The Human Impact on Changes in the Forest Range of the Silesian Beskids (Western Carpathians)
Abstract
1. Introduction
2. Materials and Methods
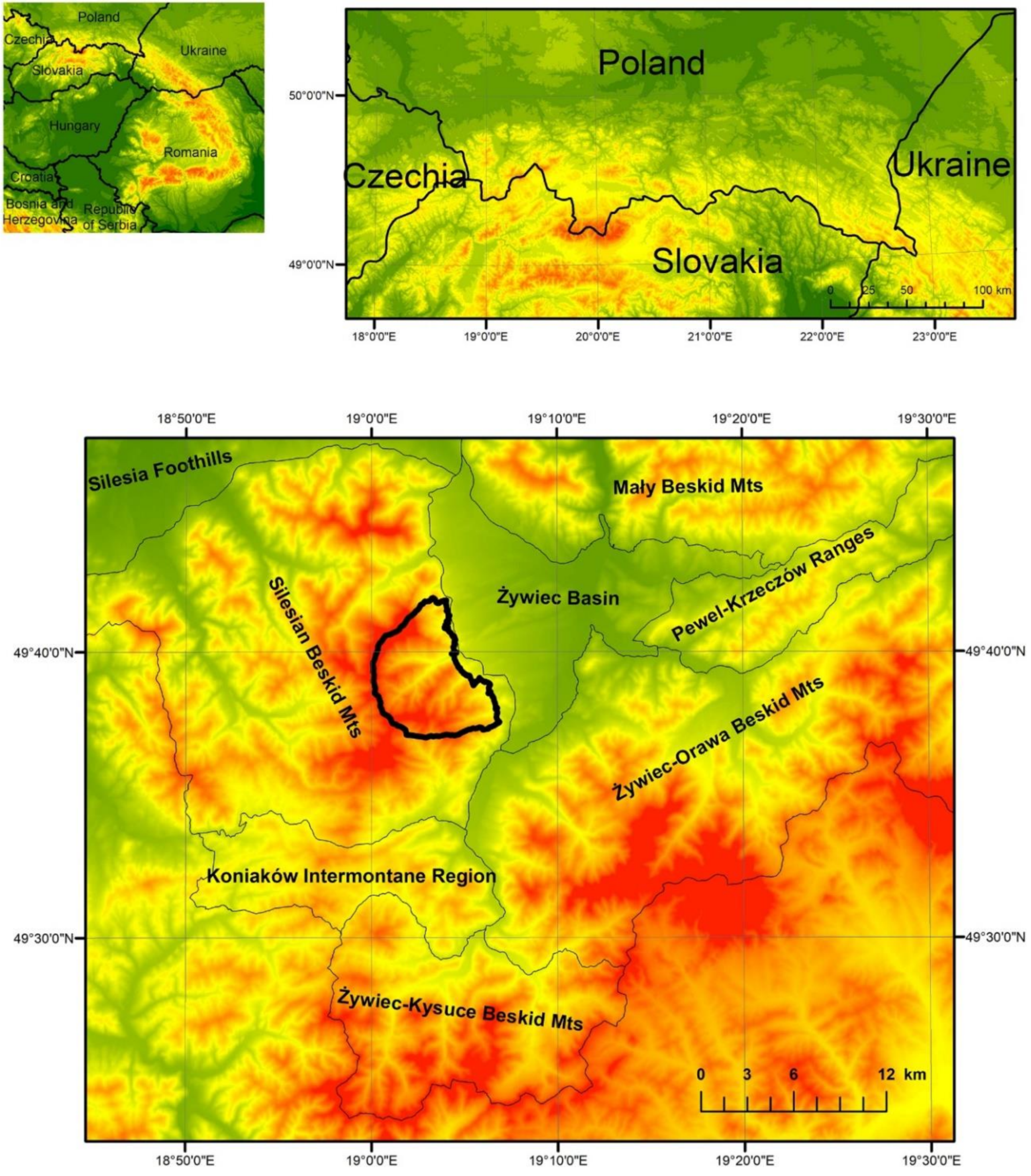
2.1. Study Area
2.2. Spatial Data
- Austrian cadastral maps from 1848 scaled at 1:2880;
- A Spezialkarte der Österreichisch-Ungarischen Monarchie (special map of the Austro-Hungarian Monarchy) from 1879 scaled at 1:75,000;
- A WIG (Military Geographical Institute of Poland) military map from 1933 scaled at 1:100,000;
- A military topographic map dated 1960 scaled at 1:25,000;
- A topographic map from 1979 scaled at 1:10,000;
- An orthophotomap dated 2015.
2.3. Cartographical Analysis
- Forest area (FA) and non-forest area (NFA);
- Percentages of forest (FP) and non-forest (NFP) areas;
- Forest core area (FCA)—equals the area within the patch that is more than the specified depth-of-edge distance from the patch perimeter; FCA has been found to be a much better predictor of site quality than patch area [56];
- Inner buffer area (IBA)—the area between the edge of the patch and the core area; it was assumed that the buffer zone is 50 m wide [57];
- Percentages of forest core (FCP) and inner buffer (IBP) areas;
- Core area index (CAI)—the percentage of a patch that constitutes the core area;
- Number of forest patches (FNP);
- Number of core areas (CAN);
- Maximal forest patch area (MFA)—the area of the largest forest patch indicated in the study area.
2.4. Analyses of Forest Sites and Communities—Field Study
- Natural—unchanged by human activity;
- Close-to-nature—those where the species composition of the stand has been changed because of human activity, but where this change did not influence the ground cover, properties of top soil horizons (mainly humus horizon), or soil water conditions;
- Anthropogenically transformed—those where the species composition of the stand has been changed because of human activity and where this change did influence the ground cover, properties of top soil horizons, and soil water conditions.
3. Results
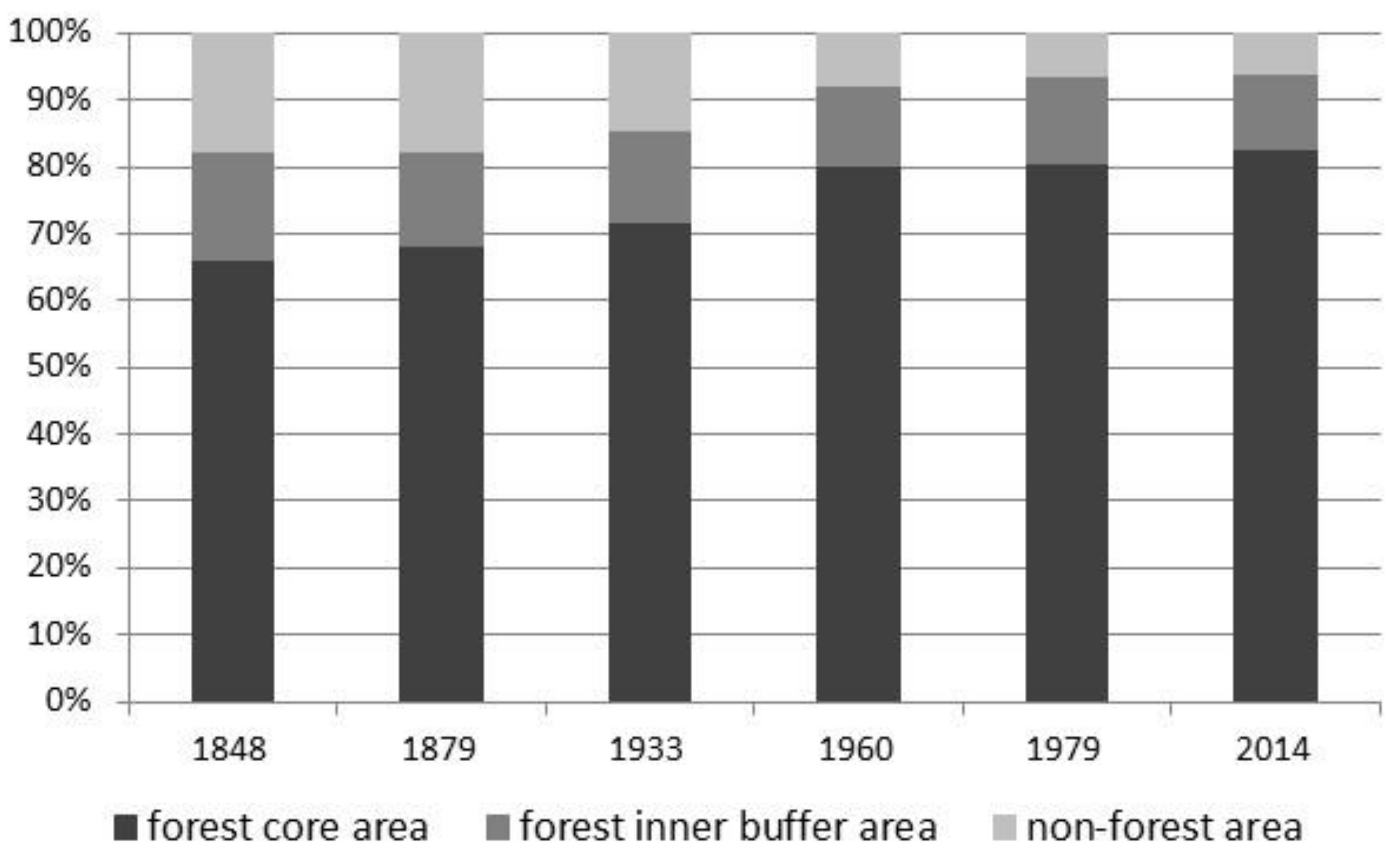
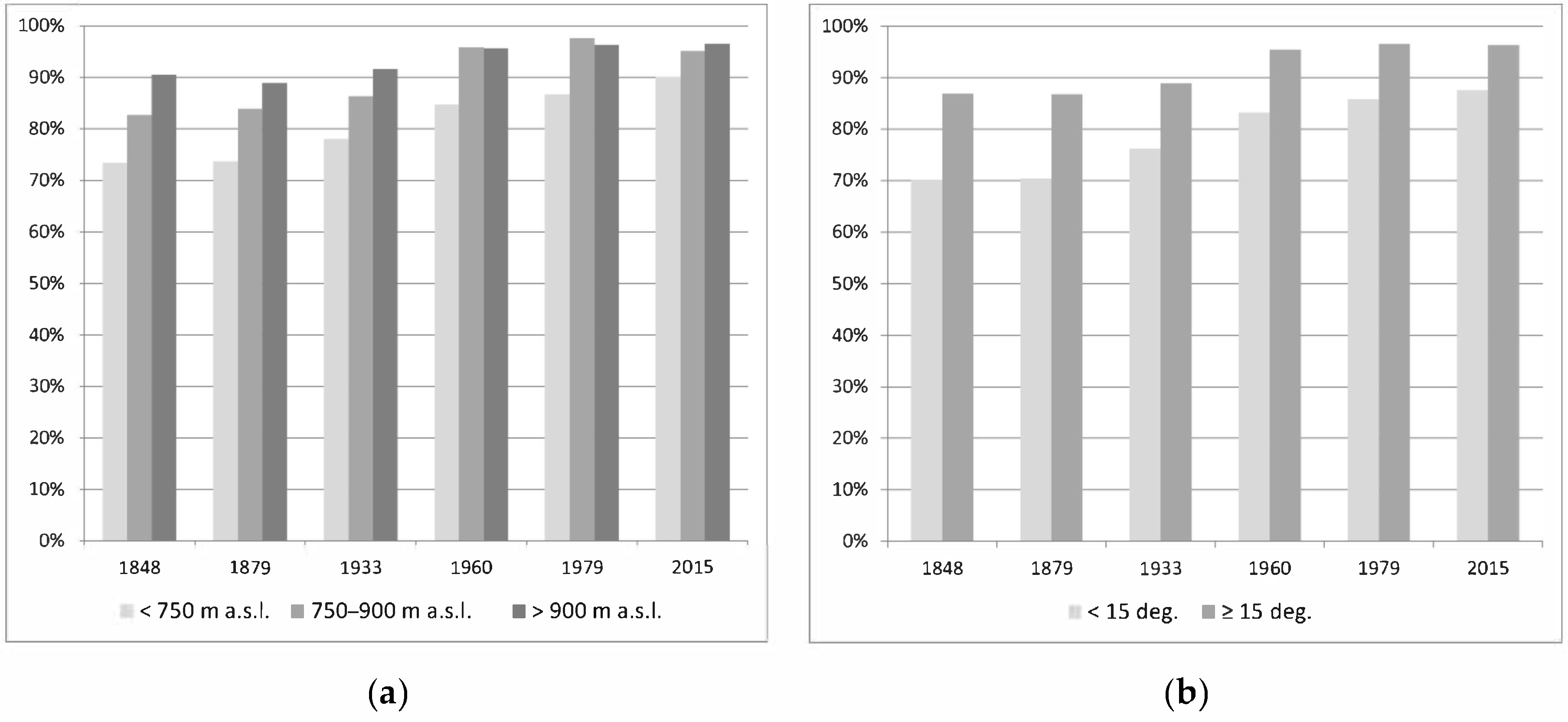
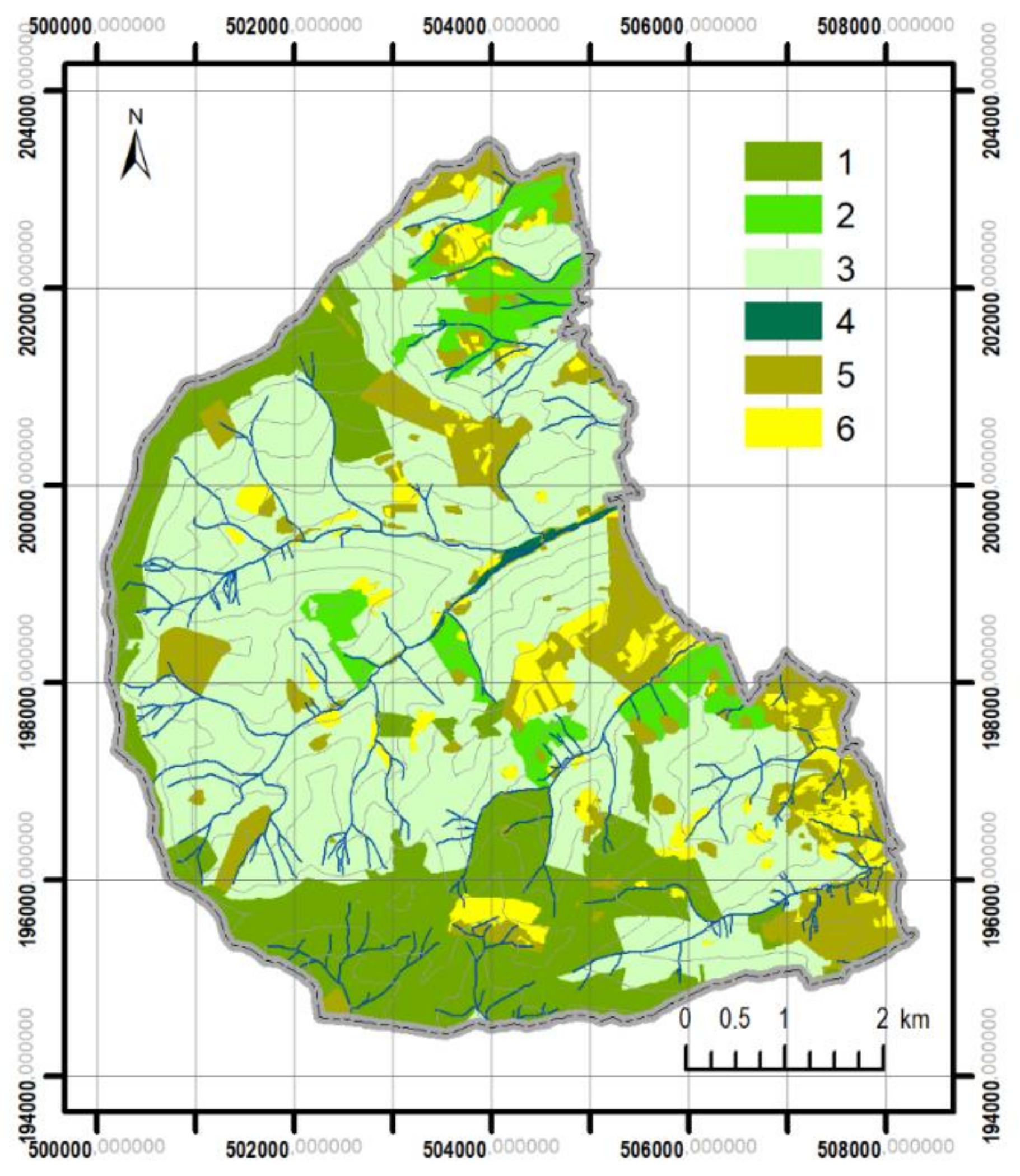
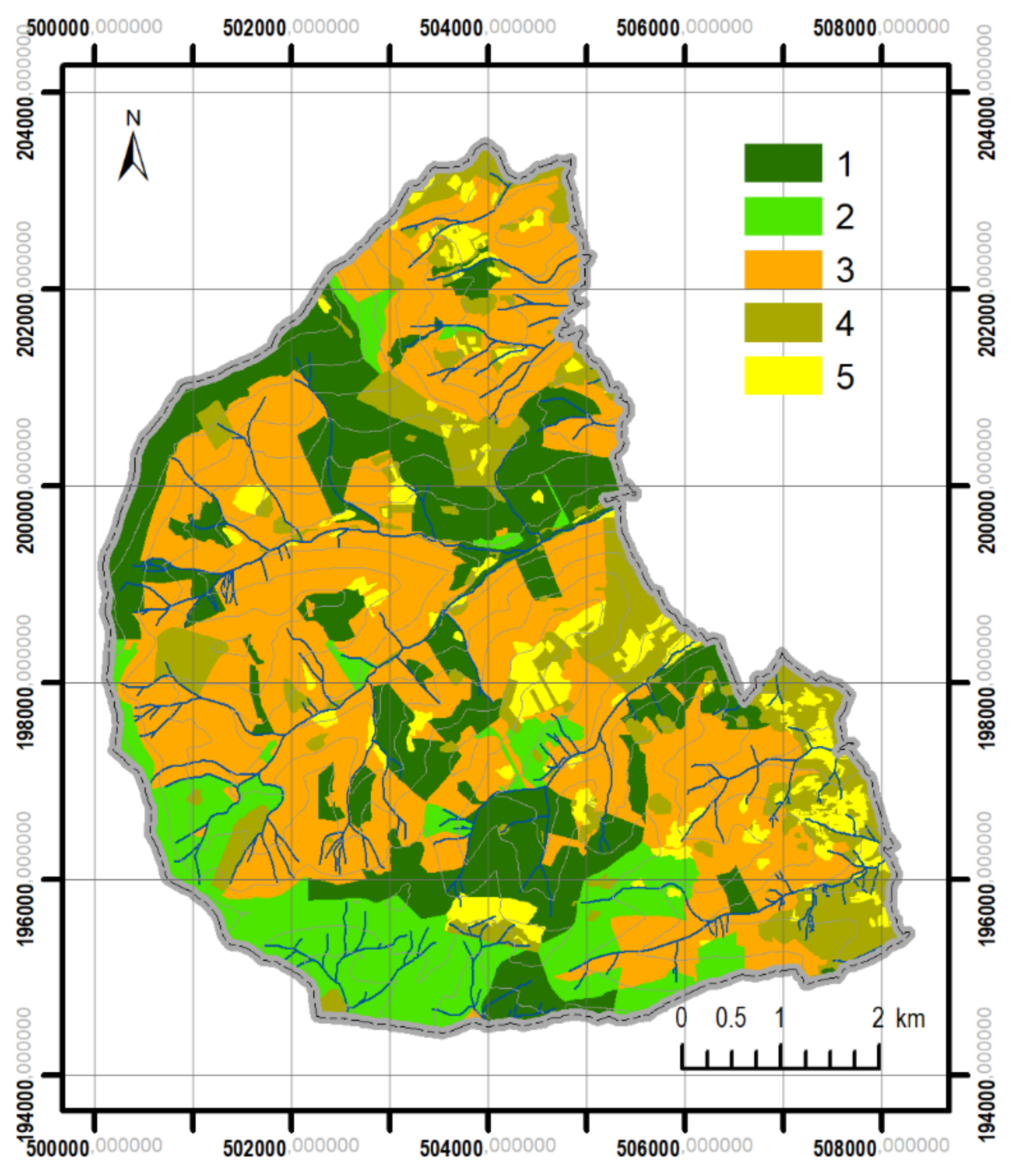
3.1. Changes in Forest Range from 1848 to the Present
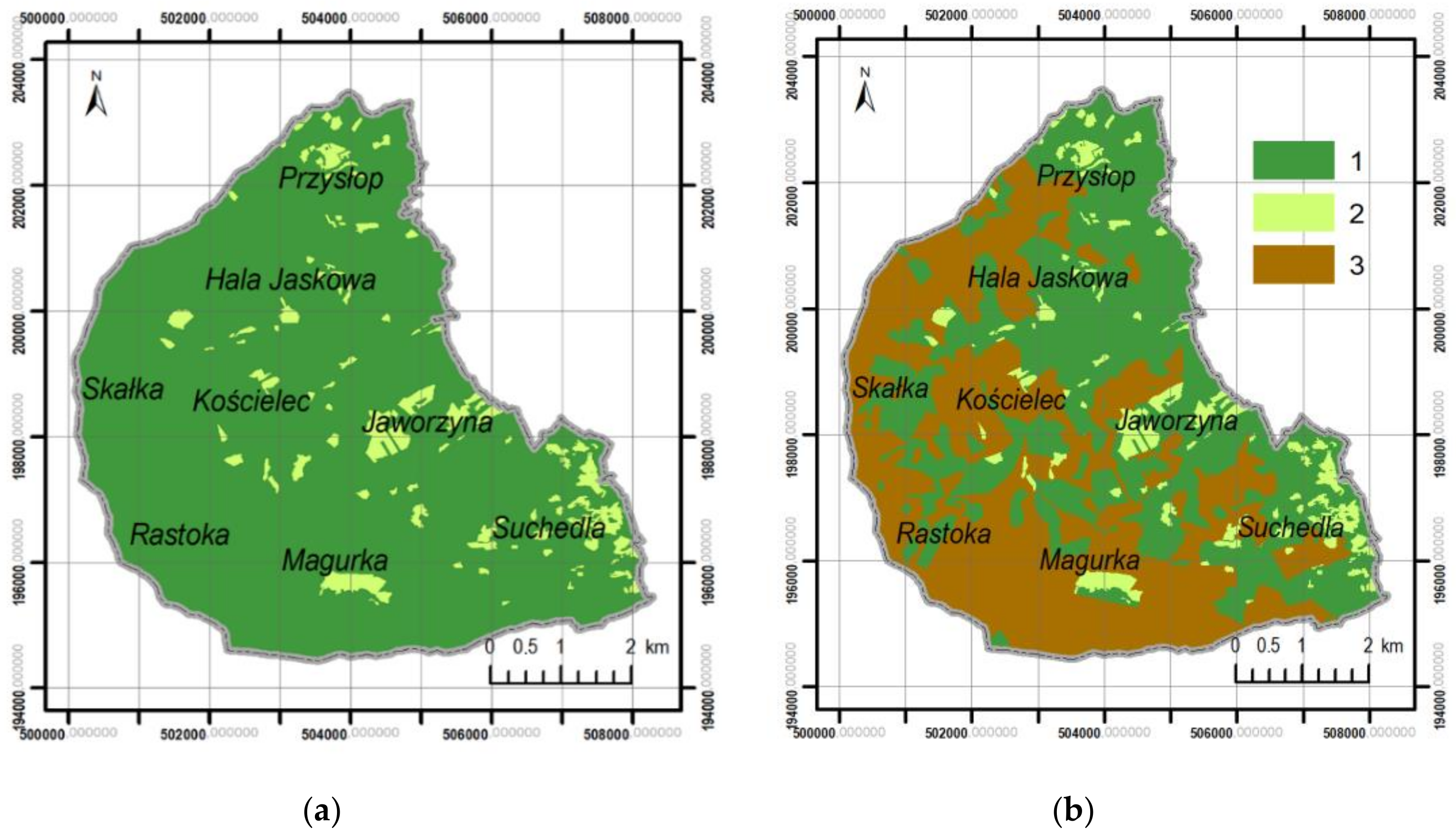
3.2. Differentiation of Contemporary Forest Ecosystems
4. Discussion
5. Conclusions
- From the mid-nineteenth century, the forest range in the Silesian Beskids has been systematically growing, from 82.1 to 93.9%. This is related to the gradual abandonment of the pastoral economy and forest regeneration, despite temporary logging resulting in forest fragmentation.
- Pastoral economy, settlements, and crop-growing pressure from the valleys did not result in a considerable fragmentation of the forests in the Silesian Beskids.
- The lack of cartographic data showing the temporary surfaces of deforestation related to forest management made it impossible to conduct a quantitative analysis of the impact of forest management on forest ecosystems fragmentation in the past. Nevertheless, it can be assumed that the impact of forest management on the condition of forests was greater than that of pastoral economy. This was reflected by changes in the species compositions of forest stands in the last 150 years. The preference for spruce resulted in the replacement of natural ecosystems with spruce monocultures. As a consequence, the massive dying-off of this type of forest was observed and large felling areas were created at the beginning of the twenty-first century covering almost 40% of the study area.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Bank. Global Economic Prospects: Managing Growth in a Volatile World; World Bank: Washington, DC, USA, 2012; Volume 5. [Google Scholar]
- Bendorf, R.; Federici, S.; Forner, C.; Pena, N.; Rametsteiner, E.; Sanz, M.; Somogyi, Z. Including land use, land-use change and forestry in future climate change agreements: Thinking outside the box Environ. Sci. Policy 2007, 10, 283–294. [Google Scholar] [CrossRef]
- Hersperger, A.M.; Bürgi, M. Going beyond landscape change description: Quantifying the importance of driving forces of landscape change in a Central Europe case study. Land Use Policy 2009, 26, 640–648. [Google Scholar] [CrossRef]
- Plieninger, T.; Drauxa, H.; Fagerholma, N.; Bielingc, C.; Bürgi, M.; Kizos, T.; Kuemmerle, T.; Primdahl, J.; Verburg, P.H. The driving forces of landscape change in Europe: A systematic review of the evidence. Land Use Policy 2016, 57, 204–214. [Google Scholar] [CrossRef]
- Sharma, R.; Rimal, B.; Baral, H.; Nehren, U.; Paudyal, K.; Sharma, S.; Rijal, S.; Ranpal, S.; Acharya, R.P.; Alenazy, A.A.; et al. Impact of Land Cover Change on Ecosystem Services in a Tropical Forested Landscape. Resources 2019, 8, 18. [Google Scholar] [CrossRef]
- Kozak, J. Forest cover change in the Western Carpathians in the past 180 years. Mt. Res. Dev. 2003, 23, 369–375. [Google Scholar] [CrossRef]
- Rahmonov, O.; Szczypek, T.; Niedźwiedź, T.; Myga-Piątek, U.; Rahmonov, M.; Snytko, V.A. The human impact on the transformation of juniper forest landscape in the western part of the Pamir-Alay range (Tajikistan). Environ. Earth Sci. 2017, 76, 324. [Google Scholar] [CrossRef]
- Rahmonov, O.; Rahmonov, M.; Opała-Owczarek, M.; Owczarek, P.; Niedźwiedź, T.; Myga-Piątek, U. Ecological and cultural importance of Juniper ecosystem in the area of Zeravshan Valley (Tajikistan) in the background of environmental condition and anthropogenic hazards. Geogr. Pol. 2017, 90, 441–461. [Google Scholar] [CrossRef]
- Sobala, M. Mountain Meadows and Glades of the Carpathians–Type or Element of Landscape? The Problem of Delimitation and Typology of Mountain Pasture Landscapes. Sustainability 2020, 12, 3707. [Google Scholar] [CrossRef]
- Popelková, R.; Mulková, M. Landscape Changes Mapping: Central Part of Ostrava Karviná Mining District, Czech Republic. J. Maps 2011, 7, 363–375. [Google Scholar] [CrossRef]
- Harvey, B.J.; Donato, D.C.; Romme, W.H.; Turner, M.G. Fire severity and tree regeneration following bark beetle outbreaks: The role of outbreak stage and burning conditions. Ecol. Appl. 2014, 24, 1608–1625. [Google Scholar] [CrossRef]
- Kanianska, R.; Kizeková, M.; Nováček, J.; Zeman, M. Land-use and land-cover changes in rural area during different political systems: A case study of Slovakia from 1782 to 2006. Land Use Policy 2014, 36, 554–566. [Google Scholar] [CrossRef]
- Ciupa, T.; Suligowski, R.; Wałek, G. Use of GIS-Supported Comparative Cartography and Historical Maps in Long-Term Forest Cover Changes Analysis in the Holy Cross Mountains (Poland). Balt. For. 2016, 22, 63–73. [Google Scholar]
- Miteva, D.A.; Loucks, C.J.; Pattanayak, S.K. Social and Environmental Impacts of Forest Management Certification in Indonesia. PLoS ONE 2015, 10, e0129675. [Google Scholar] [CrossRef] [PubMed]
- Swenson, J.J.; Carte, C.E.; Domec, J.C.; Delgado, C.I. Gold mining in the Peruvian Amazon: Global prices, deforestation, and mercury imports. PLoS ONE 2011, 6, e18875. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A. Little left to lose: Deforestation and forest degradation in Australia Since European colonization. J. Plant Ecol. 2012, 5, 109–120. [Google Scholar] [CrossRef]
- Sütő, L.; Dobány, Z.; Novák, T.; Incze, J.; Adorján, B.; Rózsa, P. Long-term changes of land use/land cover pattern in human transformed microregions—Case studies from Borsod-Abauj-Zemplén county, North Hungary. Carpath. J. Earth Environ. Sci. 2017, 12, 473–483. [Google Scholar]
- Ifrani, I.; Abby, F.A.; Barkatullah, A.H.; Nurhayati, Y.; Said, M.Y. Forest Management Based on Local Culture of Dayak Kotabaru in the Perspective of Customary Law for a Sustainable Future and Prosperity of the Local Community. Resources 2019, 8, 78. [Google Scholar] [CrossRef]
- Geist, H.J.; Lambin, E.F. Proximate causes and underlying driving forces of tropical deforestation. Bioscience 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Meyfroidt, P.; Lambin, E. Global Forest Transition: Prospects for an End to Deforestation. Annu. Rev. Environ. Resour. 2011, 36, 343–371. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.S.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 80, 570–574. [Google Scholar] [CrossRef]
- Antrop, M. Landscapes at risk: About changes in European Landscape. In Evolution of Geographical Systems and Risk Processes in the Global Context; Dostal, P., Ed.; Charles University: Prague, Czech Republic, 2008; pp. 57–79. [Google Scholar]
- Hansen, M.C.; Stehman, S.V.; Potapov, P.V. Quantification of global gross forest cover loss. Proc. Natl. Acad. Sci. USA 2010, 107, 8650–8655. [Google Scholar] [CrossRef]
- Hall, B.; Motzkin, G.; Foster, D.R.; Syfert, M.; Burk, J. Three hundred years of forest and land-use change in Massachusetts, USA. J. Biogeogr. 2002, 29, 1319–1335. [Google Scholar] [CrossRef]
- Foster, D.R.; Motzkin, G.; Slater, B. Land-Use History as Long-Term Broad-Scale Disturbance: Regional Forest Dynamics in Central New England. Ecosystems 1998, 1, 96–119. [Google Scholar] [CrossRef]
- Grozavu, A.; Marginit, M.; Niculita, C. The dynamics of land use in the middle sector of the Moldova river drainage basin (Eastern Carpathians, Romania). In Conference Abstracts of the 2nd Forum Carpaticum. From Data to Knowledge, from Knowledge to Action; Boltiziar, M., Ed.; Slovak Academy of Sciences: Bratislava, Slovakia, 2012. [Google Scholar]
- Mihai, B.; Savulescu, I.; Sandric, I.; Oprea, R. Application of change detection to the study of vegetation dynamics in the Bucegi Mountains (Southern Carpathians, Romania). Teledetection 2006, 6, 215–231. [Google Scholar]
- Feranec, J.; Ot’ahel’, J. Land cover changes in Slovakia in the period 1970–2000. Geogr. Časopis 2008, 60, 113–128. [Google Scholar]
- Krivosudsky, R. Land use development of south-east slopes of Malé Karpaty mts. In Research and Management of Historical Agricultural Landscape; Dobrovodská, M., Spulerová, J., Stefunková, D., Eds.; Institute of Landscape Ecology, Slovak Academy of Sciences: Bratislava, Slovakia, 2011; pp. 39–49. [Google Scholar]
- Baumann, M.; Kuemmerle, T.; Elbakidze, M.; Ozdogan, M.; Radeloff, V.C.; Keuler, N.S.; Prishchepov, A.V.; Kruhlov, I.; Hostert, P. Patterns and drivers of post-socialist farmland abandonment in Western Ukraine. Land Use Policy 2011, 28, 552–562. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Oloffson, P.; Chaskovsky, O.; Baumann, M.; Ostapowicz, K.; Woodcock, C.E.; Houghton, R.A.; Hostert, P.; Keeton, W.S.; Radeloff, V.C. Post-Soviet farmland abandonment, forest recovery, and carbon sequestration in western Ukraine. Global Chang. Biol. 2011, 17, 1335–1349. [Google Scholar] [CrossRef]
- Konkoly-Gyuró, E.D.; Nagy, P.; Balázs, P.; Király, G. Assessment of land cover change in western Hungarian landscapes. In TransEcoNet Workshop on Landscape History Proceedings; Balázs, P., Konkoly-Gyuró, E., Eds.; University of West Hungary Press: Sopron, Hungary, 2011; pp. 75–89. [Google Scholar]
- Biró, M.; Czúcz, B.; Horváth, F.; Révész, A.; Csatári, B.; Molnár, Z. Drivers of grassland loss in Hungary during the post-socialist transformation (1987–1999). Landsc. Ecol. 2012, 28, 789–803. [Google Scholar] [CrossRef]
- Price, B.; Kaim, D.; Szwagrzyk, M.; Ostapowicz, K.; Kolecka, N.; Schmatz, D.R.; Wypycz, A.; Kozak, J. Legacies, socio-economic and biophysical processes and drivers: The case of future forest cover expansion in the Polish Carpathians and Swiss Alps. Reg. Environ. Chang. 2017, 17, 2279–2291. [Google Scholar] [CrossRef]
- Kozak, J.; Ziółkowska, P.; Vogt, M.; Dobosz, M.; Kaim, D.; Kolecka, N.; Ostafin, K. Forest-Cover Increase Does Not Trigger Forest-Fragmentation Decrease: Case Study from the Polish Carpathians. Sustainability 2018, 10, 1472. [Google Scholar] [CrossRef]
- Sobala, M.; Rahmonov, O.; Myga-Piątek, U. Historical and contemporary forest ecosystem changes in the Beskid Mountains (southern Poland) between 1848 and 2014. iForest Biogeosci. For. 2017, 10, 939–947. [Google Scholar] [CrossRef]
- Ostafin, K. Zmiany Granicy Rolno-Leśnej w Środkowej Część Beskidu Średniego od Połowy XIX w. do 2005 r; Wyd. UJ: Kraków, Poland, 2009. [Google Scholar]
- Affek, A. Past Carpathian landscape recorded in the microtopography. Geogr. Pol. 2016, 89, 415–424. [Google Scholar] [CrossRef]
- Bucała, A. Long-term impact of socio-economic changes on agricultural land use in the Polish Carpathians. Land Use Policy 2017, 64, 391–404. [Google Scholar] [CrossRef]
- Klimo, E.; Hager, H.; Kulhavý, J. Spruce Monocultures in Central Europe—Problems and Prospects; European Forest Institute: Joensuu, Finland, 2000; Volume 33. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; Mcdowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Kandler, O.; Innesb, J.L. Air pollution and forest decline in Central Europe. Environ. Pollut. 1995, 90, 171–180. [Google Scholar] [CrossRef]
- Małek, S.; Belyazid, S.; Sverdrup, H. Modelling changes in forest soil chemistry in the oldest spruce stands in the Potok Dupnianski Catchment in Southern Poland using ForSAFE model. Folia For. Pol. A 2012, 5, 209–214. [Google Scholar] [CrossRef]
- Wiggering, H.C.; Dalchow, M.; Glemnitz, K.; Helming, K.; Muller, A.; Schultz, U.; Stachow, U.; Zander, P. Indicators for multifunctional land use—Linking socio-economic requirements with landscape potentials. Ecol. Indic. 2006, 6, 238–249. [Google Scholar] [CrossRef]
- Pinto-Correia, T.; Kristensen, L. Linking research to practice: The landscape as the basis for integrating social and ecological perspectives of the rural. Landsc. Urban Plan. 2013, 120, 248–256. [Google Scholar] [CrossRef]
- Griffiths, P.; Kuemmerle, T.; Baumann, M.; Radeloff, V.C.; Abrudan, I.V.; Lieskovsky, J.; Munteanu, C.; Ostapowicz, K.; Hostert, P. Forest disturbances, forest recovery, and changes in forest types across the Carpathian ecoregion from 1985 to 2010 based on Landsat image composites. Remote Sens. Environ. 2014, 151, 72–88. [Google Scholar] [CrossRef]
- Munteanu, C.; Kuemmerle, T.; Boltiziar, M.; Butsic, V.; Gimmig, U.; Halada, L.; Kaim, D.; Kiraly, G.; Konkoly-Gyuro, E.; Kozak, J.; et al. Forest and agricultural land change in the Carpathian region—A meta-analysis of long-term patterns and drivers of change. Land Use Policy 2014, 38, 685–697. [Google Scholar] [CrossRef]
- Boltiziar, I.; Olah, B.; Gallay, I.; Gallayova, Z. Transformation of the Slovak cultural landscape and its recent trends. In Landscape and Landscape Ecology, Proceedings of the 17th International Symposium on Landscape and Landscape Ecology, Nitra, Slovakia, 27–29 May 2015; Hlada, L., Baca, A., Boltiziar, M., Eds.; Institute of Landscape Ecology, Slovak Academy of Sciences: Bratislava, Slovakia, 2016; pp. 57–67. [Google Scholar]
- Plit, J. Przeobrażenia krajobrazów kulturowych Karpat Polskich dawniej i dziś. Prace Kom. Kraj. Kult. PTG 2004, 3, 33–42. [Google Scholar]
- Kubijowicz, W. Życie Pasterskie w Beskidach Magórskich; Nakł. Polskiej Akademji Umiejętności: Kraków, Poland, 1927. [Google Scholar]
- Kawecki, W. Lasy Żywiecczyzny a pasterstwo. Sylwan 1936, 54, 49–55. [Google Scholar]
- Sobala, M. Zastosowanie austriackich map katastralnych w badaniach użytkowania ziemi w połowie XIX wieku. Pol. Przegląd Kartogr. 2012, 44, 324–333. [Google Scholar]
- Affek, A. Georeferencing of historical maps using GIS, as exemplified by the Austrian Military Surveys of Galicia. Geogr. Pol. 2013, 86, 375–390. [Google Scholar] [CrossRef]
- Kaim, D.; Kozak, J.; Ostafin, K.; Dobosz, M.; Ostapowicz, K.; Kolecka, N.; Gimmi, U. Uncertainty in historical land-use recontructions with topographic maps. Quest. Geogr. 2014, 33, 55–63. [Google Scholar] [CrossRef]
- Maras, S.S.; Maras, H.H.; Aktug, B.; Maras, E.E.; Yildiz, F. Typological error correction of GIS vector data. Int. J. Phys. Sci. 2010, 5, 476–483. [Google Scholar]
- Temple, S. Predicting impacts of habitat fragmentation on forest birds: A comparison of two models. In Wildlife 2000: Modeling Habitat Relationships of Terrestrial Vertebrates; Verner, J., Morrison, M., Ralph, C.J., Eds.; University of Wisconsin Press: Madison, WI, USA, 1986. [Google Scholar]
- Murcia, C. Edge effects in fragmented forests: Implication for conservation. Tree 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Harper, K.A.; MacDonald, E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Essen, P. Edge Influence on Forest Structure and Composition in Fragmented Landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Saunders, D.; Hobbs, R.; Margules, C. Biological Consequences of Ecosystem Fragmentation: A Review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Święcicki, Z. Instrukcja Urządzania Lasu cz. 2. Instrukcja Wyróżniania i Kartowania w Lasach Państwowych Typów Siedliskowych Lasu Oraz Zbiorowisk Roślinnych; CILP: Warszawa, Poland, 2012. [Google Scholar]
- Braun-Blanquet, J. Pflansensoziologie; Springer: Vienna, Austria, 1964. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warszawa, Poland, 2003. [Google Scholar]
- Sobala, M. Pasture landscape durability in the Beskid Mountains (Western Carpathians, Poland). Geogr. Pol. 2018, 91, 197–215. [Google Scholar] [CrossRef]
- Bičk, I.; Kupková, L.; Jeleček, L.; Kabrda, J.; Štych, P.; Janoušek, Z.; Winklerová, J. Land Use Changes in the Czech Republic 1845–2010. Socio-Economic Driving Forces; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Kupková, L.; Bičík, I. Landscape transition after the collapse of communism in Czechia. J. Maps 2016, 12, 526–531. [Google Scholar] [CrossRef]
- Dezső, Z.; Bartholy, J.; Pongrácz, R.; Barcza, Z. Analysis of land-use/land-cover change in the Carpathian region based on remote sensing techniques. Phys. Chem. Earth 2005, 30, 109–115. [Google Scholar] [CrossRef]
- Bradshaw, R.; Mitchell, F.J.G. The palaeoecological approach to reconstructing former grazing-vegetation interactions. For. Ecol. Manag. 1999, 120, 3–12. [Google Scholar] [CrossRef]
- Pieńkowski, P. Ocena fragmentacji lasów Pomorza Zachodniego pomiędzy XV a XX w. Sylwan 2015, 159, 610–616. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Wilczek, Z. Zespoły Leśne Beskidu Śląskiego i Zachodniej Części Beskidu Żywieckiego na tle Zbiorowisk Leśnych Karpat Zachodnich; Wyd. UŚ: Katowice, Poland, 1995. [Google Scholar]
- Wilczek, Z. 2006. Fitosocjologiczne Uwarunkowania Ochrony Przyrody Beskidu Śląskiego (Karpaty Zachodnie); Wyd. UŚ: Katowice, Poland, 2006. [Google Scholar]
- Cebecauer, T.; Hoflerka, J. The consequences of land-cover changes on soil erosion distribution in Slovakia. Geomorphology 2008, 98, 187–198. [Google Scholar] [CrossRef]
- Skokanová, H.; Havlíček, M.; Borovec, R.; Demek, J.; Eremiášová, R.; Chrudina, Z.; Mackovčin, P.; Rysková, R.; Slavík, P.; Stránská, T.; et al. Development of land use and main land use change processes in the period 1836–2006: Case study in the Czech Republic. J. Maps 2012, 8, 88–96. [Google Scholar] [CrossRef]
- Forejt, M.; Skalos, J.; Pereponova, A.; Plieninger, T.; Vojta, J.; Šantrůčková, M. Changes and continuity of wood-pastures in the lowland landscape in Czechia. Appl. Geogr. 2017, 79, 235–244. [Google Scholar] [CrossRef]
- Stutzer, A. Early stages of podzolisation in young aeolian sediments, western Jutland. Catena 1998, 32, 115–129. [Google Scholar] [CrossRef]
- Augusto, L.; Turpault, M.P.; Ranger, J. Impact of tree species on feldspar weathering rates. Geoderma 2000, 96, 215–237. [Google Scholar] [CrossRef]
- Maciaszek, W.; Gruba, P.; Januszek, K.; Lasota, J.; Wanic, T.; Zwydak, M. Soil Degradation and Redegradation under the Influence of Forest Management in the Żywiec Region; Wyd. AR: Kraków, Poland, 2000. [Google Scholar]
- Rozpendowska, E.; Skiba, S. Influence of the habitat incompatible spruce vegetation on soils in Carpathians. Rocz. Bieszcz. 2006, 14, 237–245. [Google Scholar]
- Spieckier, H. Growth of Norway spruce under changing environmental conditions in Europe. In Spruce Monocultures in Central Europe—Problems and Prospects; Klimo, E., Hager, H., Kulhavý, J., Eds.; European Forest Institute: Joensuu, Finland, 2000; Volume 33. [Google Scholar]
- Pechanec, V.; Machar, I.; Sterbova, L.; Prokopova, M.; Kilianova, H.; Chobot, K.; Cudlin, P. Monetary Valuation of Natural Forest Habitats in Protected Areas. Forests 2017, 8, 427. [Google Scholar] [CrossRef]
- Małek, S.; Barszcz, J.; Majsterkiewicz, K. Changes in the threat of spruce stand disintegration in the Beskid Śląski and Żywiecki Mts in the years 2007–2010. J. For. Sci. 2012, 58, 519–529. [Google Scholar] [CrossRef]
- Čada, V.; Morrissey, R.; Michalová, Z.; Bače, R.; Janda, P.; Svoboda, M. Frequent severe natural disturbances and non-equilibrium landscape dynamics shaped the mountain spruce forest in central Europe. For. Ecol. Manag. 2016, 363, 169–178. [Google Scholar] [CrossRef]
- Janda, P.; Trotsiuka, V.; Mikolá, M.; Bačea, R.; Nagelac, T.A.; Seidld, R.; Seedrea, M.; Morrissey, R.C.; Kucbel, S.; Jaloviar, P.; et al. The historical disturbance regime of mountain Norway spruce forests in the Western Carpathians and its influence on current forest structure and composition. Ecol. Manag. 2017, 388, 67–78. [Google Scholar] [CrossRef]
- Sobala, M. Rola tworzenia wielkoobszarowych zrębów zupełnych w zmianie struktury krajobrazu wschodniej części Beskidu Śląskiego. Z Bad. Wpływem Antropopresji Śr. 2012, 13, 71–80. [Google Scholar]
- Renčo, M.; Čerevková, A. Windstorms as mediator of soil nematode community changes: Evidence from European spruce forest. Helminthologia 2017, 54, 36–47. [Google Scholar] [CrossRef][Green Version]
- Mezei, P.; Blaženec, M.; Grodzki, W.; Škvarenina, J.; Jakuš, R. Influence of different forest protection strategies on spruce tree mortality during a bark beetle outbreak. Ann. For. Sci. 2017, 74, 1–10. [Google Scholar] [CrossRef]
- Matic, S.; Anic, I.; Barievic, D. The Possibility of Converting Spruce Monocultures into Autochthonous Stands in Croatia. In Spruce Monocultures in Central Europe—Problems and Prospects; Klimo, E., Hager, H., Kulhavý, J., Eds.; European Forest Institute: Joensuu, Finland, 2000; Volume 33. [Google Scholar]
- Machar, I.; Vozenilek, V.; Simon, J.; Pechanec, V.; Brus, J.; Fulnecek, P.; Vitek, T. Joining of the historical research and future prediction as a support tool for the assessment of management strategy for European beech-dominated forests in protected areas. Nat. Conserv. 2017, 22, 51–78. [Google Scholar] [CrossRef]
- Shifley, S.R.; Moser, W.K.; Nowak, D.J.; Miles, P.D.; Butler, B.J.; Aguilar, F.X.; DeSantis, R.D.; Greenfield, E.J. Five Anthropogenic Factors That Will Radically Alter Forest Conditions and Management Needs in the Northern United States. For. Sci. 2014, 60, 914–925. [Google Scholar] [CrossRef]
- Szwagrzyk, J. Natural regeneration of forest related to the spatial structure of trees: A study of two forest communities in Western Carpathians, southern Poland. Vegetatio 1990, 89, 11–22. [Google Scholar] [CrossRef]
- Jonášová, M.; Vávrová, E.; Cudlín, P. Western Carpathian mountain spruce forest after a windthrow: Natural regeneration in cleared and uncleared areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar] [CrossRef]
- Michalik, S.; Skiba, S. Assessment of the relationship between soil cover and vegetation in the Bieszczady National Park. Rocz. Bieszcz. 1995, 4, 85–95. [Google Scholar]
- Holeksa, J. Breakdown of tree stand and spruce regeneration versus structure and dynamics of a Carpathian subalpine spruce forest. Monogr. Bot. 1998, 82, 1–209. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Socha, J.; Durło, G. How will climate change impact biomass increment by Norway spruce stands in Western Beskids? Folia For. Pol. 2012, 54, 94–108. [Google Scholar]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; Peters, M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For. Ecol. Manag. 2008, 254, 390–406. [Google Scholar] [CrossRef]
- Wear, D.N.; Huggett, R.; Li, R.; Perryman, B.; Liu, S. Forecasts of Forest Conditions in Regions of the United States under Future Scenarios: A Technical Document Supporting the Forest Service 2012 RPA Assessment; USDA For Serv., Gen. Tech. Rep. SRS-170; Southern Research Station: Asheville, NC, USA, 2013. [Google Scholar]
- Chitale, V.; Silwal, R.; Matin, M. Assessing the Impacts of Climate Change on Distribution of Major Non-Timber Forest Plants in Chitwan Annapurna Landscape, Nepal. Resources 2018, 7, 66. [Google Scholar] [CrossRef]
- Rahmonov, O.; Holbegov, M.; Szczypek, T.; Snytko, V.A.; Klys, G.; Rahmonov, M. The consequences of vegetation degradation under the influence of anthropogenic activity in the territory of the Zarafshan Range (Western Tajikistan). Geogr. Nat. Resour. 2014, 35, 193–197. [Google Scholar] [CrossRef]
- Rahmonov, O.; Rahmonov, M.; Snytko, V.A.; Szczypek, T. Anthropogenic disturbance to vegetation on the polygon-transect in the Kulikalon depression (Tajikistan). Geogr. Nat. Resour. 2011, 32, 386–393. [Google Scholar] [CrossRef]
- Frelich, L.E.; Reich, P.B. Will environmental changes reinforce the impact of global warming on the prairie–forest border of central North America? Front. Ecol. Environ. 2010, 8, 371–378. [Google Scholar] [CrossRef]
- Grodzki, W. Spatio-temporal patterns of the Norway spruce decline in the Beskid Śląski and Żywiecki (Western Carpathians) in southern Poland. J. For. Sci. 2007, 53, 38–44. [Google Scholar] [CrossRef]
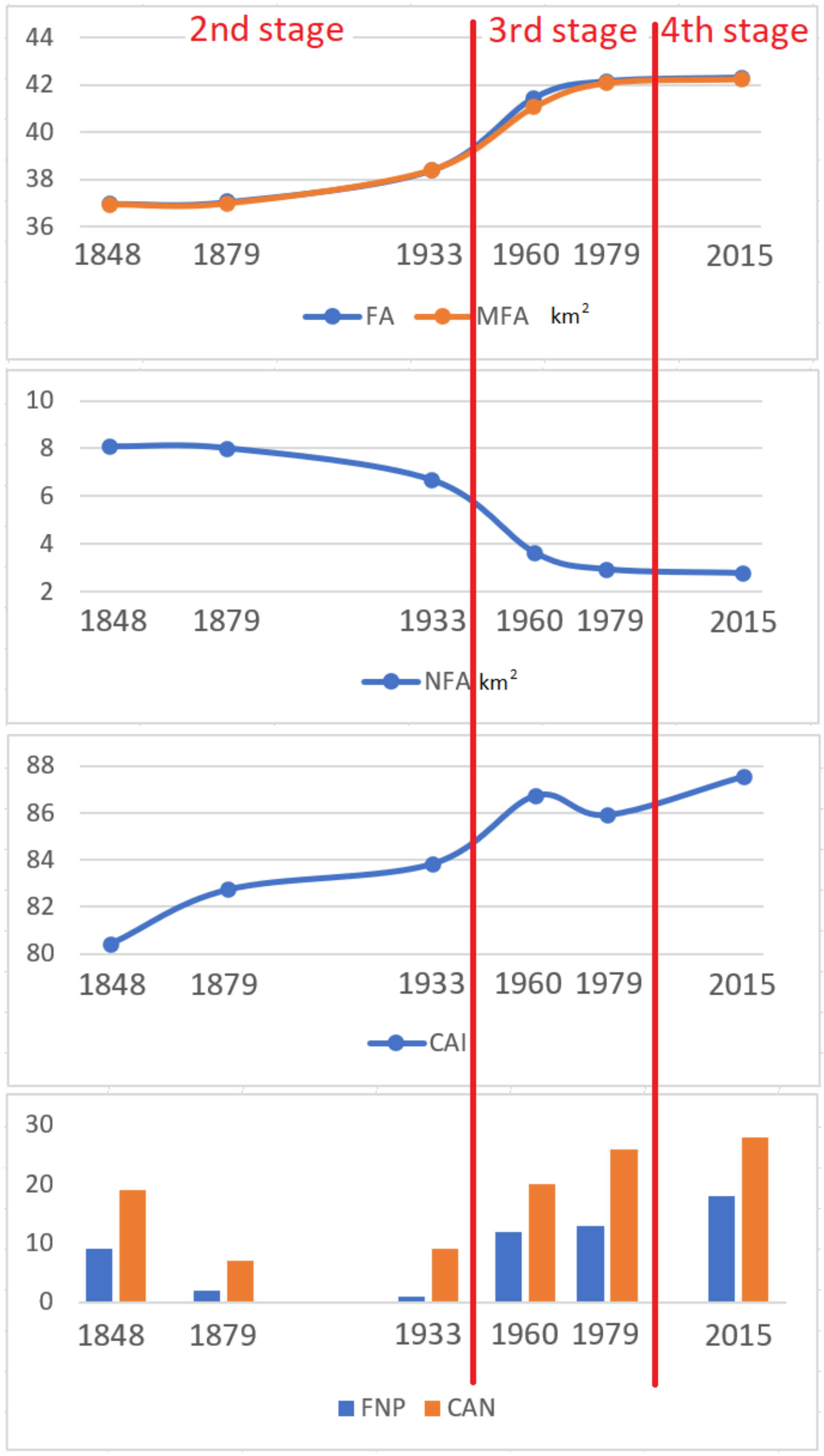
| Metric | 1848 | 1879 | 1933 | 1960 | 1979 | 2015 |
|---|---|---|---|---|---|---|
| FA (km2) | 36.99 | 37.07 | 38.41 | 41.45 | 42.14 | 42.30 |
| NFA (km2) | 8.07 | 7.99 | 6.65 | 3.61 | 2.92 | 2.76 |
| CAI | 80.41 | 82.73 | 83.83 | 86.73 | 85.91 | 87.55 |
| FNP | 9 | 2 | 1 | 12 | 13 | 18 |
| CAN | 19 | 7 | 9 | 20 | 26 | 28 |
| MFA (km2) | 36.94 | 36.99 | 38.41 | 41.08 | 42.09 | 42.26 |
| Metric | Forest Areas Excluding Felling Area (Forest Land) | Forest Areas Including Felling Area (Forests) |
|---|---|---|
| FP (%) | 93.9 | 57.7 |
| IBP (%) | 11.5 | 19.3 |
| FCP (%) | 82.3 | 38.4 |
| NFP (%) | 6.1 | 42.4 |
| CAI | 87.55 | 63.90 |
| FNP | 18 | 114 |
| CAN | 28 | 225 |
| MFA (km2) | 42.26 | 25.50 |
| Site Type | Area | Area of Sites in Different Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| (ha) | (%) | (ha) | (%) | |||||
| N1 | N2 | A | N1 | N2 | A | |||
| MCF | 968.2 | 26.6 | 439.9 | 458.7 | 15.6 | 51.0 | 47.4 | 1.6 |
| MBF | 265.0 | 7.3 | 119.2 | 39.3 | 106.5 | 45.0 | 14.8 | 40.2 |
| MMF | 2378.3 | 65.3 | 397.6 | 125.9 | 1854.8 | 16.7 | 5.3 | 78.0 |
| MRF | 12.9 | 0.4 | 12.9 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| Total | 3624.4 | 100.0 | 1023.5 | 623.9 | 1976.9 | 28.2 | 17.2 | 54.6 |
| Feature | Percentage of the Clear-Cutting Areas |
|---|---|
| Site Type | |
| Mountain coniferous forests on fresh sites | 87.5 |
| Mountain broadleaf forests in fresh sites | 8.1 |
| Mixed mountain forests on fresh sites | 44.3 |
| Mountain riparian forests | 0.0 |
| Site Condition | |
| Natural | 47.9 |
| Close-to-nature | 84.0 |
| Anthropogenically transformed | 45.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobala, M.; Rahmonov, O. The Human Impact on Changes in the Forest Range of the Silesian Beskids (Western Carpathians). Resources 2020, 9, 141. https://doi.org/10.3390/resources9120141
Sobala M, Rahmonov O. The Human Impact on Changes in the Forest Range of the Silesian Beskids (Western Carpathians). Resources. 2020; 9(12):141. https://doi.org/10.3390/resources9120141
Chicago/Turabian StyleSobala, Michał, and Oimahmad Rahmonov. 2020. "The Human Impact on Changes in the Forest Range of the Silesian Beskids (Western Carpathians)" Resources 9, no. 12: 141. https://doi.org/10.3390/resources9120141
APA StyleSobala, M., & Rahmonov, O. (2020). The Human Impact on Changes in the Forest Range of the Silesian Beskids (Western Carpathians). Resources, 9(12), 141. https://doi.org/10.3390/resources9120141





