Abstract
Chemical and biological leaching is practiced on a commercial scale for the mining of metals from ores. Although bioleaching is an environmentally-friendly alternative to chemical leaching, one of the principal shortcomings is the slow rate of leaching which needs to be addressed. The application of ultrasound in bioleaching, termed sonobioleaching, is a technique which has been reported to increase the rate and extent of metal extraction. This article reviews efforts made in the field of sonobioleaching. Since bioleaching is effectively a biological and chemical process, the effects of sonication on chemical leaching/reactions and biological processes are also reviewed. Although sonication increases metal extraction by increasing the metabolite production and enhanced mixing at a micro scale, research is limited in terms of the microorganisms explored. This paper highlights some shortcomings and limitations of existing techniques, and proposes directions for future research.
1. Introduction
Chemical and biological leaching processes are practiced in industry for the mining of metals from ores. Solid wastes often contain heavy metals which are hazardous in nature but may also be considered as secondary ores and hence are valuable from the viewpoint of resource recovery and conservation. Using the same leaching principles, these processes have been used to recover metals from solid waste. Although chemical leaching has been practiced commercially for metal mining from ores (e.g., copper and zinc), bioleaching plants are comparatively less abundant [1,2,3,4]. Bioleaching is an environmentally-friendly alternative for the extraction of heavy/valuable metals from secondary sources, such as spent catalyst, fly ash, mine tailings, and low-grade ores. Heap bioleaching, stirred tank reactor, and column bioleaching are widely used in the bioleaching of low grade ores in the industry [5,6]. Depending on the metal(s) of interest and ore mineralogy, heap bioleaching or stirred tank reactors are used on an industrial scale. Low cost heap bioleaching is affected by various environmental factors and research has focused on examining the impact of various parameters or process optimization [6]. Unfortunately, the slow rate of bioleaching often presents a barrier to commercialization [7].
Researchers have investigated various strategies to overcome this drawback [8,9,10,11,12,13]. The use of ultrasound has been investigated, although this technique is limited to only tank-type reactors. It has been observed that the enhancement in convective diffusivity in the medium and particle fragmentation caused by sonication augments the leaching reactions [14]. These ultrasound effects are due to cavitation, which increases the temperature and pressure conditions within the reactions. Ultrasonic cavitation enhances metal dissolution in the leaching reactions by chemical agents (or metabolites produced by microorganisms in the case of bioleaching). Since a higher increase in the rate of leaching reportedly occurs at low acid concentrations, this makes the application of ultrasound more promising for bioleaching where low metabolite concentration typically occurs [14]. Unfortunately, ultrasound at high intensity may adversely affect microbial growth. Owing to this, very few bioleaching studies with ultrasound have been reported. This article reviews the effect of ultrasound in chemical reaction/leaching, biological processes, and bioleaching.
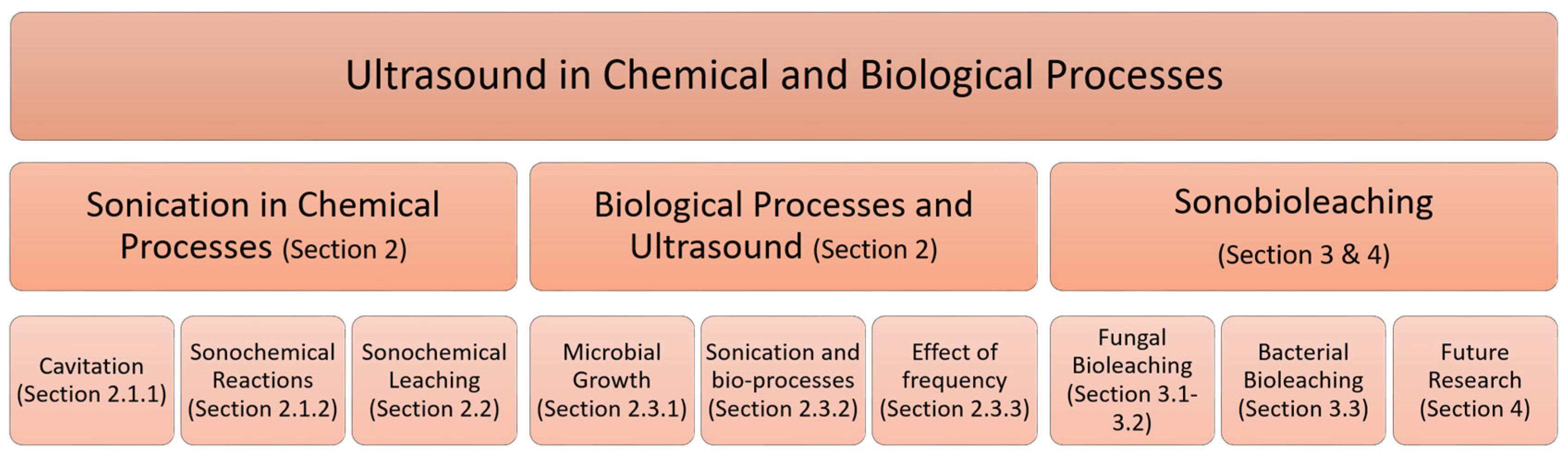
Figure 1 shows an outline of this review. In Section 2, we discuss the applications of ultrasound in chemical, and biological processes. Sonobioleaching is reviewed and discussed in Section 3 and Section 4.
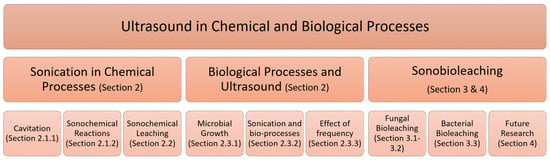
Figure 1.
Ultrasound in chemical and biological processes as categorized in the review.
2. Ultrasound in Chemical and Biological Systems
The application of ultrasound has been studied in various chemical and biological systems. The following subsections discuss the effect of ultrasound in these systems.
2.1. Sonochemical Reactions and Applications
Ultrasound-assisted chemical reactions are termed as sonochemical reactions. Various theories, such as hotspot theory and electrical theory, have been proposed to explain the effects of sonication [15,16,17]. We will discuss this in detail in the following subsection.
2.1.1. Ultrasound and Cavitation
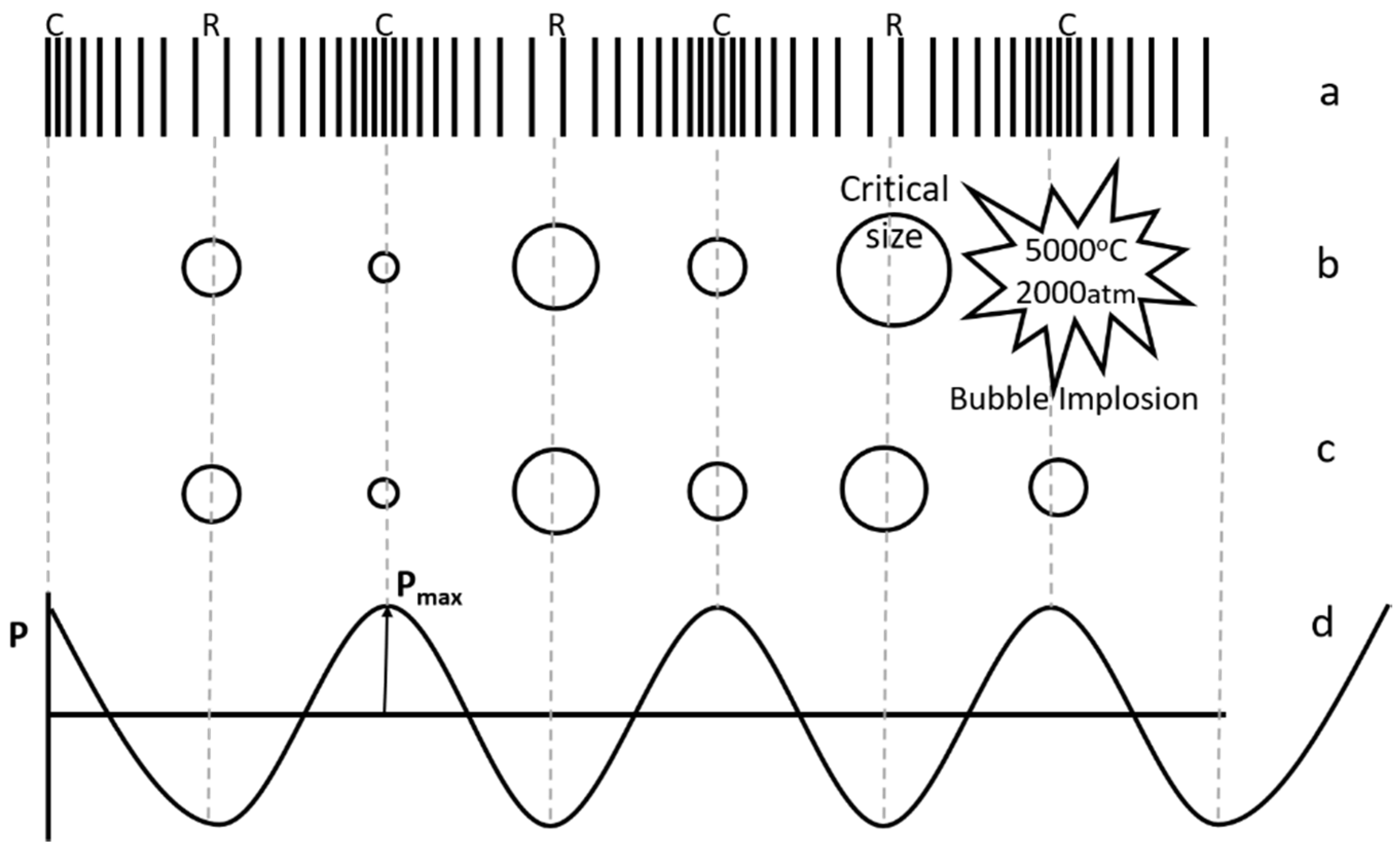
Hotspot theory, which is based on cavitation, is widely accepted in comparison with electrical theory [18,19,20]. When ultrasound propagates through any liquid medium, it compresses and stretches the molecular spacing in the medium (Figure 2a). Pressure (P) variation during compression (C, where P = +Pmax) and rarefaction/stretching (R, where P = −Pmax) can be represented as a sinusoidal wave function (Figure 2d). When the distance between the molecules of liquid exceeds the minimum molecular distance required to hold the liquid intact, cavitation bubbles containing liquid vapors and dissolved gases are formed. These bubbles increase in size with each cycle and implode after reaching a critical size during subsequent compression, leading to high temperature and pressure conditions (Figure 2b). This phenomenon, termed as cavitation, can be stable or transient, depending on ultrasonic intensity. Transient bubbles are formed at higher intensities and collapse violently after undergoing one or two acoustic cycles (Figure 2b), while bubbles formed during stable cavitation may or may not collapse after many cycles (Figure 2c). For sonochemical effects, transient cavitation is considered more important. Experimental estimates of temperature and pressure are in the range of 750–6000 K and about 500–1800 atm respectively [19,20]. High temperature and pressure conditions observed at the microscopic level provide the conditions necessary for the various reactions. Cavitation and chemical effects are not observed when the sonication intensity is lower than a minimum value, known as the cavitation threshold. A higher threshold intensity is also observed when there are no dissolved gases in the system [18].
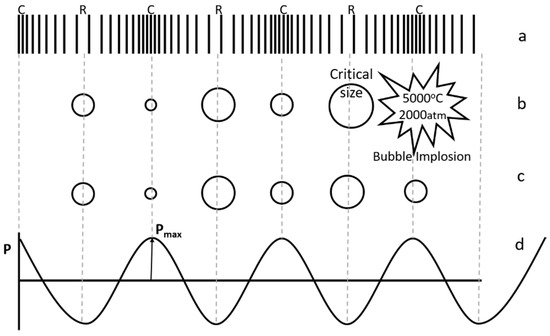
Figure 2.
Ultrasonic wave propagation (a) compression and rarefaction, (b) stable ultrasonic cavitation, (c) transient ultrasonic cavitation, (d) pressure variation during compression and rarefaction.
2.1.2. Impact of Ultrasound on Chemical Reactions
Sonochemical reactions take place in systems involving either aqueous or organic solvents. In aqueous reactions, besides temperature and pressure effects, the formation of free radicals (Equations (1)–(4)) have also been reported [21,22,23,24]. As bioleaching reactions take place in aqueous systems, free radical reactions are also likely to play a role.
In organic systems, single electron transfer (SET) mechanisms are accelerated with ultrasound where an electron-rich species gives away an electron to an electron-poor molecule and forms free radicals (cation and anion). Depending on the reaction parameters, sonication can change the reaction pathway by favoring SET mechanisms in reactions that follow either an ionic or free-radical pathway (Equations (1)–(4)) [15,25].
H2O → H• + HO•
H• + O2 → HOO•
2HO• → H2O2
2HOO• → H2O2
Ultrasound also increases the rate of the reaction between two immiscible liquids by the formation of a very fine emulsion which significantly increases the surface area available for the reaction between the two phases [15]. One example is the reduced reaction time in the preparation of biodiesel because of the cavitation and emulsification of immiscible reactants [26,27].
Ultrasound has found applications in various fields, such as chemical synthesis, polymerisation, polymer degradation, luminescence, chemical leaching, biological processes and bioleaching [11,22,28,29]. Both homogenous and heterogeneous synthesis reactions involving metals (as catalyst or reactant) and otherwise are enhanced on sonication [22]. Organometallic reactions such as transition-metal-promoted free radical reactions are also enhanced by sonication. These reactions are either oxidative or reductive where the cleavage of transition metal from organometallic compounds leads to the formation of carbon-centered free radicals [30]. Free radicals, which are formed in ultrasound, also enhance polymerisation reactions [31,32] although prolonged exposure to high intensity ultrasound has been reported to degrade the polymers [33]. In some reactions, the application of ultrasound leads to the emission of light as a result of cavitation, and is termed sonoluminescence. The emission of light increases on the addition of chemicals such as luminol and is termed sonochemical luminescence [29,34,35]. Increased luminescence owing to mechanical and thermal effects is also reported on the application of ultrasound [36].
2.2. Sonochemical Leaching
The application of ultrasound in chemical leaching is termed sonochemical leaching. Chemical leaching reactions are heterogeneous (liquid–solid) and the effect of ultrasound is mainly mechanical and is attributed to cavitation [15]. Shock waves produced by cavitation increase the momentum of solid particles, causing them to collide with great force. The particles disintegrate, with the minimum size dependent on the characteristics of the solid, the solvent, and the intensity of ultrasound. The smallest particle size attained is limited by the reduced momentum of smaller particles, which is insufficient to cause further particle breakage [15].
2.2.1. Chemical Leaching and Cavitation
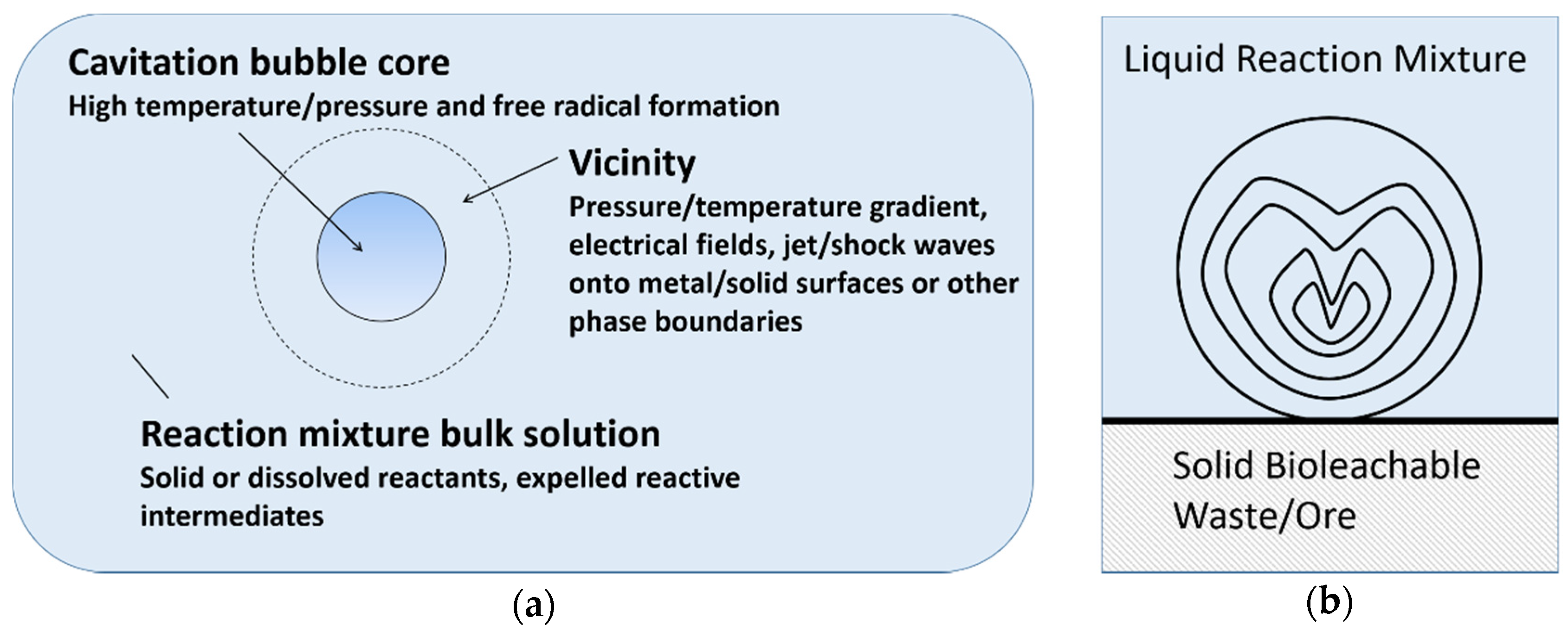
There are three main reaction sites during cavitation, namely bubble core, bubble vicinity and bulk solution [20]. As shown in Figure 3a, the formation of radical or excited molecules takes place within the bubble core, with temperature/pressure gradient, electrical field, shock waves and phase boundaries arising in the bubble vicinity. However, cavitation is different in a heterogeneous system, compared to pure liquids. Near a solid surface, bubble collapse becomes non-spherical, driving high-speed jets of liquid onto the surface (Figure 3b) which leads to shockwave damage at the surface [37]. In such systems, most of the available energy is transferred to the accelerating jet rather than the bubble wall, with this jet capable of reaching velocities of hundreds of meters per second. In addition, shockwaves created by cavity collapse in the liquid induces surface damage and the fragmentation of brittle materials. This impingement of micro-jets and shockwaves on the surface creates localized erosion, which is responsible for many of the sonochemical effects in heterogeneous reactions. This property of ultrasound can also prove useful in eroding passivating product layers, which are formed in various bioleaching systems. For instance, in spent catalyst bioleaching (without ultrasound) using Acidithiobacillus ferrooxidans, poor Mo leaching is attributed to the refractory nature of MoS2 and the formation of an elemental sulfur layer [38,39]. In this example, the application of ultrasound may enhance bioleaching by providing extreme (high temperature and pressure) conditions and eroding the product layer.
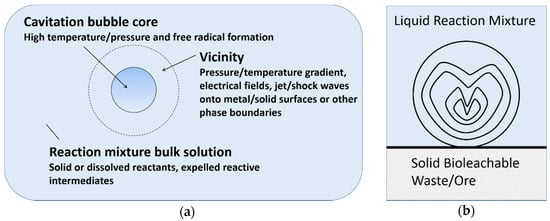
Figure 3.
(a) Reaction sites in a collapsing cavitation bubble (modified from [20]), (b) Non-spherical cavitation bubbles collapse in a solid–liquid system and liquid micro-jet formation.
2.2.2. Sonochemical Leaching of Metals
Sonication has been observed to increase the leaching of metals such as silver, gold, tungsten, titanium, nickel and other rare metals [40,41,42]. The impact of cavitation on leaching depends on various factors, such as the composition of the reaction medium, reaction conditions and the type of sonication system. The formation of hydroxyl radicals and hydrogen on the sonication of aqueous solutions [21,43] is likely to initiate chain reactions leading to an upsurge in leaching. For instance, in a study examining the effect of several variables (including ultrasound power and duration), the oxidation of silver sulfides with ultrasonically produced OH radicals reportedly increased the leaching of silver with thiourea [44].
The application of dual frequency ultrasound (20 and 40 kHz) for higher metal extraction has also been examined [42]. When dual frequency ultrasound was applied, about 92% metal extraction was observed as compared to 50% and 60% Cu recovery on the application of single frequency ultrasound at 20 kHz and 40 kHz, respectively. The increase was attributed to the promotion of a desirable reaction, faster bubble collapse, particle size reduction, a high amplitude of ultrasound, and even distribution of the sonication intensity.
The application of ultrasound reportedly increased metal (Ni, Mo, V and Al) extraction from spent catalyst [40]. For instance, compared to conventional leaching, sonication using citric acid and sulfuric acid was found to be more effective in the selective extraction of Ni, Mo and V, with minimal dissolution of alumina [40]. The impact of ultrasound and microwave was compared for the selective leaching of Mo using NaOH [45]. Although microwave was found to be more effective, ultrasound is deemed safer and more convenient. Nonetheless, research is ongoing to overcome the challenges related to microwave exposure and process limitations, such as the limited depth of microwave penetration, and non-uniform heating at a large scale [46].
Although both ultrasonic and hydrodynamic cavitation have been reported to increase uranium extraction, application of ultrasound was found to be more energy efficient in increasing metal extraction [14]. Nickel leaching in the presence of silver nitrate has also been reported to increase on sonication [47]; the addition of silver nitrate enhanced Ni extraction due to the electrochemical effect, and ultrasound further increased the oxidative leaching of nickel pyrite.
2.2.3. Sonochemical Leaching of Non-Metals
Sonication has been found beneficial for the leaching of non-metals. For instance, enhancement in the dissolution or decomposition of rock phosphate has been reported on the application of ultrasound [48,49,50]. Sonication also increased the rate of phosphate extraction using H2SO4, during the initial phase of faster leaching generally observed in extraction [48]. The same phenomenon was observed when HCl and HNO3 were used [49,50]. In these studies, the effect was attributed to an increase in the collision of H+ with the crystal lattice. The effect of ultrasound is attributed mainly to cavitation, microstreaming and instabilities produced due to the vibrating motion of particles. Similarly, the improved extraction of alkali metal and decreased fouling of boilers have been observed on ultrasonic washing of coal with water or a chemical reagent [51]. During cavitation, each collapsing bubble can be considered a micro reactor in which temperatures of several thousand degrees and pressures higher than one thousand atmospheres are created instantaneously [18]. Owing to these conditions, ultrasound has been found promising in replacing the hydrodynamic and thermal effects required in the chemical recovery of oil from sand [52].
2.3. Ultrasound in Biological Processes
Metabolites produced by microorganisms often play a role in metal extraction during bioleaching. It is therefore important to understand the effect of ultrasound on biological systems. Traditional applications of ultrasound include diagnostic imaging and the inactivation of microorganisms. Ultrasound is widely used in medical imaging at higher frequencies without cavitation. Since imaging depends on the reflected portion of ultrasound, higher frequencies (up to 15 MHz) which provide better image quality are used.
2.3.1. Microbial Growth and Sonication
The use of high power ultrasound (at 20–100 kHz) for the inactivation of microbes has been investigated since the 1960s. However, the application of ultrasound as a sole technique for the inactivation of microbes has not been established [53]. Environmental factors such as ionic strength, pressure, temperature and acid/base concentration affect the impact of sonication on microbial activity [53,54,55]. In food processing, the inactivation of microbes with ultrasound is coupled with heat and/or pressure [54]. The impact of ultrasound on the viability of bacteria has also been linked to other factors, such as the type of microbes (Gram negative or Gram positive), and its shape (rod or coccus) and size (big or small) [56,57,58]. A few studies have examined the effect of sonication on bacterial viability and growth [58,59,60,61]. For instance, a study involving three bacteria, namely Enterobacter aerogenes, Bacillus subtilis and Staphylococcus epidermidis found S. epidermidis (Gram positive, coccus) to be more resistant to sonication due to the thick biopolymer capsule layer which minimized mechanical damage [61], although another study did not find any relationship [59]. Another study with Escherichia coli and Streptococcus mutans highlighted the importance of the chemical effect of sonication (e.g., the formation of OH radicals) leading to bacterial inactivation [62].
Recently, ultrasound has also found applications in therapeutics, and in the enhancement of enzymatic/microbial activity. The thermal, physical and mechanical effects of ultrasound have led to numerous therapeutic applications over a broad frequency range of 0.02 to 20 MHz. These applications are divided into ‘thermal and non-thermal’ or ‘low and high power’ applications [63,64]. A few studies have highlighted the possible harmful effects of ultrasonic cavitation and have expressed doubts over its therapeutic effects [65,66,67]. However, there are numerous widely adopted therapeutic applications of ultrasound such as high-intensity focused ultrasound, lithotripsy, tissue healing, soft tissue stimulation, cancer therapy, stimulation of immune response, sonophoresis, sonoporation, gene therapy and bone healing [63,64,68,69,70,71].
2.3.2. Impact of Sonication on Biological Processes
Numerous studies have found that sonication shows positive effects in biological processes [72,73,74,75,76,77,78,79]. However, the coexistence of destructive and stimulating effects of sonication on microbial growth/activity remains unclear [80]. Cholesterol oxidation by Rhodococcus erythropolis (Actinobacteria) has been reported to increase on sonication in bursts of five seconds after every 10 min [79]. Changes in permeability of cell membranes and improved stirring at microscopic and macroscopic levels are responsible for increased activity [79,81,82]. Sometimes the results are due to the direct effect of sonication rather than an enhancement in biological activity. However, when sonication period was increased to 10 s, the cholesterol oxidation was comparatively smaller, which confirmed that the increase in oxidation was due to increased biological activity.
Similarly, invertase-assisted hydrolysis has been reported to increase on sonication. On ultrasonic treatment of more than 4 min, a release of invertase enzymes by the disruption of A. niger and an increase of around 28% in activity was observed [74]. However, the sonication of extracellular enzymes (only) in the system led to a reduction in activity due to its denaturation. This indicates that sonication plays a role in the release of enzymes involved in hydrolysis.
2.3.3. Biological Systems and Frequency of Ultrasound
Sonication at lower frequencies is generally preferred when the mechanical effects of ultrasound are necessary. An increase in biodegradation and sludge activity, resulting in enhanced waste water treatment have been reported on sonication at 25 kHz [83,84]. Fermentation by the bacterium Ecemothecium ashbyii reportedly increased at 24 kHz [85]. Other fermentation studies, such as the formation of lovastatin by Aspergillus terreus have also reported an increase (by 28%) on low power/intensity (957 W/m3) sonication at 24 kHz [77].
Ultrasound at higher frequencies in aqueous systems enhances reactions via the formation of free radicals [24,30,43,86,87]. Electron spin resonance studies have identified the development of free radicals in biological media on sonication at 1 MHz [86]. Numerous proteins have been shown to be susceptible to various modifications by free radicals, with effects such as altered molecular weight due to aggregation/fragmentation, the formation of peroxides of proteins, and altered net electrical charges [88,89]. Sonication at 100 kHz resulted in the nucleation of protein, with long-term irradiation causing damage to the clusters in solution [90].
3. Sonobioleaching
The effect of ultrasound in bioleaching differs when applied in one step (where the bioleachable waste is added together with the inoculum, or immediately after inoculation), and in two steps (where the bioleachable waste is added only after a high cell count or metabolite concentration has been attained).
3.1. Sonication in One-Step Bioleaching
In one of the earliest sonobioleaching studies, ultrasound was applied in one-step bioleaching of lateritic nickel ore with the fungus Aspergillus niger (A. niger) [91]. Ultrasound was applied to the growth media, using a set-up similar to an ultrasonic bath, containing the ore and fungal spores. Besides an increase in metal extraction, sonication also resulted in increased growth of the fungus. The effect of ultrasound on bioleaching was compared at three frequencies (720, 43 and 20 kHz), and the lower frequencies were found to be more effective. The duration of application (one time continuous sonication) and intensity of ultrasound were varied (at 20 kHz). In bioleaching, where the reactions are heterogeneous, the mechanical effects of ultrasound are critical. While high sonication intensity provides the desired mechanical effects leading to higher metal extraction, this may be hazardous to the microorganism. An optimum intensity (1.5 W/cm2) was found for A. niger bioleaching, above and below which the effectiveness of ultrasound was lower.
In another sonobioleaching study aimed at maximizing nickel (Ni) leaching, ultrasound was applied at 43 kHz and 1.5 W/cm2 to A. niger spores and nickel ore [92]. In one-step bioleaching, a maximum 92% of Ni and 12.5% of Fe was extracted in 20 days. Ultrasound increased both the rate and extent of leaching, with 95% of Ni leached in 14 days, along with higher selectivity (i.e., negligible leaching of Fe). The decreased Fe leaching may be attributed to the oxidation of the dissolved Fe2+ to Fe3+ on sonication, owing to the formation of free radicals and peroxide (as reported for the sonication of aqueous solutions (as growth media, Equations (1)–(4))) [24,43,86].
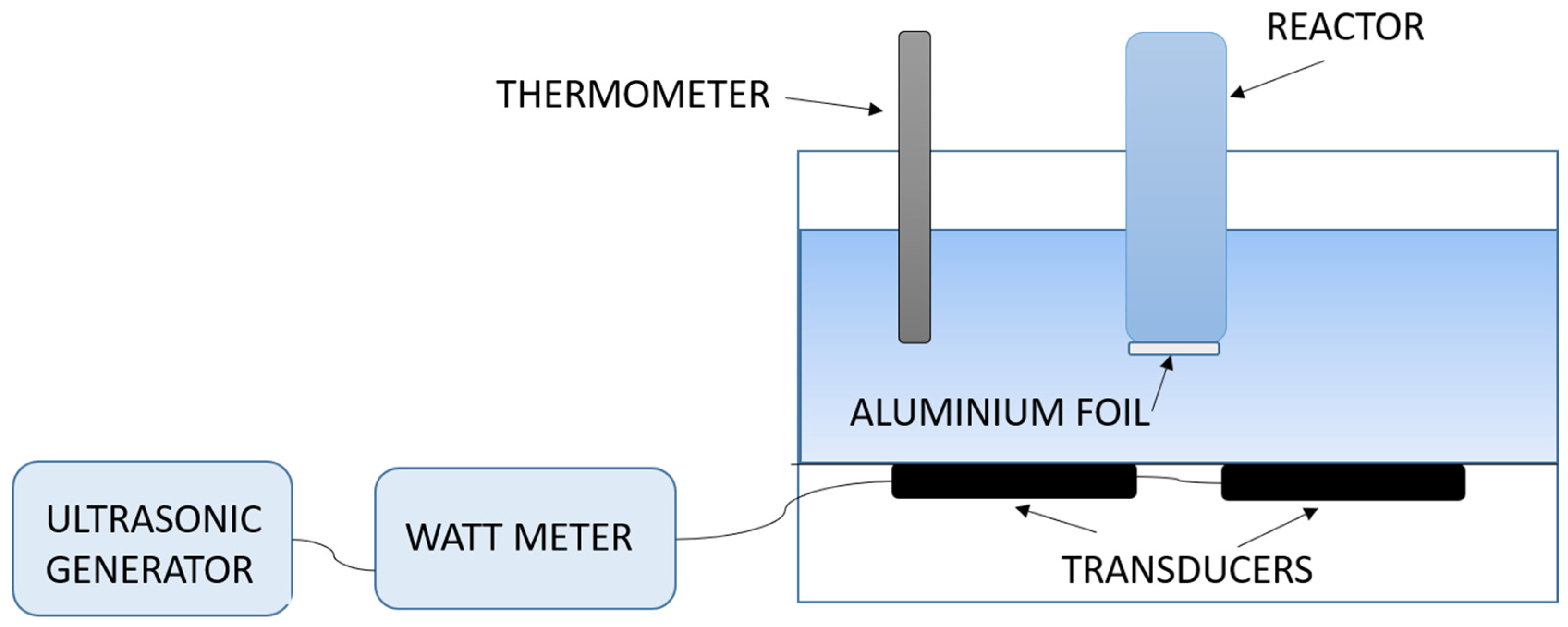
A schematic representation of the experimental set-up used for the sonobioleaching study is shown in Figure 4. An ultrasonic/signal generator is connected to a wattmeter for power measurement and piezoelectric transducers are connected at the bottom of a water bath. Aluminum foil was reportedly used at the bottom of the reaction vessel to act as a transmitter.
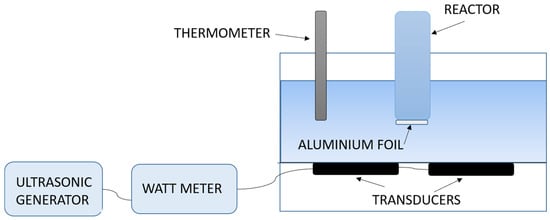
Figure 4.
Schematic of the experimental set-up used for sonobioleaching research.
It is likely that, on the introduction of an additional layer of aluminum foil, transmission will be reduced due to higher reflection and dissipation with the increased number of interfaces. The reduction in intensity promoted bioleaching, since lower intensities have been reported to be favorable for growth [60,80]. Reflectance (R) at water/glass and water/aluminum interface using Equation (5) [93] and impedance (Z) [94] was also higher with aluminum foil.
R = (Z1 − Z2)2/(Z1 + Z2)2
The percentage reflection was calculated to be 63% and 71% for the water/glass and water/aluminum interface, respectively.
Sonobioleaching increases microbial growth [11] as well as metabolite production. A higher rate and extent of leaching have also been reported in other studies [11,92,95,96,97]. However, it must be highlighted that some of the advantages are dependent on the system. For example, improvement in leaching selectivity (over non-target metals) depends on the metals and the reactions taking place in the system [95]. Hence, reduced leaching of non-target metals is not a specific effect of ultrasound on bioleaching or chemical leaching studies.
3.2. Sonication in Two-Step Bioleaching and Spent Medium Leaching
To date, all sonobioleaching studies reported with A. niger involved either one-step bioleaching and spent medium leaching. One-step bioleaching is detrimental to microbial culture growth since the bioleachable waste is introduced together with the inoculum. Sonication further increases membrane permeability of the microorganism, consequently reducing its selectivity and enhancing the toxic effect of the metals to the microorganisms. Thus, the effect of ultrasound should be examined either in two-step bioleaching (where the waste is added only after a high cell count or metabolite concentration has been attained) or in spent medium leaching (where the microorganisms are removed/filtered prior to addition of the waste).
Sonobioleaching studies with different metal wastes have been reported in two-step bioleaching and spent medium leaching using A. niger [96,97]. In a study on the sonobioleaching of black shale, ultrasound (at 40 kHz for 7 min per day) was applied during growth of the fungus (in two-step bioleaching) and spent medium leaching [96]. A higher concentration of bioleaching metabolites was obtained when ultrasound was applied during fungal growth. Leaching of heavy metals increased initially and maximum extraction was achieved within 24 days when treated ultrasonically, which was higher than extraction achieved over 36 days without sonication. Beyond 24 days, however, a decrease in metal concentration was observed with sonication, probably due to precipitation as a result of saturation of the leachate, or the activation of undesirable reactions (e.g., complexation). Metal powders have also been reported to agglomerate on prolonged sonication due to fusion at high temperature, thereby leading to reduced metal leaching [15].
Ultrasound-assisted leaching of hospital waste incinerator bottom ash using spent medium from A. niger has been reported [97]. Ultrasound applied for 10 min at 40 kHz in the spent medium led to an increase of about 20 mg/kg Al, 15 mg/kg Fe and 5 mg/kg Zn. Enhanced leaching was observed as desirable reactions were promoted by ultrasound. A previous study on bioleaching of low grade nickel ore reported a decrease in Fe leaching on sonication [92].
3.3. Sonobioleaching with Bacteria
To date, all sonobioleaching studies have been conducted and reported with the fungus A. niger. Although fungal and bacterial bioleaching have been investigated [98], the effect of ultrasound on these systems has not been compared. One study, which compared olivine ore bioleaching using Paenibacillus mucilaginosus (a bacterium) and A. niger, found fungal bioleaching to be more efficient in extraction. Unfortunately, the impact of ultrasound was examined only on the fungal bioleaching of olivine (i.e., bacterial sonobioleaching was not examined). On sonication, an increase from 9.9% to 15.7% was observed in Ni leaching over 17 days [99]. Other metals present in the ore (such as Mg, Al, Si and Cr) increased marginally. Fe extraction, however, was reduced on sonication. Similar phenomena, i.e., reduction in Fe extraction on sonication, have also been reported earlier in sonobioleaching (A. niger) of nickel lateritic ore [92].
4. Future Directions in Sonobioleaching
Research studies on sonobioleaching in various systems are summarized in Table 1, with different ultrasound frequencies (20, 43, 40 & 37 kHz) used. The frequency of ultrasound impacts the diameter of the cavitation bubble, with low frequencies resulting in large diameter cavitation and higher frequencies resulting in small diameter cavitation [100]. Thus, higher frequencies should be used for smaller particles.

Table 1.
A summary of bioleaching studies conducted with ultrasound.
Bioleaching of secondary sources such as spent catalyst, fly ash, and mine tailings has been found promising [9,101,102,103,104,105]. However, the effect of ultrasound has only been examined on bioleaching of low grade ores and bottom ash. The mechanical effects of ultrasound, such as particle disintegration, depend on the hardness characteristics of the solid, the solvent, and the intensity of ultrasound. For some bioleachable wastes, grinding can be obviated when ultrasound is applied. However, there is a limit on the smallest particle size obtained on sonication [15]. Similarly, a limit also exists on the optimal particle size in bioleaching, since very small particles (<45 µm) are harmful to bioleaching bacteria [106].
In sonobioleaching, ultrasound can be applied during the growth of the microorganism and/or leaching (Table 1) [96,99]. When applied during growth, low intensity should be used, since positive effects on bacterial growth have been observed [60,80]. The effect of ultrasound on growth should also be explored while examining sonobioleaching with a new microorganism. However, the application of ultrasound during growth is beneficial only when it is accompanied by an increase in the metabolites responsible for leaching (as has been observed with A. niger). Where sonication does not enhance microbial growth and/or metabolite production, the impact of higher intensity sonication in spent medium leaching should be evaluated. The mechanical effects of cavitation may also augment bioleaching with particle disintegration and mixing at the microscopic level. However, the effectiveness of ultrasound would also depend on factors such as the reactions involved, metabolites/reactants, the physical characteristics of the substrate, as well as the pH of media and the solubility of the product.
The use of continuous ultrasound in the discontinuous mode in sonobioleaching (Table 1, 7–15 min daily) has yielded favorable results. The application of pulsed sonication for some chemical processes have also been examined. A study on the effects of power-modulated pulse on chemical degradation processes has shown that compared to continuous sonication, a three-fold increase in the degradation rate occurred with pulsed ultrasound [107]. Such an approach should also be examined for bioleaching systems, especially during the leaching stage.
Scale-Up Prospects
In the past, the application of ultrasound on a large scale typically showed a low efficiency in conversion from electrical energy to acoustic energy [107]. However, with modern piezoelectric transducers, energy conversion efficiency has considerably increased. This has been attributed to the reduction in the dissipation of electrical power as heat and noise, so that large scale sonication may achieve an efficiency of around 80% [100,108]. Ultrasound has also found commercial applications in the food industry in a broad range of areas such as extraction, emulsification, crystallization, separation, viscosity alteration, defoaming, extrusion, microbial disinfection and cleaning [108]. This has been attributed to advantages such as improved efficiency, fast processing, easy installation, and low maintenance [108].
For large scale applications in a batch or continuous process, either a probe or a bath may be used [109,110], while for laboratory scale sonobioleaching studies, a bath reactor is likely to be preferred. Although various bath reactors may be used for sonobioleaching (e.g., standard ultrasonic bath, or a batch reactor with an external transducer or a reactor with a submersible transducer [109]), there are concerns for the cost of applications. Indeed, low cost is one of the advantages of bioleaching over other leaching processes. Energy efficiency related to sonication is comparable to other unit operations, such as homogenization, milling, and heat shock [108]. Sonication in leaching has been found to be as energy efficient as stirring [28,40,111]. For systems which involve the bioleaching of metals of high economic value (e.g., precious metals), the economics of the use of an ultrasound system are likely to be favorable.
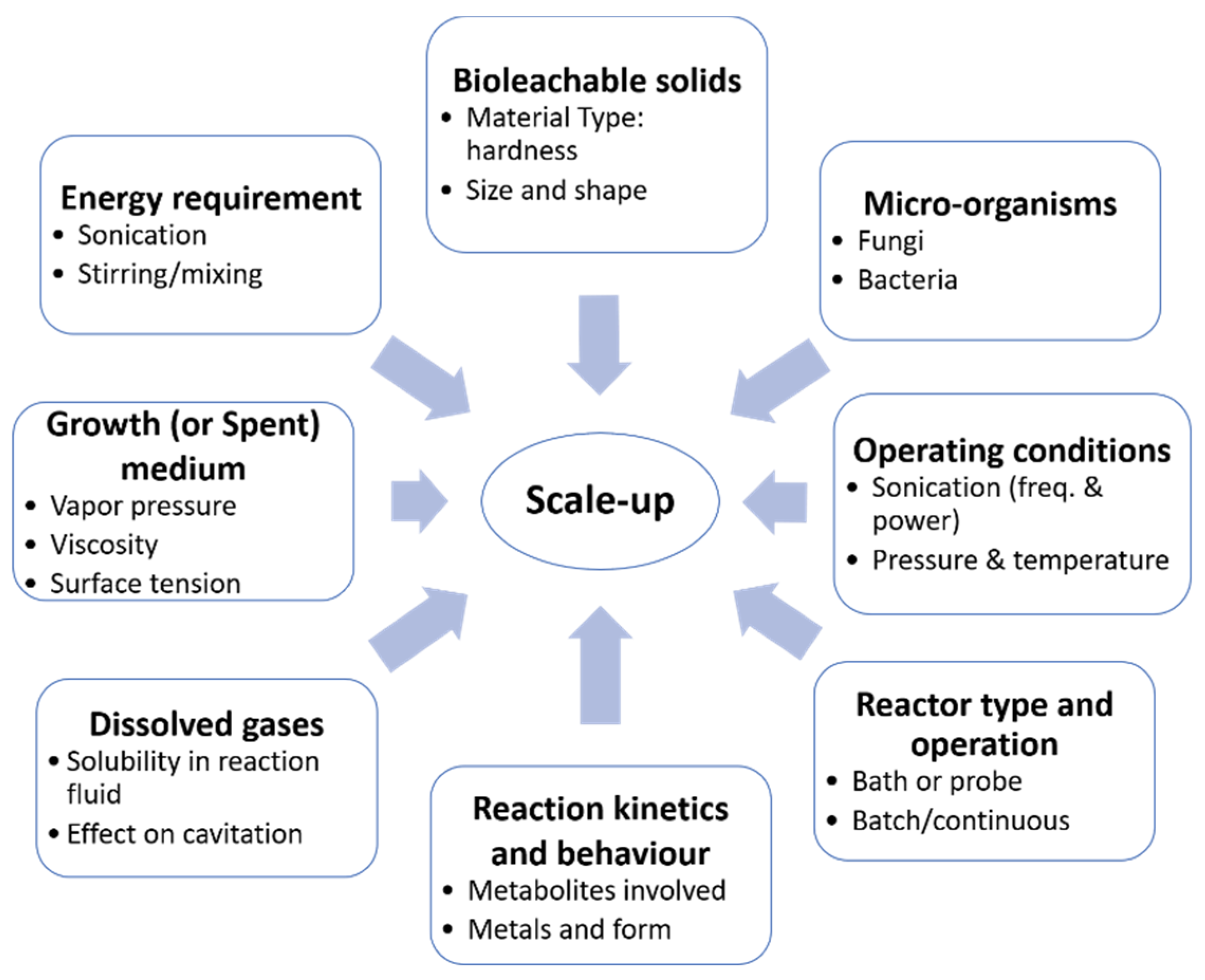
Prior to scaling up, it is important to understand the relative importance and role of chemical or physical effects in sonication. If particle degradation is the only critical effect of ultrasound, the solids can be sonicated before being placed in a conventional reactor [91,92,95]. However, if the other physical effects of ultrasound are important, such as the enhanced rate of mass transfer and/or surface renewal, then sonication would be required over the course of the reaction [15,96,97]. As illustrated in Figure 5, numerous factors need to be considered in the scale-up of an ultrasound-assisted process. The effect of ultrasound on a sonobioleaching process depends on various factors, including the type of microorganisms, solid particles, fluid properties, dissolved gases and reaction kinetics. The selection of a suitable reactor type is also essential to the study. For instance, an ultrasonic bath would likely yield different results depending on the location of the transducers (i.e., either at the bottom or on the sides of the reactor). Sonobioleaching studies have thus far used the former setup (Figure 4); the second configuration has not been explored. Transducers on the sides of the reactor may also lead to standing wave formation, which has been found effective in certain applications such as sonoporation [112]. In addition to the characteristics of the reaction mixture and the kinetics of the reaction, knowledge of the optimum system and ultrasonic conditions [15] such as the ambient reaction temperature, pressure, frequency, dissipated power, ultrasonic field, and their interactions is also essential.
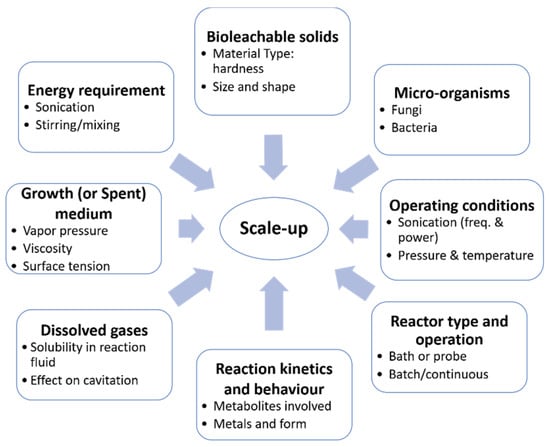
Figure 5.
Scale-up considerations for an ultrasound-assisted bioleaching process (modified from [15]).
5. Conclusions
The application of ultrasound in increasing the rate of chemical reactions, such as in chemical leaching, is widely accepted. In comparison, its application in biological processes has been limited. To date, virtually all sonobioleaching research has been reported with one microorganism, A. niger, although it has been observed that the mechanical and sonochemical effects of ultrasound increase the rate and extent of bioleaching. Since an increase in membrane permeability on sonication would lead to increased cell toxicity, ultrasound is more likely to enhance metal extraction in two-step bioleaching and spent medium leaching. Although sonobioleaching scale-up is the next obvious step following the reported increase in extraction, the economic feasibility of the system remains to be evaluated.
Author Contributions
Both authors contributed in writing this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marafi, M.; Stanislaus, A. Spent hydroprocessing catalyst management: A review: Part II. Advances in metal recovery and safe disposal methods. Resour. Conserv. Recycl. 2008, 53, 1–26. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for critical raw material recovery from primary and secondary sources: R&D priorities and future perspectives. New Biotechnol. 2015, 32, 121–127. [Google Scholar] [CrossRef]
- Brierley, J.A. A perspective on developments in biohydrometallurgy. Hydrometallurgy 2008, 94, 2–7. [Google Scholar] [CrossRef]
- Borja, D.; Lee, E.; Silva, R.A.; Kim, H.; Park, J.H.; Kim, H. Column bioleaching of arsenic from mine tailings using a mixed acidophilic culture: A technical feasibility assessment. J. Korean Inst. Resour. Recycl. 2015, 24, 69–77. [Google Scholar] [CrossRef]
- Silva, R.A.; Borja, D.; Hwang, G.; Hong, G.; Gupta, V.; Bradford, S.A.; Zhang, Y.; Kim, H. Analysis of the effects of natural organic matter in zinc beneficiation. J. Clean. Prod. 2017, 168, 814–822. [Google Scholar] [CrossRef]
- Borja, D.; Nguyen, K.A.; Silva, R.A.; Park, J.H.; Gupta, V.; Han, Y.; Lee, Y.; Kim, H. Experiences and future challenges of bioleaching research in South Korea. Minerals 2016, 6, 128. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dong, B.; Zhuang, X.; Zhao, J.; Zhang, C.; Wang, J.; Shih, K. Enhanced bioleaching efficiency of copper from waste printed circuit board driven by nitrogen-doped carbon nanotubes modified electrode. Chem. Eng. J. 2017, 324, 122–129. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. Sequential biological process for molybdenum extraction from hydrodesulphurization spent catalyst. Chemosphere 2016, 160, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Ting, Y.-P. Bioleaching of spent hydrotreating catalyst by acidophilic thermophile Acidianus brierleyi: Leaching mechanism and effect of decoking. Bioresour. Technol. 2013, 130, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.M.; Narayana, K.L.; Misra, V.N. Bioleaching with ultrasound. Ultrason. Sonochem. 2005, 12, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Silva, R.A.; Park, J.; Lee, E.; Park, J.; Kim, H. Adaptation of a mixed culture of acidophiles for a tank biooxidation of refractory gold concentrates containing a high concentration of arsenic. J. Biosci. Bioeng. 2016, 121, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Roy, S.B.; Chowdhury, S.; Hareendran, K.N.; Pandit, A.B. Enhancement of the leaching rate of uranium in the presence of ultrasound. Ind. Eng. Chem. Res. 2006, 45, 7639–7648. [Google Scholar] [CrossRef]
- Thompson, L.H.; Doraiswamy, L.K. Sonochemistry: Science and engineering. Ind. Eng. Chem. Res. 1999, 38, 1215–1249. [Google Scholar] [CrossRef]
- Margulis, M.A. Fundamental problems of sonochemistry and cavitation. Ultrason. Sonochem. 1994, 1, S87–S90. [Google Scholar] [CrossRef]
- Margulis, M.A.; Margulis, I.M. Contemporary review on nature of sonoluminescence and sonochemical reactions. Ultrason. Sonochem. 2002, 9, 1–10. [Google Scholar] [CrossRef]
- Santos, H.M.; Lodeiro, C.; Capelo-Martínez, J.-L. The power of ultrasound. In Ultrasound in Chemistry; Capelo-Martínez, J.-L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 1–16. [Google Scholar]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Peters, D. Ultrasound in materials chemistry. J. Mater. Chem. 1996, 6, 1605–1618. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms. J. Phys. Chem. 1983, 87, 1369–1377. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Sonochemistry in Environmental Protection and Remediation. In Applied Sonochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 131–156. [Google Scholar]
- Miyaji, A.; Kohno, M.; Inoue, Y.; Baba, T. Hydroxyl radical generation by dissociation of water molecules during 1.65 MHz frequency ultrasound irradiation under aerobic conditions. Biochem. Biophys. Res. Commun. 2017, 483, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Riesz, P.; Kondo, T.; Krishna, C.M. Free radical formation by ultrasound in aqueous solutions. A spin trapping study. Free Radic. Res. Commun. 1990, 10, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cella, R.; Stefani, H.A. Ultrasonic reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 343–361. [Google Scholar]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, e411–e414. [Google Scholar] [CrossRef] [PubMed]
- Stavarache, C.; Vinatoru, M.; Maeda, Y.; Bandow, H. Ultrasonically driven continuous process for vegetable oil transesterification. Ultrason. Sonochem. 2007, 14, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Avvaru, B.; Roy, S.B.; Ladola, Y.; Chowdhury, S.; Hareendran, K.N.; Pandit, A.B. Sono-chemical leaching of uranium. Chem. Eng. Process. Process Intensif. 2008, 47, 2107–2113. [Google Scholar] [CrossRef]
- Hatanaka, S.; Mitome, H.; Yasui, K.; Hayashi, S. Single-bubble sonochemiluminescence in aqueous luminol solutions. J. Am. Chem. Soc. 2002, 124, 10250–10251. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Bhatia, B.; Nayyar, N.K. Transition metal-promoted free-radical reactions in organic synthesis: The formation of carbon-carbon bonds. Chem. Rev. 1994, 94, 519–564. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Pinjari, D.V.; Sonawane, S.H.; Gogate, P.R.; B, A. Pandit, Analysis of semibatch emulsion polymerization: Role of ultrasound and initiator. Ultrason. Sonochem. 2012, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. Process Intensif. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Polymers. In Applied Sonochemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2002; pp. 157–224. [Google Scholar]
- Henglein, A.; Ulrich, R.; Lilie, J. Luminescence and chemical action by pulsed ultrasound. J. Am. Chem. Soc. 1989, 111, 1974–1979. [Google Scholar] [CrossRef]
- Tandiono; Ohl, S.-W.; Ow, D.S.; Klaseboer, E.; Wong, V.V.; Dumke, R.; Ohl, C.-D. Sonochemistry and sonoluminescence in microfluidics. Proc. Natl. Acad. Sci. USA 2011, 108, 5996–5998. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, N.; Yamada, H.; Xu, C.-N. Ultrasonic wave induced mechanoluminescence and its application for photocatalysis as ubiquitous light source. Catal. Today 2013, 201, 203–208. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Pradhan, D.; Mishra, D.; Kim, D.J.; Ahn, J.G.; Chaudhury, G.R.; Lee, S.W. Bioleaching kinetics and multivariate analysis of spent petroleum catalyst dissolution using two acidophiles. J. Hazard. Mater. 2010, 175, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Beolchini, F.; Fonti, V.; Ferella, F.; Vegliò, F. Metal recovery from spent refinery catalysts by means of biotechnological strategies. J. Hazard. Mater. 2010, 178, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Marafi, M.; Stanislaus, A. Waste catalyst utilization: extraction of valuable metals from spent hydroprocessing catalysts by ultrasonic-assisted leaching with acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Narayana, K.L.; Swamy, K.M.; Rao, K.S.; Murty, J.S. Leaching of metals from ores with ultrasound. Miner. Process. Extr. Metall. Rev. 1997, 16, 239–259. [Google Scholar] [CrossRef]
- Swamy, K.M.; Narayana, K.L. Intensification of leaching process by dual-frequency ultrasound. Ultrason. Sonochem. 2001, 8, 341–346. [Google Scholar] [CrossRef]
- Makino, K.; Mossoba, M.M.; Riesz, P. Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (.cntdot.OH and.cntdot.H) by spin trapping. J. Am. Chem. Soc. 1982, 104, 3537–3539. [Google Scholar] [CrossRef]
- Oncel, M. Leaching of silver from solid waste using ultrasound assisted thiourea method. Ultrason. Sonochem. 2005, 12, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.S.S.; Soares, H.M.V.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 2012, 129–130, 19–25. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Continuous flow through a microwave oven for the large-scale production of biodiesel from waste cooking oil. Bioresour. Technol. 2017, 224, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lu, X.; Du, Y.; Mao, W.; Wang, Y.; Li, J. Ultrasonic-assisted oxidation leaching of nickel sulfide concentrate. Chin. J. Chem. Eng. 2010, 18, 948–953. [Google Scholar] [CrossRef]
- Pandey, A.D.; Mallick, K.K.; Pandey, P.C. Intensification of the reaction between Udaipur rock phosphate and sulphuric acid by means of ultrasound. Ultrasonics 1980, 18, 115–119. [Google Scholar] [CrossRef]
- Tekin, T. Use of ultrasound in the dissolution kinetics of phosphate rock in HCl. Hydrometallurgy 2002, 64, 187–192. [Google Scholar] [CrossRef]
- Tekin, T.; Tekin, D.; Bayramoğlu, M. Effect of ultrasound on the dissolution kinetics of phosphate rock in HNO3. Ultrason. Sonochem. 2001, 8, 373–377. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Reddy, V.M.; Nagarajan, R. Ultrasonic coal washing to leach alkali elements from coals. Ultrason. Sonochem. 2015, 27, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Abramov, O.; Abramov, V.; Myasnikov, S.; Mullakaev, M. Extraction of bitumen, crude oil and its products from tar sand and contaminated sandy soil under effect of ultrasound. Ultrason. Sonochem. 2009, 16, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; de Souza Sant’Ana, A. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Hwang, G.; Han, Y.; Choi, S.Q.; Cho, S.; Kim, H. Bacterial inactivation by ultrasonic waves: Role of ionic strength, humic acid, and temperature. Water Air Soil Pollut. 2015, 226, 304. [Google Scholar] [CrossRef]
- Thacker, J. An approach to the mechanism of killing of cells in suspension by ultrasound. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1973, 304, 240–248. [Google Scholar] [CrossRef]
- Cameron, M.; McMaster, L.D.; Britz, T.J. Electron microscopic analysis of dairy microbes inactivated by ultrasound. Ultrason. Sonochem. 2008, 15, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulou, S.; Terzakis, S.; Fountoulakis, M.S.; Mantzavinos, D.; Manios, T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochem. 2009, 16, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M. Impact of Low-Frequency High-Power Ultrasound on Spoilage and Potentially Pathogenic Dairy Microbes. University of Stellenbosch, 2007. Available online: https://scholar.sun.ac.za/bitstream/handle/10019.1/1163/cameron_impact_2007.pdf?sequence=3 (accessed on 22 February 2016).
- Pitt, W.G.; Ross, S.A. Ultrasound increases the rate of bacterial cell growth. Biotechnol. Progress 2003, 19, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Miyamoto, M.; Toma, M.; Matsuoka, T.; Maebayashi, M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500 kHz. Ultrason. Sonochem. 2009, 16, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic applications of ultrasound. Progress Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Feril, L.B., Jr.; Ikeda-Dantsuji, Y. Sonodynamic therapy. Ultrasonics 2008, 48, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Child, S.Z.; Hartman, C.L.; Schery, L.A.; Carstensen, E.L. Lung damage from exposure to pulsed ultrasound. Ultrasound Med. Biol. 1990, 16, 817–825. [Google Scholar] [CrossRef]
- Holland, C.K.; Deng, C.X.; Apfel, R.E.; Alderman, J.L.; Fernandez, L.A.; Taylor, K.J.W. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med. Biol. 1996, 22, 917–925. [Google Scholar] [CrossRef]
- Baker, K.G.; Robertson, V.J.; Duck, F.A. A review of therapeutic ultrasound: biophysical effects. Phys. Ther. 2001, 81, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Mitragotri, S. Therapeutic opportunities in biological responses of ultrasound. Ultrasonics 2008, 48, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Tachibana, K. Use of ultrasound in drug delivery systems: Emphasis on experimental methodology and mechanisms. Int. J. Hyperth. 2012, 28, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Husseini, G.A.; Pitt, W.G. The use of ultrasound and micelles in cancer treatment. J. Nanosci. Nanotechnol. 2008, 8, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-L.; Zeng, W.-C.; Lu, X.-L. Influence of ultrasound to the activity of tyrosinase. Ultrason. Sonochem. 2013, 20, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.; Parsons, S.A.; Jefferson, B. The influence of ultrasound frequency and power, on the algal species Microcystis aeruginosa, Aphanizomenon flos-aquae, Scenedesmus subspicatus and Melosira sp. Environ. Technol. 2013, 34, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.H.M.; Pião, A.C.S.; Domingos, R.N.; Carmona, E.C. Ultrasound effects on invertase from Aspergillus niger. World J. Microbiol. Biotechnol. 2004, 20, 137–142. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Y.; Wang, W.; Yu, Y. Low intensity ultrasound stimulates biological activity of aerobic activated sludge. Front. Environ. Sci. Eng. China 2007, 1, 67–72. [Google Scholar] [CrossRef]
- Pérez-Elvira, S.; Fdz-Polanco, M.; Plaza, F.I.; Garralón, G.; Fdz-Polanco, F. Ultrasound pre-treatment for anaerobic digestion improvement. Water Sci. Technol. 2009, 60, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Herrán, N.S.; López, J.L.C.; Pérez, J.A.S.; Chisti, Y. Effects of ultrasound on culture of Aspergillus terreus. J. Chem. Technol. Biotechnol. 2008, 83, 593–600. [Google Scholar] [CrossRef]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Bar, R. Ultrasound enhanced bioprocesses: Cholesterol oxidation by Rhodococcus erythropolis. Biotechnol. Bioeng. 1988, 32, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason. Sonochem. 2014, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Schlicher, R.K.; Radhakrishna, H.; Tolentino, T.P.; Apkarian, R.P.; Zarnitsyn, V.; Prausnitz, M.R. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 2006, 32, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, W.L. Ultrasonic microstreaming and related phenomena. Br. J. Cancer 1982, 5, 156–160. [Google Scholar]
- Schläfer, O.; Onyeche, T.; Bormann, H.; Schröder, C.; Sievers, M. Ultrasound stimulation of micro-organisms for enhanced biodegradation. Ultrasonics 2002, 40, 25–29. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Gao, J.; Chen, Y. Using acoustic cavitation to improve the bio-activity of activated sludge. Bioresour. Technol. 2008, 99, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Bochu, W.; Chuanren, D.; Sakanishi, A. Low ultrasonic stimulates fermentation of riboflavin producing strain Ecemothecium ashbyii. Colloids Surf. B Biointerfaces 2003, 30, 37–41. [Google Scholar] [CrossRef]
- Edmonds, P.D.; Sancier, K.M. Evidence for free radical production by ultrasonic cavitation in biological media. Ultrasound Med. Biol. 1983, 9, 635–639. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Davies, K.J. Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kakinouchi, K.; Adachi, H.; Matsumura, H.; Inoue, T.; Murakami, S.; Mori, Y.; Koga, Y.; Takano, K.; Kanaya, S. Effect of ultrasonic irradiation on protein crystallization. J. Cryst. Growth 2006, 292, 437–440. [Google Scholar] [CrossRef]
- Swamy, K.M.; Sukla, L.B.; Narayana, K.L.; Kar, R.N.; Panchanadikar, V.V. Use of ultrasound in microbial leaching of nickel from laterites. Ultrason. Sonochem. 1995, 2, S5–S9. [Google Scholar] [CrossRef]
- Sukla, L.B.; Swamy, K.M.; Narayana, K.L.; Kar, R.N.; Panchanadikar, V.V. Bioleaching of Sukinda laterite using ultrasonics. Hydrometallurgy 1995, 37, 387–391. [Google Scholar] [CrossRef]
- Tole, N.M.; Ostensen, H. Basic Physics of Ultrasonographic Imaging; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Onda Corporation. Tables of Acoustic Properties of Materials, Acoustic Tables of Reference. 2013. Available online: http://www.ondacorp.com/tecref_acoustictable.shtml (accessed on 1 February 2016).
- Kar, R.N.; Sukla, L.B.; Swamy, K.M.; Panchanadikar, V.V.; Narayana, K.L. Bioleaching of lateritic nickel ore by ultrasound. Metall. Mater. Trans. B 1996, 27, 351–354. [Google Scholar] [CrossRef]
- Anjum, F.; Bhatti, H.N.; Ghauri, M.A. Enhanced bioleaching of metals from black shale using ultrasonics. Hydrometallurgy 2010, 100, 122–128. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Bukhari, S.; Potgieter, J.H. Combined ultrasonic and bioleaching treatment of hospital waste incinerator bottom ash with simultaneous extraction of selected metals. Environ. Technol. 2014, 35, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Sabra, N.; Dubourguier, H.-C.; Duval, M.-N.; Hamieh, T. Study of canal sediments contaminated with heavy metals: Fungal versus bacterial bioleaching techniques. Environ. Technol. 2011, 32, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.; Santos, R.; van Audenaerde, A.; Monballiu, A.; van Gerven, T.; Meesschaert, B. Chemoorganotrophic bioleaching of olivine for nickel recovery. Minerals 2014, 4, 553–564. [Google Scholar] [CrossRef]
- Cleaning Technologies Group. Magnetostrictive Versus Piezoelectric Transducers for Power Ultrasonic Applications. 2016. Available online: http://www.ctgclean.com/blog/technology-library/articles/magnetostrictive-versus-piezoelectric-transducers-for-power-ultrasonic-applications/ (accessed on 30 March 2016).
- Aung, K.M.M.; Ting, Y.-P. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. J. Biotechnol. 2005, 116, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-J.; Ting, Y.-P. Optimisation on bioleaching of incinerator fly ash by Aspergillus niger—Use of central composite design. Enzyme Microb. Technol. 2004, 35, 444–454. [Google Scholar] [CrossRef]
- Lee, E.; Han, Y.; Park, J.; Hong, J.; Silva, R.A.; Kim, S.; Kim, H. Bioleaching of arsenic from highly contaminated mine tailings using Acidithiobacillus thiooxidans. J. Environ. Manag. 2015, 147, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, E.; Shaik, K.; Borja, D.; Smart, M.; Park, J.H.; Kim, H.J.; Petersen, J.; Harrison, S.T.L. Investigating the bioleaching of an arsenic mine tailing using a mixed mesophilic culture. Solid State Phenom. 2017, 262, 668–672. [Google Scholar] [CrossRef]
- Park, J.; Han, Y.; Lee, E.; Choi, U.; Yoo, K.; Song, Y.; Kim, H. Bioleaching of highly concentrated arsenic mine tailings by Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2014, 133, 291–296. [Google Scholar] [CrossRef]
- Deveci, H. Effect of particle size and shape of solids on the viability of acidophilic bacteria during mixing in stirred tank reactors. Hydrometallurgy 2004, 71, 385–396. [Google Scholar] [CrossRef]
- Casadonte, D.J.; Flores, M.; Petrier, C. The use of pulsed ultrasound technology to improve environmental remediation: a comparative study. Environ. Technol. 2005, 26, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bates, D. Industrial applications of high power ultrasonics. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 599–616. [Google Scholar]
- Mason, T.; Peters, D. 4-Large scale sonochemistry. In Practical Sonochemistry, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2002; pp. 82–112. Available online: http://www.sciencedirect.com/science/article/pii/B9781898563839500096 (accessed on 24 March 2016).
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.; Srinivasakannan, C.; Peng, J.; Duan, X.; Ju, S. A comparison of the conventional and ultrasound-augmented leaching of zinc residue using sulphuric acid. Arab. J. Sci. Eng. 2013, 39, 163–173. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hynynen, K. Key factors that affect sonoporation efficiency in in vitro settings. In Proceedings of the IEEE Ultrasonics Symposium, Vancouver, BC, Canada, 3–6 October 2006; pp. 852–855. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).