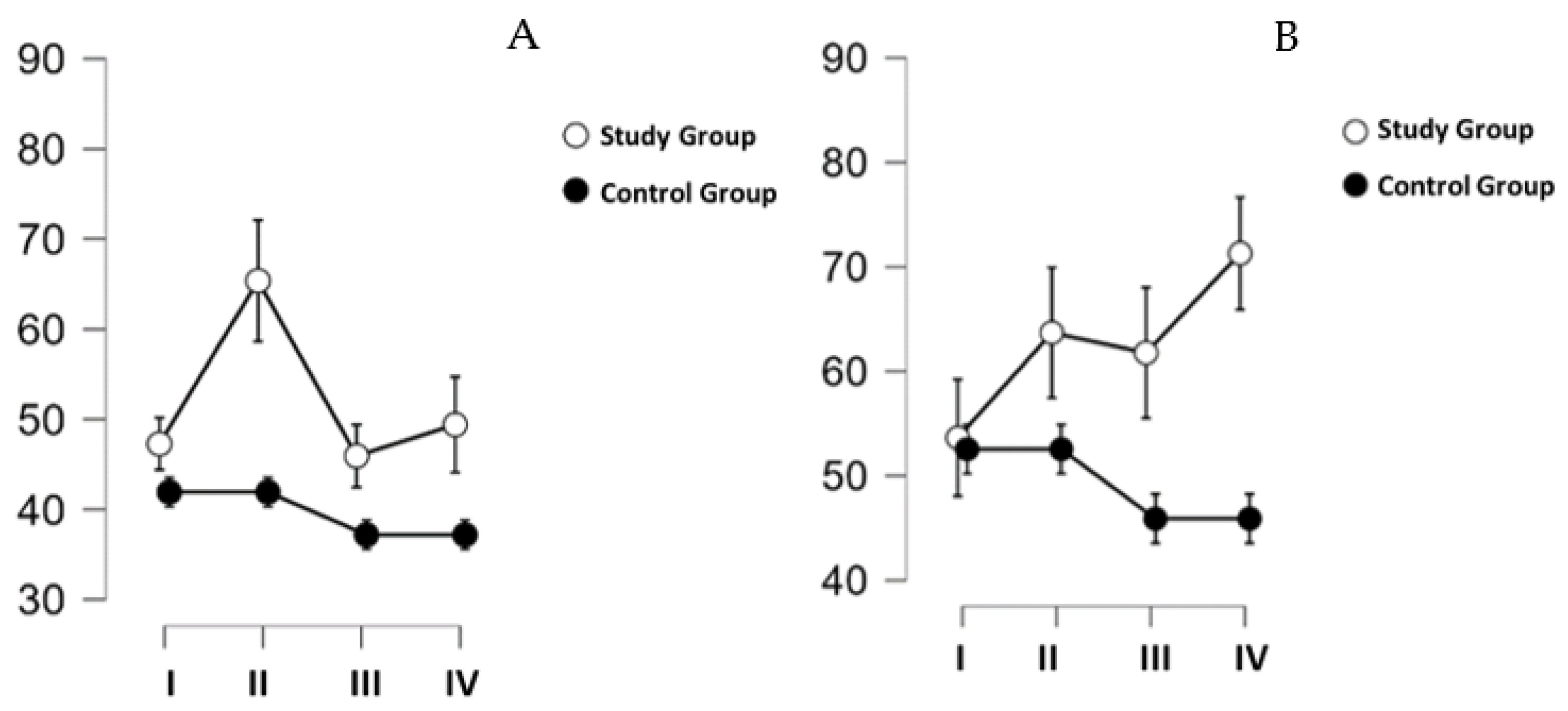
Hand corneometry results differed over time (
p = 0.019; η
2 = 0.084), and group designation was also a differentiating factor (
p = 0.045; η
2 = 0.149). The results are shown in
Figure 3A. Post hoc tests, considering only the variable time, indicated the presence of significant differences between Measure I and Measure II (
p = 0.046). The two factors, time and group, indicated significant differences between GB measurements I and II (
p = 0.006).
The results of corneometry on the mandibular skin are shown in
Figure 3B. Despite the divergent direction of change for GK and GB visible in the figure, the results did not reach the established threshold of statistical significance (time:
p = 0.442, time ✻ group:
p = 0.100). The post hoc test performed showed a significant difference between the groups (
p = 0.045).
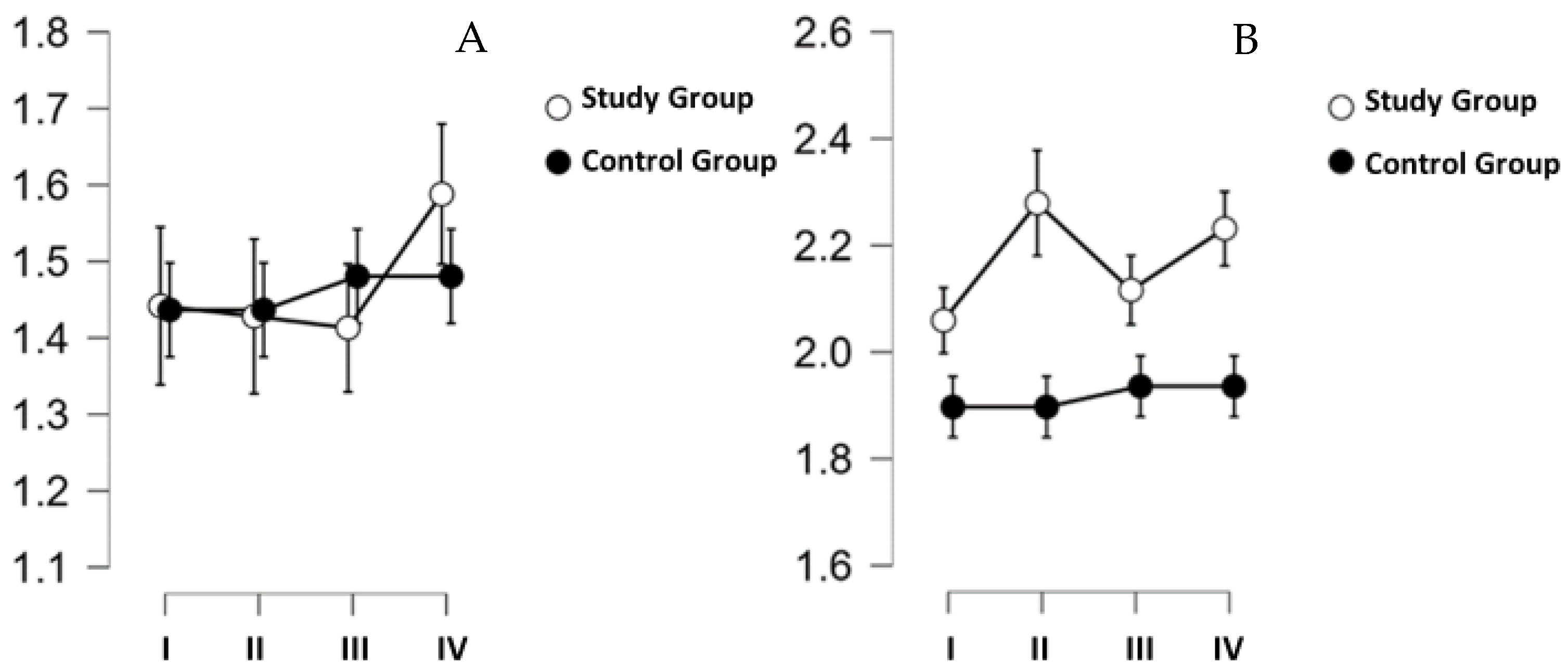
The results of the intedometric tests are shown in
Figure 4. The results of the measurements taken on the hand (
Figure 4A) did not differ in time (
p = 0.568), did not differ between groups (
p = 0.925), and no difference was indicated considering the 2 factors (time ✻ group:
p = 0.738). The results of the analysis of intedtometric measurements performed on the mandibular skin were similarly shaped, with
p = 0.332,
p = 0.114, and
p = 0.340, respectively (
Figure 4B).
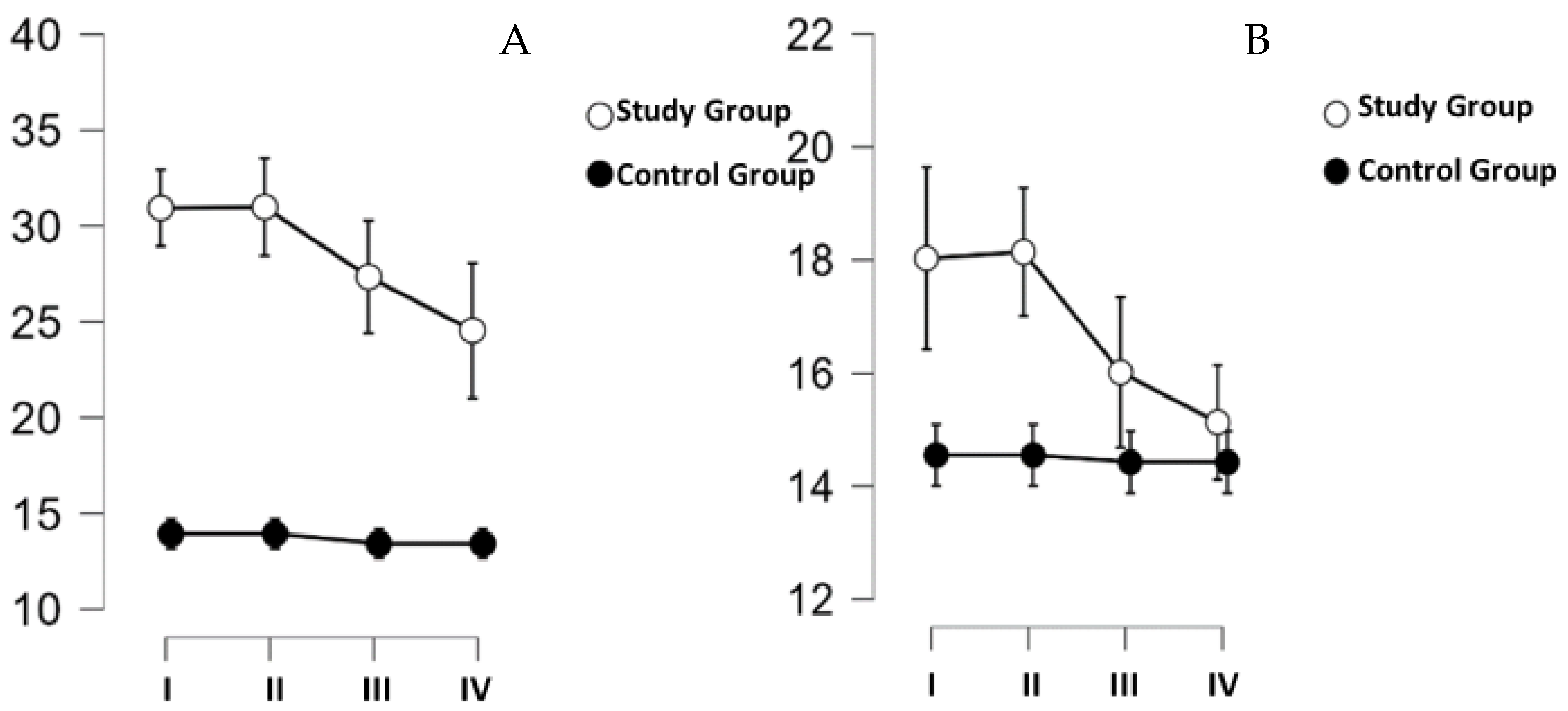
The next item was a tewametric test. The results of this test are shown in
Figure 5. As with the skin elasticity test, no effect of time (
p = 0.349) as a factor or combined time and group (
p = 0.483) as a factor on the amount of TEWL on the hand skin was indicated. However, a significant difference was indicated between the groups (
p < 0.001), where the thicker subjects had significantly higher TEWL values on the palm. High readings on the palm were observed primarily for the first two measurements taken before (I) and after (II) the first training on the cycloergometer. Measurements taken on the mandible (
Figure 5B) were not different over time (
p = 0.271) and did not differ for the selected groups (
p = 0.134), and for the time- and group-adjusted analysis, no differences with the assumed threshold of significance (
p = 0.335) were indicated either (
Figure 5B).
In the SG group, after the training series, statistically significant changes were obtained for body weight, BMI, and TBW (
Table 3). The maximum reduction in body weight was 2.8 kg. A significant decrease was observed for each variable. The magnitude of the effects obtained should be interpreted as an average. In the CG, statistically significant results were obtained for BMI and VFA (
Table 3). These variables, after a 4-week period, increased. Cohen’s d effect size measure indicates that the effect ranks at the medium level. In the control group, body weight increased by an average of 1.06; 33% of subjects decreased in weight, 11% remained the same, and 56% increased. The largest weight decrease was 0.5 kg, and the maximum increase was 6.5 kg. The result did not reach the assumed threshold of significance (
p = 0.090).
The maximum decrease in BMI in the SG was 2.1 kg/m2. The difference after 4 weeks for the whole group was statistically significant (p = 0.045). The mean BMI in the CG increased by 0.53; in 11% of subjects, it decreased, 11% remained the same, and 78% increased. The maximum increase in BMI was 2.6 kg/m2, and the largest decrease was 0.2 kg/m2. The result reached the assumed threshold of significance (p = 0.045).
In the SG, body fat mass decreased by an average of 0.26 kg; in 73% of FM participants, it decreased. The largest decrease was 3.0 kg. The result did not reach the assumed threshold of significance (p = 0.324). In contrast, in the CG, FM increased by an average of 0.49 kg. The maximum increase in fat mass was 3.0 kg, and the largest decrease was 0.5 kg. The result did not reach the assumed statistical significance (p = 0.099). In the SG, the PBF index changed by 0.39% on average. An identical difference occurred in the CG, with the fact that in the trained influenza, PBF decreased and in the control influenza, it increased. In both groups, the result did not reach the threshold of statistical significance. SLM changed by an average of 0.54 kg in the treatment group and 0.66 kg in the CG. The change for both groups was not significant.
A decrease in VFA in the SG was indicated, averaging 0.67 cm2. The maximum decrease was 10 cm2, but the change did not reach the assumed threshold of significance (p = 0.488). In the control group, the average VFA increased by 1.22 cm2, and the result is statistically significant (p = 0.042).
AC changes did not reach the threshold of significance in both groups. Based on the WHR value, aneroid (so-called abdominal) obesity was found in all participating women (100%). In the study group, the average WHR value did not change after 4 weeks of training. In the control group, the average WHR value increased, but the result did not reach the assumed threshold of statistical significance (p = 0.173).
The TBW index in the SG changed in value by an average of 0.74; it decreased in 72% of the participants. The largest increase was 0.3%, and the maximum decrease was 3.6%. In the control group, TBW changed by an average of 0.07. The maximum increase was 0.4%, and the largest decrease was 0.1%. Statistical analysis showed that a statistically significantly lower TBW value was observed in the study group (p = 0.017).
In the study group, total cholesterol concentrations changed by an average of 0.17; for 60% of participants, it increased, and for 40%, it decreased. In the control group, cholesterol concentrations increased by an average of 0.03. The change did not reach the assumed threshold of significance (
p = 0.315;
p = 0.076). In the study group, non-HDL concentrations changed by an average of 0.05; for 50% of participants, it increased, and for 50%, it decreased. The maximum increase was 0.78 mmol/L, and the largest decrease was 1.27 mmol/L (
p = 0.459). In the control group, non-HDL concentrations changed by 0.05; for 56% of participants, it increased, for 33%, it remained unchanged, and for 11%, it decreased. Statistical analysis of HDL concentration results showed no significance for both groups (
p = 0.261;
p = 0.472). In the study group, the mean change in LDL concentration was an increase of 0.32 (
p < 0.00001), and no change in the CG was observed (
p = 0.440). The TG concentration in the SG decreased by an average of 0.20 mmol/L. However, statistical analysis did not indicate significant changes for the study group (
p = 0.218) or the control group (
p = 0.497). Lipidogram results are shown in
Table 4.
The data obtained were also analyzed to indicate correlations between selected skin characteristics and body composition components, as well as between skin characteristics and factors determined in blood drawn from the participants. The results of the correlation analysis are shown in
Table 5.
4. Discussion
Currently, the vast majority of studies on the effect of hypoxia on the skin concern studies conducted in cell cultures [
19,
20]. Hypoxia is an important topic of studies seeking to implement practical solutions, where the possibility of using it as a repair and anti-aging agent is indicated [
21]. The present work, for the first time, shows how a series of workouts conducted in a hypoxic chamber affects the skin characteristics of women with obesity. The study was conducted with a control group, which helped strengthen the results obtained. It is known that the skin covering different parts of the body differs significantly in a number of physicochemical characteristics. For example, the skin in the groin is very flacid, while the skin of the arms, chest, and abdomen is less flacid despite similar concentrations of collagen, indicating that the structure of the dermis and subcutaneous tissue affects the overall elastic modulus in these areas [
22]. Hence, in this project, two skin areas were chosen to perform measurements with regard to the possible different initial skin properties and subtle differences in skin microarchitecture.
Epidermal hydration is largely determined by the composition and amount of intercellular lipids and the level of natural moisturizing factor (NMF) content. A proper level of hydration promotes protective functions by forming a physical, chemical, biological, and immunological barrier to the external environment. The skin barrier changes with age [
23], as the hydration status of the epidermis is regulated not only by water and fluids supplied from outside or inside, but equally important is the skin’s ability to retain fluid [
24]. Therefore, the present study evaluated not only the skin’s hydration level but also its barrier function as determined by the magnitude of TEWL. Dry skin is a common symptom of many diseases and also occurs spontaneously as a sign of aging. For Caucasians, it occurs in about 15–20% of the population [
23,
24]. It affects changes in the
stratum corneum and interferes with keratinization and transepidermal water metabolism [
25]. Dry skin is less elastic, more flaky, prone to infection and inflammation, and more difficult to regenerate. Our results showed that an increase in skin hydration was observed on the hands after the first workout, but the effect was not permanent. On the other hand, women in the SG significantly reduced the water content of their bodies, yet hydration on the hands or cheeks did not decrease. This is an important observation, which shows that the combined effect of the applied stimuli (training and hypoxia) allowed the skin to maintain good hydration characteristics. It can be assumed that under conditions of increased water intake by the participants, skin hydration would have increased unequivocally.
It has been indicated that the problem of dry skin is correlated with several factors, including that it is inversely correlated with BMI [
26]. This correlation was not confirmed in the present project, which is probably due to the fact that only people with a BMI indicating obesity or overweight participated. This selection of the group may not have allowed the correlation between these variables to be made visible. However, very interesting correlations with lipidogram results were indicated. Analysis of the data obtained showed that the result of the corneometric measurement is inversely correlated with TC, non-LDL, and LDL. What is important is that these results are significant both for the hydration of the skin on the hands and for the skin on the jaw. At present, this is the first report that clearly demonstrates that proper skin hydration is mosaically dependent on lipid metabolism, as assessed by measuring blood lipoprotein concentrations.
Participants in our project had concentrations of TC and its individual fractions elevated above recommended levels. Insufficiently hydrated skin becomes less resistant to the adverse effects of external factors such as wind, temperature, UV radiation, air conditioning, various detergents, or microorganisms [
23,
24]. The women in the present study specifically pointed to a number of these factors as known irritants to their skin.
Identometric measurements of the skin performed before and after the first workout and before and after the last workout in the hypoxic chamber showed no statistically significant changes in skin elasticity. This shows that training in a hypoxic chamber for a period of four weeks has no positive effect on increasing skin firmness, but also does not cause a decrease in skin firmness. Therefore, it is safe for the skin and does not cause any negative changes. This is important primarily in the context of the statistically significant TBW difference that was shown. After four weeks of training, the women performing workouts had lower TBW values than they did before the introduction of the workouts. Despite this unfavorable change, skin elasticity in the two study groups did not deteriorate.
In the case of the study group, the mean value of indentometry on the hand decreased after the first workout and had the same direction for four weeks; however, it increased after the workout on the last day compared to the measurement before this workout. On the mandible, there was an increase in the average value of indentometer measurements before and after the first and last training sessions. However, these changes were not large enough to be statistically significant. This shows that the dynamics of changes in skin elasticity under the conditions studied may vary depending on the selected body area. Very interesting correlations were also indicated. Skin elasticity on the mandible was positively correlated with body weight and WHR. In our opinion, this is directly related to the influence of subcutaneous adipose tissue on the measurement result (the ability of the measuring probe to penetrate the tissues). A significant inverse correlation with TG concentration was also indicated, which can be linked to the stiffening effect of triglycerides on not only blood vessels but also other structures. It was not possible to show significant correlations with LDL and HDL, which in our opinion would be an extremely interesting result, but it most likely requires a study with a larger group of participants. On the other hand, the fact that in the present project, with a large range of participant ages, it was possible to show the relationship between TG and skin elasticity on the mandible is one of the strengths of this work.
Koch and Wilke [
27] studied the results of measurements with the use of an indentometer for measuring the stiffness of musculo-fascial tissue and tested the reliability of the device. On the basis of their study, they concluded that the “IndentoPro” indentometer tested showed high repeatability in repeated measurements by the same investigator and sufficient reproducibility when used by different investigators. Another observation was that there was no correlation between the indentometry value and weight, height, BMI, age, gender, or skinfold thickness. In this aspect, the presented results completely confirm the reports of Koch and Wilke [
27], as we did not find significant correlations with any of the elements of body composition of the women studied. On the other hand, the fact that the correlations we observed (with VFA, WHR, and TG) were only with the results on the mandible shows that the condition of the skin on the hands will be more influenced by external (environmental) factors than the skin on the face (which is more responsive to the internal well-being of the body).
The lack of correlation between the age of the participants and skin elasticity assessed by indentometric measurement is surprising. It can be assumed that in the present study this is related to the characteristics of the participants, who, meeting the recruitment criteria, had to be characterized by altered body composition and a raised BMI. With a group selected in this way, it is impossible to make a clear assessment of the impact of BMI on the formation of skin elasticity since there are no participants with BMIs within the normal range or below normal values. If this factor modifies (or is correlated with) the result of indentometry, it will interfere with the effect of age on skin elasticity. However, as shown above, identical results have already been obtained by other researchers.
TEWL is an objective indicator for assessing skin barrier function that measures the amount of water evaporating through the
stratum corneum. Differences in TEWL are influenced by systemic diseases or dermatoses (e.g., atopic dermatitis, psoriasis), among others. In addition, great attention should be paid to the anatomical distribution of sweat glands in the skin and the effect of stress on their activation [
28,
29]. The glands will have greater excitability in the palmar area than in the mandibular area. Prior to the training series, the average TEWL measurement for the hand was 31,991 g/(m
2 h) and for the mandible, 21,836 g/(m
2 h). Moreover, significantly higher TEWL values were observed in the test group (hand: 31.991 g/(m
2 h); mandible: 21.836 g/(m
2 h)) than in the control group (hand: 16.656 g/(m
2 h); mandible: 15.544 g/(m
2 h)). No significant changes in TEWL values were observed in both groups of female participants. The differences in the baseline values of the index are explained by the presence of a stress stimulus for the study group in the form of undertaking a series of workouts in the hypoxia chamber. This hypothesis is also supported by the fact that significantly higher TEWL values in the SG were observed only on the hands and not on the face, and it is the hands that will be more likely to manifest the stress response in the form of increased sweating. The anatomical distribution of sweat glands in the skin also justifies the difference in TEWL values for the tested body areas. The study helped confirm the safety of hypoxia chamber training for skin barrier function.
When discussing the results on TEWL, it should be pointed out that once again confirmation was obtained that TEWL is positively correlated with BM, although more often authors present results based on BMI [
30]. The higher the body weight, the higher the TEWL value. In our study, we also showed that the amount of TEWL is correlated with TBW. The higher the body’s water percentage, the greater the skin’s ability to evaporate water into the external environment; thanks to this, the skin participates in thermoregulation, for example. Of course, this happens within certain limits, and the submerged barrier function of the skin will hinder the role of the skin in thermoregulatory processes [
31]. The results also indicated the presence of a correlation between SLM and multiple TEWLs. We conjecture that this is due to the high hydration of muscle tissue and, through this relationship, link the multiplicity of TEWLs to lean body mass. The literature has not previously indicated such a link, so we believe this should be replicated and the relationship between these variables should be explored in more depth.
For four weeks, the women in the study participated in physical training, which had a beneficial effect on their body weight and BMI (both variables decreased in a statistically significant manner). Unfortunately, it was also pointed out that after four weeks, the female subjects experienced a significant decrease in TBW. Despite this, skin elasticity did not decrease, and after one month, the women studied had similar results to those at baseline. This is an important observation, showing that despite the decrease in TBW, the skin maintained its baseline elasticity. This observation is also consistent with the lack of significance for the correlation between TBW and skin elasticity.
In the control group, a statistically significant increase in BMI and visceral fat was observed. These results show that overweight and obesity are dynamic conditions, and the absence of any training or dietary intervention exacerbates the pathology and leads to a worsening of the condition in those affected [
32].
Hypoxia training, or training under conditions of reduced partial pressure of oxygen in atmospheric air, has a proven positive effect on the human body [
12,
15,
16]. The beneficial effects of training in hypoxia relate to adaptive changes in organs and tissues responsible for oxygen uptake and transport, as well as vasodilators (especially nitric oxide) with antihypertensive effects, adaptive changes in the immune system, and changes in the liver detoxification system associated with activation of the cytochrome P-450 system. Training under hypoxia is characterized by a progressive increase in ventilation, hemodynamics, and erythropoiesis to increase oxygen delivery to tissues and optimize its utilization. Such training improves energy production by increasing mitochondrial morphogenesis, activating electron flux through mitochondrial respiratory complex I, and increasing the efficiency of oxidative phosphorylation. Adaptive-compensatory responses involve both tissue and cellular mechanisms. Ultrastructural changes in the mitochondria and energy metabolism resulting from hypoxia appear to be of great importance. These effects are central to the adaptive response to hypoxia. However, knowledge of the negative effects of training in hypoxia on cellular adaptive mechanisms remains limited [
32].
Training under hypoxic conditions increases vascular endothelial growth factor activity, so that the blood vessel network is expanded. As a result, adipose tissue is better oxygenated, and the process of lipolysis is increased, which causes the breakdown of triglycerides and a faster reduction in body fat. Kayser et al. (2013) also pointed out that under hypoxia, basal metabolism is increased and appetite is demarcated [
33]. The combination of the hypoxia factor with individually intensity-tailored physical training achieved favorable changes in the body composition of female participants assigned to the SG. An unfavorable change, however, was a decrease in TBW. This may be due to an inadequate water supply and increased perspiration due to high physical activity.
Physical activity also plays an important role in the treatment of obesity [
7,
34,
35,
36]. Regular physical activity has been proven to control body weight and favorably alter the lipid profile [
37,
38,
39]. In the present project, this was highlighted only in the context of triglycerides. The direction of change for LDL was just the opposite, and an increase in this lipoprotein fraction was observed.
Study Limitation
The results of this study and the conclusions drawn from them have limitations due to the research protocol used. First, only women (both menstruating and postmenopausal) participated in the study. Another limitation is the limited number of women. Therefore, conclusions can only be drawn for one gender. All of the women studied were of the same race (Caucasian), which will limit conclusions to only those of that ethnic group. Another limitation is the lack of measurements over a period of time after the removal of the training factor and the thermoclimatic factor. This makes it impossible to gain insight into the distant changes occurring in the skin’s condition after training conducted under hypoxic conditions.