Geochemistry of Monazite within Carbonatite Related REE Deposits
Abstract
:1. Introduction
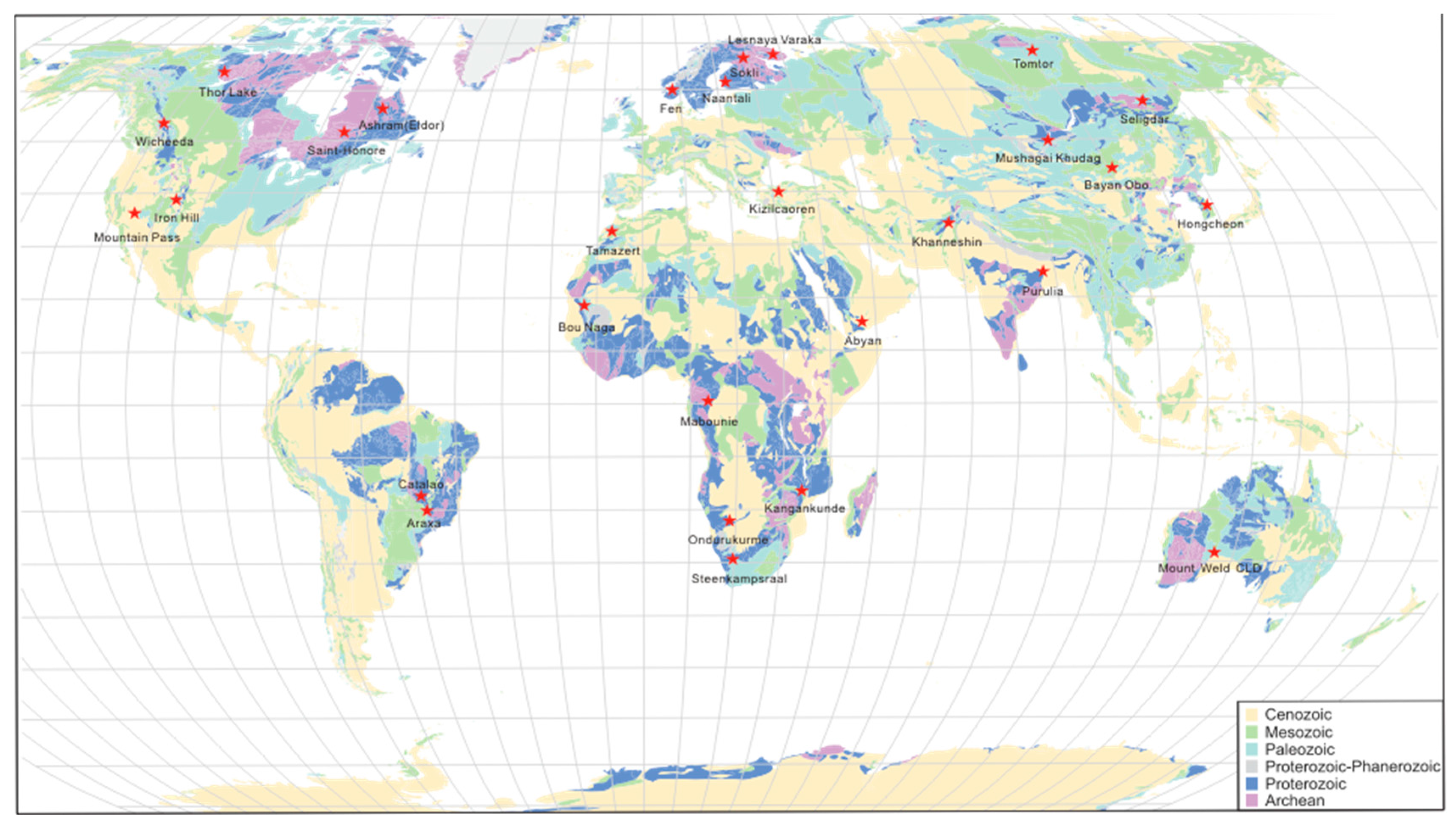
2. Distribution of Monazite Dominated Carbonatite REE Deposits
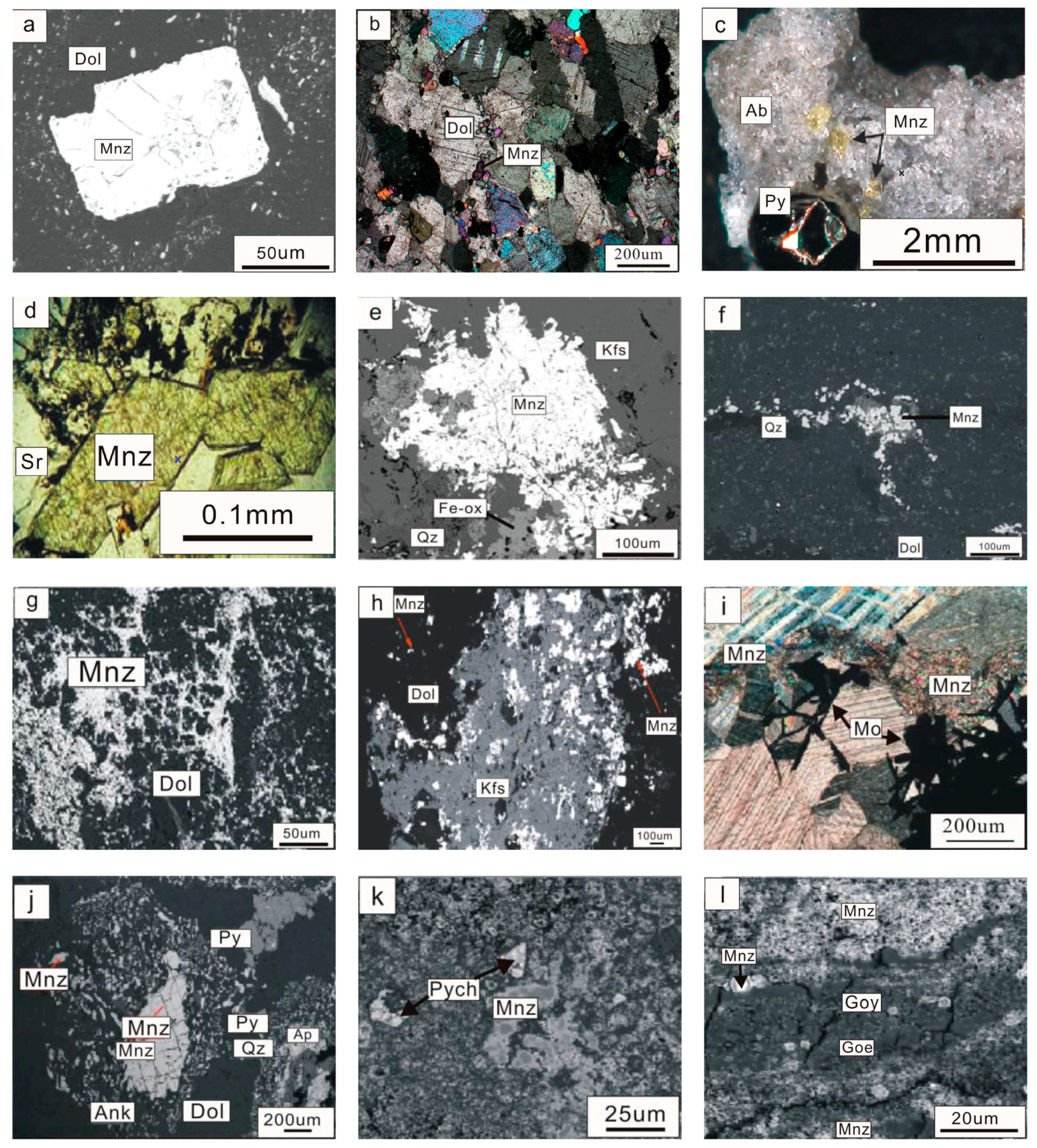
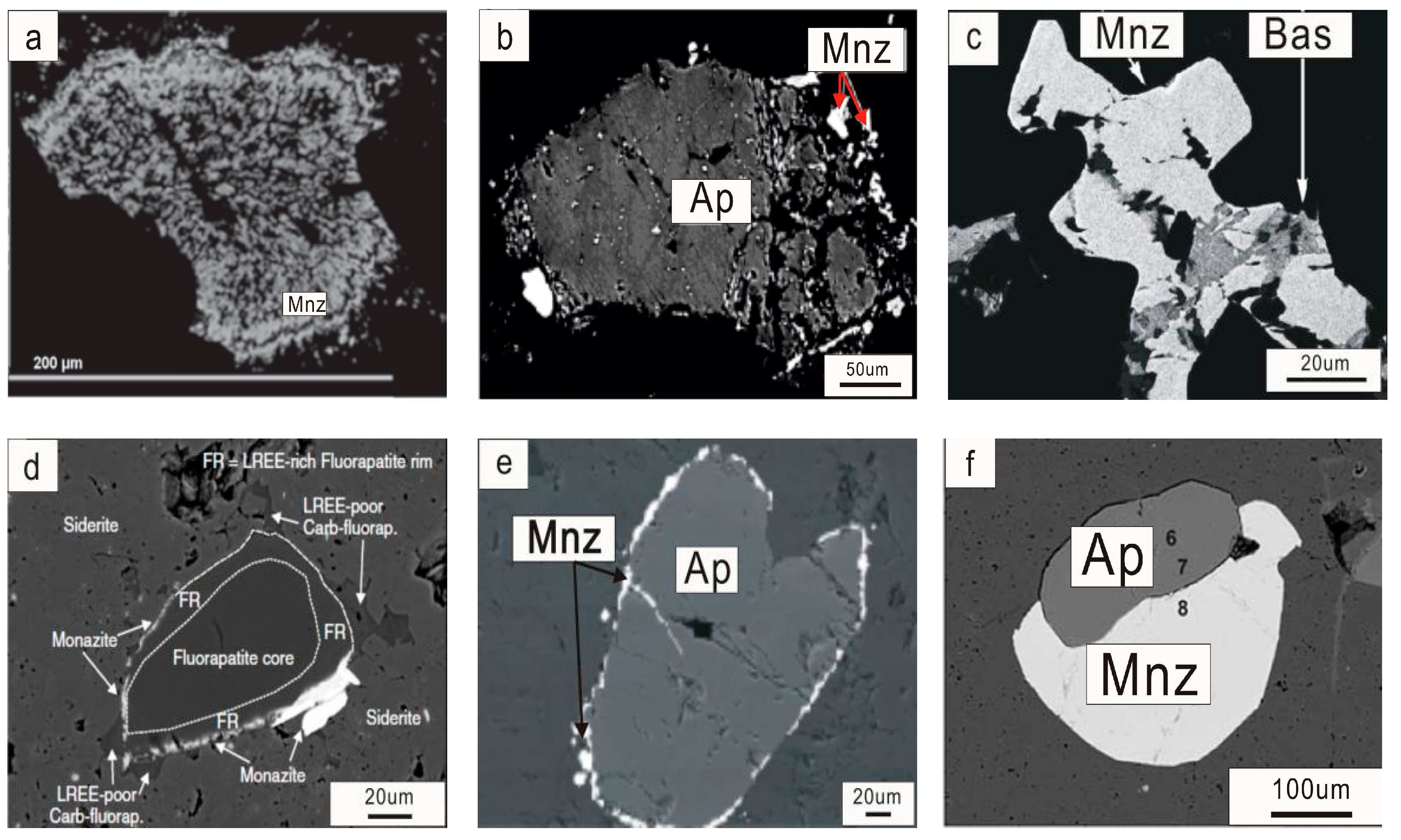
3. Texture Characteristics of Monazite
4. Chemical Compositions
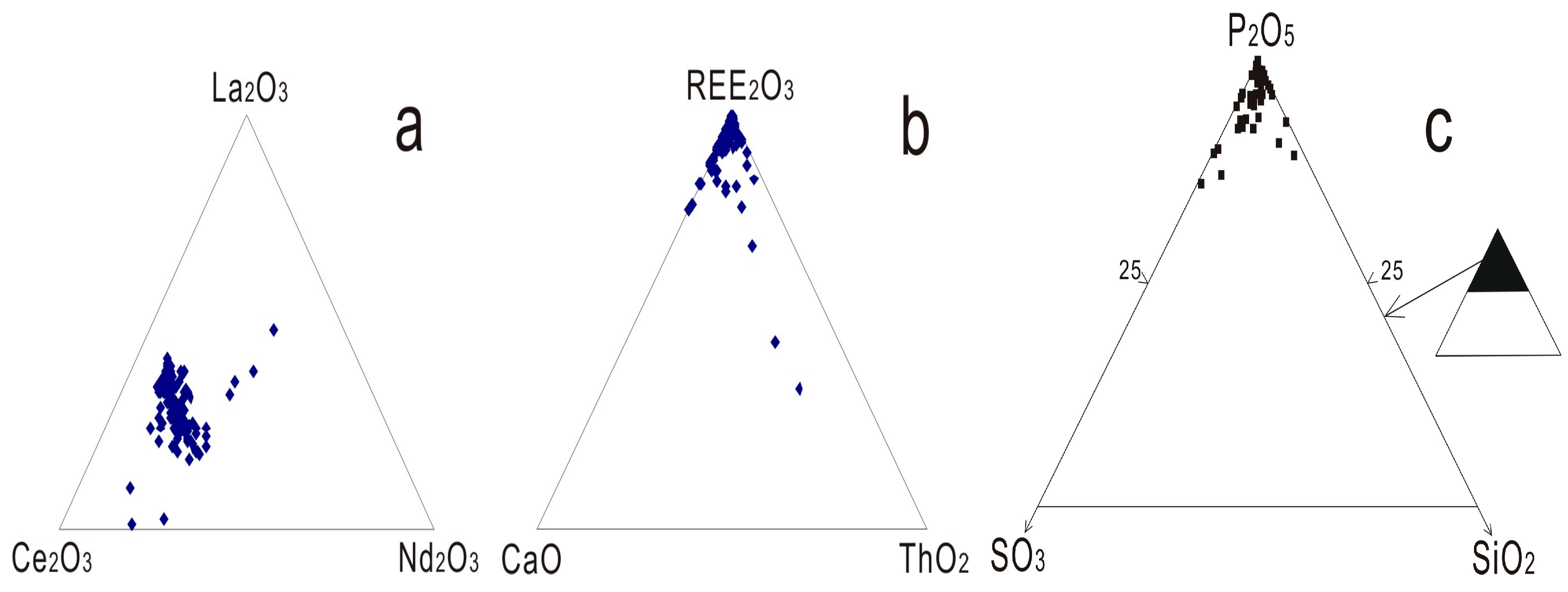
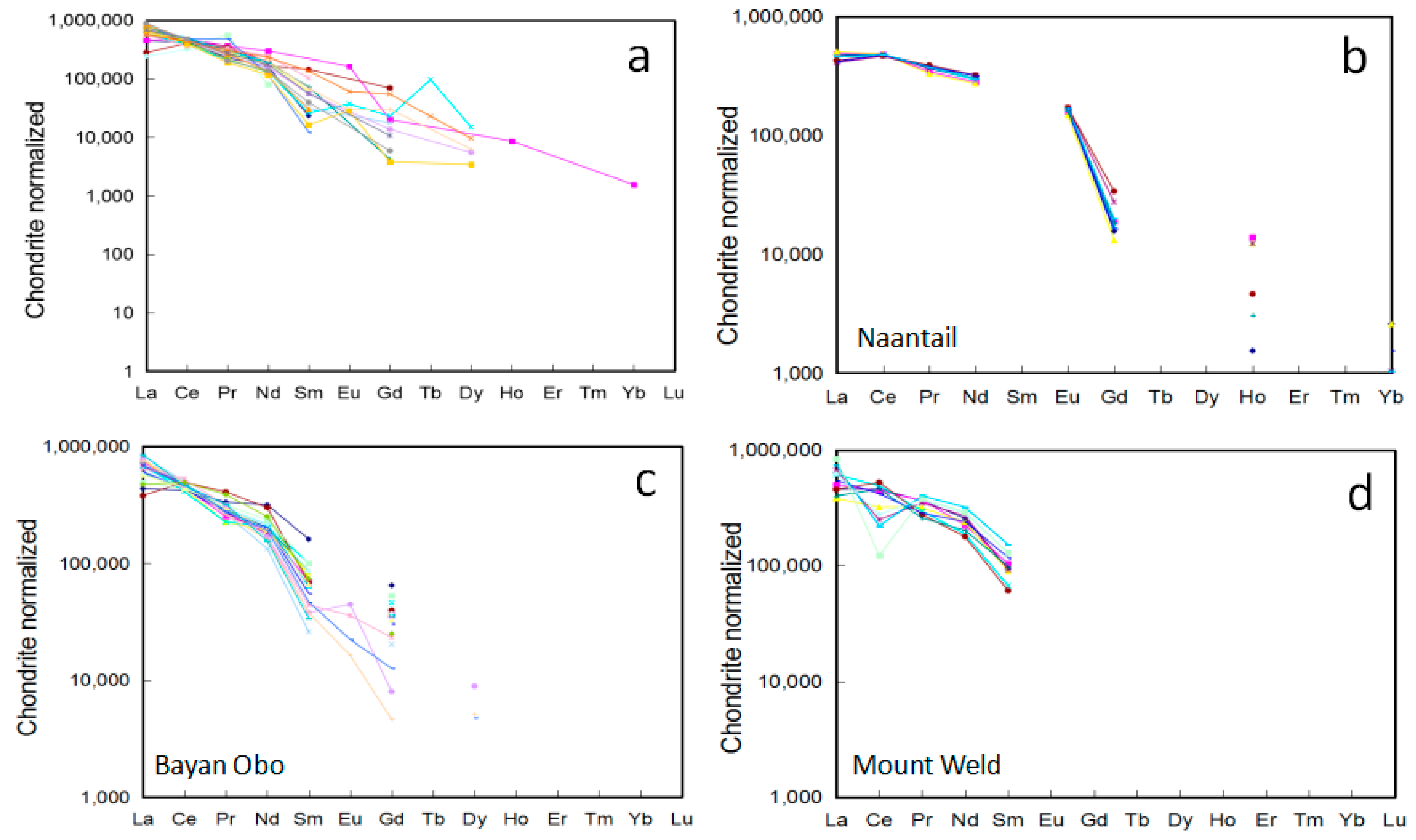
4.1. REE Compositions
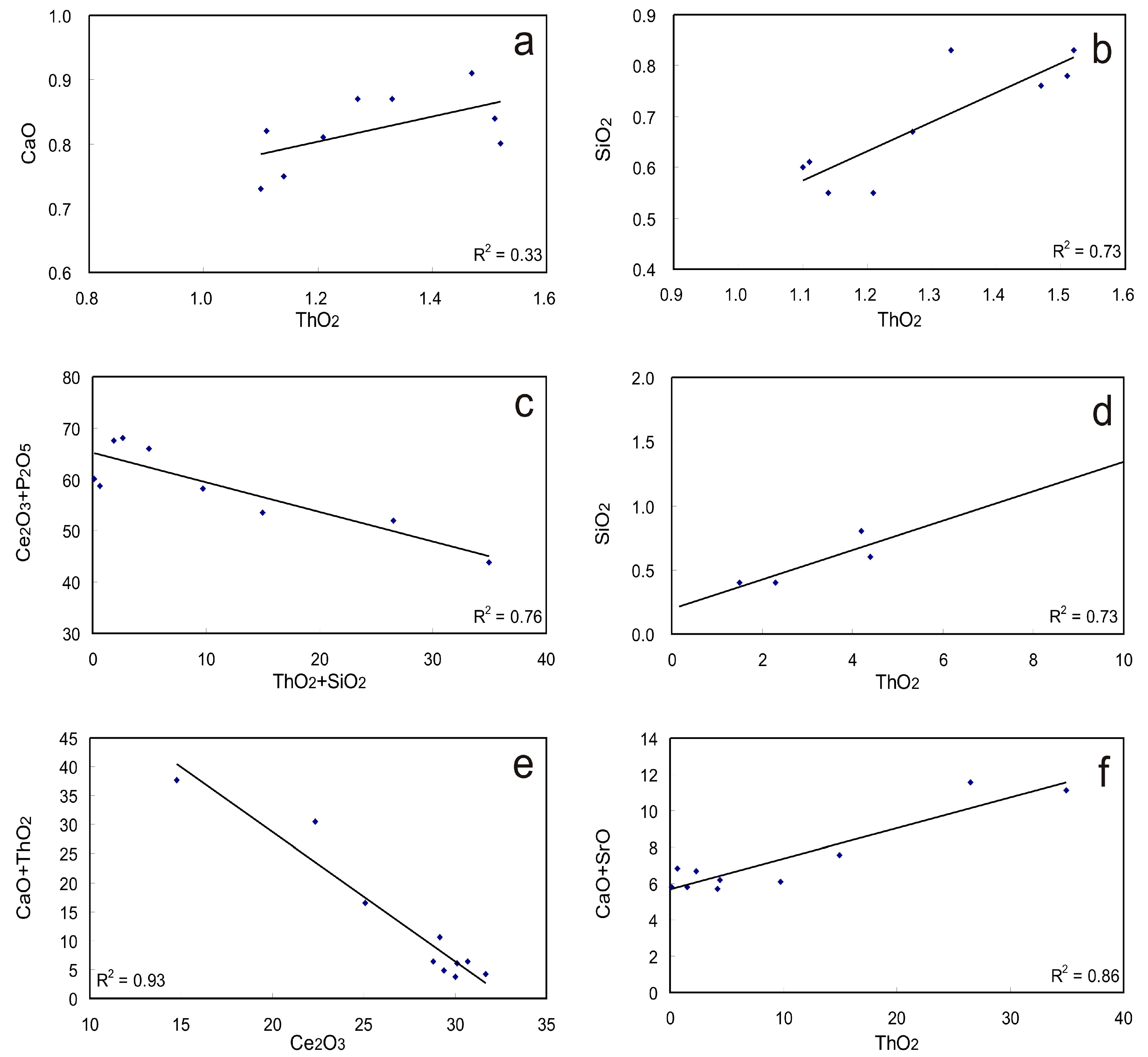
4.2. Various Element Accommodations in Monazite
5. Isotope Geochemistry
6. Geochronology
7. Beneficiation of Monazite
8. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Verplank, P.L. The role of fluids in the formation of rare earth element deposits. Procedia Earth Planet. Sci. 2017, 17, 758–761. [Google Scholar] [CrossRef]
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare earth mineralization in igneous rocks: Sources and processes. Elements 2012, 8, 347–354. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Mackay, D.A.R.; Simandl, G.J.; Ma, W.; Redfearn, M.; Gravel, J. Indicator mineral-based exploration for carbonatites and related specialty metal deposits—A QEMSCAN orientation survey, British Columbia, Canada. J. Geochem. Explor. 2016, 165, 159–173. [Google Scholar] [CrossRef]
- Decheux, N.; Clavier, N.; Podor, R. Monazite as a promising long-term radioactive waste matrix: Benefits of high-structural flexibility and chemical durability. Am. Mineral. 2013, 98, 833–847. [Google Scholar] [CrossRef]
- Mariano, A.N. Nature of Economic Mineralization in Carbonatites and Related Rocks. In Carbonatites: Genesis and Evolution; Unwin: London, UK, 1989. [Google Scholar]
- Hurai, V.; Paquette, J.L.; Huraiova, M.; Slobodnik, M.; Hvozdara, P.; Siegfried, P.; Gajdosova, M.; Milovska, S. New insights into the origin of the Evate apatite-iron oxide-carbonate deposit, Northeastern Mozambique, constrained by mineralogy, textures, thermochronometry, and fluid inclusions. Ore Geol. Rev. 2017, 80, 1072–1091. [Google Scholar] [CrossRef]
- Dill, H.G. A review of mineral resources in Malawi: With special reference to aluminium variation in mineral deposits. J. Afr. Earth Sci. 2007, 47, 153–173. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Doroshkevich, A.G.; Ponomarchuk, A.V.; Sergeev, S.A. Mineralogy, age and genesis of apatite-dolomite ores at the Seligdar apatite deposit (Central Aldan, Russia). Ore Geol. Rev. 2017, 81, 296–308. [Google Scholar] [CrossRef]
- Trofanenko, J.; William-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The nature and origin of the REE mineralization in the Wicheeda carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Chen, C.; Zhang, S.; Sun, X.; Yang, Z.; Liang, W. Geochemical and mineralogical characteristics of weathered ore in the Dalucao REE deposit, Mianning–Dechang REE Belt, western Sichuan Province, southwestern China. Ore Geol. Rev. 2015, 71, 437–456. [Google Scholar] [CrossRef]
- Kim, N.; Cheong, A.C.; Yi, K.; Jeong, Y.J.; Koh, S.M. Post-collisional carbonatite-hosted rare earth element mineralization in the Hongcheon area, central Gyeonggi massif, Korea: Ion microprobe monazite U-Th-Pb geochronology and Nd-Sr isotope geochemistry. Ore Geol. Rev. 2016, 79, 78–87. [Google Scholar] [CrossRef]
- Ani, T.A.; Sarapaa, O. Geochemistry and mineral phases of REE in Jammi carbonatite veins and fenites, southern end of the Sokli complex, NE Finland. Geochem. Explor. Environ. Anal. 2013, 13, 217–224. [Google Scholar] [CrossRef]
- Woodard, J.; Hetherignton, C.J. Carbonatite in a post-collisional tectonic setting: Geochronology and emplacement conditions at Naantali, SW Finland. Precambrian Res. 2014, 240, 94–107. [Google Scholar] [CrossRef]
- Basu, S.K.; Bhattacharyya, T. Petrography and mineral chemistry of alkaline-carbonatite complex in Singhbum crustal province, Purulia region, eastern India. J. Geol. Soc. India 2014, 83, 54–70. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Ba-bttat, M.A.O.; Taylor, R.N.; Milton, J.A.; Windley, B.F.; Evins, P.M. The carbonatite-marble dykes of Abyan Province, Yemen Republic: The mixing of mantle and crustal carbonate materials revealed by isotope and trace element analysis. Mineral. Petrol. 2004, 82, 105–135. [Google Scholar] [CrossRef]
- Liou, J.G.; Zhang, R.V.; Ernst, W.G.; Rumble, D.I.; Maruyama, S. High-pressure minerals from deeply subducted metamorphic rocks. Rev. Mineral. 1998, 37, 33–96. [Google Scholar]
- Wall, F.; Zaitsev, A.N. Rare earth minerals in Kola carbonatites. Phoscorites and Carbonatites from Mantle to Mine: The Key Example of the Kola Alkaline Province. Mineral. Soc. Ser. 2004, 10, 341–373. [Google Scholar]
- Poletti, J.E.; Cottle, J.M.; Hagen-Peter, G.A.; Lackey, J.S. Petrochronological constraints on the origin of Mountain Pass ultrapotassic and carbonatite intrusive suite, California. J. Petrol. 2016, 57, 1555–1598. [Google Scholar] [CrossRef]
- Decree, S.; Boulvais, P.; Tack, L.; Andre, L.; Baele, J.M. Fluorapatite in carbonatite-related phosphate deposits: The case of the Matongo carbonatite (Burundi). Miner. Deposita 2016, 51, 453–466. [Google Scholar] [CrossRef]
- Lazareva, E.V.; Zhmodik, S.M.; Dobretsov, N.L. Main minerals of abnormally high-grade ores of the Tomtor deposit (Arctic Siberia). Russ. Geol. Geophys. 2015, 56, 844–873. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Rare-earth element mineralization within the Mt. Weld carbonatite laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- De Toledo, M.C.M.; de Oliveira, S.M.B.; Fontan, F.; Ferrari, V.C.; de Parseval, P. Mineralogia, morfologia e cristaloquímica da monazita de Catalão I (GO, Brasil). Revista Brasileira de Geociências 2004, 34, 135–146. [Google Scholar]
- Castor, S.B.; Hedrick, J.B. Rare earth elements. In Industrial Minerals Volume, 7th ed.; Society for Mining, Metallurgy and Exploration Littleton: Englewood, IL, USA, 2006; pp. 769–792. [Google Scholar]
- Mariano, A.N.; Mariano, A. Rare earth mining and exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Ribeiro, C.C.; Brod, J.A.; Junqueira-Brod, T.C.; Gaspar, J.C.; Petrinovic, I.A. Mineralogical and field aspects of magma fragmentation deposits in a carbonate-phosphate magma chamber: Evidence from the Catalao I complex, Brazil. J. S. Am. Earth Sci. 2005, 18, 355–369. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Lueshite, pyrochlore and monazite-(Ce) from apatite-dolomite carbonatite, Lesnaya Varaka complex, Kola Peninsula, Russia. Mineral. Mag. 1998, 62, 769–782. [Google Scholar] [CrossRef]
- Levinson, A.A. A system of nomenclature for rare earth minerals. Am. Mineral. 1966, 51, 152–158. [Google Scholar]
- Nickel, E.H.; Mandarino, J.A. Revised nomenclature for rare-earth-elements minerals. Procedures involving the IMA Commission on New Minerals and Mineral Names, and guidelines on mineral nomenclature. Am. Mineral. 1987, 72, 1031–1042. [Google Scholar]
- Graeser, S.; Schwander, H. Gasparite-(Ce) and monazite-(Nd): Two new minerals to the monazite group from the Alps. Schweiz. Mineral. Petrogr. M. 1987, 67, 103–113. [Google Scholar]
- Cressey, G.; Wall, F.; Cressey, B.A. Differential REE uptake by sector growth of monazite. Mineral. Mag. 1999, 63, 813–828. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, H.K.; Yin, J.; Park, J.K. Chemistry and origin of monazites from carbonatite dikes in the Hongcheon–Jaeun district, Korea. J. Asian Earth Sci. 2005, 25, 57–67. [Google Scholar] [CrossRef]
- Zhu, X.K.; O’Nions, R.K. Monazite chemical composition: Some implications for monazite geochrology. Mineral. Petrol. 1999, 137, 351–363. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Keller, J.; Tao, K.J.; Wall, F.; Williams, C.T.; Zhang, P.S. Carbonatite dykes at Bayan Obo, Inner Mongolia, China. Mineral. Petrol. 1992, 46, 195–228. [Google Scholar] [CrossRef]
- Smith, M.P.; Henderson, P.; Campbell, L.S. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta 1999, 64, 3141–3160. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Ripp, G.; Viladkar, S.G.; Vladykin, N.V. The arshan REE carbonatites, Southwestern Transbaikalia, Russia: Mineralogy, Paragenesis and evolution. Can. Mineral. 2008. [Google Scholar] [CrossRef]
- Rose, D. Brabantite, CaTh[PO4]2, a new mineral of the monazite group. N. Jahrb. Mineral.-Monatshefte 1980, 6, 247–257. [Google Scholar]
- Laurent, A.T.; Seydoux-Guillaume, A.M.; Duchene, S.; Bingen, B.; Bosse, V.; Datas, L. Sulphate incorporation in monazite lattice and dating the cycle of sulphur in metamorphic belts. Mineral. Petrol. 2016, 171, 94. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.; Chakhmouradian, A.R.; Qi, L.; Song, W.L. A unique Mo deposit associated with carbonatites in the Qinling orogenic belt, central China. Lithos 2010, 118, 50–60. [Google Scholar] [CrossRef]
- Enkhbayar, D.; Seo, J.; Choi, S.G.; Lee, Y.L.; Batmunkh, E. Mineral chemistry of REE-rich apatite and sulfur-rich monazite from the Mushgai Khudag, alkaline volcanic-plutonic complex, South Mongolia. Int. J. Geosci. 2016, 7, 20–31. [Google Scholar] [CrossRef]
- Wall, F.; Niku-paavola, V.N.; Story, C.; Muller, A.; Jeffries, T. Xenotime-(Y) from carbonatite dykes at Lofdal, Namibia: Unusually low LREE: HREE ratio in carbonatite, and the first dating of xenotime overgrowths on zircon. Can. Mineral. 2008. [Google Scholar] [CrossRef]
- Forster, H.J. The chemical composition of REE-Y-Th-U-rich accessory minerals from peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany. Part I: The monazite-(Ce)—brabantite solid solution series. Am. Mineral. 1998, 83, 259–272. [Google Scholar] [CrossRef]
- Förster, H.J.; Harlov, D.E.; Milke, R. Composition and Th–U–total Pb ages of huttonite and thorite from Gillespie’s Beach, South Island, New Zealand. Can. Mineral. 2000, 38, 675–684. [Google Scholar] [CrossRef]
- Bulakh, A.G.; Nesterov, A.R.; Zaitsev, A.N.; Pilipuk, A.N.; Wall, F.; Kirillov, A.S. Sulfur-containing monazite-(Ce) from late-stage mineral assemblages at the Kandaguba and Vuorijarvi carbonatite complexes, Kola Peninsula, Russia. N. Jahrb. Mineral.-Monatshefte 2000, 5, 217–233. [Google Scholar]
- Doroshkevich, A.G.; Ripp, G.S.; Moore, K.R. Genesis of the Khaluta alkaline-basic Ba-Srcarbonatite complex (West Transbaikala, Russia). Mineral. Petrol. 2010, 98, 245–268. [Google Scholar] [CrossRef]
- Williams, M.L.; Jercinovic, M.J.; Hetherington, C.J. Microprobe monazite geochronology: Understanding geologic processes by integrating composition and chronology. Annu. Rev. Earth Planet. Sci. 2007, 35, 137–175. [Google Scholar] [CrossRef]
- Zhu, X.K.; Sun, J.; Pan, C.X. Sm-Nd isotopic constraints on rare-earth mineralization in the Bayan Obo ore deposit, Inner Mongolia, China. Ore Geol. Rev. 2015, 64, 543–553. [Google Scholar] [CrossRef]
- Poitrasson, F.; Chenery, S.; Shepherd, T.J. Electron microprobe and LA-ICP-MS study of monazite hydrothermal alteration: Implications for U–Th–Pb geochronology and nuclear ceramics. Geochim. Cosmochim. Acta 2000, 64, 3283–3297. [Google Scholar] [CrossRef]
- Bosse, V.; Boulvais, P.; Gautier, P. Fluid-induced disturbance of the monazite Th-Pb chronometer: In situ dating and element mapping in pegmatites from the Rhodope (Greece, Bulgaria). Chem. Geol. 2009, 261, 286–302. [Google Scholar]
- Seydoux-Guillaume, A.M.; Paquette, J.L.; Wiedenbeck, M.; Montel, J.M.; Heinrich, W. Experimental resetting of the U–Th–Pb systems in monazite. Chem. Geol. 2002, 191, 165–181. [Google Scholar] [CrossRef]
- Poitrasson, F.; Chenery, S.; Bland, D.J. Contrasted monazite hydrothermal alteration mechanisms and their geochemical implications. Earth Planet. Sci. Lett. 1996, 145, 79–96. [Google Scholar] [CrossRef]
- Wang, J.; Tatsumoto, M.; Li, X.; Premo, W.R.; Chao, E.C.T. A precise 232Th-208Pb chronology of fine-grained monazite: age of the Bayan Obo REE-Fe-Nb ore deposit, China. Geochim. Cosmochim. Acta 1994, 58, 3155–3169. [Google Scholar] [CrossRef]
- Downes, P.J.; Dunkley, D.J.; Fletcher, I.R.; McNaughton, N.J.; Rasmussen, B.; Jaques, A.L.; Verral, M.; Sweetapple, M.T. Zirconolite, zircon and monazite-(Ce) U-Th-Pb age constraints on the emplacement, deformation and alteration history of the Cummins Range carbonatite complex, Halls Creek Orogen, Kimberley region, Western Australia. Mineral. Petrol. 2016, 110, 199–222. [Google Scholar] [CrossRef]
- Ferron, G.A. Estimation of the deposition of polydisperse hyroscpic aerosol-particles in the respiratory-track. J. Aerosol Sci. 1991, 6. [Google Scholar] [CrossRef]
- Kettanah, Y.A.; Ismail, S.A. Heavy mineral concentrations in the sandstones of Amij formation with particular emphasis on the mineral chemistry and petrogenetic characteristics of monazite, western desert of Iraq. J. Afr. Earth Sci. 2016, 123, 350–369. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Aly, M.I.; Masry, B.A.; Gasser, M.S.; Khalifa, N.A.; Daoud, J.A. Extraction of Ce (IV), Yb (III) and Y (III) and recovery of some rare earth elements from Egyptian monazite using CYANEX 923 in kerosene. Int. J. Miner. Process. 2016, 153, 71–79. [Google Scholar] [CrossRef]
- Rydberg, J.; Musikas, C.G. Principles and Practices of Solvent Extraction; Marcel Dekker Inc.: New York, NY, USA, 1992. [Google Scholar]
- El-Hefny, N.E.; Daoud, J.A. Extraction and separation of thorium (IV) and praseodymium (III) with CYANEX 301 and CYANEX 302 from nitrate medium. J. Radioanal. Nucl. Chem. 2004, 261, 357–363. [Google Scholar] [CrossRef]
- El-Hefny, N.E.; El-Nadi, Y.A.; Daoud, J.A. Extraction of lanthanum and samarium from nitrate medium by some commercial organophosphorus extractants. Solvent Extr. Ion Exch. 2007, 25, 1–16. [Google Scholar]
- Masry, B.A.; Aly, M.I.; Khalifa, N.A.; Zikry, A.A.F.; Gasser, M.S.; Daoud, J.A. Liquid-liquid extraction and separation of Pr (III), Nd (III), Sm (III) from nitric acid medium by CYANEX 923 in kerosene. Arab J. Nucl. Sci. Appl. 2015, 48, 1–16. [Google Scholar]
- El-Nadi, Y.A.; Daoud, J.A.; Aly, H.F. Modified leaching and extraction of uranium from hydrous oxide cake of Egyptian monazite. Int. J. Miner. Process. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Maes, S.; Zhuang, W.-Q.; Rabaey, K.; Alvarez-Cohen, L.; Hennebel, T. Concomitant leaching and electrochemical extraction of rare earth elements from monazite. Environ. Sci. Technol. 2017, 51, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Honghui, H.; Bai, T.; Jiang, S. Geochemistry of Monazite within Carbonatite Related REE Deposits. Resources 2017, 6, 51. https://doi.org/10.3390/resources6040051
Chen W, Honghui H, Bai T, Jiang S. Geochemistry of Monazite within Carbonatite Related REE Deposits. Resources. 2017; 6(4):51. https://doi.org/10.3390/resources6040051
Chicago/Turabian StyleChen, Wei, Huang Honghui, Tian Bai, and Shaoyong Jiang. 2017. "Geochemistry of Monazite within Carbonatite Related REE Deposits" Resources 6, no. 4: 51. https://doi.org/10.3390/resources6040051
APA StyleChen, W., Honghui, H., Bai, T., & Jiang, S. (2017). Geochemistry of Monazite within Carbonatite Related REE Deposits. Resources, 6(4), 51. https://doi.org/10.3390/resources6040051




