Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy
Abstract
1. Introduction
2. Materials and Methods
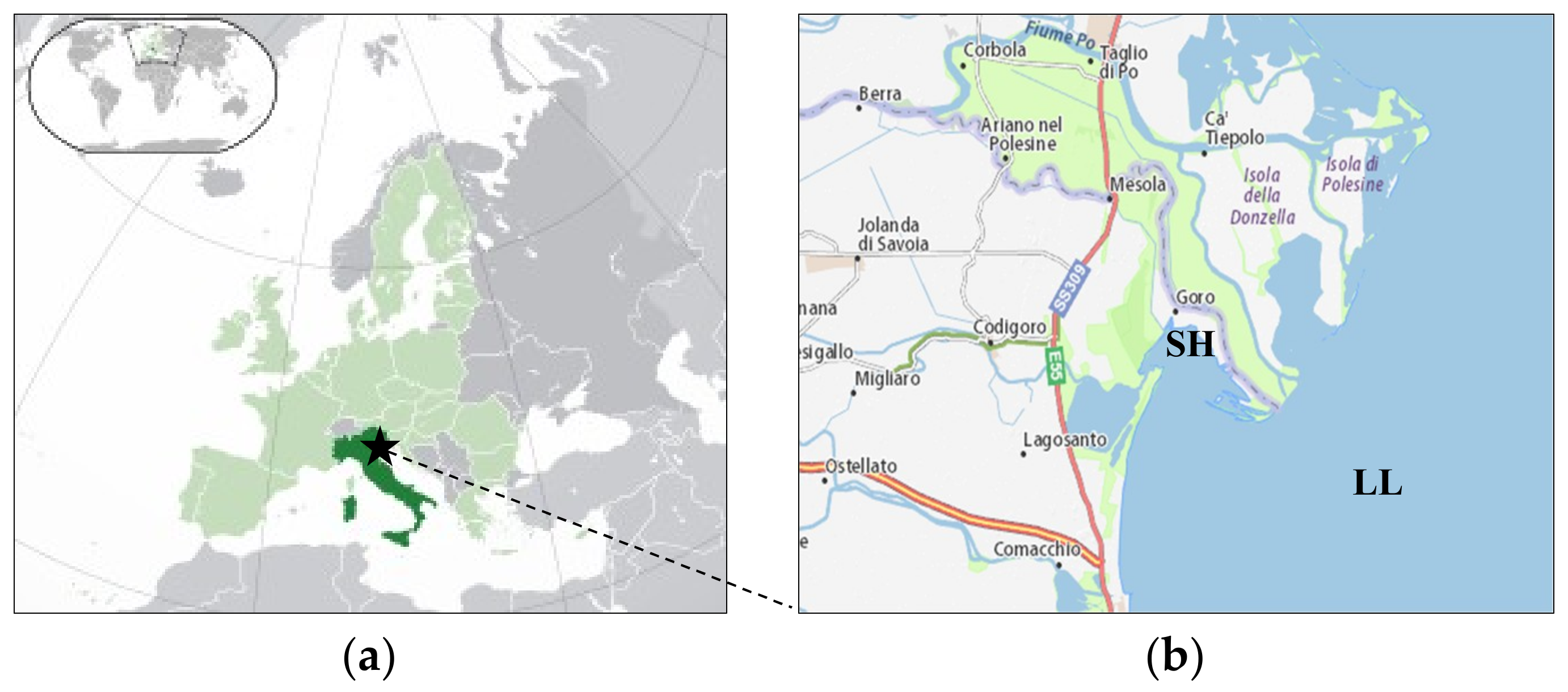
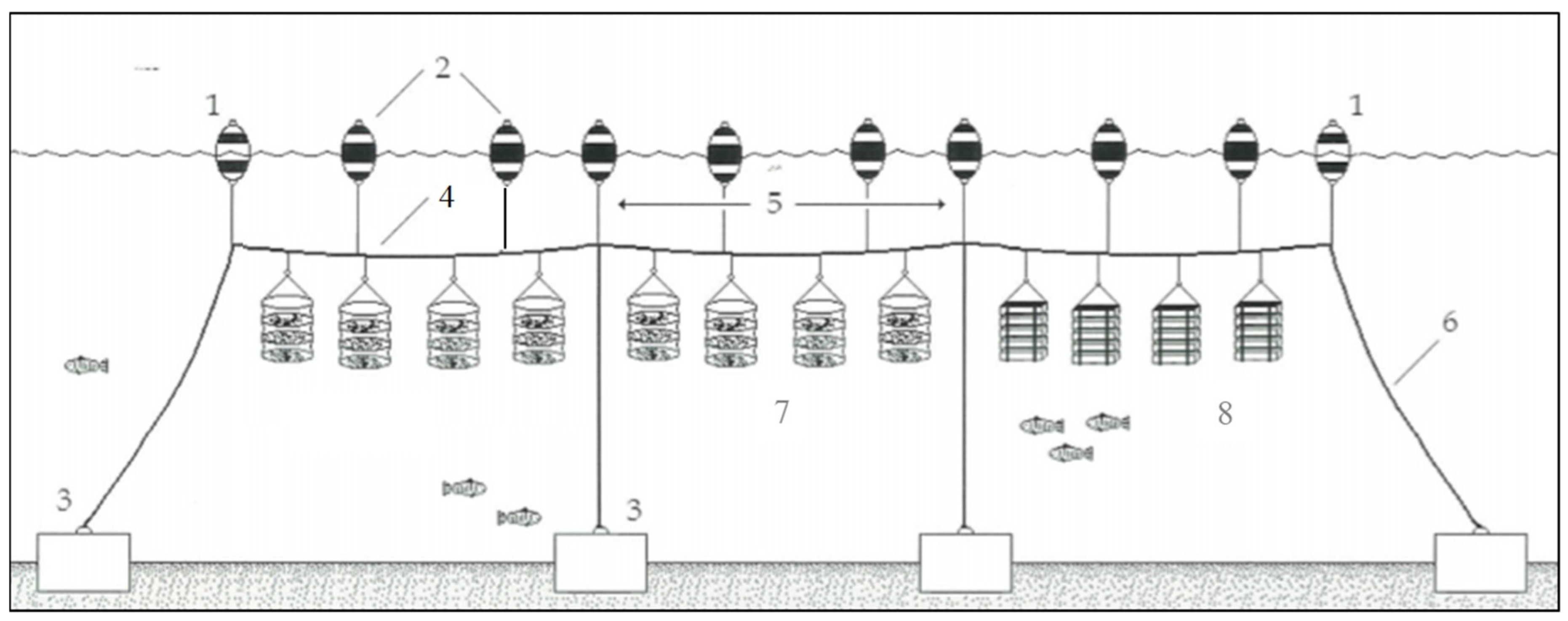
2.1. Description of the Case Study
2.2. The LCA Framework
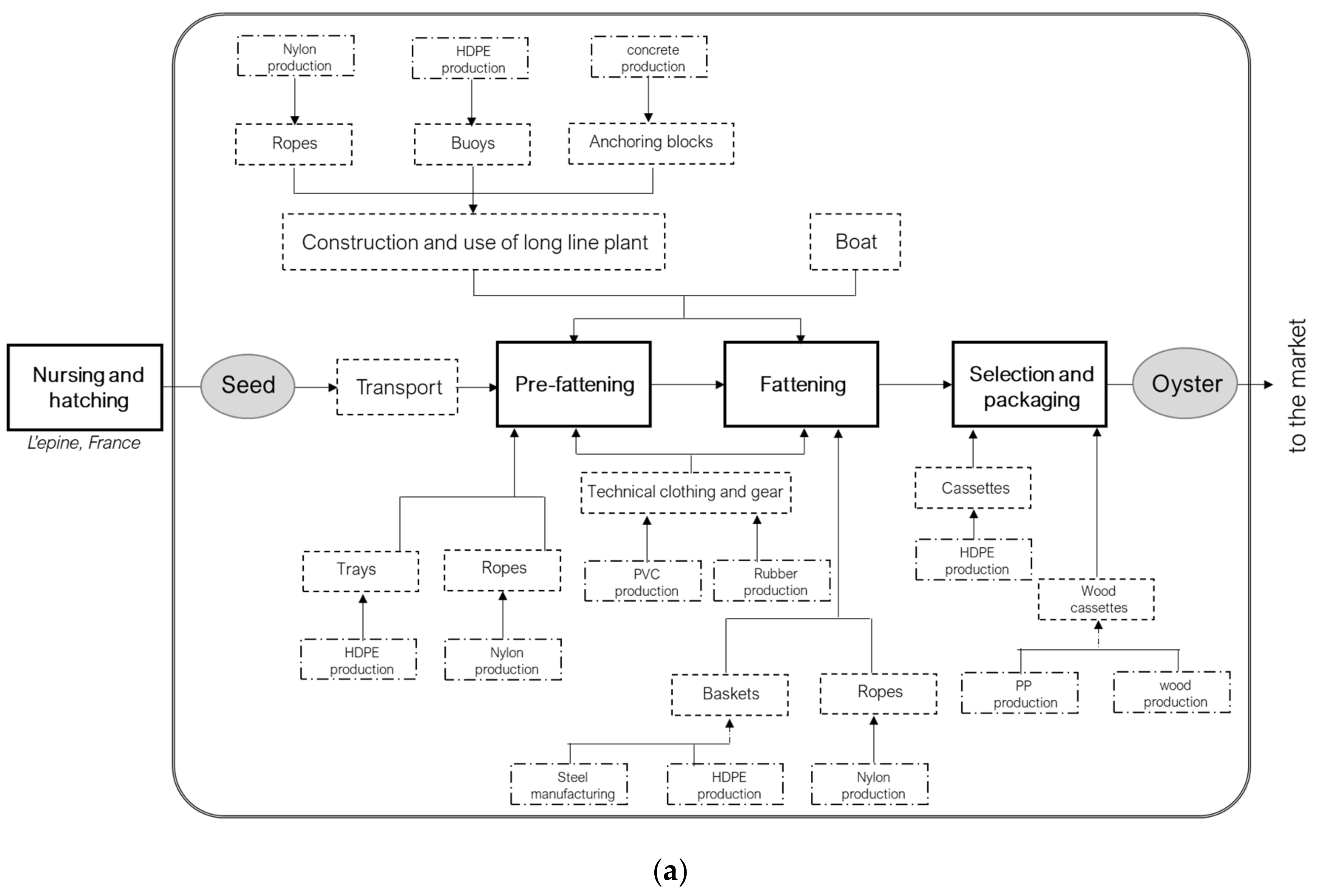
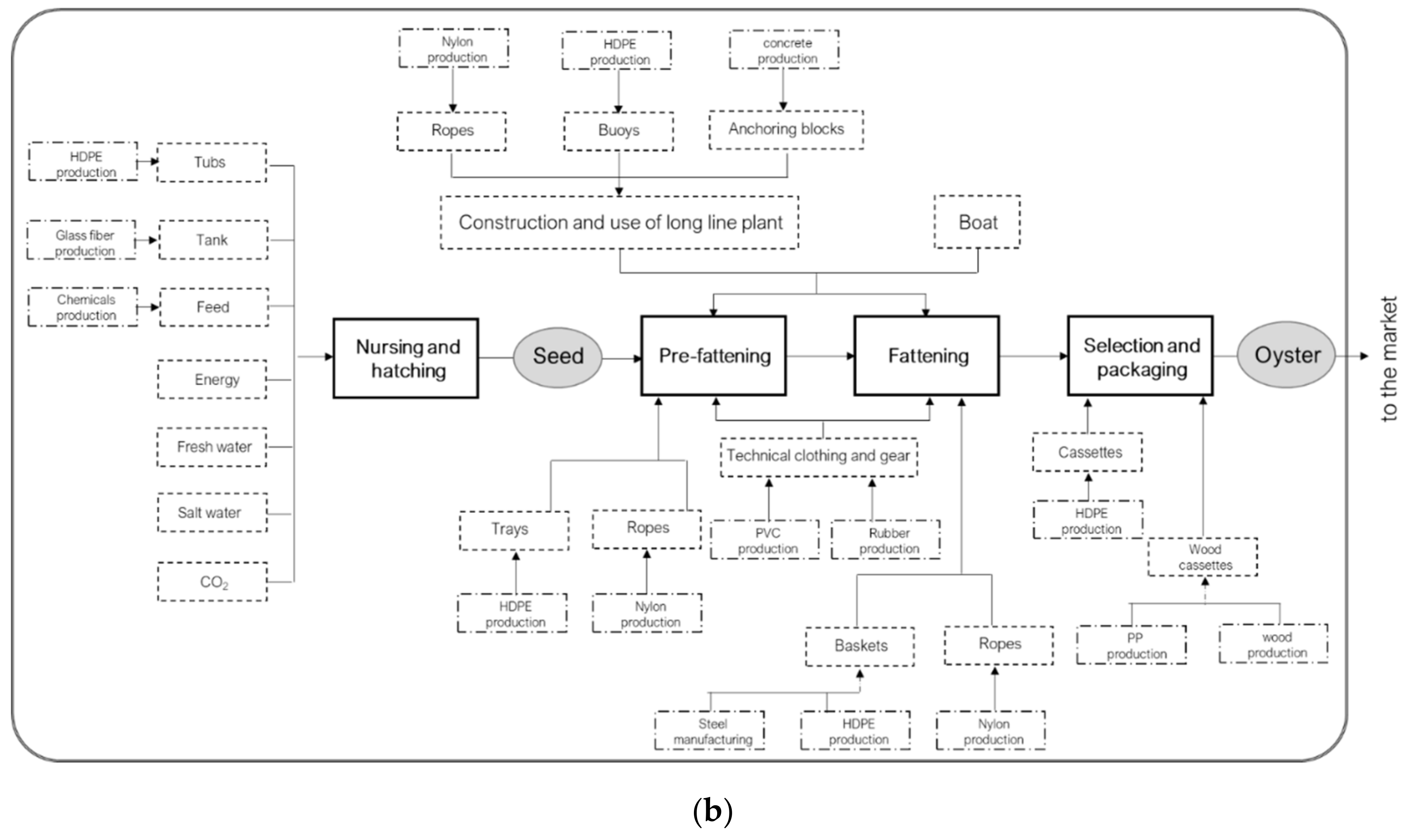
2.2.1. Goal and Scope Definition
- -
- Identification of the main hotspots existing under the current farming conditions described above; and
- -
- Comparison of the current scenario of oyster seed supply from France to an alternative in situ hatching.
2.2.2. Life Cycle Inventory
2.2.3. Life Cycle Inventory Assessment (LCIA)
2.2.4. Uncertainty Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brooks, J.; Blandford, D. Chapter 2: Policies for Global Food Security. In Global Challenges for Future Food and Agricultural Policies, 1st ed.; Blandford, D., Hassapoyannes, K., Eds.; World Scientific Publishing Co Pte Ltd.: Singapore, 2019; Volume 1, pp. 5–38. [Google Scholar]
- Duarte, C.M.; Holmer, M.; Olsen, Y.; Soto, D.; Marbà, N.; Guiu, J.; Karakassis, I. Will the oceans help feed humanity? BioScience 2009, 59, 967–976. [Google Scholar] [CrossRef]
- Godfray, H.C.J. The debate over sustainable intensification. Food Secur. 2015, 7, 199–208. [Google Scholar] [CrossRef]
- Bringezu, S.; Ramaswami, A.; Schandl, H.; O’Brien, M.; Pelton, R.; Acquatella, J.; Ayuk, E.; Chiu, A.; Flanegin, R.; Fry, J.; et al. Assessing Global Resource Use: A Systems Approach to Resource Efficiency and Pollution Reduction; A Report of the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2017. [Google Scholar]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.J. Reducing water use for animal production through aquaculture. J. Water Resour. Dev. 2006, 22, 101–113. [Google Scholar] [CrossRef]
- Marra, J. When will we tame the oceans? Nature 2005, 436, 175–176. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; Available online: http://www.fao.org/3/i9540en/i9540en.pdf (accessed on 10 August 2019).
- Ding, Q.; Chen, X.; Chen, Y.; Tian, S. Estimation of catch losses resulting from overexploitation in the global marine fisheries. Acta Oceanol. Sin. 2017, 36, 37–44. [Google Scholar] [CrossRef]
- Hilborn, R.; Costello, C. The potential for blue growth in marine fish yield, profit and abundance of fish in the ocean. Mar. Policy 2018, 87, 350–355. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Nash, K.L.; Halpern, B.S.; Remenyi, T.A.; Corney, S.P.; Fleming, A.; Blanchard, J.L. Food production shocks across land and sea. Nat. Sustain. 2019, 2, 130–137. [Google Scholar] [CrossRef]
- Morris, J.P.; Humphreys, M.P. Modelling seawater carbonate chemistry in shellfish aquaculture regions: Insights into CO2 release associated with shell formation and growth. Aquaculture 2019, 501, 338–344. [Google Scholar] [CrossRef]
- EEA. Aquaculture Production. 2019. Available online: https://www.eea.europa.eu/data-and-maps/indicators/ aquaculture-production-4/assessment (accessed on 23 July 2019).
- Bostock, J.; Lane, A.; Hough, C.; Yamamoto, K. An assessment of the economic contribution of EU aquaculture production and the influence of policies for its sustainable development. Aquac. Int. 2016, 24, 699–733. [Google Scholar] [CrossRef]
- Feinman, S.G.; Farah, Y.R.; Bauer, J.M.; Bowen, J.L. The influence of oyster farming on sediment bacterial communities. Estuaries Coasts 2018, 41, 800–814. [Google Scholar] [CrossRef]
- Khan, B.M.; Liu, Y. Marine Mollusks: Food with Benefits. CRFSFS 2019, 18, 548–564. [Google Scholar] [CrossRef]
- Lane, A.; Chatain, B.; Roque D’Orbacastel, E. Aquaculture in Occitanie, France. World Aquac. 2018, 49, 12–17. [Google Scholar]
- Marzano, A. Harvesting the Sea: The Exploitation of Marine Resources in the Roman Mediterranean; Oxford University Press: Oxford, UK, 2013; pp. 173–195. [Google Scholar]
- Oyster Trade, Product Trade, Exporters and Importers-OEC. Available online: https://oec.world/en/profile/hs92/030710/ (accessed on 24 July 2019).
- Orban, E.; Di Lena, G.; Masci, M.; Nevigato, T.; Casini, I.; Caproni, R.; Pellizzato, M. Growth, nutritional quality and safety of oysters (Crassostrea gigas) cultured in the lagoon of Venice (Italy). J. Sci. Food Agric. 2004, 84, 1929–1938. [Google Scholar] [CrossRef]
- Henriksson, P.J.; Guinée, J.B.; Kleijn, R.; de Snoo, G.R. Life cycle assessment of aquaculture systems—A review of methodologies. Int. J. Life Cycle Assess. 2012, 17, 304–313. [Google Scholar] [CrossRef]
- ISO 14040. Environmental Management—Life Cycle Assessment—Principles and Framework International Organization for Standardization, Geneva, Switzerland. 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 24 July 2019).
- De Luca, A.I.; Iofrida, N.; Leskinen, P.; Stillitano, T.; Falcone, G.; Strano, A.; Gulisano, G. Life cycle tools combined with multi-criteria and participatory methods for agricultural sustainability: Insights from a systematic and critical review. Sci. Total Environ. 2017, 595, 352–370. [Google Scholar] [CrossRef]
- Tamburini, E.; Pedrini, P.; Marchetti, M.; Fano, E.; Castaldelli, G. Life cycle based evaluation of environmental and economic impacts of agricultural productions in the Mediterranean area. Sustainability 2015, 7, 2915–2935. [Google Scholar] [CrossRef]
- Pelletier, N.L.; Ayer, N.W.; Tyedmers, P.H.; Kruse, S.A.; Flysjo, A.; Robillard, G.; Sonesson, U. Impact categories for life cycle assessment research of seafood production systems: Review and prospectus. Int. J. Life Cycle Assess. 2007, 12, 414–421. [Google Scholar] [CrossRef]
- Besson, M.; Aubin, J.; Komen, H.; Poelman, M.; Quillet, E.; Vandeputte, M.; De Boer, I.J.M. Environmental impacts of genetic improvement of growth rate and feed conversion ratio in fish farming under rearing density and nitrogen output limitations. J. Clean Prod. 2016, 116, 100–109. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Iribarren, D.; Moreira, M.T.; Feijoo, G. Combined application of life cycle assessment and data envelopment analysis as a methodological approach for the assessment of fisheries. Int. J. Life Cycle Assess. 2010, 15, 272–283. [Google Scholar] [CrossRef]
- Ziegler, F.; Hornborg, S.; Green, B.S.; Eigaard, O.R.; Farmery, A.K.; Hammar, L.; Vázquez-Rowe, I. Expanding the concept of sustainable seafood using Life Cycle Assessment. Fish Fish. 2016, 17, 1073–1093. [Google Scholar] [CrossRef]
- García García, B.; Rosique Jiménez, C.; Aguado-Giménez, F.; García García, J. Life cycle assessment of gilthead seabream (Sparus aurata) production in offshore fish farms. Sustainability 2016, 8, 1228. [Google Scholar] [CrossRef]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Life Cycle Assessment of fresh and canned mussel processing and consumption in Galicia (NW Spain). Resour. Conserv. Recycl. 2010, 55, 106–117. [Google Scholar] [CrossRef]
- Lozano, S.; Iribarren, D.; Moreira, M.T.; Feijoo, G. Environmental impact efficiency in mussel cultivation. Resour. Conserv. Recycl. 2010, 54, 1269–1277. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global production of marine bivalves. Trends and challenges. In Goods and Services of Marine Bivalves; Smaal, A., Ferreira, J., Grant, J., Petersen, J., Strand, Ø., Eds.; Springer: Cham, Switzerland, 2019; pp. 7–26. [Google Scholar]
- Meyhoff Fry, J. Carbon Footprint of Scottish Suspended Mussels and Intertidal Oysters; Report Commissioned by SARF078 (Scottish Aquaculture Research Forum); Scottish Aquaculture Research Forum: Pitlochry, UK, 2012; ISBN 978-1-907266-44-7. [Google Scholar]
- De Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Nisticò, R. Aquatic-derived biomaterials for a sustainable future: A European opportunity. Resources 2017, 6, 65. [Google Scholar] [CrossRef]
- Turolla, E.; Savorelli, F.; Palazzi, D.; Gelli, F. Impiego di tecniche di induzione all’emissione dei gameti in Crassostrea gigas per l’esecuzione di test di embriotossicita. Boll. Malacol. 2008, 43, 73. (In Italian) [Google Scholar]
- Walne, P.R. The culture of marine bivalve larvae. Physiol. Mollusca 1946, 1, 197–210. [Google Scholar]
- Ecoinvent Database®. Available online: https://www.ecoinvent.org/database/database.html (accessed on 9 October 2019).
- Blanc, I.; Friot, D.; Margni, M.; Jolliet, O. Towards a new index for environmental sustainability based on a DALY weighting approach. Sustain. Dev. 2008, 16, 251–260. [Google Scholar] [CrossRef]
- ReCiPe®. Available online: https://www.pre-sustainability.com/recipe (accessed on 19 September 2019).
- Reap, J.; Roman, F.; Duncan, S.; Bras, B. A survey of unresolved problems in life cycle assessment. Int. J. Life Cycle Asses. 2008, 13, 374–388. [Google Scholar] [CrossRef]
- Avadí, A.; Fréon, P. Life cycle assessment of fisheries: A review for fisheries scientists and managers. Fish. Res. 2013, 143, 21–38. [Google Scholar] [CrossRef]
- Murray, C.J.; Lopez, A.D. Evidence-based health policy—lessons from the Global Burden of Disease Study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Klepper, O.; Bakker, J.; Traas, T.P.; van de Meent, D. Mapping the potentially affected fraction (PAF) of species as a basis for comparison of ecotoxicological risks between substances and regions. J. Hazard Mater. 1998, 61, 337–344. [Google Scholar] [CrossRef]
- Goedkoop, M.; Spriensma, R. The Eco-Indicator 99 a Damage Oriented Method for Life Cycle Impact Assessment; PRé Consultants: Amersfoort, The Netherlands, 2001. [Google Scholar]
- Gronroos, J.; Seppala, J.; Silvenius, F.; Makinen, T. Life cycle assessment of Finnish cultivated rainbow trout. Boreal Environ. Res 2006, 11, 401–414. [Google Scholar]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Life Cycle Assessment of Aquaculture Feed and Application to the Turbot Sector. Int. J. Environ. Res. 2012, 6, 837–848. [Google Scholar]
- Iribarren, D.; Moreira, M.T.; Feijoo, G. Revisiting the life cycle assessment of mussels from a sectorial perspective. J. Clean. Prod. 2010, 18, 101–111. [Google Scholar] [CrossRef]
- European Commission—Joint Research Centre—Institute for Environment and Sustainability. International Reference Life Cycle Data System (ILCD) Handbook—General Guide for Life Cycle Assessment—Detailed Guidance, 1st ed.; EUR 24708 EN; Publications Office of the European Union: Luxembourg, Luxembourg, 2010. [Google Scholar]
- Jerbi, M.A.; Aubin, J.; Garnaoui, K.; Achour, L.; Kacem, A. Life cycle assessment (LCA) of two rearing techniques of sea bass (Dicentrarchus labrax). Aquac. Eng. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Nagel, F.; Meyer, S.; Schroeder, J.P.; Schulz, C. Comparative life cycle assessment (LCA) of raising rainbow trout (Oncorhynchus mykiss) in different production systems. Aquac. Eng. 2013, 54, 85–92. [Google Scholar] [CrossRef]
- Abdou, K.; Aubin, J.; Romdhane, M.S.; Le Loc’h, F.; Lasram, F.B.R. Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: A case study of a Tunisian aquaculture farm. Aquaculture 2017, 471, 204–212. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; Van der Werf, H.M.G.; Petit, J.; Morvan, Y.M. Characterisation of the environmental impact of a turbot (Scophthalmus maximus) re-circulating production system using Life Cycle Assessment. Aquaculture 2016, 261, 1259–1268. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P.; Sonesson, U.; Scholz, A.; Ziegler, F.; Flysjo, A.; Silverman, H. Not All Salmon are Created Equal: Life Cycle Assessment (LCA) of Global Salmon Farming Systems. Environ. Sci. Technol. 2009, 43, 8730–8736. [Google Scholar] [CrossRef]
- Filgueira, R.; Byron, C.J.; Comeau, L.A.; Costa-Pierce, B.; Cranford, P.J.; Ferreira, J.G.; McKindsey, C.W. An integrated ecosystem approach for assessing the potential role of cultivated bivalve shells as part of the carbon trading system. Mar. Ecol. Prog. Ser. 2015, 518, 281–287. [Google Scholar] [CrossRef]
- Rice, M.A. Environmental impacts of shellfish aqua-culture: Filter feeding to control eutrophication. In Proceedings of the Workshop on Aquaculture and the Marine Environment: A Meeting for Stakeholders in the Northeast, University of Massachusetts, Boston, MA, USA, 11–13 January 2001; pp. 1–11. [Google Scholar]
- Mallet, A.L.; Carver, C.E.; Landry, T. Impact of suspended and off-bottom Eastern oyster culture on the benthic environment in eastern Canada. Aquaculture 2006, 255, 362–373. [Google Scholar] [CrossRef]
- Huntington, T.C.H.; Roberts, N.; Cousins, V.; Pitta, N.; Marchesi, A.; Sanmamed, T.; Hunter-Rowe, T.F.; Fernandes, P.; Tett, J.; Cue, M.; et al. Some Aspects of the Environmental Impact of Aquaculture in Sensitive Areas; Report to the DG Fish and Maritime Affairs of the European Commission; Poseidon Aquatic Resource Management Ltd.: Hampshire, UK, 2006. [Google Scholar]
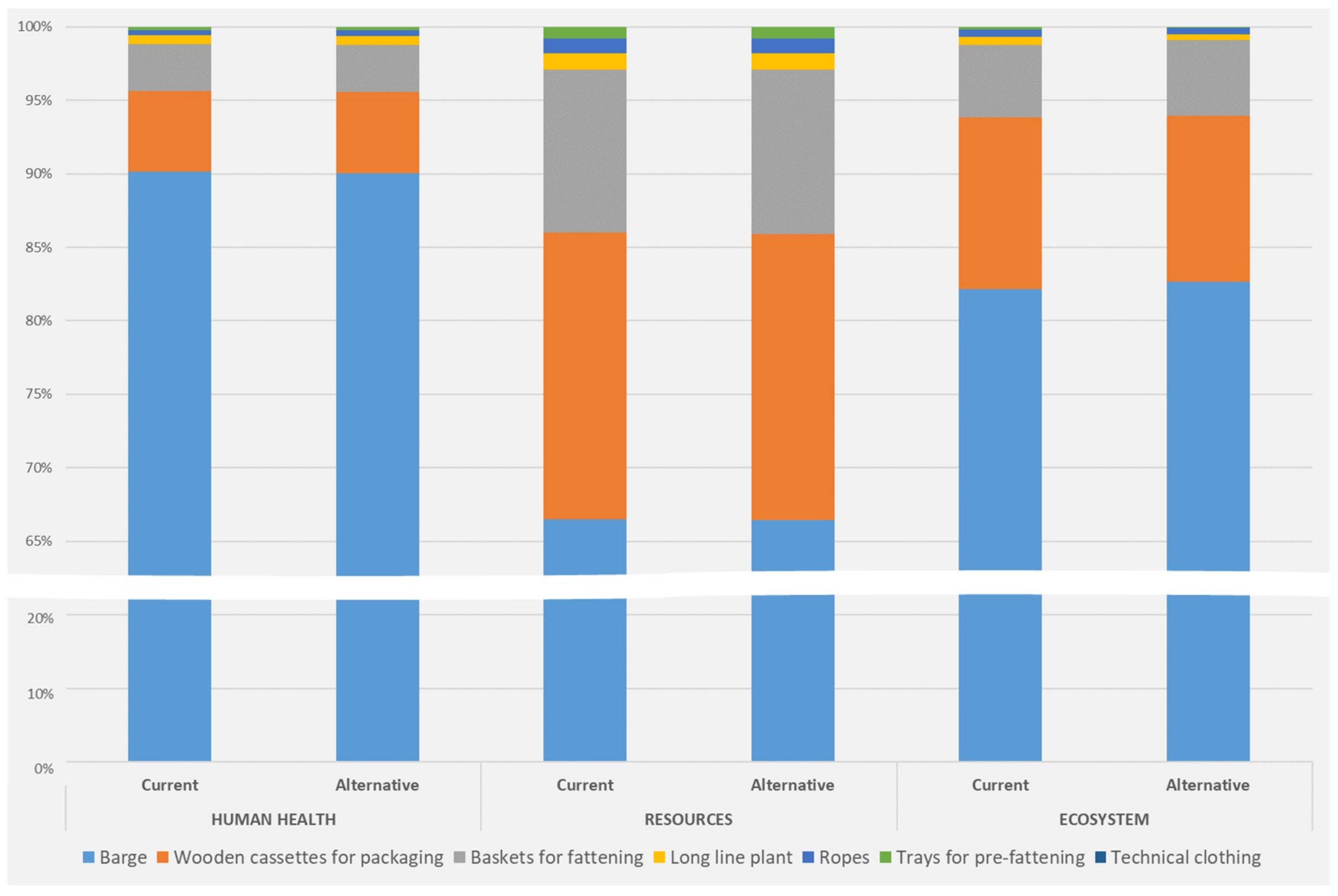
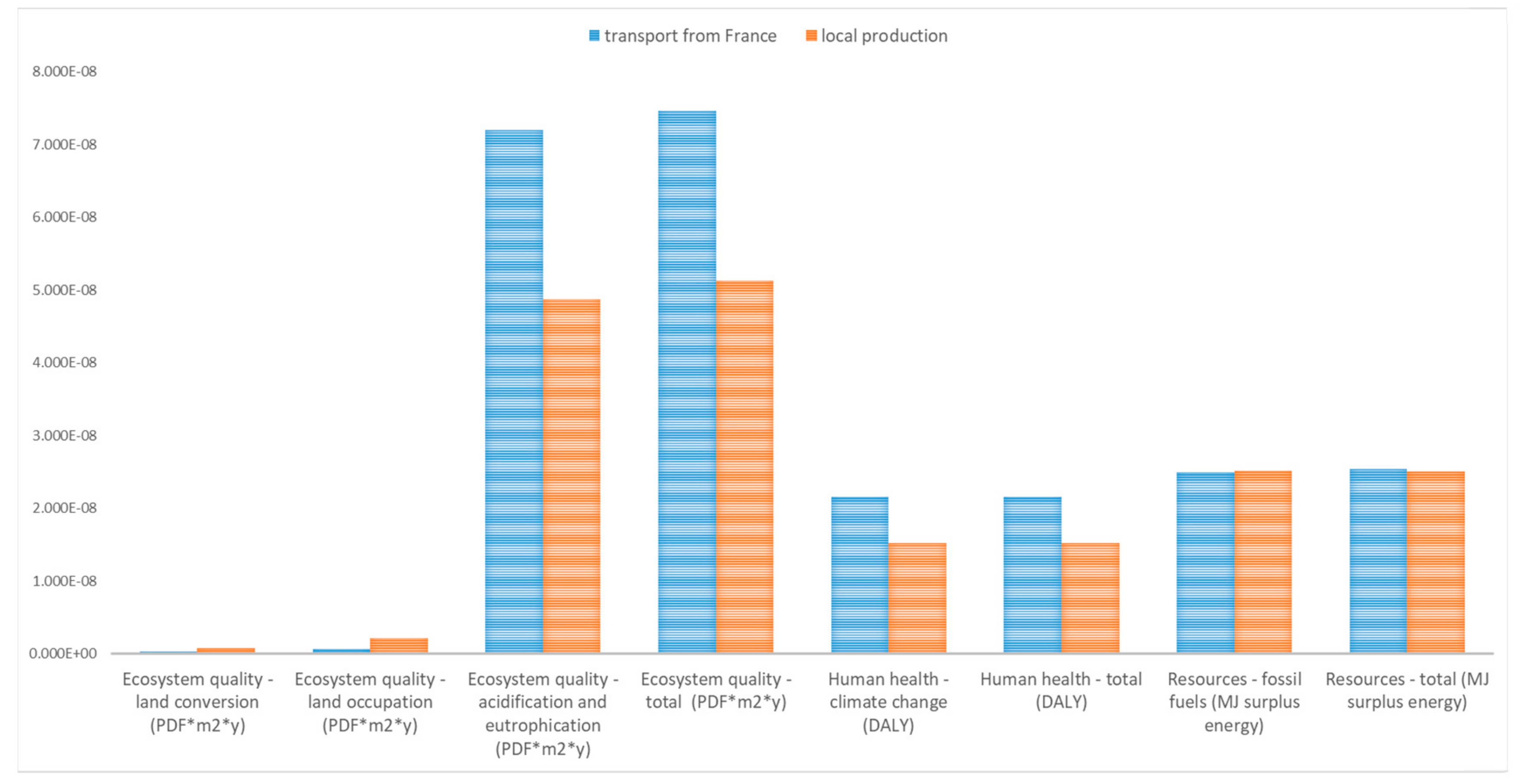
| Inputs | Seed from France | Local Seed |
|---|---|---|
| Resources | ||
| Sea use (m2 year−1) | - | 120,000 |
| Seawater (m3) | - | 160 |
| Freshwater (m3) | - | 16 |
| Materials and fuel | ||
| High-density polyethylene (HDPE) (kg) | - | 181.3 |
| Polypropylene (PP) (kg) | - | 16 |
| Polyvinyl chloride (PVC) (kg) | - | 1.24 |
| Rubber (kg) | - | 0.95 |
| Glass fiber (kg) | - | 2.7 |
| Nylon (kg) | - | 63 |
| Concrete (kg) | - | 144 |
| Steel (kg) | - | 0.6 |
| Diesel for boat (l) | - | 800 |
| Wood (kg) | - | 160 |
| Chemicals | ||
| Salt solution for feed (g) | - | 40 |
| Vitamins for feed (g) | - | 6 |
| CO2 (L) | - | 90 |
| Energy | ||
| Electrical energy (kWh) | 34.2 * | 1400 |
| Vehicles | ||
| Boat (no. of items) | - | 0.033 |
| Transport from suppliers to Goro | ||
| Seed from L’Epine, France to Goro (tons km) | 160 | 0 |
| Tanks for seed production (tons km) | - | 40.3 |
| Prefattening trays (tons km) | - | 2.23 |
| Ropes (tons km) | - | 0 |
| PVC and rubber clothing (tons km) | - | 0 |
| Fattening baskets (tons km) | - | 24.8 |
| Cassettes for selection (tons km) | - | 0.82 |
| Wood cassettes for packaging (tons km) | - | 0 |
| Emissions to air | ||
| Carbon dioxide (kg) | 0.506 | 0.001 |
| Nitrous oxide (kg) | 0.0014 | 5.29 × 10−6 |
| Sulfur dioxide (kg) | 0.0008 | 0.0006 |
| Methane (kg) | 3.26 × 10−6 | 3.48 × 10−6 |
| Nonmethane volatile organic carbon (NMVOC) (kg) | 0.0025 | 0.0025 |
| Particulates <2.5 μ (kg) | 0.00023 | 0.00023 |
| Particulates >10 μ (kg) | 4.78 × 10−5 | 4.51 × 10−5 |
| Particulates >2.5 μ and <10 μ (kg) | 6.86 × 10−5 | 6.63 × 10−5 |
| Emissions to water | ||
| Adsorbable organic halogen as Cl (AOX) (kg) | 1.84 × 10−9 | |
| Biochemical oxygen demand (BOD) (kg) | 0.00062 | |
| Heat, waste (MJ) | 6.79 × 10−5 | |
| Nitrate (kg) | 2.21 × 10−6 |
| Impact Category | Unit |
|---|---|
| Human health: total | DALY * |
| Human health: climate change | DALY |
| Human health: carcinogenic | DALY |
| Human health: respiratory effects caused by chemical substances | DALY |
| Human health: ozone layer depletion | DALY |
| Ecosystem quality: total | PDF m2 year ** |
| Ecosystem quality: sea conversion | PDF m2 year |
| Ecosystem quality: sea occupation | PDF m2 year |
| Ecosystem quality: acidification and eutrophication | PDF m2 year |
| Ecosystem quality: ecotoxicity | PDF m2 year |
| Resources: total | MJ surplus energy *** |
| Resources: fossil fuels | MJ surplus energy |
| Resources: minerals | MJ surplus energy |
| Impact Category | Current (Seeds from France) | Alternative (Seeds In Situ) | Unit |
|---|---|---|---|
| Human health: total | 0.0104 | 0.0104 | DALY |
| Human health: climate change | 0.0101 | 0.0101 | DALY |
| Human health: carcinogenic | 7.26 × 10−6 | 7.26 × 10−6 | DALY |
| Human health: respiratory effects caused by chemical substances | 2.31 × 10−6 | 2.31 × 10−6 | DALY |
| Human health: ozone layer depletion | 1.26 × 10−10 | 1.26 × 10−10 | DALY |
| Ecosystem quality: total | 0.0298 | 0.0298 | PDF m2 year |
| Ecosystem quality: sea conversion | 0.0011 | 0.0011 | PDF m2 year |
| Ecosystem quality: sea occupation | 0.0023 | 0.0023 | PDF m2 year |
| Ecosystem quality: acidification and eutrophication | 0.0316 | 0.0315 | PDF m2 year |
| Ecosystem quality: ecotoxicity | 0.0002 | 0.0002 | PDF m2 year |
| Resources: total | 0.7544 | 0.7543 | MJ surplus energy |
| Resources: fossil fuels | 0.7467 | 0.7466 | MJ surplus energy |
| Resources: minerals | 0.0077 | 0.0077 | MJ surplus energy |
| Impact Category | Mean | SD | CV% | Min | Max | Median | 5% | 95% |
|---|---|---|---|---|---|---|---|---|
| Current Scenario | ||||||||
| Human health: total | 1.11 × 10−2 | 1.64 × 10−3 | 13% | 8.40 × 10−3 | 1.64 × 10−2 | 1.11 × 10−2 | 8.64 × 10−3 | 1.38 × 10−2 |
| Human health: climate change | 1.11 × 10−2 | 1.64 × 10−3 | 13% | 8.39 × 10−3 | 1.63 × 10−2 | 1.10 × 10−2 | 8.63 × 10−3 | 1.38 × 10−2 |
| Human health: carcinogenics | 9.52 × 10−6 | 4.14 × 10−6 | 50% | 2.40 × 10−6 | 2.63 × 10−5 | 9.00 × 10−6 | 4.78 × 10−6 | 1.69 × 10−5 |
| Human health: respiratory effects | 3.40 × 10−6 | 6.14 × 10−7 | 25% | 2.18 × 10−6 | 5.23 × 10−6 | 3.33 × 10−6 | 2.54 × 10−6 | 4.65 × 10−6 |
| Human health: ozone layer depletion | 1.78 × 10−1 | 6.00 × 10−11 | 29% | 8.32 × 10−11 | 4.64 × 10−10 | 1.71 × 10−10 | 9.87 × 10−11 | 2.86 × 10−10 |
| Ecosystems: total | 4.38 × 10−2 | 1.03 × 10−2 | 36% | 2.79 × 10−2 | 9.14 × 10−2 | 4.21 × 10−2 | 3.15 × 10−2 | 6.31 × 10−2 |
| Ecosystem quality: sea conversion | 1.43 × 10−3 | 6.75 × 10−4 | 42% | 6.61 × 10−4 | 5.03 × 10−3 | 1.26 × 10−3 | 7.69 × 10−4 | 3.22 × 10−3 |
| Ecosystem quality: sea occupation | 2.61 × 10−3 | 7.99 × 10−4 | 27% | 1.24 × 10−3 | 5.25 × 10−3 | 2.48 × 10−3 | 1.66 × 10−3 | 4.19 × 10−3 |
| Ecosystem quality: acidification and eutrophication | 3.98 × 10−2 | 1.01 × 10−2 | 39% | 2.53 × 10−2 | 8.73 × 10−2 | 3.74 × 10−2 | 2.77 × 10−2 | 6.02 × 10−2 |
| Ecosystem quality: ecotoxicity | 4.22 × 10−4 | 2.91 × 10−4 | 64% | 1.33 × 10−4 | 2.77 × 10−3 | 3.58 × 10−4 | 2.20 × 10−4 | 8.25 × 10−4 |
| Resources: total | 8.23 × 10−1 | 1.31 × 10−1 | 14% | 5.57 × 10−1 | 1.17 | 7.99 × 10−1 | 6.69 × 10−1 | 1.04 |
| Resources: fossil fuels | 8.15 × 10−1 | 1.30 × 10−1 | 14% | 5.50 × 10−1 | 1.16 | 7.92 × 10−1 | 6.62 × 10−1 | 1.04 |
| Resources: minerals | 8.03 × 10−3 | 9.29 × 10−4 | 11% | 6.23 × 10−3 | 1.09 × 10−2 | 7.93 × 10−3 | 6.62 × 10−3 | 9.63 × 10−3 |
| Alternative Scenario | ||||||||
| Human health: total | 1.11 × 10−2 | 1.41 × 10−3 | 15% | 7.69 × 10−3 | 1.49 × 10−2 | 1.10 × 10−2 | 9.14 × 10−3 | 1.36 × 10−2 |
| Human health: climate change | 1.11 × 10−2 | 1.41 × 10−3 | 15% | 7.68 × 10−3 | 1.49 × 10−2 | 1.10 × 10−2 | 9.13 × 10−3 | 1.36 × 10−2 |
| Human health: carcinogenics | 1.01 × 10−5 | 5.00 × 10−6 | 43% | 3.06 × 10−6 | 3.62 × 10−5 | 8.80 × 10−6 | 4.60 × 10−6 | 1.96 × 10−5 |
| Human health: respiratory effects | 3.50 × 10−6 | 8.60 × 10−7 | 18% | 2.20 × 10−6 | 9.20 × 10−6 | 3.34 × 10−6 | 2.58 × 10−6 | 5.00 × 10−6 |
| Human health: ozone layer depletion | 1.73 × 10−10 | 5.01 × 10−11 | 34% | 7.64 × 10−11 | 4.43 × 10−10 | 1.67 × 10−10 | 1.14 × 10−10 | 2.64 × 10−10 |
| Ecosystem quality: total | 4.59 × 10−2 | 1.67 × 10−2 | 23% | 2.87 × 10−2 | 1.51 × 10−1 | 4.23 × 10−2 | 3.14 × 10−2 | 6.82 × 10−2 |
| Ecosystem quality: sea conversion | 1.44 × 10−3 | 6.09 × 10−4 | 47% | 6.88 × 10−4 | 4.98 × 10−3 | 1.30 × 10−3 | 8.07 × 10−4 | 2.75 × 10−3 |
| Ecosystem quality: sea occupation | 2.51 × 10−3 | 6.87 × 10−4 | 31% | 1.19 × 10−3 | 4.68 × 10−3 | 2.37 × 10−3 | 1.69 × 10−3 | 4.02 × 10−3 |
| Ecosystem quality: acidification and eutrophication | 4.20 × 10−2 | 1.65 × 10−2 | 25% | 2.62 × 10−2 | 1.48 × 10−1 | 3.78 × 10−2 | 2.80 × 10−2 | 6.43 × 10−2 |
| Ecosystem quality: ecotoxicity | 4.19 × 10−4 | 2.68 × 10−4 | 69% | 1.49 × 10−4 | 1.94 × 10−3 | 354 × 10−4 | 2.04 × 10−4 | 8.31 × 10−4 |
| Resources: total | 836 × 10−1 | 1.19 × 10−1 | 16% | 5.71 × 10−1 | 1.16 | 8.27 × 10−1 | 6.70 × 10−1 | 1.06 |
| Resources: fossil fuels | 8.28 × 10−1 | 1.19 × 10−1 | 16% | 5.62 × 10−1 | 1.15 | 8.20 × 10−1 | 6.63 × 10−1 | 1.05 |
| Resources: minerals | 8.26 × 10−3 | 9.27 × 10−4 | 12% | 6.33 × 10−3 | 1.04 × 10−2 | 8.26 × 10−3 | 6.87 × 10−3 | 1.01 × 10−2 |
| Impact Category | Alternative Scenario (Seed in situ) | Unit |
|---|---|---|
| Climate change | 1.85 | Kg CO2 eq |
| Terrestrial acidification | 9.29 × 10−3 | Kg SO2 eq |
| Marine and freshwater eutrophication | 1.38 × 10−3 | Kg PO4 eq |
| Water depletion | 31.76 | l |
| Title | Climate Change (kg CO2 eq) | Eutrophication (kg PO4 eq) | Acidification (kg SO2 eq) | Water Dependence (m3) | Ref. |
|---|---|---|---|---|---|
| Sea bass | 11.00–17.00 | 180–240 × 10−3 | 50–80 × 10−3 | 190–396 | Jerdi et al. [49] |
| Rainbow trout | 2.24–13.62 | 4.04–60.36 × 10−3 | 10.43–40.72 × 10−3 | - | Samuel-Fitwi et al. [50] |
| Sea bream | 3.67 | 98.86 | 21.61 | - | Adbou et al. [51] |
| Sea bass | 3.18 | 91.03 | 18.85 | - | |
| Turbot | 6.02 | 80 × 10−3 | 50 × 10−3 | - | Aubin et al. [52] |
| Salmon | 2.16 | 49 × 10−3 | 20.4 × 10−3 | - | Pellettier et al. [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamburini, E.; Fano, E.A.; Castaldelli, G.; Turolla, E. Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy. Resources 2019, 8, 170. https://doi.org/10.3390/resources8040170
Tamburini E, Fano EA, Castaldelli G, Turolla E. Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy. Resources. 2019; 8(4):170. https://doi.org/10.3390/resources8040170
Chicago/Turabian StyleTamburini, Elena, Elisa Anna Fano, Giuseppe Castaldelli, and Edoardo Turolla. 2019. "Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy" Resources 8, no. 4: 170. https://doi.org/10.3390/resources8040170
APA StyleTamburini, E., Fano, E. A., Castaldelli, G., & Turolla, E. (2019). Life Cycle Assessment of Oyster Farming in the Po Delta, Northern Italy. Resources, 8(4), 170. https://doi.org/10.3390/resources8040170








