1. Introduction
With the world’s energy demands growing, oil recovery has become a global priority. The development of oil extraction techniques has gradually changed and improved in order to increase oil recovery. Worldwide, oil production only recovers on average about a third of the oil originally present in reservoirs, which is the amount of oil recovered economically using conventional methods. The remaining two-thirds of residual oil in the reservoirs is the focus of Enhanced Oil Recovery (EOR) methods [
1].
Microbial enhanced oil recovery (MEOR) represents the use of microbes in oil recovery, which is a tertiary EOR technique still under development. MEOR is widely appropriate in carbonate and sandstone reservoirs for both light and heavy crude oil. MEOR has been proposed as an effective and cheap alternative for EOR, and involves utilising the metabolic products the microorganisms produce to aid the oil production. In general, there are seven types of bioproducts, which include biopolymers, bio-surfactants, biomass, solvents, acids, emulsifiers, and gases. It is noted that bio-surfactants are more attractive compared to other bioproducts due to their low toxicity, stability, and impact on the wettability alteration to increase enhanced oil recovery [
2].
Bio-surfactants are amphiphilic, consisting of both hydrophobic and hydrophilic parts. Bio-surfactants have the potential to lower the surface and interfacial tension (IFT) by gathering at the interface between the two immiscible fluids. This reduces the repulsive forces and allows the two phases to interact and mix more easily; it also improves the mobility and solubility of the insoluble or hydrophobic organic compounds [
3,
4].
Rhamnolipids are a type of bio-surfactant that are produced mainly by
Pseudomonas aeruginosa. It should be noted that because of their low toxicity, production from renewable sources, and antimicrobial (particularly antifungal) activity, the use of rhamnolipid bio-surfactants shows great promise for broad commercial application [
5].
The concentration of a bio-surfactant is effective until the critical micelle concentration (CMC) is obtained. CMC is associated with micelle formation, which allows bio-surfactants to reduce the IFT and increase the solubility and bioavailability of a hydrophobic compound. CMC is known as the efficiency of the bio-surfactant, where less bio-surfactant is required to lower the IFT; an effective bio-surfactant will have a low CMC [
6]. The remaining oil residual in reservoirs is generally located in restricted access regions where oil is trapped in pores by capillary pressure. Bio-surfactants can lower the IFT between oil/rock and water/oil and also alter the wettability to water-wet the system—this reduces the capillary forces, which allows water to move through the rock pores [
7].
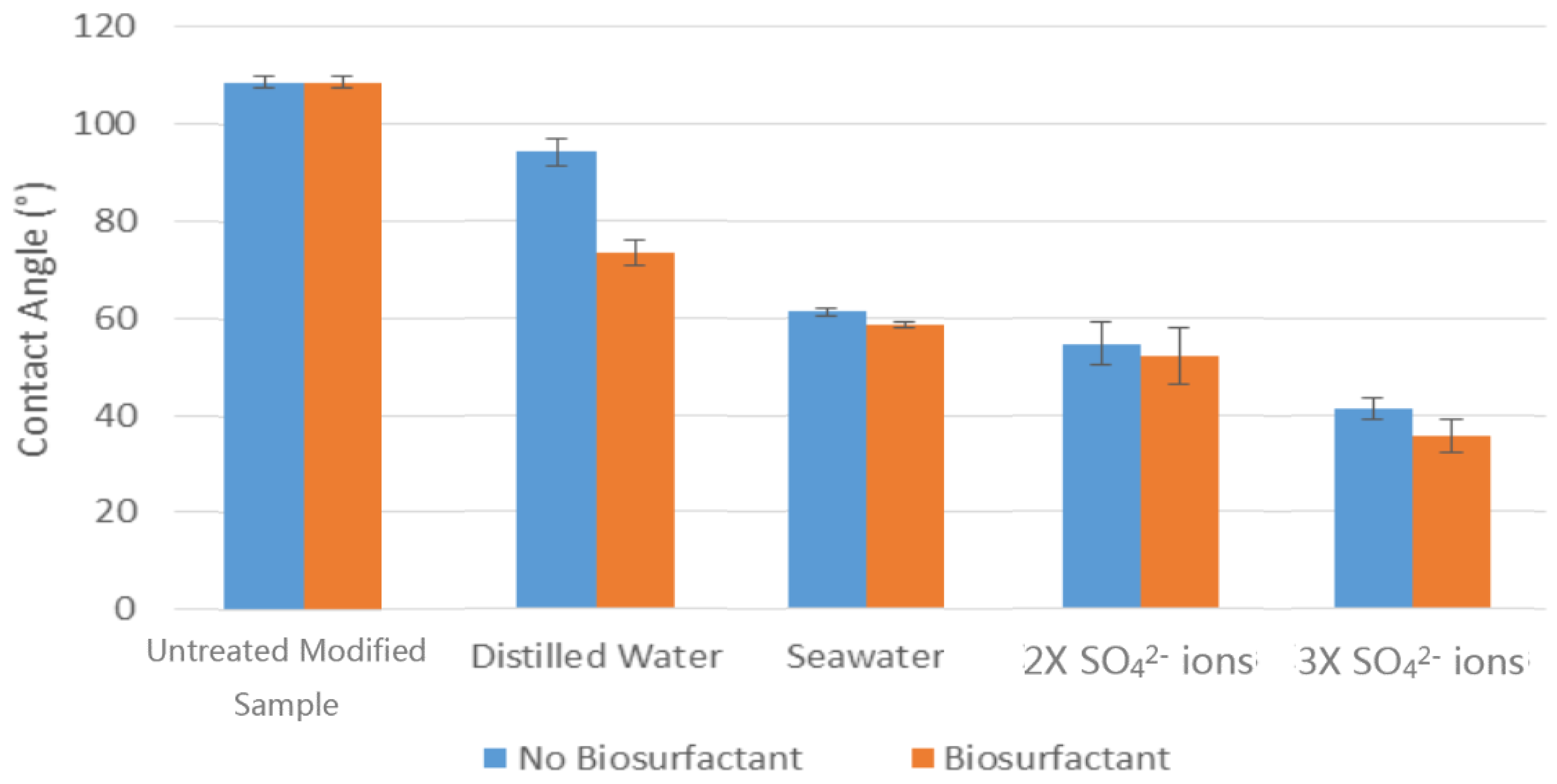
Al-Sulaimani et al. [
8] found that the maximum production of residual oil went up by 50% by using bio-surfactant and chemical surfactant in a 50:50 mixing ratio. The study also looked at the influence of the bio-surfactant on the wettability of calcite surface by studying contact angle measurements. The results showed that a 0.25% treated (
w/
v) bio-surfactant solution changed to greater water-wetting as the angle decreased from 70.6 to 25.32°. Other studies [
9,
10,
11] presented similar trends in the use of different bio-surfactants for the displacement of oil by water.
Wettability tests performed on carbonate reservoirs at elevated temperature have showed that increasing temperatures alter the wettability towards greater water-wetting, which was attributed to the de-attachment of oil-wet particles (i.e., calcium stearate) from the calcite surface, making the surface more water-wet [
12,
13,
14]. In general, wettability and IFT are considered to be the main controlling parameters of fluid flow in carbonate rocks, particularly for high temperature reservoir conditions.
Water salinity has also been shown to have a significant impact via an increase of the capillary force, lowering of the IFT, and altering of the wettability of carbonate reservoirs [
15,
16]. Seawater is an example of brine water that contains different active ions such as calcium (Ca
2+), magnesium (Mg
2+), and sulphate (SO
42−) ions [
17,
18]. By injecting brine water into the rock fracture, SO
42− ions are adsorbed onto the positive calcite surface. This decreases the rock positive charges, which also reduces the electrostatic repulsion, thereby allowing cation ions to move closer to the surface. The presence of cation ions will bind with the negatively charged carboxylic acids and remove them from the rock surface. As the temperature increases, the concentration of Ca
2+ and SO
42− near the surface increases, increasing the efficiency of the displacement of carboxylic groups. The removal of adsorbed carboxylic groups from the rock surface reduces the capillary forces, which improves oil recovery [
19].
The main objective of this research was to assess the alteration in wettability of modified oil-wet calcium carbonate and IFT reduction under the influence of bio-surfactants. Moreover, this study investigated the effectiveness of bio-surfactants in the wetting alteration process as well as the fluid/fluid interfacial tension in the presence of most active sulphate ions at elevated temperature.
4. Conclusions
Many research studies have been carried out to find cheap, effective, and environmentally friendly methods to recover the remaining oil residual in the reservoirs. The objective of this research was to obtain more detailed information on the mechanism and effects of a bio-surfactant (rhamnolipids), on interfacial activities of oil/water and wettability of calcium carbonate as a substitute for carbonate rock in presence of salt and at elevated temperature.
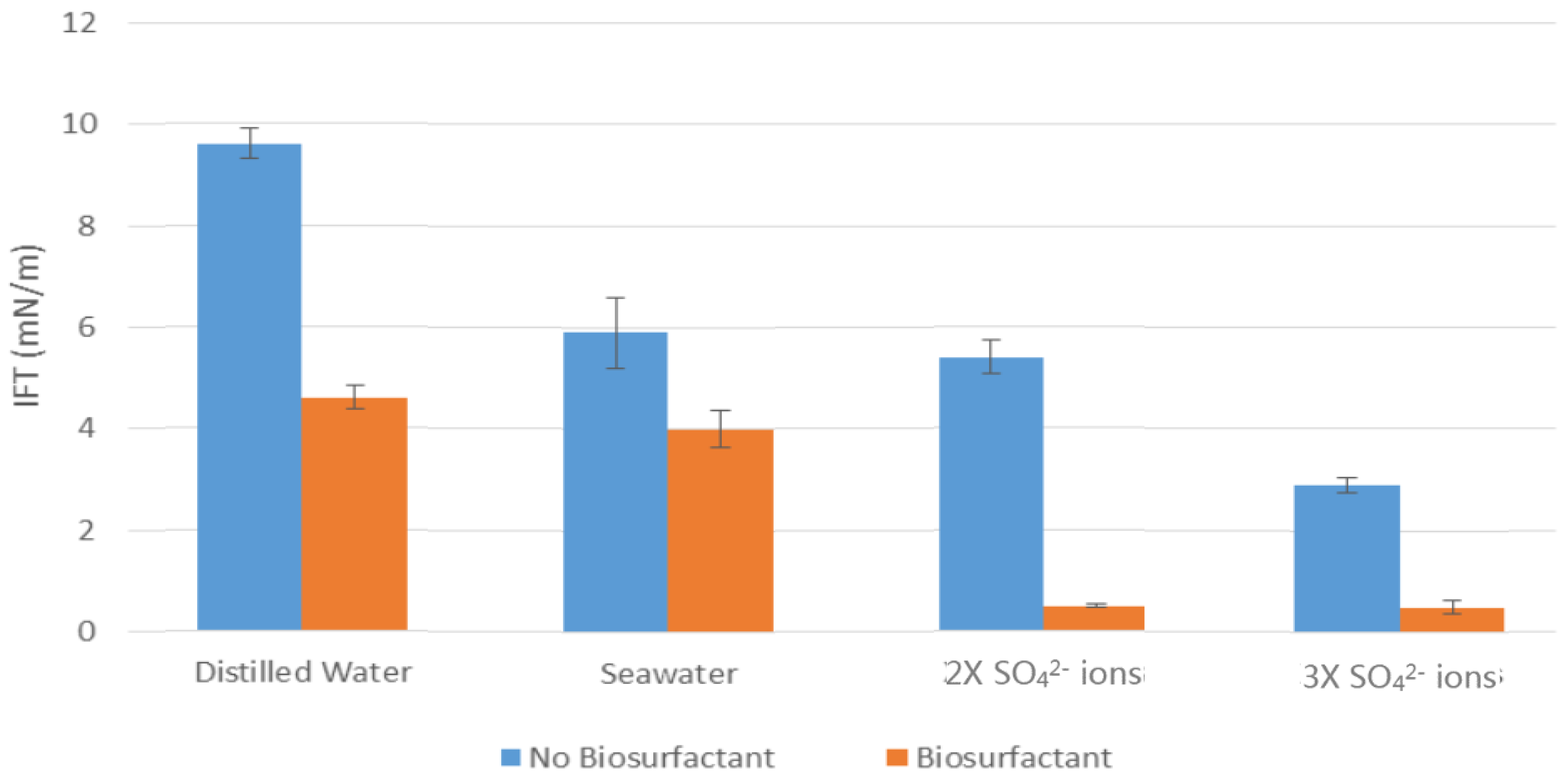
The results showed that rhamnolipids is capable to alter the wettability of carbonate rocks and reduce the IFT between oil and water. Rhamnolipids successfully improved the modified oil-wet rock to water-wet rock, and the observation of the IFT showed the existence of a large decrease even under the disturbance of different brine waters. The increase of SO42− showed a positive impact on the rocks wettability and IFT, which also helps to lower the IFT and modify the rocks more towards water-wet. The resultant diagrams showed that presence of salt had a greater effect on the contact angle reduction, whereas rhamnolipids were better at reducing the IFT.
The results showed that increasing temperature conditions had undesired effects on the wettability and IFT, as well as on the performance of the bio-surfactant. Even though addition of biosurfactant to water solutions under higher temperature showed the rock surface remaining oil-wet, but the salinity were not affected as the results still demonstrates a reduction in the contact angle and IFT measurement after increase in temperature.