Tree Crops, a Permanent Agriculture: Concepts from the Past for a Sustainable Future
Abstract
:1. Introduction
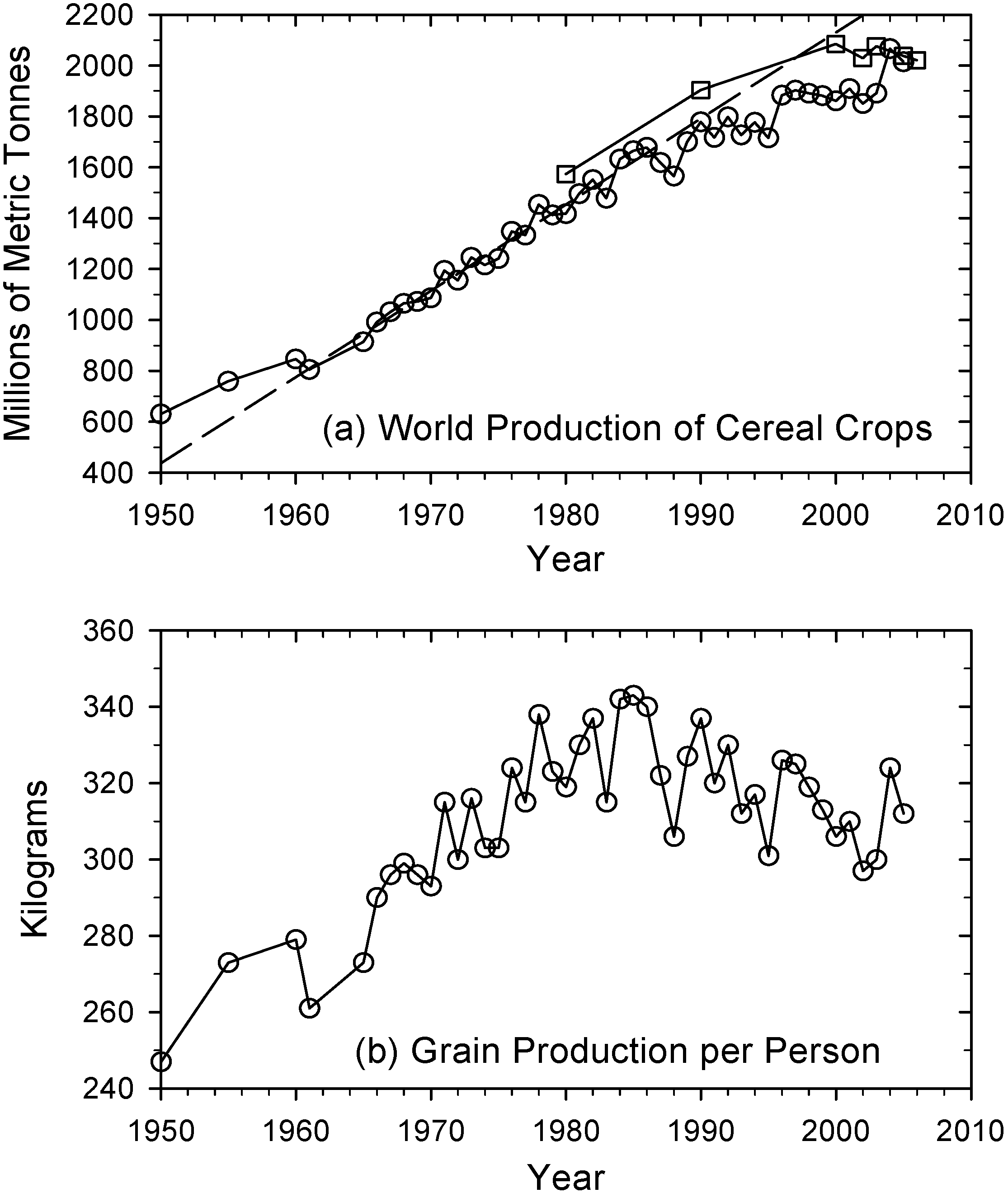
2. Perennial Crops Remain an Underutilized Resource
3. Nut Trees for Human Food and Animal Feed
3.1. Juglans regia
3.2. Juglans regia
3.3. Carya spp.
3.4. Corylus spp.
4. Food and Bioenergy Tree Crops in Warmer Climates
5. The Proposal
6. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Gill, V.; Dale, T. Topsoil and Civilization; University of Oklahoma Press: Norman, OK, USA, 1974. [Google Scholar]
- Perlin, J. A Forest Journey, the Role of Wood in the Development of a Civilization; Harvard University Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Hillel, D. Out of the Earth—Civilization and the Life of the Soil; University of California Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Evans, L.T. Feeding the Ten Billion—Plants and Population Growth; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Williams, M. Deforesting the Earth—From Prehistory to Global Crisis; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef]
- Smith, J.R. Tree Crops: A Permanent Agriculture; Devin-Adair: New York, NY, USA, 1950. [Google Scholar]
- Food and Agriculture Organization of the United Nations Web Page. Available online: http://faostat.fao.org (accessed on 27 March 2013).
- About Us. United States Department of Agriculture. Available online: http://www.ars.usda.gov/AboutUs/AboutUs.htm?modecode=66-06-05-00 (accessed on 16 August 2013).
- Xu, Y.X.; Hanna, M.A. Evaluation of Nebraska hybrid hazelnuts: Nut/kernel characteristics, kernel proximate composition, and oil and protein properties. Ind. Crops Prod. 2010, 31, 84–91. [Google Scholar] [CrossRef]
- Reid, W.; Coggeshall, M.V.; Hunt, K.L. Black walnut cultivars for nut production. Annu. Rep. North. Nut Grow. Assoc. 2004, 95, 65–77. [Google Scholar]
- Mehlenbacher, S.A. Progress and prospects in nut breeding. Acta Hortic. 2003, 622, 57–79. [Google Scholar]
- Fruit & Tree Nuts. United States Department of Agriculture. Available online: http://www.ers.usda.gov/topics/crops/fruit-tree-nuts/background.aspx#.UeMQXKx4lgE (accessed on 26 May 2012).
- MacDaniels, L.H.; Lieberman, A.S. Tree crops: A neglected source of food and forage from marginal lands. Bioscience 1979, 29, 173–175. [Google Scholar] [CrossRef]
- Brown, L.R.; Renner, M.; Halweil, B. Vital Signs 2000,the Environmental Trends That Are Shaping Our Future; Worldwatch Institute & W. W. Norton & Company: New York, NY, USA, 2000. [Google Scholar]
- Rosenzweig, C.; Hillel, D. Climate Changes and the Global Harvest—Potential Impacts of the Greenhouse Effect on Agriculture; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Lal, R.; Griffin, M.; Apt, J.; Love, L.; Morgan, M.G. Managing soil carbon. Science 2004, 304, 393. [Google Scholar] [CrossRef]
- Meehl, G.A.; Washington, W.M.; Collins, W.D.; Arblaster, J.M.; Hu, A.; Buja, L.E.; Strand, W.G.; Teng, H. How much more global warming and sea level rise? Science 2005, 307, 1769–1772. [Google Scholar] [CrossRef]
- Cohen, J.E. Human population: The next half century. Science 2003, 302, 1172–1175. [Google Scholar] [CrossRef]
- Conway, G. The Doubly Green Revolution: Food for All in the Twenty-First Century; Cornell University Press: Ithaca, NY, USA, 1998. [Google Scholar]
- Organization for Economic Co-operation and Development Food and Agriculture Organization of the United Nations. OECD-FAO Agricultural Outlook: 2006–2015. Available online: ftp://ftp.fao.org/docrep/fao/009/a0621e/a0621e00.pdf (accessed on 27 March 2013).
- Pretty, J.N. Regenerating Agriculture—Policies and Practices for Sustainability and Self-Reliance; Joseph Henry Press: Washington, DC, USA, 1995. [Google Scholar]
- Federoff, N.V.; Cohen, J.E. Plants and population: Is there time? Proc. Nat. Acad. Sci. USA 1999, 96, 5903–5907. [Google Scholar] [CrossRef]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Nat. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhammer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–676. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T.; Yang, H. Meeting cereal demand while protecting natural resources and improving environmental quality. Annu. Rev. Environ. Resour. 2003, 28, 315–358. [Google Scholar] [CrossRef]
- Glover, J.D. The necessity and possibility of perennial grain production systems. Renew. Agric. Food Syst. 2005, 20, 1–4. [Google Scholar] [CrossRef]
- Conway, G.R.; Pretty, J.N. Unwelcome Harvest—Agriculture and Pollution; Earthscan: London, UK, 1991. [Google Scholar]
- Randall, G.W.; Huggins, D.R.; Russelle, M.P.; Fuchs, D.J.; Nelson, W.W.; Anderson, J.L. Nitrate losses through subsurface tile drainage in conservation reserve program, alfalfa, and row crop systems. J. Environ. Qual. 1997, 26, 1240–1247. [Google Scholar]
- Pimentel, D.; Harvey, C.; Resosudarmo, P.; Sinclair, K.; Kurz, D.; McNair, M.; Crist, S.; Shpritz, L.; Saffouri, R.; Blair, R. Environmental and economic costs of soil erosion and conservation benefits. Science 1995, 267, 1117–1123. [Google Scholar] [CrossRef]
- Pimentel, D.; Houser, J.; Preiss, E.; White, O.; Fang, H.; Mesnick, L.; Barsky, T.; Tariche, S.; Schreck, J.; Alpert, S. Water resources: Agriculture, the environment, and society. Bioscience 1997, 47, 97–106. [Google Scholar] [CrossRef]
- Postel, S.L. Pillar of Sand: Can the Irrigation Miracle Last? W. W. Norton & Company: New York, NY, USA, 1999. [Google Scholar]
- Randall, G.W.; Mulla, D.J. Nitrate nitrogen in surface waters as influenced by climatic conditions and agricultural practices. J. Environ. Qual. 2001, 30, 337–344. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Harlan, J.R. The Living Fields: Our Agricultural Heritage; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Stokstad, E. Deadly wheat fungus threatens world’s breadbaskets. Science 2007, 315, 1786–1787. [Google Scholar] [CrossRef]
- Pennisi, E. Armed and dangerous. Science 2010, 327, 804–805. [Google Scholar] [CrossRef]
- Deffeyes, K.S. Hubbert’s Peak—The Impending World Oil Shortage; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Deffeyes, K.S. Beyond Oil: The View from Hubbert’s Peak; Macmillan: London, UK, 2006. [Google Scholar]
- Hall, D.O.; Mynick, H.E.; Williams, R.H. Cooling the greenhouse with bioenergy. Nature 1991, 353, 11–12. [Google Scholar] [CrossRef]
- Hall, D.O.; Scrase, J.I. Will biomass be the environmentally friendly fuel of the future? Biomass Bioenerg. 1998, 15, 357–637. [Google Scholar] [CrossRef]
- Sims, R.E.H. Climate Change Solutions from Biomass, Bioenergy, and Biomaterials. Available online: http://www.ecommons.cornell.edu/bitstream/1813/10341/1/Invited%20Overview%20Ralph%20Sims%209Sept2003.pdf (accessed on 27 March 2013).
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.F.; Graham, R.L.; Stokes, B.J.; Erbach, D.C. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2005. [Google Scholar]
- Brown, L.R. Plan B: 2.0 Rescuing a Planet Under Stress and a Civilization in Trouble; W. W. Norton & Company: New York, NY, USA, 2006. [Google Scholar]
- Moreira, J.R. Global biomass energy potential. Mitig. Adapt. Strateg. Glob. Clim. Chang. 2006, 11, 313–341. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Somerville, C. The billion-ton biofuels vision. Science 2006, 312, 1277. [Google Scholar] [CrossRef]
- Assadourian, E.; Bender, M.; Berner, C.; Carrus, K.; Chafe, Z.; Eckerle, K.; Flavin, C.; French, H.; Gardner, G.; Greer, L.; et al. Vital Signs 2006–2007; Worldwatch Institute & W. W. Norton & Company: New York, NY, USA, 2006; p. 23. [Google Scholar]
- Brown, L.R.; Flavin, C.; Kane, H. Vital Signs 1996; Worldwatch Institute & W. W. Norton & Company: New York, NY, USA, 1996; p. 25. [Google Scholar]
- Production of Cereals and Share in World. Available online: ftp://ext-ftp.fao.org/ES/Reserved/essb/ess/ftp_essb/john&Ozan/pdf/b01.pdf (accessed on 20 February 2012).
- Crop Prospects and Food Situation. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/docrep/009/J8104e/j8104e03.htm (accessed on 20 February 2012).
- Richardson, S.D. Forests and Forestry in China; Island Press: Washington, DC, USA, 1990. [Google Scholar]
- Zhang, P.; Shao, G.; Zhao, G.; Master, D.L.; Parker, G.R.; Dunning, J.B., Jr.; Li, Q. China’s forestry policy for the 21st century. Science 2000, 288, 2135–2136. [Google Scholar] [CrossRef]
- State of the World Forests. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/docrep/007/y5574e/y5574e00.htm (accessed on 27 March 2013).
- An edible landscape can be defined as the use of various food-producing plants in the residential landscape in place of, or in combination with, more traditional ornamental landscape plants. Edible landscapes could combine adapted perennial fruit and nut trees, berry bushes, brambles and vines, vegetables, herbs, etc., along with other plants, into attractive and functional designs. Plantings provide abundant food and other related products of a diversity of types over the growing season, as well as shade and beauty. Edible landscapes are not a new concept in areas of the world that have undergone repeated food shortages. Several of the authors were greatly impressed by the diversity and density of edible plants found in the residential landscapes across much of Eastern Europe, southern Russia, and Central Asia, when travelling in these regions for germplasm collections and other research activities.
- Cassman, K.G.; Harwood, R.R. The nature of agricultural systems: Food security and environmental balance. Food Policy 1995, 20, 439–454. [Google Scholar] [CrossRef]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef]
- Mukunda, H.S.; Dasappa, S.; Shrinivasa, U. Open-top wood gasifiers. In Renewable Energy Sources for Fuels and Electricity; Johansson, T.B., Burnham, L., Eds.; Island Press: Washington, DC, USA, 1993; pp. 699–728. [Google Scholar]
- Williams, R.H.; Larson, E.D. Advanced gasification-based biomass power generation. In Renewable Energy Sources for Fuels and Electricity; Johansson, T.B., Burnham, L., Eds.; Island Press: Washington, DC, USA, 1993; pp. 729–785. [Google Scholar]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Chynoweth, D.P. Renewable biomethane from land and ocean energy crops and organic wastes. HortScience 2005, 40, 283–286. [Google Scholar]
- Jackson, R.B.; Schlesinger, W.H. Curbing the U.S. carbon deficit. Proc. Nat. Acad. Sci. USA 2004, 101, 15827–15829. [Google Scholar] [CrossRef]
- Hall, D.O.; Rosillo-Calle, R.; Williams, R.H.; Woods, J. Biomass for bioenergy: Supply prospects. In Renewable Energy Sources for Fuels and Electricity; Johansson, T.B., Burnham, L., Eds.; Island Press: Washington, DC, USA, 1993; pp. 592–651. [Google Scholar]
- Hakkila, P.; Parikka, M. Fuel resources from the forest. In Bioenergy from Sustainable Energy; Richardson, J., Björheden, R., Hakkila, P., Lowe, A.T., Smith, C.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 19–48. [Google Scholar]
- Tilman, D.J.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Curtis, F.; Ehrenfeld, D. The new geography of trade: Globalization’s decline may stimulate local recovery. Solut. J. 2012, 3, 35–40. [Google Scholar]
- Moreira, J.R. Sugarcane for energy—Recent results and progress in Brazil. Energy Sustain. Dev. 2000, 4, 43–54. [Google Scholar] [CrossRef]
- Basiron, Y. Palm oil production through sustainable plantations. Eur. J. Lipid Sci. Technol. 2007, 109, 289–295. [Google Scholar] [CrossRef]
- Corley, R.H.V. Oil palm: A major tropical crop. Burotrop Bull. 2003, 19, 5–8. [Google Scholar]
- Danielsen, F.; Beukema, H.; Burgess, N.D.; Parish, F.; Brühl, C.A.; Donald, P.F.; Murdiyarso, D.; Phalan, B.; Reijnders, L.; Struebig, M.; et al. Biofuel plantations on forested lands: Double jeopardy for biodiversity and climate. Conserv. Biol. 2009, 23, 348–358. [Google Scholar] [CrossRef]
- Koh, L.P.; Wilcove, D.S. Oil palm: Disinformation enables deforestation. Trends Ecol. Evol. 2009, 24, 67–68. [Google Scholar] [CrossRef]
- Koh, L.P.; Wilcov, D.S. Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 2008, 1, 60–64. [Google Scholar] [CrossRef]
- Laurance, W.F.; Koh, L.P.; Butler, R.; Sodhi, N.S.; Bradshaw, C.J.A.; Neidel, J.D.; Consunji, H.; Mateo, V.J. Improving the performance of the roundtable on sustainable palm oil for nature conservation. Conserv. Biol. 2010, 24, 377–381. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J. Agroforestry as a solution to the oil palm debate. Conserv. Biol. 2008, 22, 1368–1369. [Google Scholar] [CrossRef]
- Stone, R. Can palm oil plantations come clean? Science 2013, 317, 1491. [Google Scholar] [CrossRef]
- Naylor, R.L.; Falcon, W.P.; Goodman, R.M.; Jahn, M.M.; Sengooba, T.; Tefera, H.; Nelson, R.J. Biotechnology in the developing world: A case for increased investments in orphan crops. Food Policy 2004, 29, 15–44. [Google Scholar] [CrossRef]
- Falcon, W.P.; Naylor, R.L. Rethinking food security for the 21st century. Am. J. Agric. Econ. 2005, 87, 1113–1127. [Google Scholar] [CrossRef]
- Kean, S. Besting Johnny Appleseed. Science 2010, 328, 301–303. [Google Scholar] [CrossRef]
- The Fagaceae Genome Web Home Page. Available online: http://www.fagaceae.org (accessed on 26 September 2013).
- Genome Database for Rosaceae Home Page. Available online: http://www.rosaceae.org (accessed on 26 September 2013).
- International Populus Genome Consortium Home Page. Available online: http://www.ornl.gov/sci/ipgc (accessed on 26 September 2013).
- The Generic Genome Browser. Available online: http://hazelnut.cgrb.oregonstate.edu/ (accessed on 26 September 2013).
- Jannink, J.L.; Lorenz, A.J.; Iwata, H. Genomic selection in plant breeding: From theory to practice. Brief. Funct. Genomics 2010, 9, 166–177. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.P.; Teichmann, T. Poplar genomics is getting popular: The impact of the poplar genome project on tree research. Plant Biol. 2004, 6, 2–4. [Google Scholar] [CrossRef]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef]
- Foster, G.R.; Young, R.A.; Ronkens, M.J.M.; Onstad, C.A. Processes of soil erosion by water. In Soil Erosion and Crop Productivity; Stewart, F.R.F., Stewart, B.A., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1985; pp. 137–162. [Google Scholar]
- Troeh, F.R.; Hobbs, J.A.; Donahue, R.L. Soil and Water Conservation, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1991. [Google Scholar]
- Sanchez, P.A.; Buresh, R.J.; Leakey, R.R.B. Trees, soils, and food security. Philos. Trans. Biol. Sci. 1997, 352, 949–960. [Google Scholar] [CrossRef]
- Ryan, D.F.; Bormann, F.H. Nutrient resorption in northern hardwood forests. BioScience 1982, 32, 29–32. [Google Scholar] [CrossRef]
- Braun, L.C.; Gillman, J.H.; Russelle, M.P. Fertilizer nitrogen timing and uptake efficiency of hybrid hazelnuts in the Upper Midwest, USA. HortScience 2009, 44, 1688–1693. [Google Scholar]
- Leakey, R.R.B.; Tomich, T.P. Domestication of tropical trees: From biology to economics and policy. In Agroforestry in Sustainable Agricultural Systems; Buck, L.E., Lassoie, J.P., Fernanders, E.C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 319–338. [Google Scholar]
- Cox, T.S.; Bender, M.; Picone, C.; van Tassel, D.L.; Holland, J.B.; Brummer, E.C.; Zoeller, B.E.; Paterson, A.H.; Jackson, W. Breeding perennial grain crops. Crit. Rev. Plant Sci. 2002, 21, 59–91. [Google Scholar] [CrossRef]
- Cox, T.S.; Glover, J.D.; van Tassel, D.L.; Cox, C.M.; DeHaan, L.R. Prospects for developing perennial grain crops. Bioscience 2006, 56, 649–659. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B.H. The Oil Palm; Blackwell Science: Malden, MA, USA, 2003. [Google Scholar]
- DeHaan, L.R.; van Tassel, D.L.; Cox, T.S. Perennial grain crops: A synthesis of ecology and plant breeding. Renew. Agric. Food Syst. 2005, 20, 5–14. [Google Scholar] [CrossRef]
- Glover, J.D. Harvested perennial grasslands: Ecological models for farming’s perennial future. Agric. Ecosyst. Environ. 2010, 137, 1–2. [Google Scholar] [CrossRef]
- Glover, J.D.; Culman, S.W.; DuPont, S.T.; Broussard, W.; Young, L.; Mangan, M.E.; Mai, J.G.; Crews, T.E.; DeHaan, L.R.; Buckley, D.H.; et al. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agric. Ecosyst. Environ. 2010, 137, 3–12. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Crews, T.E. Perennial crops and endogenous nutrient supplies. Renew. Agric. Food Syst. 2005, 20, 25–37. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Wiseman, W.J., Jr. Gulf of Mexico hypoxia, a.k.a. “The Dead Zone”. Annu. Rev. Ecol. Syst. 2002, 33, 235–263. [Google Scholar] [CrossRef]
- Funk, C.R.; White, J.F., Jr. Use of natural and transformed endophytes for turf improvement. In Endophyte/Grass Interactions; Hill, N., Bacon, C.W., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 229–239. [Google Scholar]
- Postgate, J.R. The Fundamentals of Nitrogen Fixation; Cambridge University Press: New York, NY, USA, 1982. [Google Scholar]
- Saito, M.; Marumoto, T. Inoculation with arbuscular mycorrhizal fungi: The status quo in Japan and the future prospects. Plant Soil 2002, 244, 273–279. [Google Scholar] [CrossRef]
- Emerich, D.W.; Krishnan, H.B. Nitrogen Fixation in Crop Production; Agronomy Monograph Series 52; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America (ASA-CSSA-SSSA): Madison, WI, USA, 2009. [Google Scholar]
- Fischer, R.A.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, S85–S98. [Google Scholar]
- Garnett, T.; Appleby, M.C.; Balmford, A.; Bateman, I.J.; Benton, T.G.; Bloomer, P.; Burlingame, B.; Dawkins, M.; Dolan, L.; Fraser, D.; et al. Sustainable intensification in agriculture: Premises and policies. Science 2013, 341, 33–34. [Google Scholar] [CrossRef]
- Sanchez, P.A.; Swaminathan, M.S. Cutting world hunger in half. Science 2005, 307, 357–359. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Waliyar, F.; Jamnadas, R.H.; Moustafa, A.; Andrade, M.; Drechsel, P.; Hughes, J.D.A.; Kadirvel, P.; Luther, K. Relearning old lessons for the future of food—By bread alone no longer: Diversifying diets with fruits and vegetables. Crop Sci. 2010, 50, S-51–S-62. [Google Scholar]
- Phillips, R.L. Mobilizing science to break yield barriers. Crop Sci. 2010, 50, S-99–S-108. [Google Scholar] [CrossRef]
- Sanchez, P.A. Soil fertility in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Lehmann, J.; Kern, D.C.; German, L.A.; McCann, J.; Martins, G.C.; Moreira, A. Soil fertility and production potential. In Amazonian Dark Earths: Origin, Properties, Management; Lehmann, J., Kern, D.C., Glaser, B., Woods, W.I., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 105–124. [Google Scholar]
- Jackson, W. New Roots for Agriculture; University of Nebraska Press: Lincoln, NE, USA, 1980. [Google Scholar]
- Wagoner, P. Perennial grain development: Past efforts and potential for the future. Crit. Rev. Plant Sci. 1990, 9, 381–408. [Google Scholar] [CrossRef]
- Waggoner, P. Perennial grain: New use for intermediate wheatgrass. J Soil Water Conserv. 1990, 45, 81–82. [Google Scholar]
- Pimentel, D.; Jackson, W.; Bender, M.; Pickett, W. Perennial grains: An ecology of crops. Interdiscip. Sci. Rev. 1986, 11, 42–49. [Google Scholar] [CrossRef]
- Jakubziner, M.M. New Wheat Species. In Proceedings of the 1st International Wheat Genetics Symposium, Winnipeg, Canada, 11–15 August 1959; pp. 207–220.
- Vinall, H.N.; Hein, M.A. Breeding miscellaneous grasses. In Yearbook of Agriculture 1937; U.S. Department of Agriculture: Washington, DC, USA, 1937; p. 1059. [Google Scholar]
- Scheinost, P.L.; Lammer, D.L.; Cai, X.; Murray, T.; Jones, S.S. Perennial wheat: The development of a sustainable cropping system for the U.S., Pacific Northwest. Am. J. Altern. Agric. 2001, 16, 147–151. [Google Scholar] [CrossRef]
- Bell, L.W.; Byrne, F.; Ewing, M.A.; Wade, L.J. A preliminary whole-farm economic analysis of perennial wheat in an Australian dryland farming system. Agric. Syst. 2008, 96, 166–174. [Google Scholar] [CrossRef]
- Piper, J.K. Growth and seed yield of three perennial grains within monocultures and mixed stands. Agric. Ecosyst. Environ. 1998, 68, 1–11. [Google Scholar] [CrossRef]
- Sculte, L.A.; Liebman, M.; Asbjornsen, H.; Crow, T.R. Agroecosystem restoration through strategic integration of perennials. J. Soil Water Conserv. 2006, 61, 164–169. [Google Scholar]
- Pimentel, D.; Cerasale, D.; Stanley, R.C.; Perlman, R.; Newman, E.M.; Brent, L.C.; Mullan, A.; Chang, D.T.I. Annual vs perennial grain production. Agric. Ecosyst. Environ. 2012, 161, 1–9. [Google Scholar] [CrossRef]
- Glover, J.D.; Reganold, J.P.; Bell, L.W.; Borevitz, J.; Brummer, E.C.; Buckler, E.S.; Cox, C.M.; Cox, T.S.; Crews, T.E.; Culman, S.W.; et al. Increased food and ecosystem security via perennial grains. Science 2010, 328, 1638–1639. [Google Scholar] [CrossRef]
- Pimentel, D.; Wilson, C.; McCullum, C.; Huang, R.; Dwen, P.; Flack, J.; Tran, Q.; Saltman, T.; Cliff, B. Economic and environmental effects of biodiversity. BioScience 1997, 47, 747–757. [Google Scholar] [CrossRef]
- Jackson, W. Natural systems agriculture: A truly radical alternative. Agric. Ecosyst. Environ. 2002, 88, 111–117. [Google Scholar] [CrossRef]
- Tilman, D.J.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef]
- Tilman, D.J.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef]
- Rosengarten, F., Jr. The Book of Edible Nuts; Walker & Company: New York, NY, USA, 1984. [Google Scholar]
- Garret, H.E.; Harper, L.S. The science and practice of black walnut agroforestry in Missouri, USA. In Agroforestry in Sustainable Agricultural Systems; Buck, L.E., Lassoie, J.P., Fernanders, E.C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 97–110. [Google Scholar]
- Thompson, M.M.; Lagerstedt, H.B.; Mehlenbacher, S.A. Hazelnuts. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1996; Volume 3, pp. 125–184. [Google Scholar]
- Mamadjanov, D.K. Walnut fruit forests and diversity of walnut trees (Juglans regia L.) in Kyrgyzstan. Acta Hort. 2006, 705, 173–176. [Google Scholar]
- Alasalvar, C.; Shahidi, F. Tree Nuts: Composition, Phytochemicals, and Health Effects; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Reid, W. Eastern black walnut: Potential for commercial nut producing cultivars. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 327–331. [Google Scholar]
- Thompson, T.E.; Madden, G.D. Pecans. In A Guide to Nut Tree Culture in North America; Fulbright, D.W., Ed.; Northern Nut Growers Association: Hamden, CT, USA, 2003; Volume 1, pp. 79–105. [Google Scholar]
- Sparks, D. Pecan Cultivars—The Orchard’s Foundation; Pecan Production Innovations: Watkinsville, GA, USA, 1992. [Google Scholar]
- Grauke, L.J.; Thompson, T.E. Pecans and hickories. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1996; pp. 185–240. [Google Scholar]
- McGranahan, G.; Leslie, C. Walnuts (Juglans). In Genetic Resources of Temperate Fruit and Nut Crops; Moore, J.N., Ballington, J.R., Eds.; International Society for Horticultural Science: Leuven, Belgium, 1991; pp. 907–951. [Google Scholar]
- Tsao, R.; Li, L. Phytochemical profiles and potential health benefits of heartnut (Juglans ailantifolia var. cordiformis): A comparison with the common walnut (Juglans regia L.). In Tree Nuts—Composition, Phytochemicals and Health Effects; Alasalvar, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 237–248. [Google Scholar]
- McGranahan, G.; Leslie, C. Breeding walnuts (Juglans regia). In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 254–278. [Google Scholar]
- Leslie, C.A.; McGranahan, G.H. The origin of the walnut. In Walnut Production Manual; Ramos, D., Ed.; University of California Agriculture and Natural Resources Publications: Richmond, CA, USA, 1998; pp. 3–7. [Google Scholar]
- O’Rourke, F.L.S. The Carpathian (Persian) walnut. In Handbook of North American Nut Trees; Jaynes, R.A., Ed.; Northern Nut Growers Association: Geneva, NY, USA, 1969; pp. 232–239. [Google Scholar]
- McGranahan, G.H.; Leslie, C. Advances in genetic improvement of walnut at the University of California, Davis. Acta Hort 2006, 705, 117–122. [Google Scholar]
- Thompson, M.M. Exploration and exploitation of new fruit and nut germplasm. In New Crops; Janick, J., Simon, J.E., Eds.; John Wiley and Sons: New York, NY, USA, 1993; pp. 155–160. [Google Scholar]
- Popov, S.I. Diversity of walnut in the walnut-fruit forests and its practical value. In Biodiversity and Sustainable Use of Kyrgyzstan’s Walnut-Fruit Forests; Blaser, J., Carter, J., Gilmour, D., Eds.; International Union for Conservation of Nature (IUCN): Gland, Switzerland; Cambridge, UK; INTERCOOPERATION: Bern, Switzerland, 1995; pp. 117–119. [Google Scholar]
- Germain, E.; Delort, F.; Kanivets, V. Precocious maturing walnut populations originating from Central Asia: Their behavior in France. Acta Hort 1997, 422, 83–89. [Google Scholar]
- Forde, H.I.; McGranahan, G.H. Walnuts. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1996; Volume 3, pp. 241–273. [Google Scholar]
- Vahdati, K.; McKenna, J.R.; Dandekar, A.M.; Leslie, C.A.; Uratsu, S.L.; Hackett, W.P.; Negri, P.; McGranahan, G.H. Rooting and other characteristics of a transgenic walnut hybrid (Juglans hindsii × J. regia) rootstock expressing rolABC. J. Am. Soc. Hortic. Sci. 2002, 127, 724–728. [Google Scholar]
- Dangl, G.S.; Woeste, K.; Aradhya, M.K.; Koehmstedt, A.; Simon, C.; Potter, D.; Leslie, C.A.; McGranahan, G. Characterization of 14 microsatellite markers for genetic analysis and cultivar identification of walnut. J. Am. Soc. Hortic. Sci. 2005, 130, 348–354. [Google Scholar]
- Molnar, T.J.; Zaurov, D.E.; Capik, J.M.; Eisenman, S.W.; Ford, T.; Nikolyi, L.V.; Funk, C.R. Persian walnuts (Juglans regia L.) in Central Asia. Annu. Rep. North. Nut. Grow. Assoc. 2011, 101, 56–69. [Google Scholar]
- Pollock, S.; Perez, A. Fruit and Tree Nuts Situation and Outlook Yearbook; U.S. Department of Agriculture: Washington, DC, USA, 2005.
- Simopoulos, A.P. Omega-3 fatty acids in wild plants, nuts and seeds. Asia Pac. J. Clin. Nutr. 2002, 11, S163–S173. [Google Scholar] [CrossRef]
- Gleason, H.A.; Cronquist, A. Manual of Vascular Plants of Northeastern United States and Adjacent Canada; New York Botanical Garden: Bronx, NY, USA, 1998. [Google Scholar]
- Prindle, J. Black walnut crop purchased by Hammons Products Company 2007–2009. Personal communication, Hammons Products Company: Stockton, MO, USA, 16 July 2010. [Google Scholar]
- Chenoweth, B. Black Walnut—The History, Use, and Unrealized Potential of a Unique American Renewable Natural Resource; Sagamore Publishing: Champaign, IL, USA, 1995. [Google Scholar]
- Victory, E.R. History of black walnut genetics research in North America. In Black Walnut in a New Century, Proceedings of the 6th Walnut Council Research Symposium, Lafayette, IN, USA, 25–28 July 2004; Michler, C.H., Pijut, P.M., van Sambeek, J.W., Coggeshall, M.V., Seifert, J., Woeste, K., Overton, R., Ponder, F., Jr., Eds.; North Central Research Station, Forest Service, U.S. Department of Agriculture: Washington, DC, USA, 2004; pp. 1–8. [Google Scholar]
- Beineke, W.F. Twenty years of black walnut genetic improvement at Purdue University. North. J. Appl. For. 1989, 6, 68–71. [Google Scholar]
- Victory, E.R.; Glaubitz, J.C.; Rhodes, O.E., Jr.; Woeste, K.E. Genetic homogeneity in Juglans nigra (Juglandaceae) at nuclear microsatellites. Am. J. Bot. 2006, 93, 118–126. [Google Scholar] [CrossRef]
- Reid, W.; Coggeshall, M.V.; Hunt, K.L. Cultivar Evaluation and Development for Black Walnut Orchards. In Black Walnut in a New Century, Proceedings of the 6th Walnut Council Research Symposium, Lafayette, IN, USA, 25–28 July 2004; Michler, C.H., Pijut, P.M., van Sambeek, J.W., Coggeshall, M.V., Seifert, J., Woeste, K., Overton, R., Ponder, F., Jr., Eds.; North Central Research Station, Forest Service, U.S. Department of Agriculture: Washington, DC, USA; pp. 18–24.
- Reid, W.; Coggeshall, M.; Garret, H.E.; van Sambeek, J. Growing Black Walnut for Nut Production. Available online: http://www.centerforagroforestry.org/pubs/walnutNuts.pdf (accessed on 27 March 2013).
- Coggeshall, M.V. Black walnut cultivar improvement program at the University of Missouri. Annu. Rep. North. Nut. Grow. Assoc. 2002, 93, 93–96. [Google Scholar]
- Woeste, K.E.; McKenna, J.R. Walnut Genetic Improvement at the Start of a New Century. In Black Walnut in a New Century, Proceedings of the 6th Walnut Council Research Symposium, Lafayette, IN, USA, 25–28 July 2004; Michler, C.H., Pijut, P.M., van Sambeek, J.W., Coggeshall, M.V., Seifert, J., Woeste, K., Overton, R., Ponder, F., Jr., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2004; pp. 9–17. [Google Scholar]
- The Northern Nut Growers Association Home Page. Available online: http://www.nutgrowing.org (accessed on 27 March 2013).
- Hanson, B. Black walnut cultivar performance. Annu. Rep. North. Nut. Grow. Assoc. 2003, 94, 120–132. [Google Scholar]
- Gordon, J.H. Nut Growing Ontario Style; Society of Ontario Nut Growers: Niagara-on-the-Lake, Ontario, Canada, 1993. [Google Scholar]
- Utley, C.; Nguyen, T.; Roubtsova, T.; Coggeshall, M.; Ford, T.M.; Grauke, L.J.; Graves, A.D.; Leslie, C.A.; McKenna, J.; Woeste, K.; et al. Susceptibility of walnut and hickory species to Geosmithia morbida. Plant Dis. 2013, 97, 601–607. [Google Scholar] [CrossRef]
- Brison, F.R. Pecan Culture; Capital Printing: Austin, TX, USA, 1974. [Google Scholar]
- Grauke, L.J. Yunnan hickory. Annu. Rep. North. Nut. Grow. Assoc. 2006, 97, 57–69. [Google Scholar]
- MacDaniels, L.H. Hickories. In Nut Tree Culture in North America; Jaynes, R.A., Ed.; Northern Nut Growers Association: Hamden, CT, USA, 1979; pp. 35–50. [Google Scholar]
- Potts, B.M.; Dungey, H.S. Interspecific hybridization of Eucalyptus: Key issues for breeders and geneticists. New For. 2004, 27, 115–138. [Google Scholar] [CrossRef]
- Thompson, T.E.; Grauke, L.J. Pecans and other hickories (Carya). In Genetic Resources of Temperate Fruit and Nut Crops; Moore, J.N., Ballington, J.R., Eds.; International Society for Horticultural Science: Wageningen, The Netherlands, 1991; pp. 837–904. [Google Scholar]
- Mehlenbacher, S.A. Hazelnuts (Corylus). In Genetic Resources of Temperate Fruit and Nut Crops; Moore, J.N., Ballington, J.R., Eds.; International Society for Horticultural Science: Leuven, Belgium, 1991; pp. 789–836. [Google Scholar]
- Erdogan, V.; Mehlenbacher, S.A. Interspecific hybridization in hazelnut (Corylus). J. Am. Soc. Hortic. Sci. 2000, 125, 489–497. [Google Scholar]
- Molnar, T.J.; Goffreda, J.C.; Funk, C.R. Developing hazelnuts for the eastern United States. Acta Hortic. 2005, 68, 609–617. [Google Scholar]
- United States Department of Agriculture Web Page. National Clonal Germplasm Repository Database. Available online: http://www.ars.usda.gov/Main/docs.htm?docid=11035 (accessed on 27 March 2013).
- Fuller, A.S. The Nut Culturist; Orange Judd Company: New York, NY, USA, 1896. [Google Scholar]
- Mehlenbacher, S.A.; Thompson, M.M.; Cameron, H.R. Occurrence and inheritance of resistance to eastern filbert blight in “Gasaway” hazelnut. HortScience 1991, 26, 410–411. [Google Scholar]
- Lunde, C.F.; Mehlenbacher, S.A.; Smith, D.C. Survey of hazelnut cultivars for response to eastern filbert blight inoculation. HortScience 2000, 35, 729–731. [Google Scholar]
- Chen, H.; Mehlenbacher, S.A.; Smith, D.C. Hazelnut accessions provide new sources of resistance to eastern filbert blight. HortScience 2007, 42, 466–469. [Google Scholar]
- Molnar, T.J.; Zaurov, D.E.; Goffreda, J.C.; Mehlenbacher, S.A. Survey of hazelnut germplasm from Russia and Crimea for response to eastern filbert blight. HortScience 2007, 42, 51–56. [Google Scholar]
- Molnar, T.J.; Goffreda, J.C.; Funk, C.R. Survey of Corylus resistance to Anisogramma anomala from different geographic locations. HortScience 2010, 45, 832–836. [Google Scholar]
- Mehlenbacher, S.A.; Smith, D.C.; McCluskey, R.L. “Yamhill” hazelnut. HortScience 2009, 44, 845–847. [Google Scholar]
- Mehlenbacher, S.A.; Smith, D.C.; McCluskey, R.L. “Jefferson” hazelnut. HortScience 2011, 46, 662–664. [Google Scholar]
- Mehlenbacher, S.A. Hazelnuts. In A Guide to Nut Tree Culture in North America; Fulbright, D.W., Ed.; Northern Nut Growers Association: Hamden, CT, USA, 2003; Volume 1, pp. 183–215. [Google Scholar]
- Ebrahem, K.S.; Richardson, D.G.; Tetley, R.M.; Mehlenbacher, S.A. Oil content, fatty acid composition, and vitamin E concentration of 17 hazelnut varieties, compared to other types of nuts and oil seeds. Acta Hortic. 1994, 351, 685–692. [Google Scholar]
- Leakey, R.R.B. Potential for novel food products from agroforestry trees: A review. Food Chem. 1999, 66, 1–14. [Google Scholar] [CrossRef]
- Jain, S.M.; Priyadarshan, P.M. (Eds.) Breeding Plantation Tree Crops: Tropical Species; Springer: New York, NY, USA, 2009.
- Batugal, P.; Bourdeix, R.; Baudouin, L. Coconut breeding. In Breeding Plantation Tree Crops: Temperate Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 327–375. [Google Scholar]
- Sambanthamurthi, R.; Singh, R.; Kadir, A.P.G.; Abdullah, M.O.; Kushairi, A. Opportunities for the oil palm via breeding and biotechnology. In Breeding Plantation Tree Crops: Tropical Species; Jain, S.M., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 377–421. [Google Scholar]
- Leakey, R.R.B.; Newton, A.C. Domestication of “Cinderella” species as the start of a woody-plant revolution. In Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources; Leakey, R.R., Newton, A.C., Eds.; HMSO: London, UK, 1994; pp. 3–6. [Google Scholar]
- Brewbaker, J.L.; Sorenssen, C.T. Domestication of lesser-known species of the genus Leucaena. In Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources; Leakey, R.R., Newton, A.C., Eds.; HMSO: London, UK, 1994; pp. 195–204. [Google Scholar]
- Pandey, V.C.; Kumar, A. Leucaena leucocephala: An underutilized plant for pulp and paper production. Genet. Resour. Crop Evol. 2013, 60, 1165–1171. [Google Scholar] [CrossRef]
- Nirsatmanto, A.; Leksono, B.; Kurinobu, S.; Shiraishi, A. Realized genetic gain observed in second generation seedling seed orchards of Acacia mangium in South Kalimantan, Indonesia. J. For. Res. 2004, 9, 265–269. [Google Scholar]
- Brockwell, J.; Searle, S.D.; Jeavons, A.C.; Waayers, M. Nitrogen Fixation in Acacias: An Untapped Resource for Sustainable Plantations, Farm Forestry and Land Reclamation; ACIAR Monograph Series 115; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2005; pp. 1–132.
- Eldridge, K.; Davidson, J.; Hardwood, C.; van Wyk, G. Eucalypt Domestication and Breeding; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Camphinos, E. Sustainable plantations of high-yield shape Eucalyptus trees for production of fiber: The Aracruz case. New For. 1999, 7, 129–143. [Google Scholar]
- Calder, I.R.; Risier, P.T.W.; Prasanna, K.T.; Parameswarappa, S. Eucalyptus water use greater than rainfall input—A possible explanation from southern India. Hydrol. Earth Syst. Sci. 1997, 1, 249–256. [Google Scholar] [CrossRef]
- Geary, T.F. Afforestation in Uruguay: Study of a changing landscape. J. For. 2001, 99, 35–39. [Google Scholar]
- Jawjit, W.; Kroeze, C.; Soontaranun, W.; Hordijk, L. Options to reduce the environmental impact by eucalyptus-based Kraft pulp industry in Thailand: Model description. J. Clean. Prod. 2007, 15, 1827–1839. [Google Scholar] [CrossRef]
- Jawjit, W.; Kroeze, C.; Soontaranun, W.; Hordijk, L. An analysis of the environmental pressure exerted by the eucalyptus-based Kraft pulp industry in Thailand. Environ. Dev. Sustain. 2006, 8, 289–311. [Google Scholar] [CrossRef]
- Liu, H.; Li, J. The study of ecological oproblems of Eucalyptus plantation and sustainable development in Maoming Xiaoliang. J. Sustain. Dev. 2010, 3, 197–201. [Google Scholar]
- Binkley, D.; Stape, J.L. Sustainable Management of Eucalyptus Plantations in a Changing World. In Proceedings of the IUFRO Conference—Eucalyptus in a Changing WorldBorralho N.M.G.Pereira J.S.Marques C., Aveiro, Portugal, 11–15 October 2004; Borralho, N.M.G., Pereira, J.S., Marques, C., Coutinho, J., Madeira, M., Tomé, M., Eds.;
- Bragança, M.; DeSouza, O.; Zanuncio, J.C. Environmental heterogeneity as a strategy for pest management in Eucalyptus plantations. For. Ecol. Manag. 1998, 102, 9–12. [Google Scholar] [CrossRef]
- Moffat, A.S. Resurgent forests can be greenhouse gas sponges. Science 1997, 277, 315–316. [Google Scholar] [CrossRef]
- Fearnside, P.M. Global warming and tropical land-use change: Greenhouse gas emissions from biomass burning, decomposition and soils in forest conversion, shifting cultivation and secondary vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- The Global Partnership on Forest and Landscape Restoration Web Page. Available online: http://www.ideastransformlandscapes.org/ (accessed on 26 September 2013).
- Murgueitio, E.; Calle, Z.; Uribe, F.; Calle, A.; Solorio, B. Native trees and shrubs for productive rehabilitation of tropical cattle ranching lands. For. Ecol. Manag. 2011, 261, 1654–1663. [Google Scholar] [CrossRef]
- Calle, Z.; Murgueitio, E.; Chará, J. Integrating forestry, sustainable cattle ranching and landscape restoration. Unasylva 239 2012, 63, 31–40. [Google Scholar]
- Kahn, P.C.; Molnar, T.; Zhang, G.G.; Funk, C.R. Investing in perennial crops to sustainably feed the world. Issues Sci. Technol. 2011, 27, 75–81. [Google Scholar]
- Forest Stewardship Council Home Page. Available online: http://www.fsc.org/en (accessed on 27 March 2013).
- Roundtable on Sustainable Palm Oil (RSPO) Home Page. Roundtable on Sustainable Palm Oil Production. Available online: http://www.rspo.org/ (accessed on 27 March 2013).
- World Bank Web Page. World Development Report 2008: Agriculture for Development. Available online: http://siteresources.worldbank.org/INTWDR2008/Resources/2795087-1192111580172/WDROver2008-ENG.pdf (accessed on 27 March 2013).
- Deane, C.; Ejeta, G.; Rabbinge, R.; Sayer, J. Science for development: Mobilizing global partnerships. Crop Sci. 2010, 50, v–viii. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Molnar, T.J.; Kahn, P.C.; Ford, T.M.; Funk, C.J.; Funk, C.R. Tree Crops, a Permanent Agriculture: Concepts from the Past for a Sustainable Future. Resources 2013, 2, 457-488. https://doi.org/10.3390/resources2040457
Molnar TJ, Kahn PC, Ford TM, Funk CJ, Funk CR. Tree Crops, a Permanent Agriculture: Concepts from the Past for a Sustainable Future. Resources. 2013; 2(4):457-488. https://doi.org/10.3390/resources2040457
Chicago/Turabian StyleMolnar, Thomas J., Peter C. Kahn, Timothy M. Ford, Clarence J. Funk, and C. Reed Funk. 2013. "Tree Crops, a Permanent Agriculture: Concepts from the Past for a Sustainable Future" Resources 2, no. 4: 457-488. https://doi.org/10.3390/resources2040457
APA StyleMolnar, T. J., Kahn, P. C., Ford, T. M., Funk, C. J., & Funk, C. R. (2013). Tree Crops, a Permanent Agriculture: Concepts from the Past for a Sustainable Future. Resources, 2(4), 457-488. https://doi.org/10.3390/resources2040457





