The LCA Methodology for Ceramic Tiles Production by Addition of MSWI BA
Abstract
1. Introduction
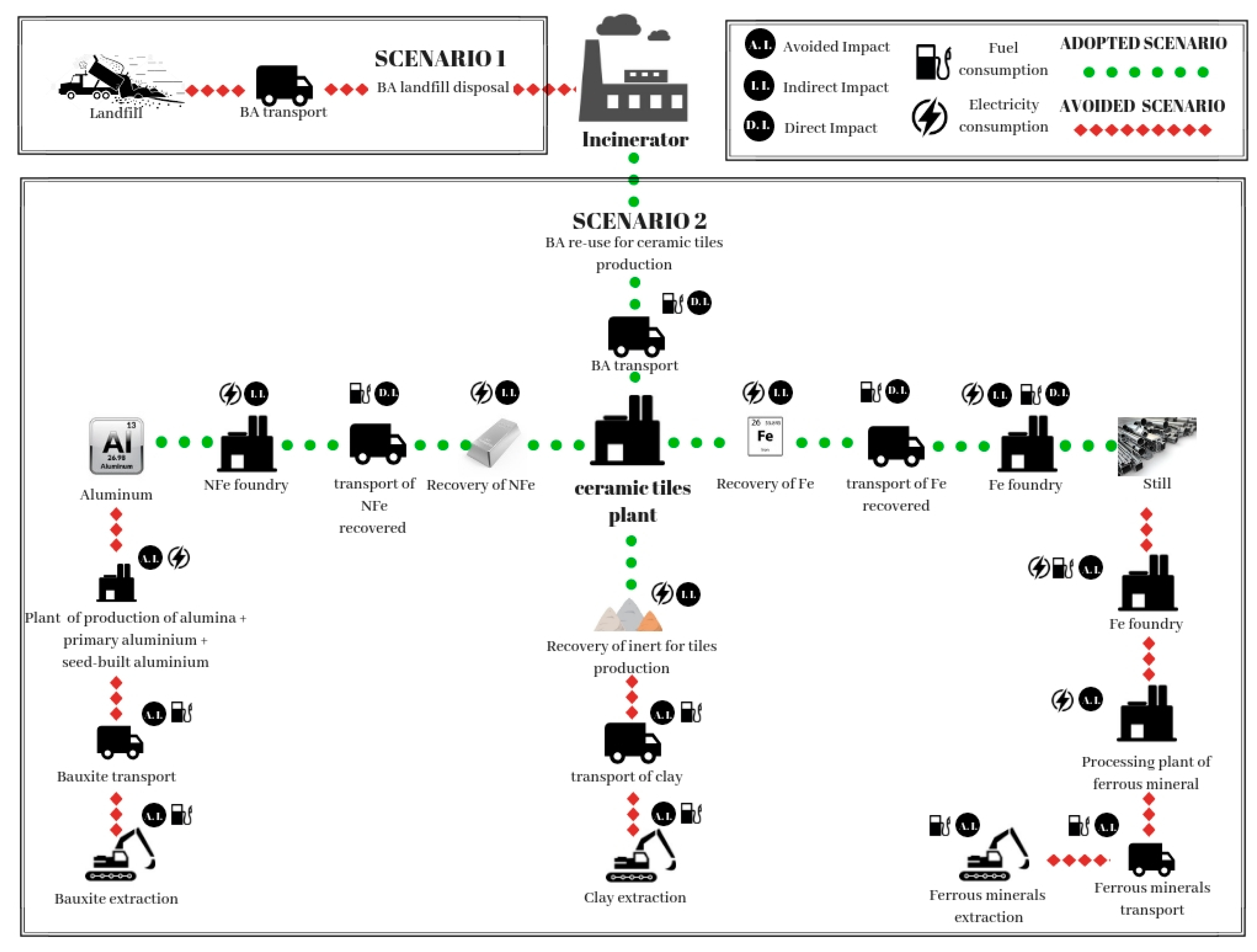
2. Case Study: Production Plant of Ceramic Tiles with Recovery of MSWI BA
MSWI BA Composition
3. LCA Procedure
3.1. Goal and Scope Definition
3.2. Modeling Framework and Life Cycle Inventory
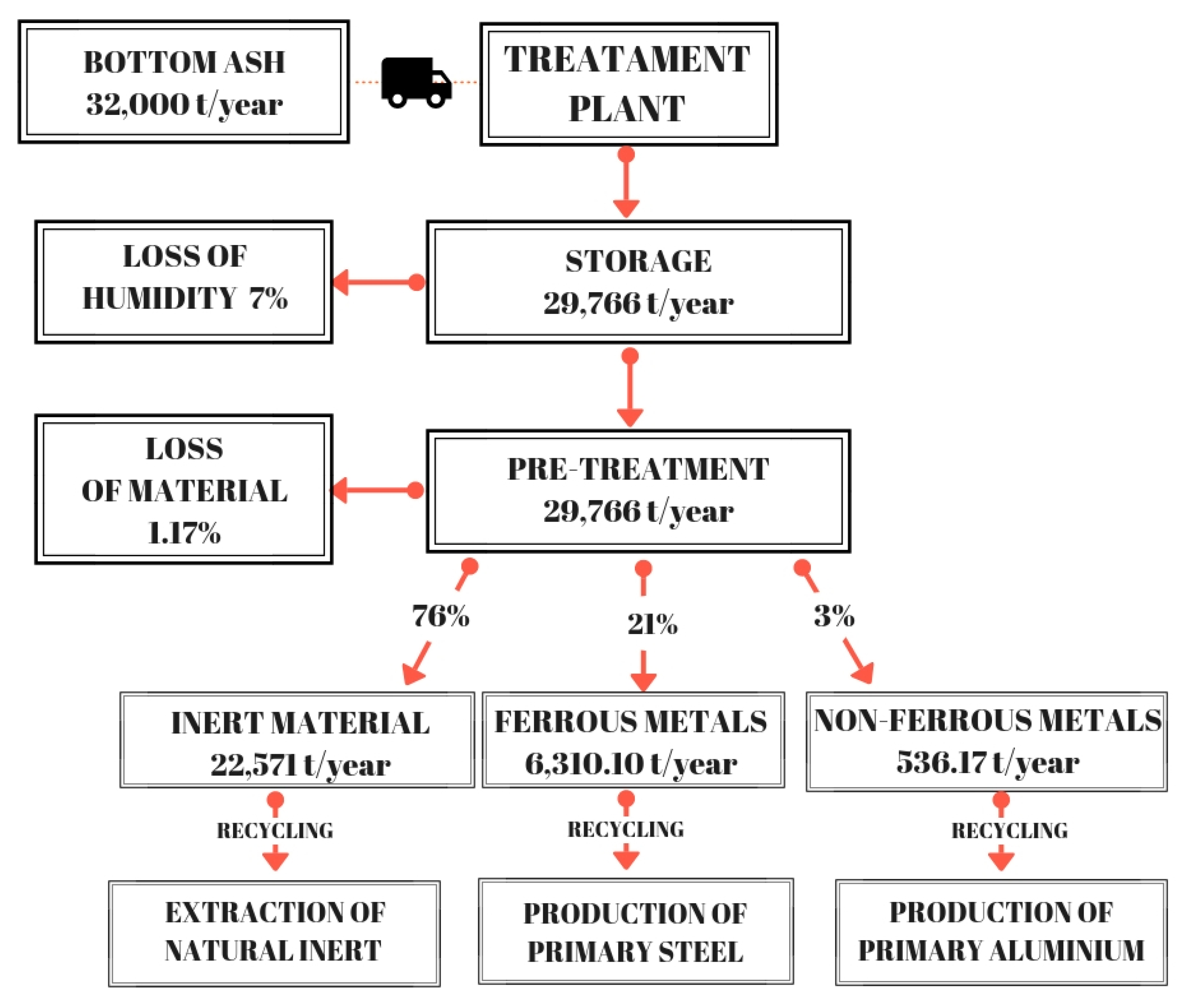
- BA arriving at the industrial plant contain 13% of humidity. During storage, they lose 7% of initial humidity by evaporation and leaching. The produced leachate represents 10% of humidity loss and it is collected through a sewage system.
- After BA storage, metals are separated by the ashes. A mass of Fe corresponding to 21% of the total ashes is recovered using a magnetic separator belt and a magnetic separator drum. Recovery of NFe is about 3% of the total ashes and was obtained by an Eddy Current Separator (ECS).
- The residual BA is utilized to produce the ceramic tiles.
3.3. Life Cycle Impact Assessment
- selection of impact categories;
- classification;
- characterization.
- Global Warming Potential (GWP);
- Photochemical Ozone Creation Potential (POCP);
- Acidification Potential (AP);
- Eutrophication Potential (EP);
- Human Toxicity Potential (HTP);
- Resources Depletion Potential (RDP).
- Direct impacts, which account for effects directly related to a given impact class;
- Indirect impacts, which account for possible effects associated to a given impact category due to a transformation after primary emission;
- Avoided impacts, which consider the saved impacts due to the presence of profitable outputs. They are equivalent to the impacts that would have occurred in actual production of the same amount of recovered energy and they need to be deducted from the impacts caused by other processes.
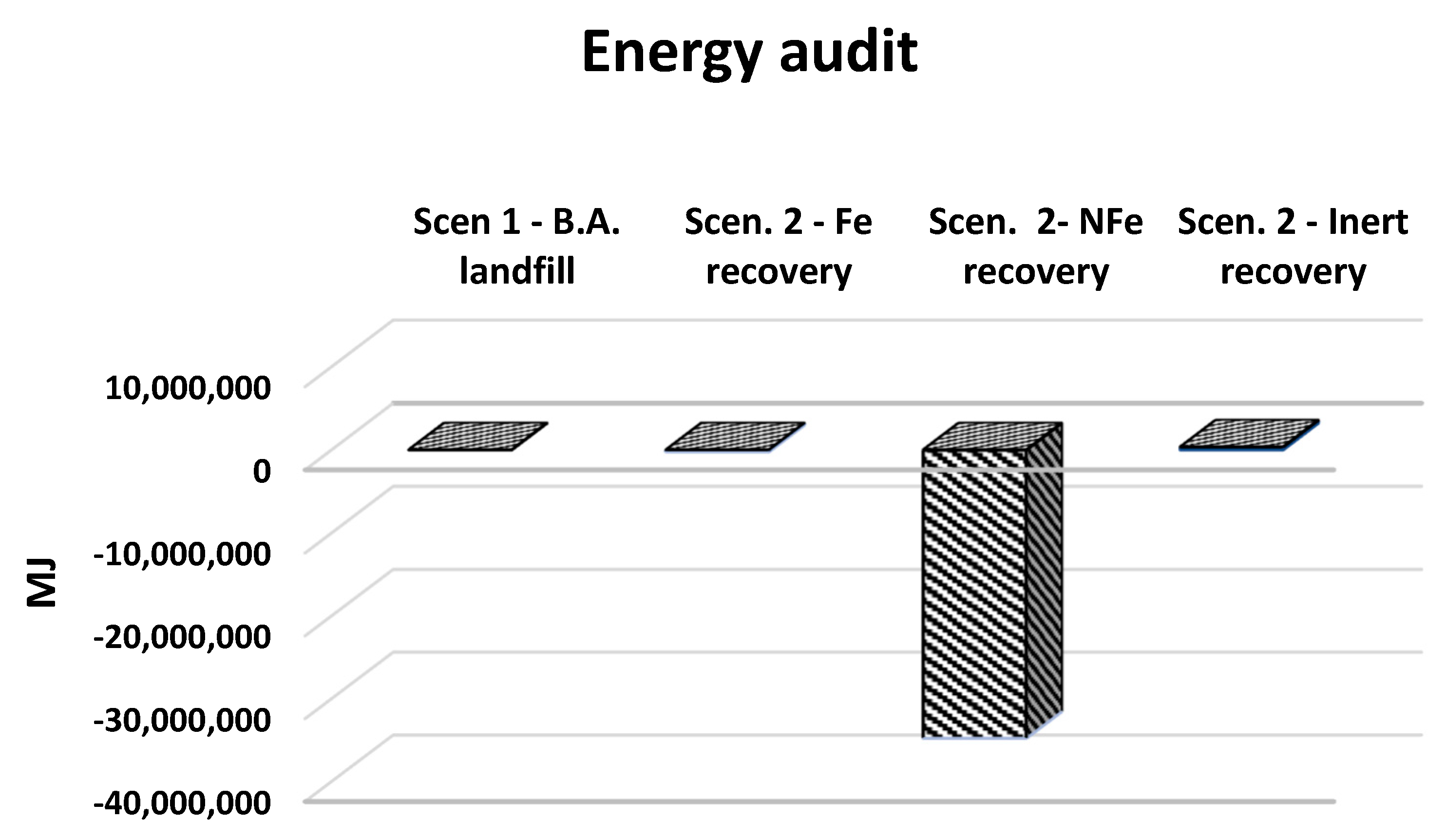
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP | Acidification Potential |
| BA | Bottom Ash |
| ECS | Eddy Current Separator |
| EF | Equivalence Factor |
| EFs | Equivalence Factors |
| EfP | Effect Potential |
| EDIP | Environmental Design of Industrial Products |
| EP | Eutrophication Potential |
| Fe | Ferrous metals |
| GWP | Global Warming Potential |
| HTP | Human Toxicity Potential |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LCIA | Life Cycle Impact Assessment |
| MSWI | Municipal Solid Waste Incineration |
| NFe | Non-ferrous metals |
| POCP | Photochemical Ozone Creation Potential |
| VOC | Volatile Organic Compounds |
References
- Lombardi, F.; Lategano, E.; Cordiner, S.; Torretta, V. Waste incineration in rotary kilns: A new simulation combustion’s tool to support design and technical change. Waste Manag. Res. 2013, 31, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Viotti, P.; Luciano, A.; Fino, D. On the ASR and ASR thermal residues characterization of full scale treatment plant. Waste Manag. 2014, 34, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Viotti, P.; Luciano, A.; Raboni, M.; Fino, D. Full scale treatment of ASR wastes in a modified rotary kiln. Waste Manag. 2014, 34, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Torretta, V.; Ionescu, G.; Raboni, M.; Merler, G. The mass and energy balance of an integrated solution for municipal solid waste treatment. Wit Trans. Ecol. Environ. 2014, 180, 151–161. [Google Scholar]
- Raboni, M.; Torretta, V.; Urbini, G.; Viotti, P. Automotive shredder residue: A survey of the hazardous organic micro-pollutants spectrum in landfill biogas. Waste Manag. Res. 2015, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rapporto Rifiuti Speciali 2017 (Report on Special Waste); ISPRA: Rome, Italy, 2017. Available online: http://www.isprambiente.gov.it/it/pubblicazioni/rapporti/rapporto-rifiuti-speciali-edizione-2017 (accessed on 3 September 2018).
- Brunner, P.H.; Monch, H. The flux of metals through municipal solid waste incinerators. Waste Manag. Res. 1986, 4, 105–119. [Google Scholar] [CrossRef]
- Chang, N.B.; Wang, H.P.; Huang, W.L.; Lin, K.S. The assessment of reuse potential for municipal solid waste and refuse-derived fuel incineration ashes. Resour. Conserv. Recycl. 1999, 25, 255–270. [Google Scholar] [CrossRef]
- Bruder-Hubscher, V.; Lagarde, F.; Leroy, M.J.F. Utilisation of bottom ash in road construction: Evaluation of the environmental impact. Waste Manag. Res. 2002, 19, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.P.; McKay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Tang, P.; Yu, Q.L.; Yu, R.; Brouwers, H.J.H. The application of MSWI bottom ash fines in high performance concrete. In Proceedings of the 1st International Conference on the Chemistry of Construction Materials, Berlin, Germany, 7–9 October 2013. [Google Scholar]
- Tao, Z.; Zengzeng, Z. Optimal Use of MSWI Bottom Ash in Concrete. Int. J. Concr. Struct. Mater. 2014, 8, 173–182. [Google Scholar]
- Kockum, P.F.; Lindqvist, J.E.; Loorent, K.; Arm, M. Microstructure of aged MSWI bottom ash in road constructions. In Proceedings of the WASCON 2012 Proceedings, Gothenburg, Sweden, 30 May–1 June 2012. [Google Scholar]
- Lynn, C.J.; Ghataora, G.S.; Dhir, R.K. Municipal incinerated bottom ash (MIBA) characteristics and potential for use in road pavements. Int. J. Pavement Res. Technol. 2017, 10, 185–201. [Google Scholar] [CrossRef]
- Alhassan, H.M.; Tanko, A.M. Characterization of Solid Waste Incinerator Bottom Ash and the Potential for its Use. Int. J. Eng. Res. Appl. 2012, 2, 516–522. [Google Scholar]
- Sappa, G.; (La Sapienza University of Rome, Rome, Italy. Iter procedurale per il raggiungimento della conformità tecnica di piastrelle per pavimentazioni). Personal communication, 2016.
- Ergul, S.; Sappa, G.; Magaldi, D.; Pisciella, P.; Pelino, M. Microstructural and phase transformations during sintering of a phillipsite rich zeolitic tuff. Ceram. Int. 2011, 37, 1843–1850. [Google Scholar] [CrossRef]
- Ferronato, N.; Rada, E.C.; Gorritty, M.P.; Cioca, L.I.; Ragazzi, M.; Torretta, V. Introduction of the circular economy within developing regions: A comparative analysis of advantages and opportunities for waste valorization. J. Environ. Manag. 2019, 230, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G. Environmental and Economic Analysis of Aluminum Recycling Using the LCA Method; ICMET case, Doc. ENEA-PROT–P135–009; ICMET: Bologna, Italy, 2004. [Google Scholar]
- Crillesen, K.; Skaarup, J. Management of Bottom Ash from WTE Plants—An Overview of Management Options and Treatment Methods; Report of the International Solid Waste Association (ISWA); ISWA: London, UK, 2006. [Google Scholar]
- Bartoli, A. La valorizzazione delle scorie da incenerimento di rifiuti solidi urbani nelle infrastrutture stradali: La sperimentazione di Meta s.pa. Modena. Master’s Thesis, University of Modena and Reggio Emilia, Modena, Italy, 2003. [Google Scholar]
- Seniunaite, J.; Vasarevicius, S. Leaching of Copper, lead and zinc from municipal solid waste incineration bottom ash. Energy Procedia 2017, 113, 442–449. [Google Scholar] [CrossRef]
- Guidelines for Life-Cycle Assessment: A ‘Code of Practice’; SETAC: Brussels, Belgium, 1993.
- Rebitzera, G.; Ekvallb, T.; Frischknechtc, R.; Hunkelerd, D.; Norrise, G.; Rydbergf, T.; Schmidtg, W.-P.; Suhh, S.; Weidemai, B.P.; Pennington, D.W. Life cycle assessment Part 1: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 2004, 30, 701–720. [Google Scholar]
- Baldo, G.L.; Marino, M.; Rossi, S. Analisi Del Ciclo di Vita LCA. Gli Strumenti per la Progettazione Sostenibile di Materiali, Prodotti e Processi; Edizione Ambiente: Milan, Italy, 2008. [Google Scholar]
- Joint Research Centre-JRC. General Guide for Life Cycle Assessment—Detailed Guidance. ILCD Handbook (International Reference Life Cycle Data System Handbook); European Commission, Joint Research Centre, Institute for Environmental and Sustainability: Luxembourg City, Grand Duchy of Luxembourg, 2010. [Google Scholar]
- International Standard Organization. ISO 14040: Environmental Management-Life Cycle Assessment-Principles and Framework; ISO: Geneva, Switzerland, 1997. [Google Scholar]
- International Standard Organization. ISO 14042: Environmental Management-Life-Cycle Assessment-Life Cycle Impact Assessment; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Colombo, D. Il Riciclaggio Delle Lattine di Alluminio: Da Rifiuto a Risorsa; University of Trento: Trento, Italy, 2001; Available online: http://www.ing.unitn.it/~colombo/VAIADINO/HTM/Pres.htm (accessed on 10 January 2019).
- Barthelmy, D. Magnetite Mineral Data. Mineralogy Database. 2018. Available online: http://www.webmineral.com (accessed on 5 August 2018).
- Clarence, H. Lorig Holger Gruner. Mineral Processing. Encyclopedia Britannica Inc. 1998. Available online: https://www.britannica.com/technology/mineral-processing (accessed on 3 February 2019).
- Biganzoli, L. Aluminium Recovery from MSWI Bottom Ash. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2012; p. 159. [Google Scholar]
- Nakajima, K.; Takeda, O.; Miki, T.; Matsubae, K.; Nakamura, S.; Nagasaka, T. Thermodynamic analysis of contamination by alloying elements in aluminum recycling. Environ. Sci. Technol. 2010, 44, 5594–5600. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Infrastructures and Transport—MIT. Average Cost Per Kilometer for Diesel Consumption of Road Haulage Companies, for Third Parties; MIT: Rome, Italy, 2016. [Google Scholar]
- CiAl, Polytechnic of Milan. Separation and Recovery of Metals and Valorization of Waste Slag from Urban Waste; Federambiente: Milan, Italy, 2010. [Google Scholar]
- Guastalegname, L.; Trani, M.L. On the Energy Consumption of Construction Equipment. Master’s Thesis, Polytechnic of Milan, Milan, Italy, 2011. [Google Scholar]
- Bourtsalas, A.C.; Cheeseman, C.R.; Fthenakis, V.M. Life Cycle Analysis of Processes for Resource Recovery from Waste-To-Energy Bottom. Available online: http://www.seas.columbia.edu/earth/wtert/newwtert/meetings/meet2016/Proceedings/posters/Xu.pdf (accessed on 10 November 2018).
- Muchova, L. Wet Physical Separation of MSWI Bottom Ash. Ph.D. Thesis, Technical University of Delft, Delft, The Netherlands, 2010. [Google Scholar]
- Environmental and economic life cycle assessment of aluminum-silicon alloys production: A case study in China. J. Clean. Prod. 2012, 24, 11–19. [CrossRef]
- Energy Technology Systems Analysis Program (ETSAP). Aluminium Production. Technology Brief I10. 2012. Available online: www.etsap.org (accessed on 10 November 2018).
- Hauschild, M.; Wenzel, H.; Alting, L. Environmental Assessment of Products: Methodology, Tools and Case Studies in Product Development; Chapman and Hall. Kluwer Academic Publisher: London, UK, 1997; Volume 1. [Google Scholar]
- Hauschild, M.; Wenzel, H.; Alting, L. Environmental Assessment of Products: Scientific Background; Chapman and Hall. Kluwer Academic Publisher: London, UK, 1998; Volume 2. [Google Scholar]
- ENEA. Life Cycle Assessment: Development of Specific Indicators for Italy for the Impact Assessment Phase; Masoni, P., Scimia, E., Eds.; ENEA: Rome, Italy, 1999. [Google Scholar]
- Heijungs, R.; Guinée, J.B.; Huppes, G.; Lankreijer, R.M.; Udo de Haes, H.A.; Wegener Sleeswijk, A.; Ansems, A.M.M.; Eggels, P.G.; van Duin, R.; de Goede, H.P. Environmental Life Cycle Assessment of Products: Guide and Backgrounds; CML: Leiden, The Netherlands, 1992. [Google Scholar]
- Allegrini, E.; Vadenbo, C.; Boldrin, A.; Astrup, T.F. Life cycle assessment of resource recovery from municipal solid waste incineration bottom ash. J. Environ. Manag. 2015, 151, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, J.O. Life Cycles Assessments and Solid Waste—Guidelines for Solid Waste Treatment and Disposal in LCA; Swedish Environmental Research Institute: Stockolm, Sweden, 1999. [Google Scholar]
- Birgisdóttir, H. Life Cycle Assessment Model for Road Construction and Use of Residues from Waste Incineration. Ph.D. Thesis, Institute of Environment & Resources, Technical University of Denmark, Lyngby, Denmark, 2015. [Google Scholar]
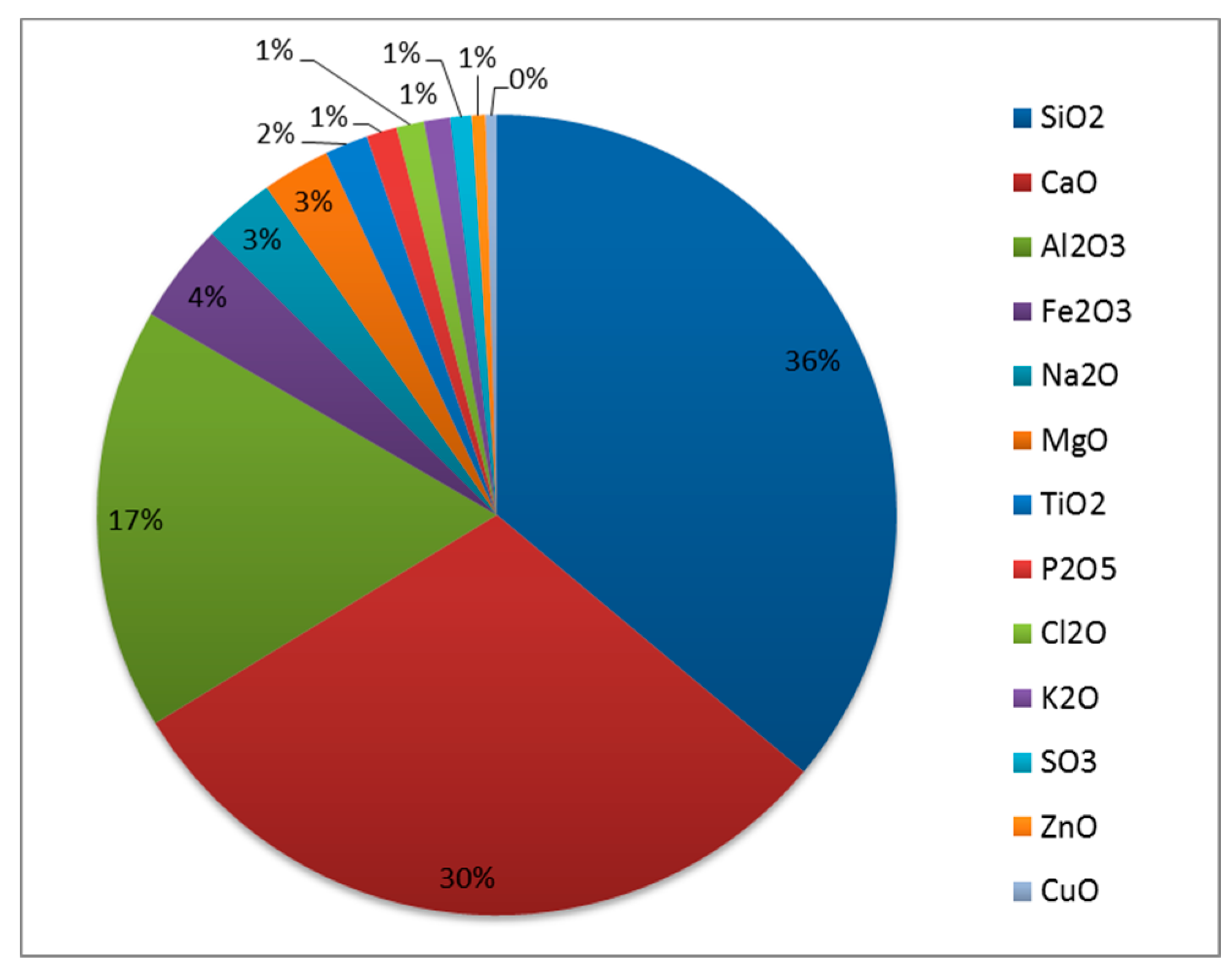
| Composition of MSWI BA | |
|---|---|
| Quantity | Elements |
| >10,000 mg/kg | Si, Fe, Ca, Al, Na, K, C |
| 1000 mg/kg > x > 10,000 mg/kg | Mg, Ti, Cl, Mn, Ba, Zn, Cu, Pb, Cr |
| <1000 mg/kg | Sn, Sb, V, Mo, As, Se, Sr, Ni, Co, Ag, Mg, B, Br, F, and I |
| Oxide | Amount (% wt) | |||||||
|---|---|---|---|---|---|---|---|---|
| Spain | Italy | Germany | Netherlands | Japan | China | Taiwan | USA | |
| SiO2 | 43.30 | 33.70 | 55.70 | 54.23 | 31.93 | 59.59 | 50.30 | 23.64 |
| CaO | 16.90 | 35.00 | 11.90 | 13.45 | 33.40 | 7.58 | 15.27 | 23.82 |
| Fe2O3 | 14.10 | 5.37 | 8.80 | 13.83 | 5.97 | 5.50 | 7.72 | 17.05 |
| Na2O | 7.58 | 2.27 | 1.40 | 2.81 | 2.53 | 1.32 | 1.30 | 1.70 |
| Al2O3 | 5.80 | 13.31 | 14.10 | 7.86 | 16.65 | 18.61 | 16.43 | 14.25 |
| MgO | 2.22 | 4.62 | 2.70 | 1.81 | 3.33 | 1.32 | n.d. | 1.85 |
| K2O | 1.11 | 1.66 | 1.20 | 0.88 | 2.22 | 2.29 | 2.14 | 0.42 |
| Scenario 1—Landfill Disposal of BA | ||||
| Process | Resource | Value | Unit | Impact |
| BA transport from incinerator to landfill [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Direct |
| Leachate (data obtained from the experimental test) | - | 10% lost humidity | l/year | Direct |
| Scenario 2—Treatment of BA for Production of Ceramic Tiles for Fe and NFe Recycling | ||||
| Process | Resource | Value | Unit | Impact |
| BA transport from incinerator to industrial plant [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Direct |
| BA treatment [35] | Electric energy | 4 | kWh/t | Indirect |
| Transport of recovered NFe from industrial plant to foundry [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Direct |
| Transport of recovered Fe from industrial plant to foundry [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Direct |
| Aluminum secondary production [19] | Electric energy | 0.75 | kWh/kg | Indirect |
| Clay transport [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Avoided |
| Clay extraction [36] | Fuel | 17.3-machine potential 80 kW | l/h | Avoided |
| Bauxite extraction [36] | Fuel | 17.3-machine potential 80 kW | l/h | Avoided |
| Bauxite transport to foundry [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Avoided |
| Bayer treatment [19] | Electric energy | 4 | kWh/kg | Avoided |
| Electrolytic process [19] | Electric energy | 20 | kWh/kg | Avoided |
| Ferrous mineral extraction [36] | Fuel | 30.3-machine potential 140 kW | l/h | Avoided |
| Ferrous mineral transport from quarry to foundry [34] | Fuel | 2.8-machine capacity 28 t | Km/l | Avoided |
| Ferrous mineral treatment [31] | Electric energy | 10 | kWh/t | Avoided |
| Process | Resource | Value | Unit | References |
|---|---|---|---|---|
| BA treatment | Electric energy | 4 * | kWh/t | [35] |
| 10 | [37] | |||
| 8 | [38] | |||
| Leachate | - | 10% lost humidity* | l/year | ** |
| 7% lost humidity | [35] | |||
| Aluminum secondary production | Electric energy | 0.75 * | kWh/kgaluminum | [19] |
| 2 | [39] | |||
| Electrolytic process | - | 20 * | kWh/kgaluminum | [19] |
| 13–17 | [39] | |||
| 12.9–15.5 | [40] |
| Global Warming (Time = 100 years) “EDIP” | |||
| Formula | Substance | Equivalence Factor | |
| Value | U.M. (100 years) | ||
| CO2 | Carbon dioxide | 1 | g CO2/g substance |
| N2O | Nitrous oxide | 320 | g CO2/g substance |
| CH4 | Methane | 25 | g CO2/g substance |
| CFCl3 | CFC-11 | 4000 | g CO2/g substance |
| CF2Cl2 | CFC-12 | 8500 | g CO2/g substance |
| CF3Cl | CFC-13 | 11,700 | g CO2/g substance |
| CF2ClCF2Cl | CFC-114 | 9300 | g CO2/g substance |
| CFC-116 | 12,500 | g CO2/g substance | |
| CCl4 | Tetrachloromethane | 1400 | g CO2/g substance |
| HCFC22 | HCFC22 | 1700 | g CO2/g substance |
| HCFC141b | HCFC141b | 630 | g CO2/g substance |
| HCFC142b | HCFC142b | 2000 | g CO2/g substance |
| CO | Carbon Monoxide | 2 | g CO2/g substance |
| CF3Br | Halon 1301 | 6200 | g CO2/ g substance |
| Acidification (“EDIP”) | |||
| Formula | Substance | Equivalence Factor | |
| Value | U.M. (100 years) | ||
| SO2 | Sulfur dioxide | 1 | g SO2/g substance |
| SO3 | Sulfur trioxide | 0.8 | g SO2/g substance |
| NO2 | Nitrogen dioxide | 0.7 | g SO2/g substance |
| NO | Nitrogen monoxide | 1.07 | g SO2/g substance |
| HNO3 | Nitric acid | 0.51 | g SO2/g substance |
| H2SO4 | Sulfuric acid | 0.65 | g SO2/g substance |
| H3PO4 | Phosphoric acid | 0.98 | g SO2/g substance |
| H2S | Hydrogen sulfide | 1.88 | g SO2/g substance |
| HF | Hydrofluoric acid | 1.6 | g SO2/g substance |
| HCl | Hydrochloric acid | 0.88 | g SO2/g substance |
| NH3 | Ammonia | 1.88 | g SO2/g substance |
| Eutrophication (“EDIP”) | |||
| Formula | Substance | Equivalence Factor | |
| Value | U.M. (100 years) | ||
| cyanide | Cyanide | 2.38 | g NO3/g substance |
| Ntot | Total nitrogen | 4.43 | g NO3/g substance |
| N2O | Nitrous oxide | 2.82 | g NO3/g substance |
| NH3 | Ammonia | 3.64 | g NO3/g substance |
| Ptot | Total phosphorus | 32.03 | g NO3/g substance |
| Photochemical Ozone Creation (“EDIP”) | |||
| Formula | Substance | Equivalence Factor | |
| Value | U.M. (100 years) | ||
| CH4 | Methane | 0.03 | g Ethylene/g substance |
| Ethane | 0.3 | g Ethylene/g substance | |
| Propane | 1.2 | g Ethylene/g substance | |
| Butane | 1.2 | g Ethylene/g substance | |
| Hexane | 1.5 | g Ethylene/g substance | |
| Heptane | 1.7 | g Ethylene/g substance | |
| Alkane | 1.2 | g Ethylene/g substance | |
| CHarom. | 0.048 | g Ethylene/g substance | |
| Methanol | 0.21 | g Ethylene/g substance | |
| Acetone | 0.27 | g Ethylene/g substance | |
| Butene | 1.2 | g Ethylene/g substance | |
| Benzene | 0.45 | g Ethylene/g substance | |
| Toluene | 0.83 | g Ethylene/g substance | |
| EthilBenzene | 1.1 | g Ethylene/g substance | |
| Formaldehyde | 0.58 | g Ethylene/g substance | |
| Acetaldehyde | 1.2 | g Ethylene/g substance | |
| Aldehyde | 1.3 | g Ethylene/g substance | |
| VOC | 0.808 | g Ethylene/g substance | |
| Human toxicity (USES 2.0) | |||
| Formula | Substance | Equivalence Factor | |
| Value | U.M. (100 years) | ||
| air | |||
| Sb | Antimony | 6200 | geq1-4-dichlorobenzene |
| As | Arsenic | 370,000 | geq1-4-dichlorobenzene |
| Ba | Barium | 710 | geq1-4-dichlorobenzene |
| Cd | Cadmium | 160,000 | geq1-4-dichlorobenzene |
| Co | Cobalt | 19000 | geq1-4-dichlorobenzene |
| Cu | Copper | 4700 | geq1-4-dichlorobenzene |
| Pb | Lead | 360 | geq1-4-dichlorobenzene |
| Hg | Mercury | 1200 | geq1-4-dichlorobenzene |
| Mo | Molybdenum | 4900 | geq1-4-dichlorobenzene |
| Ni | Nickel | 38,000 | geq1-4-dichlorobenzene |
| Se | Selenium | 43,000 | geq1-4-dichlorobenzene |
| Sn | Tin | 1.2 | geq1-4-dichlorobenzene |
| V | Vanadium | 6000 | geq1-4-dichlorobenzene |
| Zn | Zinc | 110 | geq1-4-dichlorobenzene |
| NH3 | Ammonia | 1 | geq1-4-dichlorobenzene |
| H2S | Hydrogen sulfide | 0.77 | geq1-4-dichlorobenzene |
| HCl | Hydrogen chloride | 2.40 | geq1-4-dichlorobenzene |
| C2H4 | Ethylene | 0.69 | geq1-4-dichlorobenzene |
| CH2O | Formaldehyde | 0.91 | geq1-4-dichlorobenzene |
| C6H6 | Benzene | 2000 | geq1-4-dichlorobenzene |
| C6H5CH3 | Toluene | 0.36 | geq1-4-dichlorobenzene |
| C6H5OH | Phenols | 0.57 | geq1-4-dichlorobenzene |
| CHCl3 | Chloroform | 12 | geq1-4-dichlorobenzene |
| 1,2CH2ClCH2Cl | Dichloroethane | 7 | geq1-4-dichlorobenzene |
| water | |||
| As | Arsenic | 880 | geq1-4-dichlorobenzene |
| Ba | Barium | 570 | geq1-4-dichlorobenzene |
| Cd | Cadmium | 23 | geq1-4-dichlorobenzene |
| Cr | Chrome | 2.1 | geq1-4-dichlorobenzene |
| Co | Cobalt | 99 | geq1-4-dichlorobenzene |
| Cu | Copper | 1.3 | geq1-4-dichlorobenzene |
| Pb | Lead | 12 | geq1-4-dichlorobenzene |
| Hg | Mercury | 250 | geq1-4-dichlorobenzene |
| Mo | Molybdenum | 5000 | geq1-4-dichlorobenzene |
| Ni | Nickel | 310 | geq1-4-dichlorobenzene |
| Se | Selenium | 51,000 | geq1-4-dichlorobenzene |
| Sn | Tin | 0.017 | geq1-4-dichlorobenzene |
| V | Vanadium | 2900 | geq1-4-dichlorobenzene |
| Zn | Zinc | 0.57 | geq1-4-dichlorobenzene |
| CH2O | Formaldehyde | 0.04 | geq1-4-dichlorobenzene |
| C6H6 | Benzene | 1900 | geq1-4-dichlorobenzene |
| C6H5CH3 | Toluene | 0.33 | geq1-4-dichlorobenzene |
| C6H5OH | Phenols | 0.054 | geq1-4-dichlorobenzene |
| soil | |||
| As | Arsenic | 490 | geq1-4-dichlorobenzene |
| Cd | Cadmium | 90 | geq1-4-dichlorobenzene |
| Co | Cobalt | 61 | geq1-4-dichlorobenzene |
| Cu | Copper | 3.2 | geq1-4-dichlorobenzene |
| Pb | Lead | 180 | geq1-4-dichlorobenzene |
| Hg | Mercury | 200 | geq1-4-dichlorobenzene |
| Mo | Molybdenum | 2800 | geq1-4-dichlorobenzene |
| Ni | Nickel | 160 | geq1-4-dichlorobenzene |
| Se | Selenium | 25,000 | geq1-4-dichlorobenzene |
| TI | Thallium | 100,000 | geq1-4-dichlorobenzene |
| Sn | Tin | 32 | geq1-4-dichlorobenzene |
| V | Vanadium | 1600 | geq1-4-dichlorobenzene |
| Zn | Zinc | 0.35 | geq1-4-dichlorobenzene |
| C6H5Cl | Chlorobenzene | 7.1 | geq1-4-dichlorobenzene |
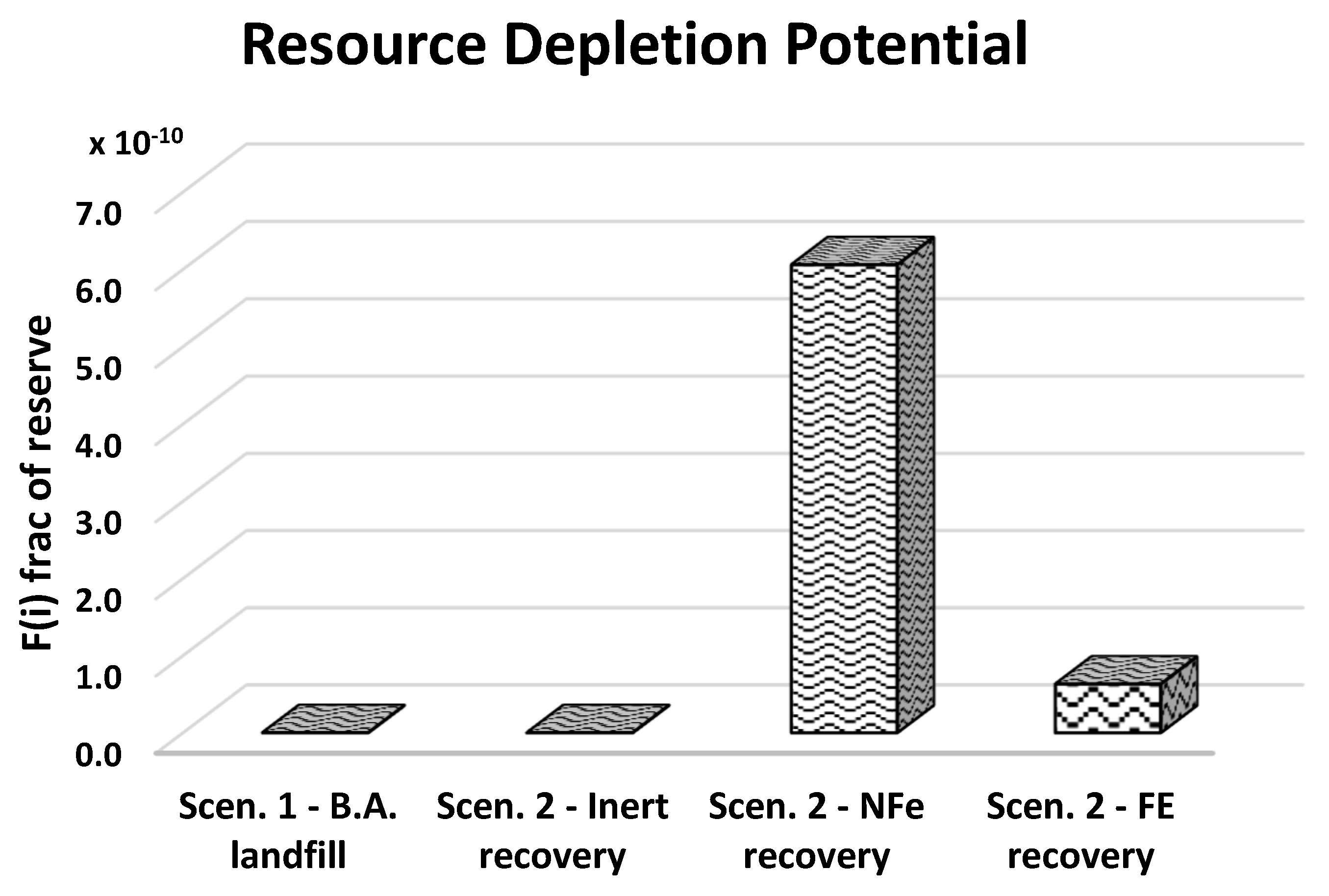
| Depletion of non-renewable resources | |||
| Formula | Substance | Reserve available | |
| value | U.M. (100 years) | ||
| R(i) | F(i): frac of reserve | ||
| Oil (in ground) | 2.4 × 1014 | 4.17 × 10−15 | |
| Natural gas | 1.3 × 1014 | 7.69 × 10−15 | |
| U | Uranium (hours) | 1.3 × 1010 | 7.69 × 10−11 |
| Cu | Copper (hours) | 6.1 × 1011 | 1.64 × 10−12 |
| Pb | Lead (hours) | 1.2 × 1011 | 8.33 × 10−12 |
| Ni | Nickel | 1.1 × 1011 | 9.09 × 10−−12 |
| Zn | Zinc | 3.3 × 1011 | 3.03 × 10−12 |
| Al2O3 | Bauxite | 2.8 × 1013 | 3.57 × 10−14 |
| Fe | Iron | 1.0 × 1014 | 1.00 × 10−14 |
| Mn | Manganese | 5.0 × 1012 | 2.00 × 10−13 |
| Ag | Silver | 4.2 × 108 | 2.38 × 10−9 |
| Coal (in ground) | 3.0 × 1015 | 3.33 × 10−16 | |
| Unit | Scen. 1-BA Landfill | Scen. 2-Inert Recovery | Scen. 2-NFe Recovery | Scen. 2-FE Recovery | |
| GWP | |||||
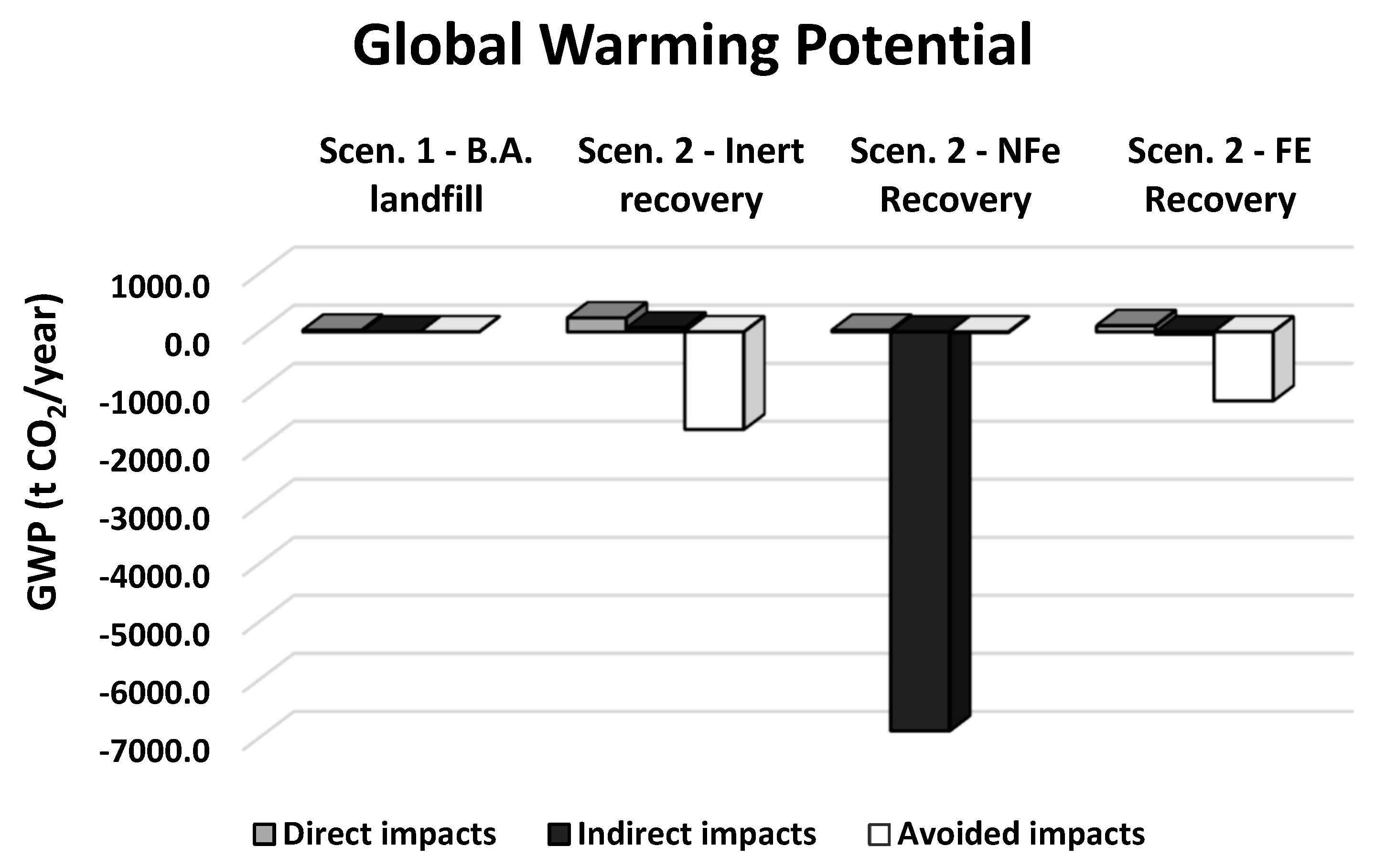
| Direct impacts | t CO2eq/year | 28.500 | 241.000 | 29.500 | 107.000 |
| Indirect impacts | t CO2eq/year | 0.000 | 69.100 | −6880.000 | −42.900 |
| Avoided impacts | t CO2eq/year | 0.000 | −1680.000 | −12.100 | −1190.000 |
| Total impacts | t CO2eq/year | 28.500 | −1370.000 | −6860.000 | −1120.000 |
| AP | |||||
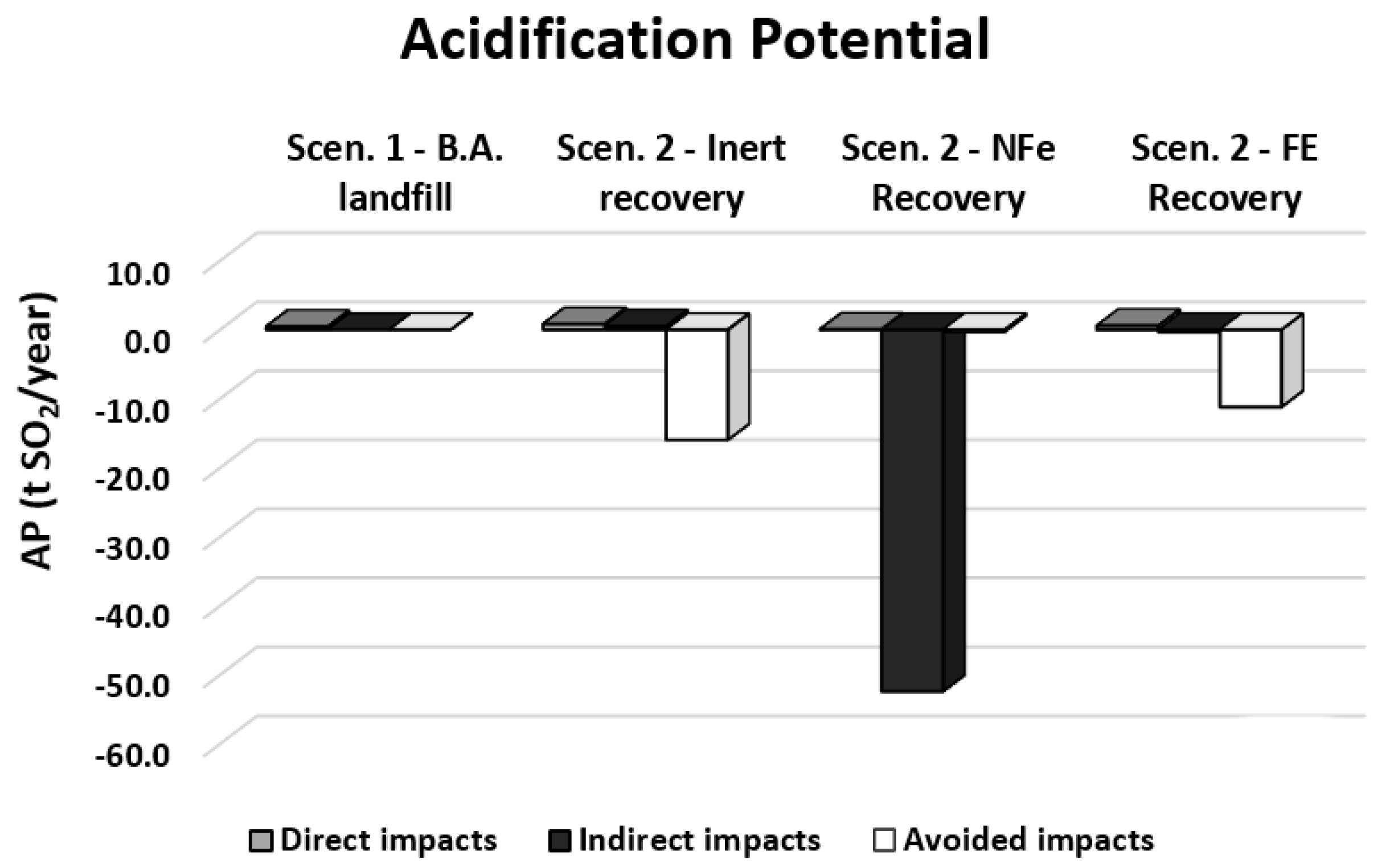
| Direct impacts | t SO2eq/year | 0.460 | 0.838 | 0.062 | 0.613 |
| Indirect impacts | t SO2eq/year | 0.000 | 0.527 | −52.400 | −0.327 |
| Avoided impacts | t SO2eq/year | 0.000 | −16.000 | −0.297 | −11.200 |
| Total impacts | t SO2eq/year | 0.460 | −14.600 | −52.600 | −10.900 |
| EP | |||||
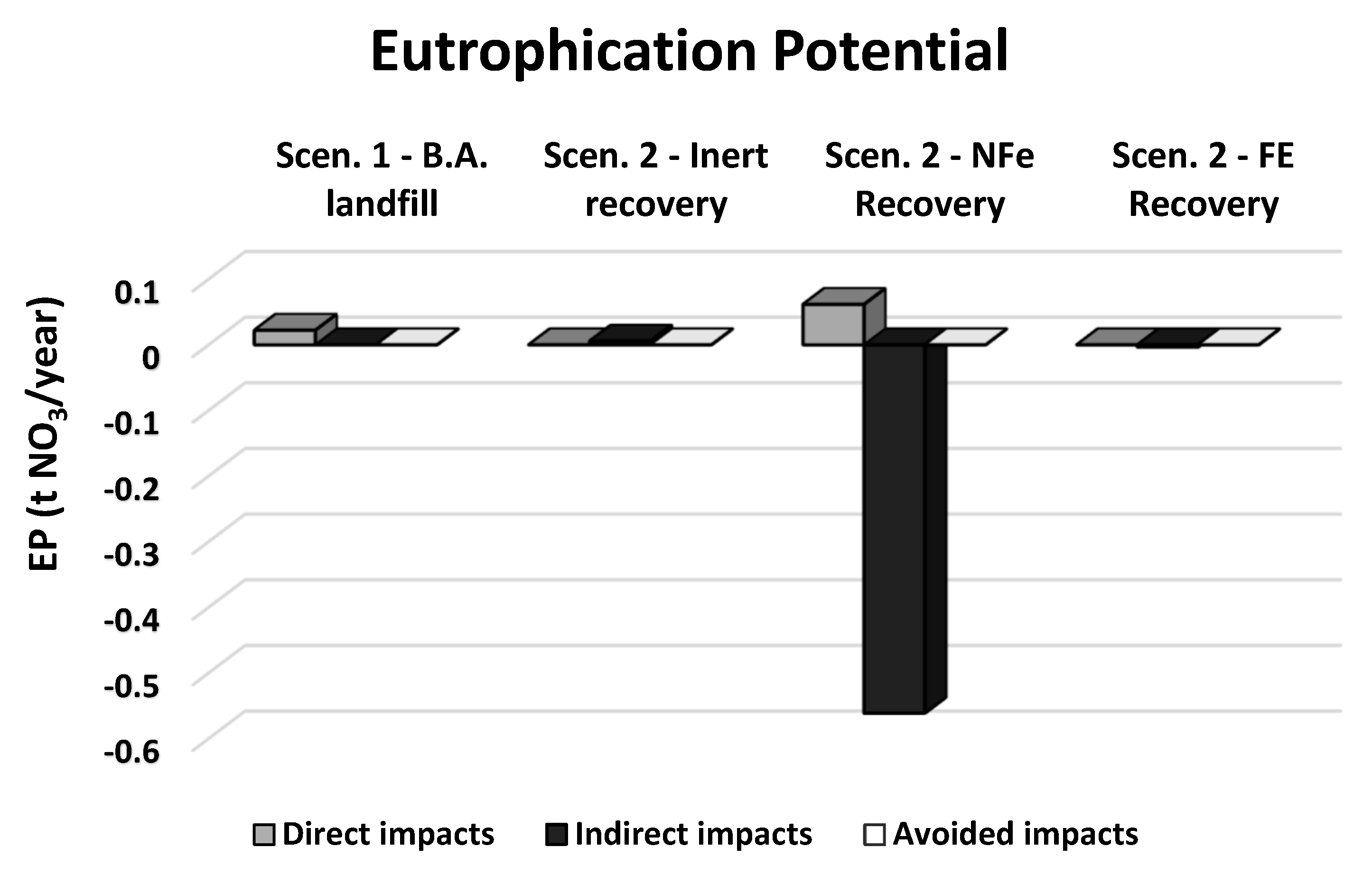
| Direct impacts | t NO3eq/year | 0.022 | 0.000 | 0.062 | 0.000 |
| Indirect impacts | t NO3eq/year | 0.000 | 0.006 | −0.561 | −0.004 |
| Avoided impacts | t NO3eq/year | 0.000 | 0.000 | 0.000 | 0.000 |
| Total impacts | t NO3eq/year | 0.022 | 0.006 | −0.500 | −0.004 |
| POCP | |||||
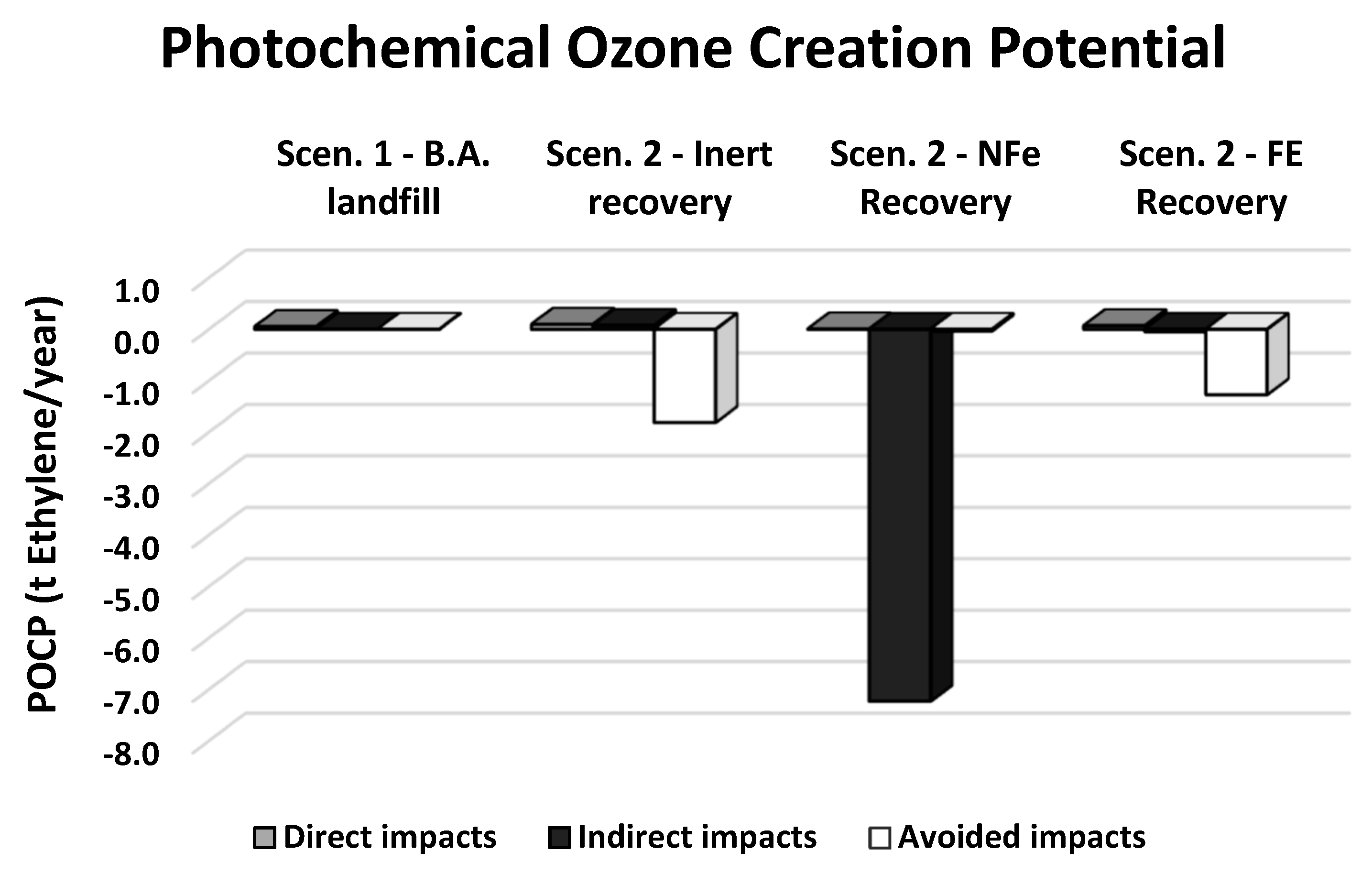
| Direct impacts | t Ethyleneeq/year | 0.052 | 0.095 | 0.007 | 0.070 |
| Indirect impacts | t Ethyleneeq/year | 0.000 | 0.073 | −7.230 | −0.045 |
| Avoided impacts | t Ethyleneeq/year | 0.000 | −1.810 | −0.034 | −1.270 |
| Total impacts | t Ethyleneeq/year | 0.052 | −1.640 | −7.260 | −1.250 |
| HTP | |||||
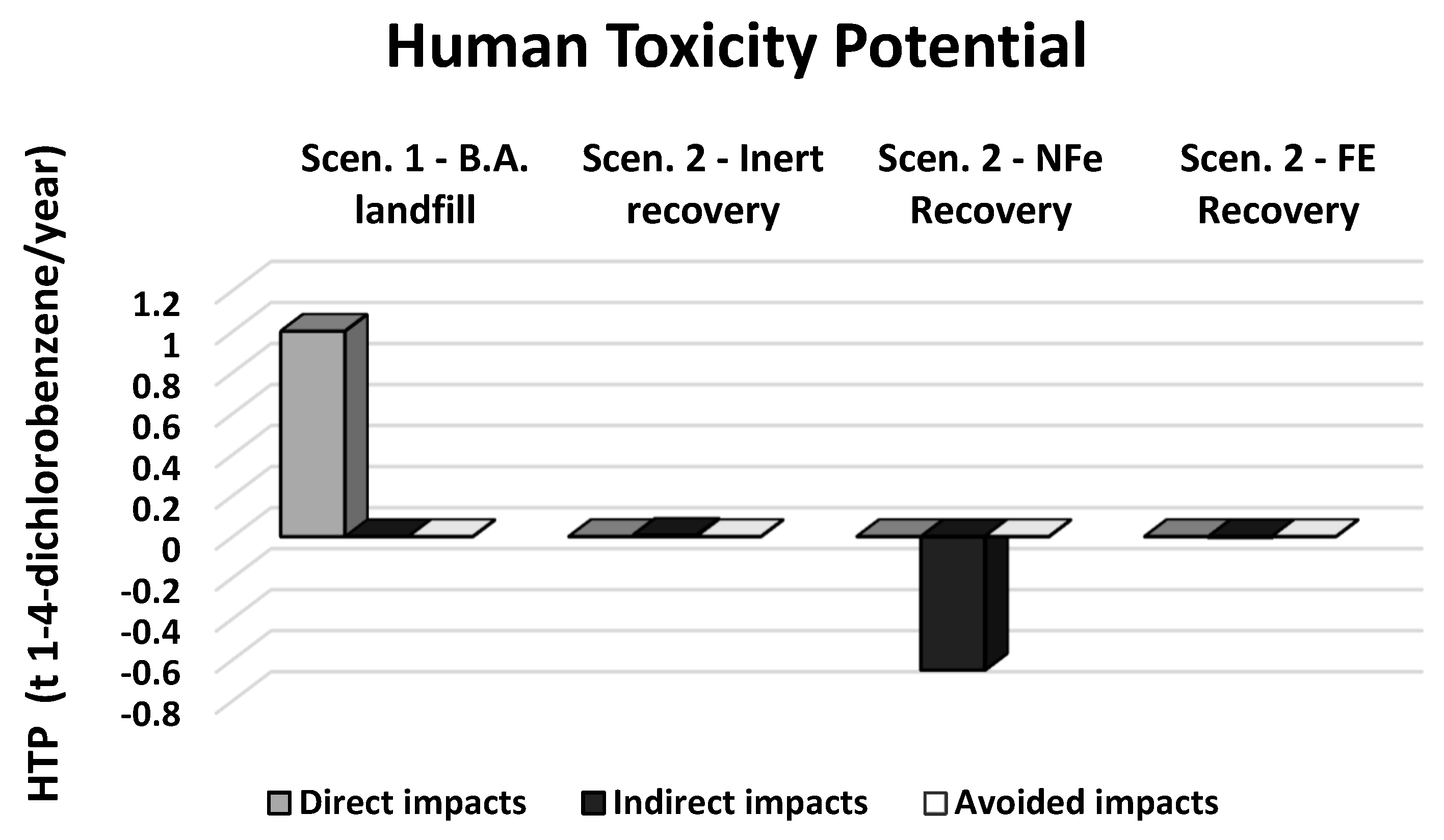
| Direct impacts | t 1-4-dichlorobenzeneeq/year | 1.000 | 0.000 | 0.000 | 0.000 |
| Indirect impacts | t 1-4-dichlorobenzeneeq/year | 0.000 | 0.007 | −0.651 | −0.004 |
| Avoided impacts | t 1-4-dichlorobenzeneeq/year | 0.000 | 0.000 | 0.000 | 0.000 |
| Total impacts | t 1-4-dichlorobenzeneeq/year | 1.000 | 0.007 | −0.651 | −0.004 |
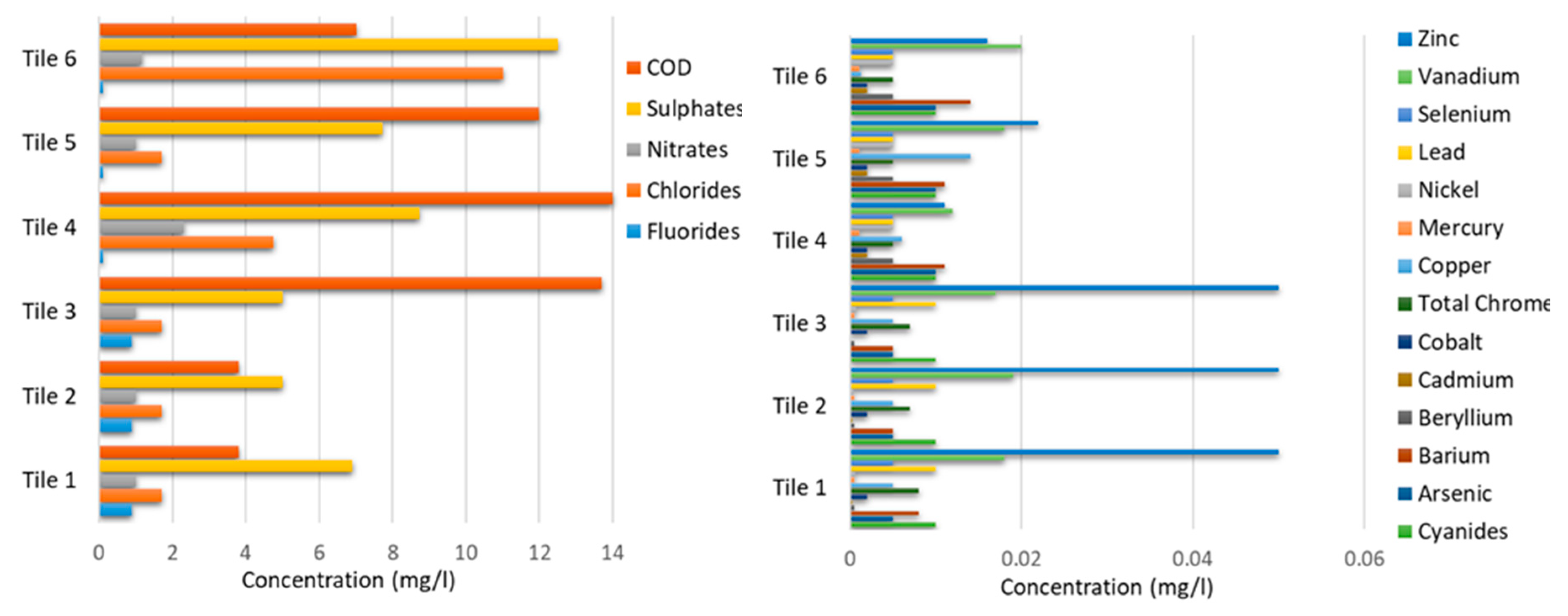
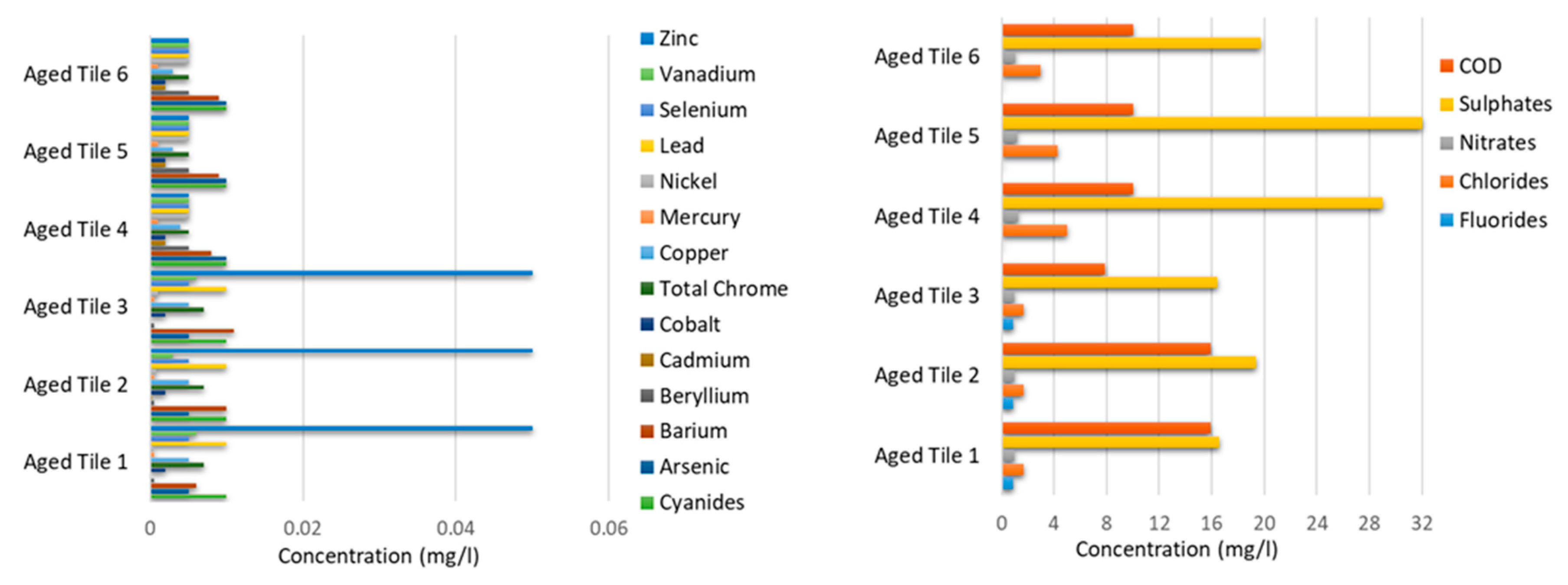
| Parameter | Unit | Limit Values | Bottom Ashes 1 | Bottom Ashes 2 | Bottom Ashes 3 | Bottom * Ashes 4 | Bottom * Asesh 5 | Bottom * Ashes 6 |
|---|---|---|---|---|---|---|---|---|
| Fluorides | mg/L | ≤1.5 | 0.121 | 0.138 | 0.16 | <0.9 | <0.9 | <0.9 |
| Chlorides | mg/L | ≤100 | 78.3 | 113 | 165 | 78.9 | 173 | 168 |
| Nitrates | mg/L | ≤50 | 2.27 | 2.24 | 2.44 | 4.3 | <1 | <1 |
| Sulphates | mg/L | ≤250 | 3.53 | 3.64 | 4.32 | <5 | <5 | 8.8 |
| Cyanides | mg/L | ≤0.05 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Arsenic | mg/L | ≤0.05 | <0.01 | <0.01 | <0.01 | 0.005 | <0.005 | <0.005 |
| Barium | mg/L | ≤1 | 4.18 | 2.76 | 2.5 | 4.4 | 3.4 | 4.2 |
| Beryllium | mg/L | ≤0.01 | <0.005 | <0.005 | <0.005 | <0.0005 | <0.0005 | <0.0005 |
| Cadmium | mg/L | ≤0.005 | <0.002 | <0.002 | <0.002 | 0.0003 | <0.0001 | <0.0001 |
| Cobalt | mg/L | ≤0.25 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 |
| Total Chrome | mg/L | ≤0.05 | 0.009 | 0.011 | 0.013 | 0.008 | 0.016 | 0.011 |
| Copper | mg/L | ≤0.05 | 0.085 | 0.095 | 0.07 | 0.059 | 0.08 | 0.076 |
| Mercury | mg/L | ≤0.001 | <0.001 | <0.001 | <0.001 | <0.0005 | <0.0005 | <0.0005 |
| Nickel | mg/L | ≤0.01 | <0.005 | <0.005 | <0.005 | <0.0004 | <0.0004 | <0.0004 |
| Lead | mg/L | ≤0.05 | 2.3 | 1.39 | 1.13 | 3.8 | 3.6 | 7.2 |
| Selenium | mg/L | ≤0.01 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 | <0.005 |
| Vanadium | mg/L | ≤0.25 | 0.007 | 0.007 | <0.005 | <0.003 | <0.003 | <0.003 |
| Zinc | mg/L | ≤3 | 1.11 | 0.64 | 0.53 | 1.3 | 0.87 | 1.2 |
| COD | mg/L | ≤30 | 59 | 45 | 80 | 69 | 113 | 97.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sappa, G.; Iacurto, S.; Ponzi, A.; Tatti, F.; Torretta, V.; Viotti, P. The LCA Methodology for Ceramic Tiles Production by Addition of MSWI BA. Resources 2019, 8, 93. https://doi.org/10.3390/resources8020093
Sappa G, Iacurto S, Ponzi A, Tatti F, Torretta V, Viotti P. The LCA Methodology for Ceramic Tiles Production by Addition of MSWI BA. Resources. 2019; 8(2):93. https://doi.org/10.3390/resources8020093
Chicago/Turabian StyleSappa, Giuseppe, Silvia Iacurto, Adelaide Ponzi, Fabio Tatti, Vincenzo Torretta, and Paolo Viotti. 2019. "The LCA Methodology for Ceramic Tiles Production by Addition of MSWI BA" Resources 8, no. 2: 93. https://doi.org/10.3390/resources8020093
APA StyleSappa, G., Iacurto, S., Ponzi, A., Tatti, F., Torretta, V., & Viotti, P. (2019). The LCA Methodology for Ceramic Tiles Production by Addition of MSWI BA. Resources, 8(2), 93. https://doi.org/10.3390/resources8020093









