Morais Ultramafic Complex: A Survey towards Nickel Phytomining
Abstract
1. Introduction
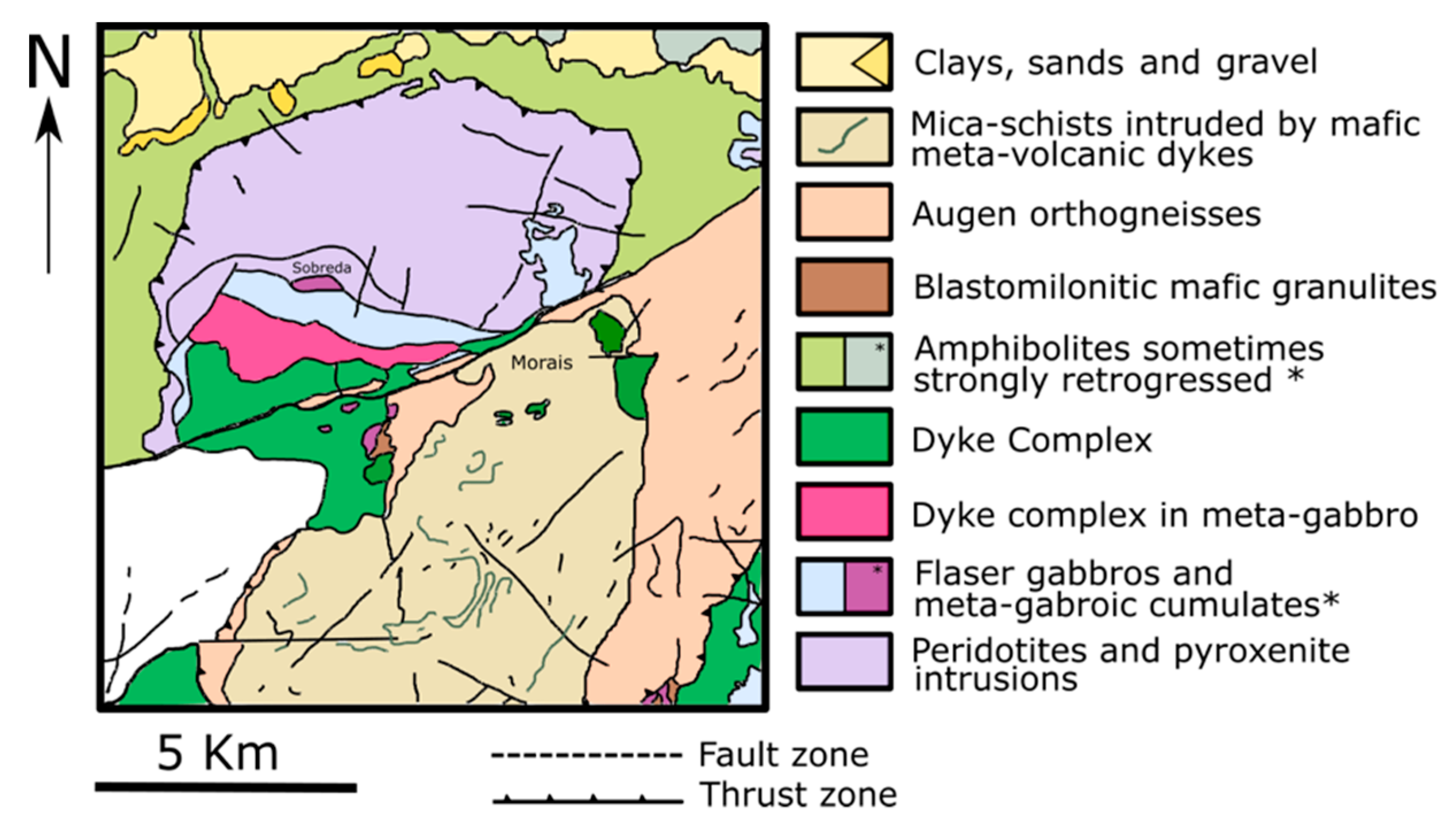
Morais Massif Location and Geology
2. Materials and Methods
3. Results and Discussion
3.1. Soil
3.2. Plants
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kidd, P.S.; Bani, A.; Benizri, E.; Gonnelli, C.; Hazotte, C.; Kisser, J.; Konstantinou, M.; Kuppens, T.; Kyrkas, D.; Laubie, B.; et al. Developing Sustainable Agromining Systems in Agricultural Ultramafic Soils for Nickel Recovery. Front. Environ. Sci. 2018, 6, 44. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. Geology and Plant Life: The Effects of Landforms and Rock Types on Plants; University of Washington Press: Seattle, WA, USA, 2004; ISBN 9780295984520. [Google Scholar]
- Alves, S.; Trancoso, M.A.; de Lurdes Simões Gonçalves, M.; Correia dos Santos, M.M. A nickel availability study in serpentinised areas of Portugal. Geoderma 2011, 164, 155–163. [Google Scholar] [CrossRef]
- Reeves, R.D. Hyperaccumulation of nickel by serpentine plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 253–277. [Google Scholar]
- Sobczyk, M.K.; Smith, J.A.C.; Pollard, A.J.; Filatov, D.A. Evolution of nickel hyperaccumulation and serpentine adaptation in the Alyssum serpyllifolium species complex. Heredity 2017, 118, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, P.N.; Baker, A.J.M.; Chaney, R.L.; Erskine, P.D.; Echevarria, G.; Morel, J.L.; van der Ent, A. Current status and challenges in developing nickel phytomining: An agronomic perspective. Plant Soil 2016, 406, 55–69. [Google Scholar] [CrossRef]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Dioscorides Press: Portland, OR, USA, 1988; Volume 40. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Whiting, S.N.; Reeves, R.D.; Baker, A.J.M. Mining, metallophytes and land reclamation. Min. Environ. Manag. 2002, 10, 11–16. [Google Scholar]
- Alves, S.; Nabais, C.; de Lurdes Simões Gonçalves, M.; Correia dos Santos, M.M. Nickel speciation in the xylem sap of the hyperaccumulator Alyssum serpyllifolium ssp. lusitanicum growing on serpentine soils of northeast Portugal. J. Plant Physiol. 2011, 168, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Díez Lázaro, J.; Kidd, P.S.; Monterroso Martínez, C. A phytogeochemical study of the Trás-os-Montes region (NE Portugal): Possible species for plant-based soil remediation technologies. Sci. Total Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Gonçalves, M.T.; Gonçalves, S.C.; Portugal, A.; Silva, S.; Sousa, J.P.; Freitas, H. Effects of nickel hyperaccumulation in Alyssum pintodasilvae on model arthropods representatives of two trophic levels. Plant Soil 2007, 293, 177–188. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Tappero, R.V.; Maugel, T.K.; Erbe, E.F.; Sparks, D.L.; Chaney, R.L. Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum. Plant Soil 2009, 314, 35–48. [Google Scholar] [CrossRef]
- Goolsby, E.W.; Mason, C.M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.S.; Martens, S.N. The raison d’être for metal hyperaccumulation by plants. In The Vegetation of Ultra- mafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Limited: Andover, MA, USA, 1992; pp. 279–289. [Google Scholar]
- Minguzzi, C.; Vergnano, O. Il contenuto di nichel nelle ceneri di Alyssum bertolonii. Atti della Soc. Toscana di Sci. Nat. A 1948, 55, 49–77. [Google Scholar]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffre, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Dudley, T.R. A new Portuguese subspecies of Alyssum serpyllifolium Desfontaines. Agron. Lusit. 1967, 28, 69–76. [Google Scholar]
- Pinto da Silva, A.R. A flora e a vegetação das áreas ultrabásicas do nordeste transmontano. Agron. Lusit. 1970, 30, 175–364. [Google Scholar]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; da Silva, E.F. Phytomining of Rare and Valuable Metals. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., R. Lanza, G., Newman, L., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 469–486. ISBN 978-3-319-52379-8. [Google Scholar]
- Brooks, R.R.; Chambers, M.F.; Nicks, L.J.; Robinson, B.H. Phytomining. Trends Plant Sci. 1998, 3, 359–362. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Chaney, R.L.; Anderson, C.W.N.; Meech, J.A.; Erskine, P.D.; Simonnot, M.-O.; Vaughan, J.; Morel, J.L.; et al. Agromining: Farming for Metals in the Future? Environ. Sci. Technol. 2015, 49, 4773–4780. [Google Scholar] [CrossRef]
- Ribeiro, A.; Quesada, C.; Dallmeyer, R.D. Geodynamic evolution of the Iberian Massif. In Pre-Mesozoic Geology of Iberia; Dallmeyer, R.D., Martínez-Garcia, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 397–410. [Google Scholar]
- Santos, F.J.; Ibarguchi, J.I.G.; Pin, C.; Paquette, J.L. Composite origin of an early Variscan transported suture: Ophiolitic units of the Morais Nappe Complex (north Portugal). Tectonics 2006, 25, 1–19. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Ghaderian, S.M.; Rodríguez-Garrido, B.; Prieto-Fernández, Á.; Kidd, P.S. Plant species-specificity and effects of bioinoculants and fertilization on plant performance for nickel phytomining. Plant Soil 2018, 425, 265–285. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Robinson, B.H.; Chiarucci, A.; Brooks, R.R.; Petit, D.; Kirkman, J.H.; Gregg, P.E.H.; De Dominicis, V. The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J. Geochem. Explor. 1997, 59, 75–86. [Google Scholar] [CrossRef]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780849302060. [Google Scholar]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Phytomining: A review. Miner. Eng. 2009, 22, 1007–1019. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Olmedo-Cobo, J.; Gómez-Zotano, J. Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain). Minerals 2018, 8, 447. [Google Scholar] [CrossRef]
- Vithanage, M.; Kumarathilaka, P.; Oze, C.; Karunatilake, S.; Seneviratne, M.; Hseu, Z.-Y.; Gunarathne, V.; Dassanayake, M.; Ok, Y.S.; Rinklebe, J. Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: A critical review. Environ. Int. 2019, 131, 104974. [Google Scholar] [CrossRef]
- van der Ent, A.; Nkrumah, P.N.; Tibbett, M.; Echevarria, G. Evaluating soil extraction methods for chemical characterization of ultramafic soils in Kinabalu Park (Malaysia). J. Geochem. Explor. 2019, 196, 235–246. [Google Scholar] [CrossRef]
- Davies, F.T.; Puryear, J.D.; Newton, R.J.; Egilla, J.N.; Saraiva Grossi, J.A. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J. Plant Physiol. 2001, 158, 777–786. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, Z.X.; He, Z.Y. Pollution behaviour of organic Cr(III) complexes in soil–plant system. Chin. J. Appl. Ecol. 1994, 5, 187–191. [Google Scholar]
- Deng, T.-H.-B.; van der Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel hyperaccumulation mechanisms: A review on the current state of knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef]
- Groeber, S.; Przybyłowicz, W.; Echevarria, G.; Montarges-Pelletier, E.; Barnabas, A.; Mesjasz-Przybyłowicz, J. Fate of nickel and calcium in seedlings of the hyperaccumulator Berkheya coddii during germination. Biol. Plant. 2015, 59, 560–569. [Google Scholar] [CrossRef]
- Novo, A.B. Plants to harvest rhenium: Scientific and economic viability. Environ. Chem. Lett. 2015, 13, 439–445. [Google Scholar] [CrossRef]
- Bani, A.; Echevarria, G.; Sulçe, S.; Morel, J.L. Improving the Agronomy of Alyssum murale for Extensive Phytomining: A Five-Year Field Study. Int. J. Phytoremediation 2015, 17, 117–127. [Google Scholar] [CrossRef]
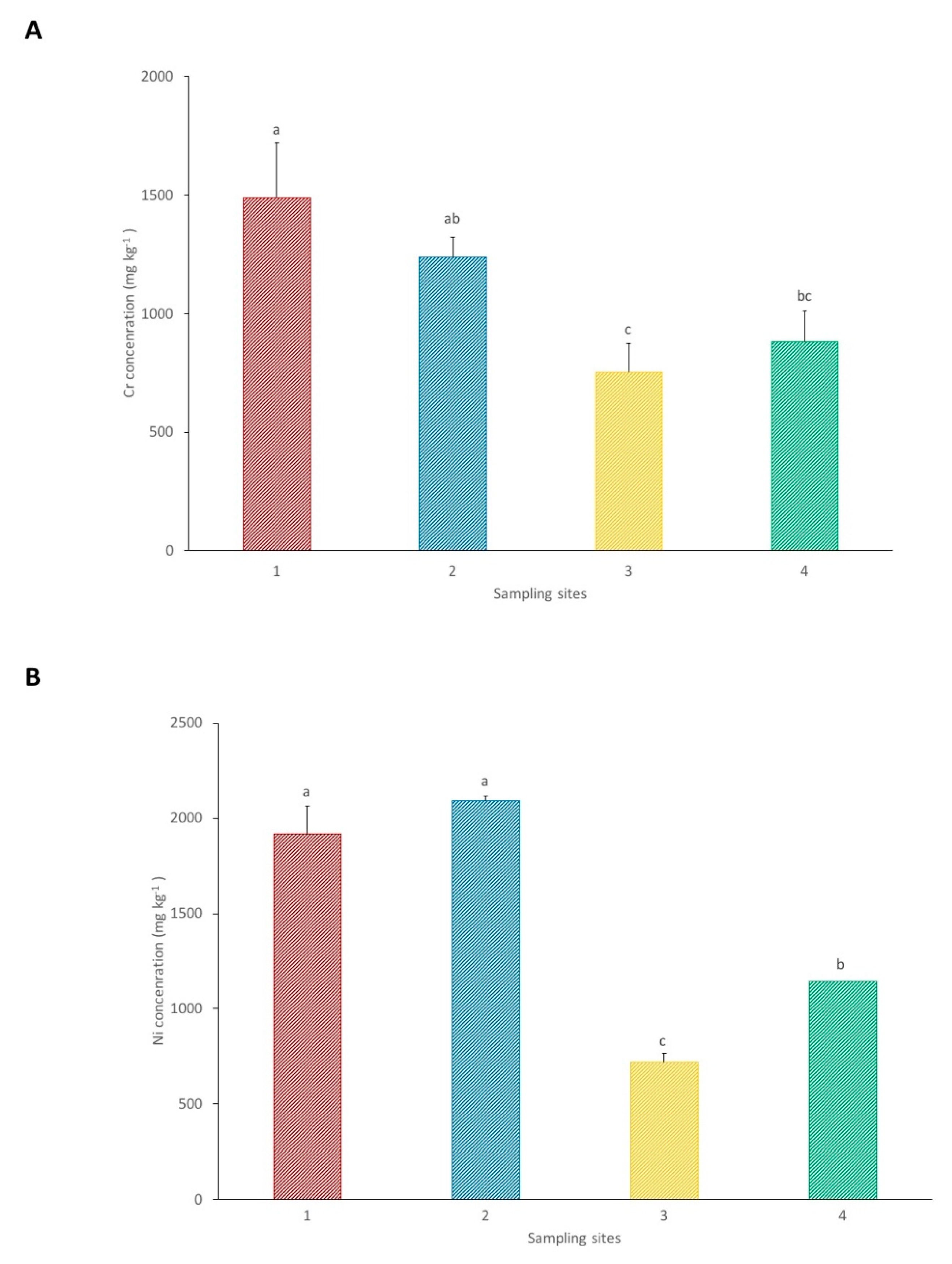

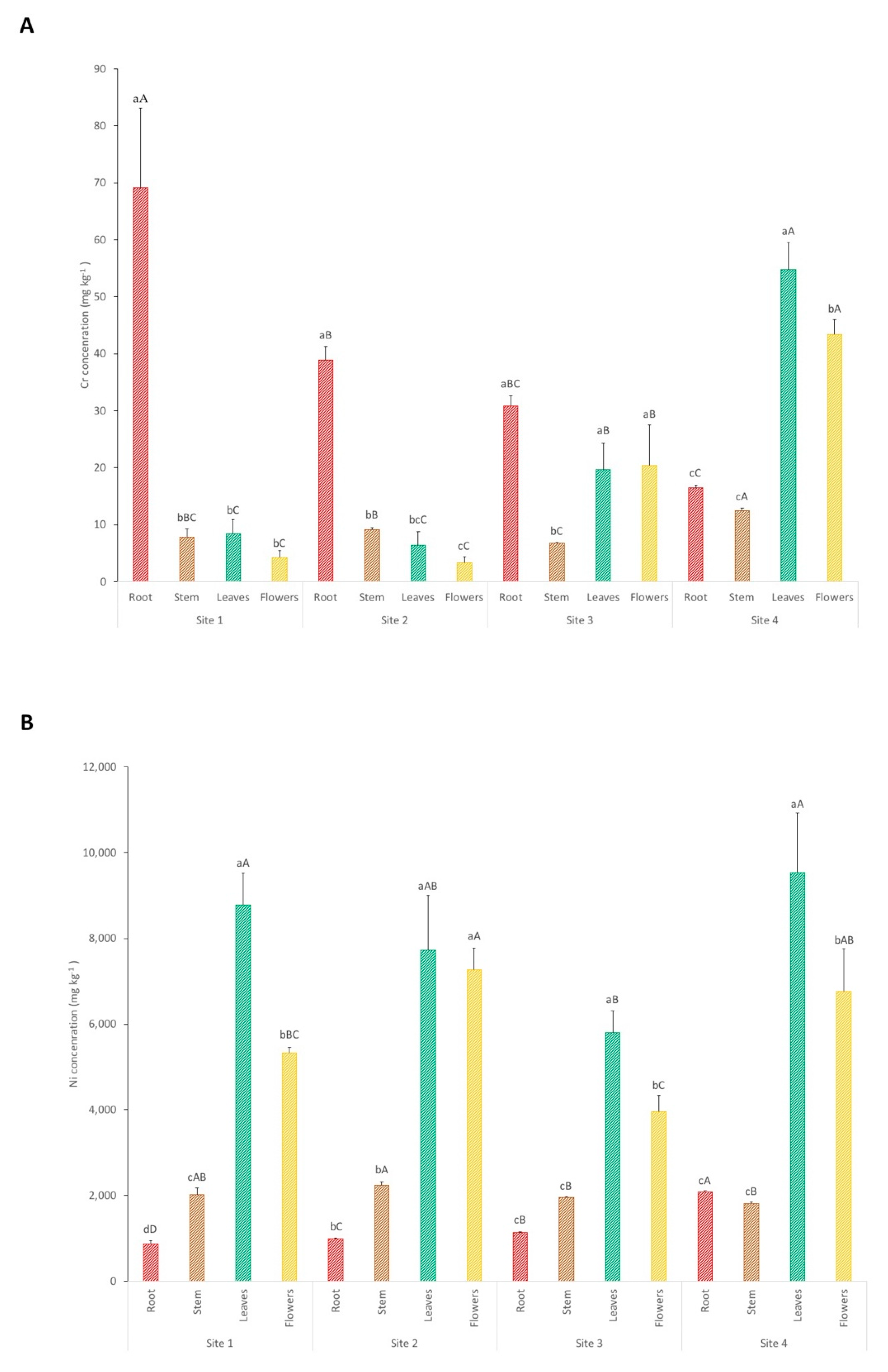
| Site | pH | Conductivity (µS/cm) | Ca (mg kg−1) | Mg (mg kg−1) | Ca:Mg | P (mg kg−1) |
|---|---|---|---|---|---|---|
| 1 | 6.81 ± 0.03a | 84.70 ± 51.42a | 1359 ± 460b | 22,577 ± 2647a | 0.059 ± 0.014b | 295 ± 21a |
| 2 | 6.65 ± 0.02b | 101.30 ± 14.66a | 927 ± 133b | 26,028 ± 2105a | 0.036 ± 0.003b | 304 ± 25a |
| 3 | 6.50 ± 0.02c | 46.47 ± 12.16a | 3482 ± 712a | 23,606 ± 2325a | 0.147 ± 0.018a | 160 ± 9b |
| 4 | 6.39 ± 0.03d | 117.70 ± 112a | 3376 ± 672a | 21,117 ± 2594a | 0.159 ± 0.013a | 257 ± 13a |
| Site | pH | Ca:Mg | Ni (mg kg−1) | Cr (mg kg−1) |
|---|---|---|---|---|
| Portugal (Morais) | 6.6 | 0.036 | 2092 | 1240 |
| Spain (Melide) | 5.8 | 0.169 | 967 | 1263 |
| Austria | 6.1 | 0.065 | 1450 | 1840 |
| Greece | 7.2 | 0.050 | 2347 | - |
| Albania | 7.5 | 0.065 | 3140 | 1600 |
| Site | HA (mg) | TF | BF |
|---|---|---|---|
| 1 | 88.36 ± 4.62a | 4.91 ± 0.22a | 2.05 ± 0.34b |
| 2 | 93.80 ± 8.64a | 4.53 ± 0.35a | 1.98 ± 0.21b |
| 3 | 67.44 ± 1.05b | 2.84 ± 0.05b | 4.18 ± 0.20a |
| 4 | 95.56 ± 5.43a | 2.21 ± 0.11c | 3.77 ± 0.18a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.R.A.; Silva, E.F.; Novo, L.A.B. Morais Ultramafic Complex: A Survey towards Nickel Phytomining. Resources 2019, 8, 144. https://doi.org/10.3390/resources8030144
Alves ARA, Silva EF, Novo LAB. Morais Ultramafic Complex: A Survey towards Nickel Phytomining. Resources. 2019; 8(3):144. https://doi.org/10.3390/resources8030144
Chicago/Turabian StyleAlves, Ana R. A., Eduardo F. Silva, and Luís A. B. Novo. 2019. "Morais Ultramafic Complex: A Survey towards Nickel Phytomining" Resources 8, no. 3: 144. https://doi.org/10.3390/resources8030144
APA StyleAlves, A. R. A., Silva, E. F., & Novo, L. A. B. (2019). Morais Ultramafic Complex: A Survey towards Nickel Phytomining. Resources, 8(3), 144. https://doi.org/10.3390/resources8030144







