Struvite Precipitation from Centrate—Identifying the Best Balance Between Effectiveness and Resource Efficiency
Abstract
1. Introduction
- Is it necessary to maintain a high pH level in centrates at a high SI due to existing ion concentration?
- What is the optimum pH level balancing low PO4-P concentration after precipitation, while avoiding excessive use of lye for pH adjustment?
- 3.
- How do temperature variations, combined with previously mentioned variations in pH and ion concentration, affect the precipitation process?
2. Materials and Methods
2.1. Matrix
2.2. Design of the Experiments
2.3. Procedure of the Experiments
2.4. Mixing, Temperature and pH Control
2.5. Chemicals
2.6. Analytics
2.7. Software
3. Results
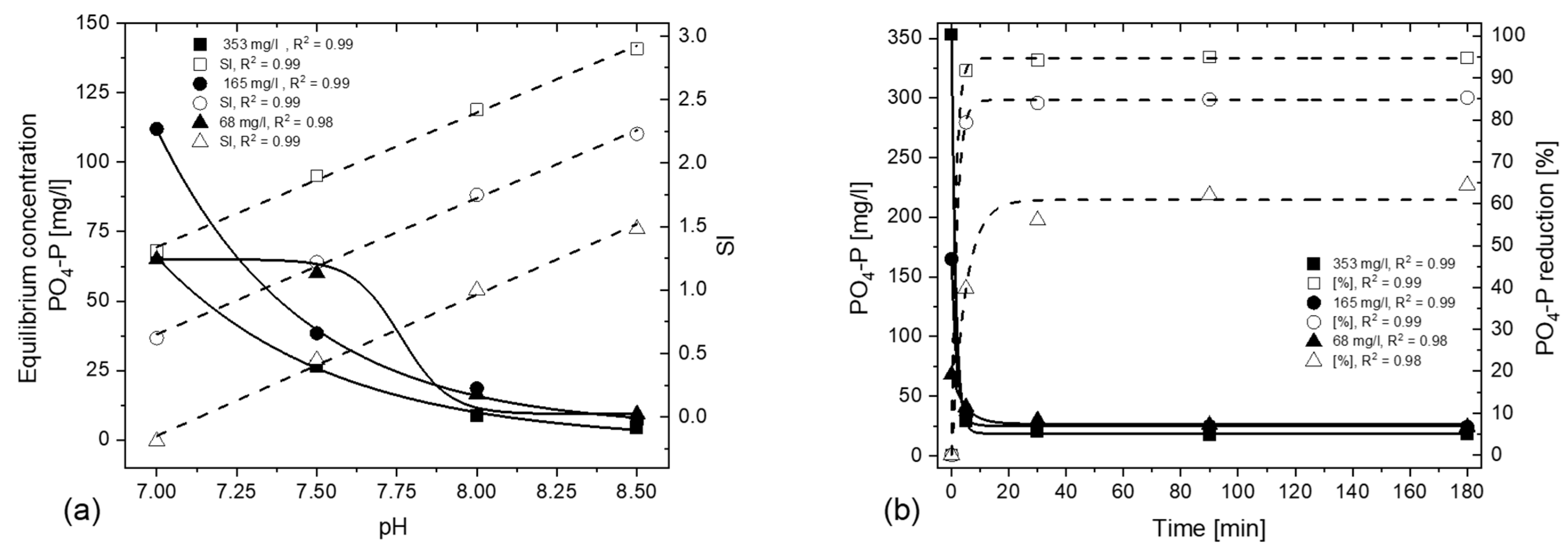
3.1. Effect of Initial Ion Concentration, Applied pH Value and Temperature
3.1.1. Effect of Initial Ion Concentration and Applied pH Value
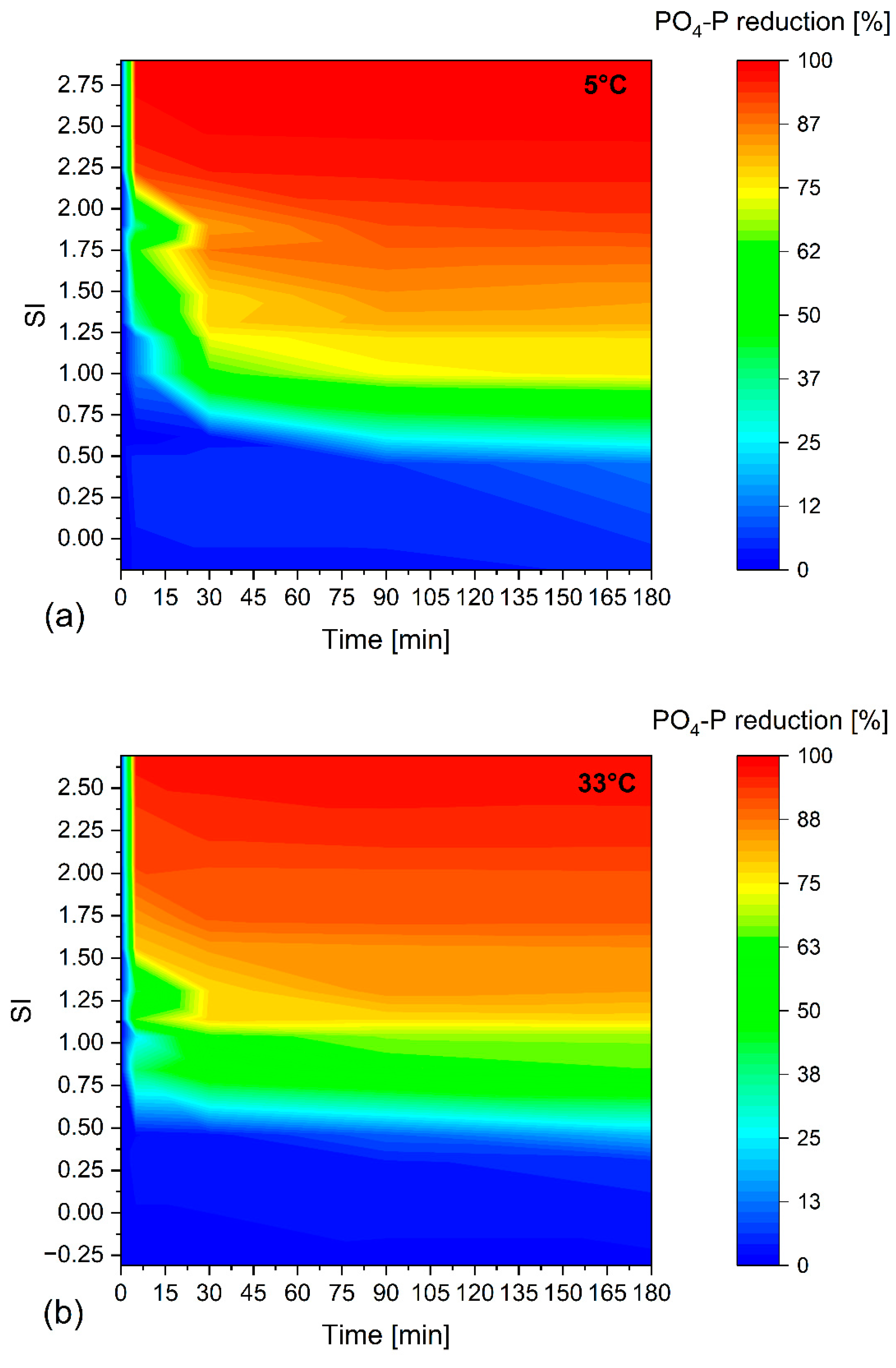
3.1.2. Effect of Temperature
3.1.3. Influence on Reaction Kinetics
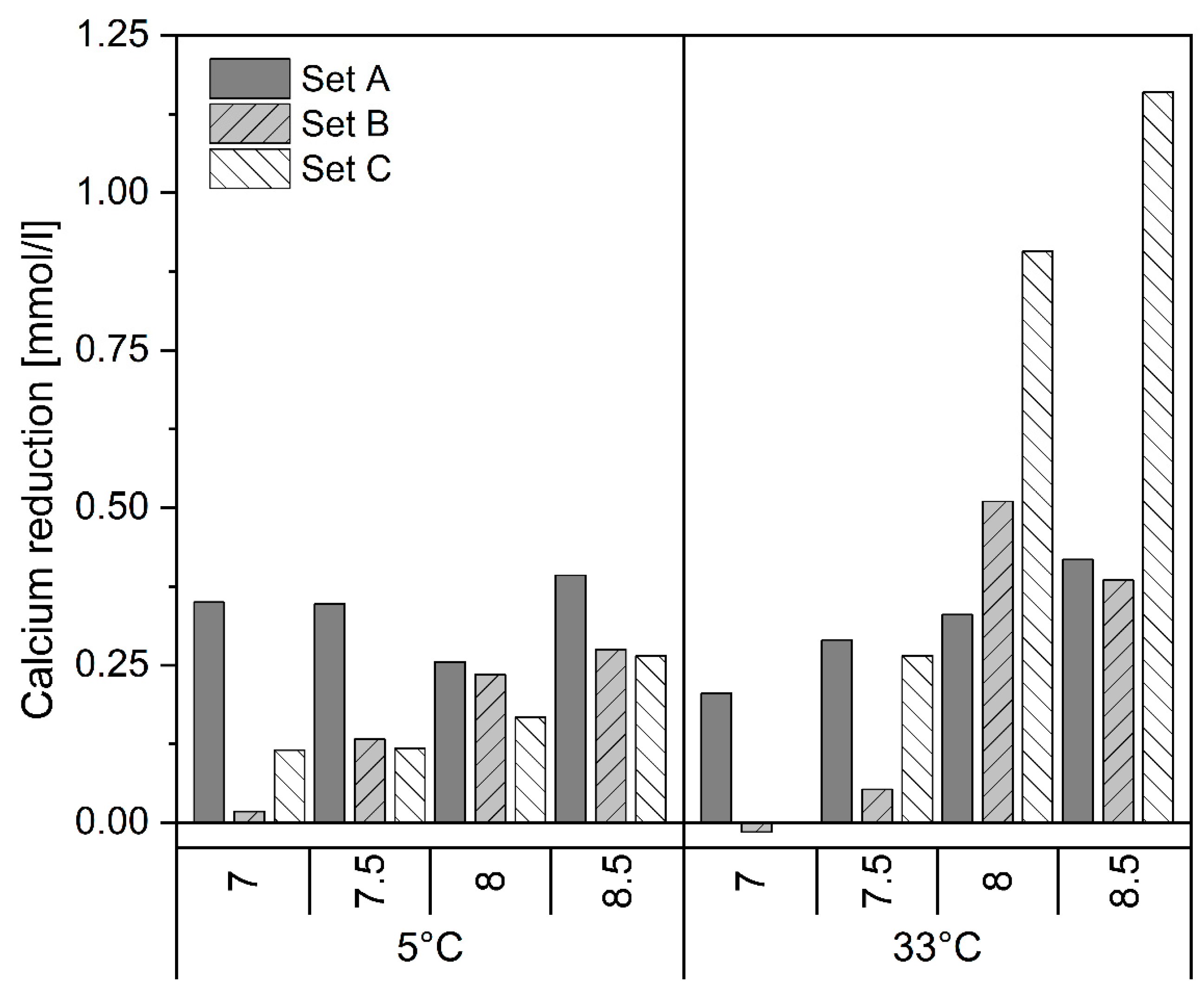
3.2. Fate of Other Ions
3.3. Crystals
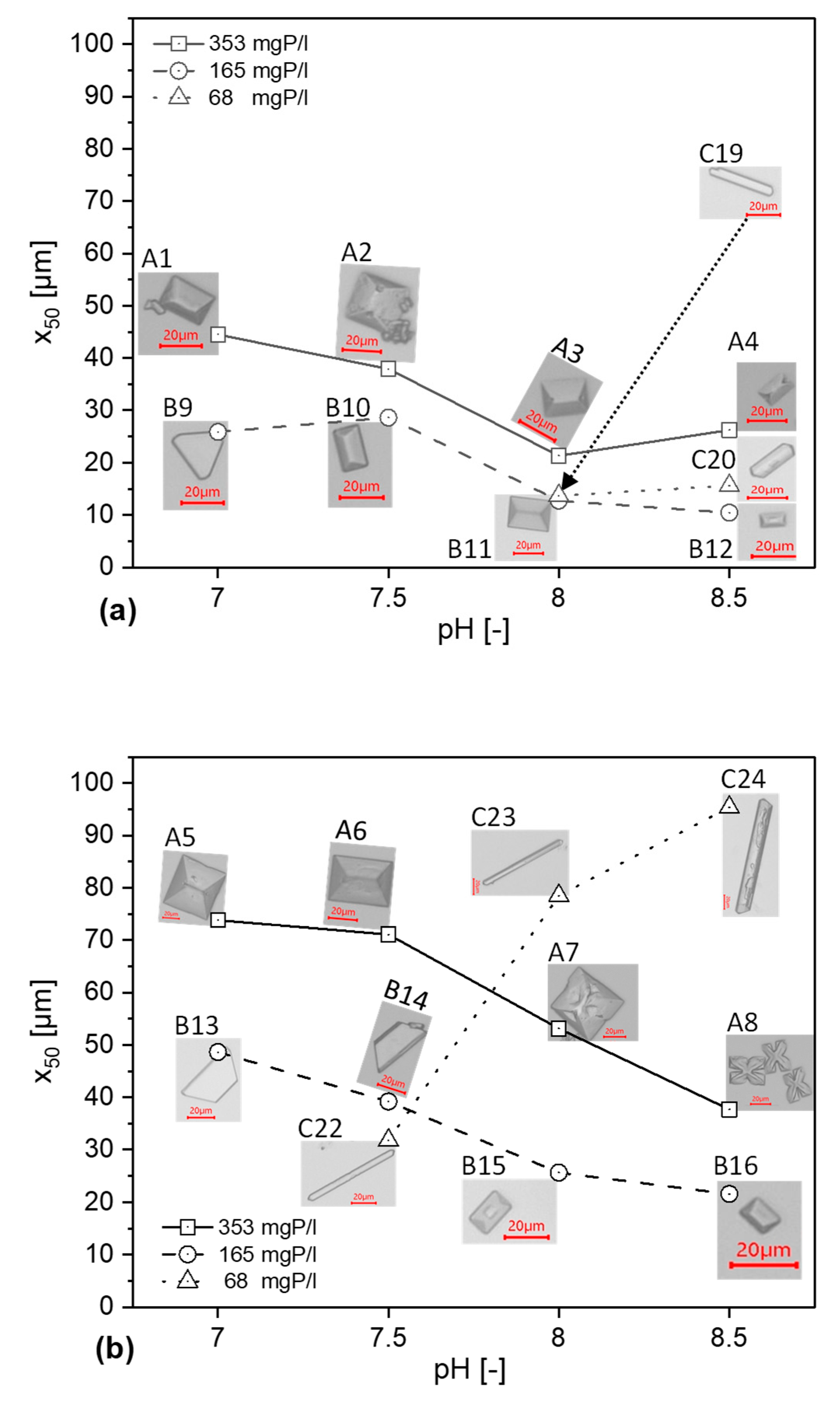
3.3.1. Influence on Particle Size Distribution
3.3.2. Morphology of the Crystals
4. Conclusions
- Increasing the pH accelerates the precipitation process.
- PO4-P strives for a state of equilibrium, provided there is a sufficient SI. The resulting PO4-P concentration depends on the applied pH, independent of the initial ion concentration.
- The size of the recovered crystals decreases with increasing pH at high and moderate concentrations in set A and B, whilst doing the opposite for low concentrations in set C.
- Higher percentual PO4-P elimination is possible with a higher initial PO4-P concentration for the same pH and temperature conditions; by decreasing the initial ion concentration, operators must increase the applied pH of the process in order to achieve the same percentual P-removal.
- Temperature has the least effect on the PO4-P removal. P removal is slightly better but slower within the first 30 min at 5 °C.
- Higher temperature has a positive effect on the resulting crystal size compared to the same conditions at 5 °C.
- An SI above 1 is required for a noticeable precipitation effect. No further PO4-P reduction occurs after 90 min at an SI of above 1.
- An SI of 1.0–1.15 will precipitate about 75% of the PO4-P for 5 °C and 33 °C, respectively. To precipitate 90% of the PO4-P, an SI of 1.7 is required for 5 °C and 33 °C.
- Identify optimal operating conditions for specific centrate compositions.
- Effectively control struvite precipitation in real-world applications.
- Balance process effectiveness with resource efficiency.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Critical Raw Materials Resilience: Charting a Path Towards Greater Security and Sustainability, COM(2020) 474 Final; Office for Official Publications of the European Communities: Brussels, Belgium, 2020. [Google Scholar]
- Dockhorn, T. Stoffstrommanagement und Ressourcenökonomie in der Kommunalen Abwasserwirtschaft; Gesellschaft zur Förderung des Institutes für Siedlungswasserwirtschaft an der Technischen Universität Braunschweig: Braunschweig, Germany, 2007. [Google Scholar]
- Achilleos, P.; Roberts, K.R.; Williams, I.D. Struvite precipitation within wastewater treatment: A problem or a circular economy opportunity? Heliyon 2022, 8, e09862. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Dombinov, V.; Robles-Aguilar, A.A.; Jablonowski, N.D.; Ribeiro, C. Struvite-based composites for slow-release fertilization: A case study in sand. Sci. Rep. 2022, 12, 14176. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Ellis, N.; Mavinic, D.S. Effects of various process parameters on struvite precipitation kinetics and subsequent determination of rate constants. Water Sci. Technol. 2008, 57, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bouropoulos, N.C.; Koutsoukos, P.G. Spontaneous precipitation of struvite from aqueous solutions. J. Cryst. Growth 2000, 213, 381–388. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Abarca, R.R.M.; de Luna, M.D.G.; Huang, Y.-H.; Lu, M.-C. Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: Effects of pH, phosphate concentration and coexisting ions. Chemosphere 2017, 173, 466–473. [Google Scholar] [CrossRef]
- Kofina, A.N.; Koutsoukos, P.G. Spontaneous Precipitation of Struvite from Synthetic Wastewater Solutions. Cryst. Growth Des. 2005, 5, 489–496. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. Environ. Sci. Pollut. Res. Int. 2019, 26, 6725–6734. [Google Scholar] [CrossRef]
- Song, Y.-H.; Qiu, G.-L.; Yuan, P.; Cui, X.-Y.; Peng, J.-F.; Zeng, P.; Duan, L.; Xiang, L.-C.; Qian, F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190, 140–149. [Google Scholar] [CrossRef]
- Wilsenach, J.A.; Schuurbiers, C.A.H.; van Loosdrecht, M.C.M. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. Struvite precipitation thermodynamics in source-separated urine. Water Res. 2007, 41, 977–984. [Google Scholar] [CrossRef]
- Ueno, Y.; Fujii, M. Three years experience of operating and selling recovered struvite from full-scale plant. Environ. Technol. 2001, 22, 1373–1381. [Google Scholar] [CrossRef]
- Battistoni, P.; de Angelis, A.; Pavan, P.; Prisciandaro, M.; Cecchi, F. Phosphorus removal from a real anaerobic supernatant by struvite crystallization. Water Res. 2001, 35, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Natividad-Marin, L.; Burns, M.W.; Schneider, P. A comparison of struvite precipitation thermodynamics and kinetics modelling techniques. Water Sci. Technol. 2023, 87, 1393–1422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y.; Yuan, P.; Peng, J.; Fan, M. Modeling the crystallization of magnesium ammonium phosphate for phosphorus recovery. Chemosphere 2006, 65, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.G. Crystallization principles and techniques. In Crystallization Process Systems; Elsevier: Amsterdam, The Netherlands, 2002; pp. 58–79. [Google Scholar] [CrossRef]
- Hanhoun, M.; Montastruc, L.; Azzaro-Pantel, C.; Biscans, B.; Frèche, M.; Pibouleau, L. Temperature impact assessment on struvite solubility product: A thermodynamic modeling approach. Chem. Eng. J. 2011, 167, 50–58. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Beckie, R.D. A solubility and thermodynamic study of struvite. Environ. Technol. 2007, 28, 1015–1026. [Google Scholar] [CrossRef]
- Ohlinger, K.N.; P.E.; Young, T.M.; Schroeder, E.D. Kinetics Effects on Preferential Struvite Accumulation in Wastewater. J. Environ. Eng. 1999, 125, 730–737. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mavinic, D.S.; Bhuiyan, M.I.H.; Koch, F.A. Exploring the determination of struvite solubility product from analytical results. Environ. Technol. 2006, 27, 951–961. [Google Scholar] [CrossRef]
- Ali, M.I.; Schneider, P.A. An approach of estimating struvite growth kinetic incorporating thermodynamic and solution chemistry, kinetic and process description. Chem. Eng. Sci. 2008, 63, 3514–3525. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef]
- Buchanan, J.R.; Mote, C.R.; Robinson, R.B. Thermodynamics of struvite formation. Am. Soc. Agric. Eng. 1994, 37, 617–621. [Google Scholar]
- Corona, F.; Hidalgo, D.; Martín-Marroquín, J.M.; Antolín, G. Study of the influence of the reaction parameters on nutrients recovering from digestate by struvite crystallisation. Environ. Sci. Pollut. Res. Int. 2021, 28, 24362–24374. [Google Scholar] [CrossRef] [PubMed]
- Kabdaşlı, I.; Atalay, Z.; Tünay, O. Effect of solution composition on struvite crystallization. J. Chem. Technol. Biotechnol. 2017, 92, 2921–2928. [Google Scholar] [CrossRef]
- Bareha, Y.; Saoudi, M.; Santellani, A.C.; Le Bihan, A.; Picard, S.; Mebarki, C.; Cunha, M.; Daumer, M.-L. Use of fermentation processes for improving the dissolution of phosphorus and its recovery from waste activated sludge. Environ. Technol. 2022, 43, 1307–1317. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.M.; Boiarkina, I.; Yu, W.; Huang, Y.F.; Wang, G.Q.; Young, B.R. Phosphorus recovery through struvite crystallisation: Recent developments in the understanding of operational factors. J. Environ. Manag. 2019, 248, 109254. [Google Scholar] [CrossRef]
- Abbona, F.; Calleri, M.; Ivaldi, G. Synthetic struvite, MgNH4PO4.6H2O: Correct polarity and surface features of some complementary forms. Acta Crystallogr. B Struct. Sci. 1984, 40, 223–227. [Google Scholar] [CrossRef]
- Mohajit; Bhattarai, K.K.; Taiganides, E.; Yap, B.C. Struvite deposits in pipes and aerators. Biol. Wastes 1989, 30, 133–147. [Google Scholar] [CrossRef]
- Booker, N.A.; Priestley, A.J.; Fraser, I.H. Struvite Formation in Wastewater Treatment Plants: Opportunities for Nutrient Recovery. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Münch, E.V.; Barr, K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001, 35, 151–159. [Google Scholar] [CrossRef]
- Quintana, M.; Sánchez, E.; Colmenarejo, M.F.; Barrera, J.; García, G.; Borja, R. Kinetics of phosphorus removal and struvite formation by the utilization of by-product of magnesium oxide production. Chem. Eng. J. 2005, 111, 45–52. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- European Union. Commission Implementing Regulation (EU) 2023/121 of 17 January 2023 Amending and Correcting Implementing Regulation (EU) 2021/1165 Authorising Certain Products and Substances for Use in Organic Production and Establishing Their Lists. In Official Journal of the European Union; Implementing Regulation EU 2023/121; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Fattah, K.P.; Sinno, S.; Atabay, S.; Khan, Z.; Al-Dawood, Z.; Yasser, A.K.; Temam, R. Impact of Magnesium Sources for Phosphate Recovery and/or Removal from Waste. Energies 2022, 15, 4585. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, T. User’s Guide to PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; US Geological Survey: Baltimore, MD, USA, 1999. [Google Scholar]
- Battistoni, P.; Pavan, P.; Prisciandaro, M.; Cecchi, F. Struvite crystallization: A feasible and reliable way to fix phosphorus in anaerobic supernatants. Water Res. 2000, 34, 3033–3041. [Google Scholar] [CrossRef]
- Celen, I.; Türker, M. Recovery of ammonia as struvite from anaerobic digester effluents. Environ. Technol. 2001, 22, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Marchi, A.; Geerts, S.; Weemaes, M.; Schiettecatte, W.; Vanhoof, C. Full-scale phosphorus recovery from digested waste water sludge in Belgium—Part I: Technical achievements and challenges. Water Sci. Technol. 2015, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ye, Z.-L.; Lou, Y.; Pan, S.; Wang, X.; Wang, M.K.; Chen, S. A comprehensive understanding of saturation index and upflow velocity in a pilot-scale fluidized bed reactor for struvite recovery from swine wastewater. Powder Technol. 2016, 295, 16–26. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Mavinic, D.S.; Koch, F.A. Phosphorus recovery from wastewater through struvite formation in fluidized bed reactors: A sustainable approach. Water Sci. Technol. 2008, 57, 175–181. [Google Scholar] [CrossRef]
- González-Morales, C.; Fernández, B.; Molina, F.J.; Naranjo-Fernández, D.; Matamoros-Veloza, A.; Camargo-Valero, M.A. Influence of pH and Temperature on Struvite Purity and Recovery from Anaerobic Digestate. Sustainability 2021, 13, 10730. [Google Scholar] [CrossRef]
- Eko, A.; Ang, H.M.; Sen, T. Effect of initial solution pH on solubility and morphology of struvite crystals. In Proceedings of the Chemeca 2011 Conference, Sydney, Australia, 18–21 September 2011; pp. 18–21. [Google Scholar]
- Mehta, C.M.; Batstone, D.J. Nucleation and growth kinetics of struvite crystallization. Water Res. 2013, 47, 2890–2900. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Siciliano, A.; Limonti, C.; Tünay, O. Is K-Struvite Precipitation a Plausible Nutrient Recovery Method from Potassium-Containing Wastes?—A Review. Sustainability 2022, 14, 11680. [Google Scholar] [CrossRef]
- Hoagland, D.R. Optimum nutrient solutions for plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, S.; Ucar, S.; Andreassen, J.-P.; Østerhus, S.W. Engineering of struvite crystals by regulating supersaturation—Correlation with phosphorus recovery, crystal morphology and process efficiency. J. Environ. Chem. Eng. 2019, 7, 102918. [Google Scholar] [CrossRef]





| A | B | C | ||
|---|---|---|---|---|
| PO4-P | mg/L | 353 | 165 | 68 |
| NH4-N | 1042 | 542 | 320 | |
| Mg | 8.5 | 8.0 | 7.6 | |
| Ca | 35 | 46 | 71 | |
| Na | 119 | 108 | 70.5 | |
| K | 398 | 222 | 102 |
| Set | # | PO4-P [mg/L] | NH4-N [mg/L] | T. [°C] | pH [-] |
|---|---|---|---|---|---|
| A | 1 | 353 | 1024 | 5 | 7.0 |
| A | 2 | 353 | 1024 | 5 | 7.5 |
| A | 3 | 353 | 1024 | 5 | 8.0 |
| A | 4 | 353 | 1024 | 5 | 8.5 |
| A | 5 | 353 | 1024 | 33 | 7.0 |
| A | 6 | 353 | 1024 | 33 | 7.5 |
| A | 7 | 353 | 1024 | 33 | 8.0 |
| A | 8 | 353 | 1024 | 33 | 8.5 |
| B | 9 | 165 | 542 | 5 | 7.0 |
| B | 10 | 165 | 542 | 5 | 7.5 |
| B | 11 | 165 | 542 | 5 | 8.0 |
| B | 12 | 165 | 542 | 5 | 8.5 |
| B | 13 | 165 | 542 | 33 | 7.0 |
| B | 14 | 165 | 542 | 33 | 7.5 |
| B | 15 | 165 | 542 | 33 | 8.0 |
| B | 16 | 165 | 542 | 33 | 8.5 |
| C | 17 | 68 | 320 | 5 | 7.0 |
| C | 18 | 68 | 320 | 5 | 7.5 |
| C | 19 | 68 | 320 | 5 | 8.0 |
| C | 20 | 68 | 320 | 5 | 8.5 |
| C | 21 | 68 | 320 | 33 | 7.0 |
| C | 22 | 68 | 320 | 33 | 7.5 |
| C | 23 | 68 | 320 | 33 | 8.0 |
| C | 24 | 68 | 320 | 33 | 8.5 |
| Set | # | PO4-P [mg/L] | NH4-N [mg/L] | T [°C] | pH [-] | X10 [µm] | X50 [µm] | X90 [µm] |
|---|---|---|---|---|---|---|---|---|
| A | 1 | 353 | 1024 | 5 | 7 | 20.79 | 43.1 | 63.15 |
| A | 2 | 353 | 1024 | 5 | 7.5 | 19.48 | 36.78 | 56.80 |
| A | 3 | 353 | 1024 | 5 | 8 | 8.98 | 16.13 | 28.67 |
| A | 4 | 353 | 1024 | 5 | 8.5 | 25.25 | 38.2 | 56.27 |
| A | 5 | 353 | 1024 | 33 | 7 | 28.43 | 71.08 | 103.25 |
| A | 6 | 353 | 1024 | 33 | 7.5 | 41.61 | 71.79 | 104.68 |
| A | 7 | 353 | 1024 | 33 | 8 | 30.64 | 51.92 | 78.7 |
| A | 8 | 353 | 1024 | 33 | 8.5 | 22.41 | 38.11 | 58.52 |
| B | 9 | 163 | 542 | 5 | 7 | 9.05 | 24.35 | 41.6 |
| B | 10 | 163 | 542 | 5 | 7.5 | 12.67 | 28.06 | 44.5 |
| B | 11 | 163 | 542 | 5 | 8 | 7.1 | 12.26 | 20.66 |
| B | 12 | 163 | 542 | 5 | 8.5 | 6.48 | 9.87 | 15.41 |
| B | 13 | 163 | 542 | 33 | 7 | 24.51 | 47.31 | 82.61 |
| B | 14 | 163 | 542 | 33 | 7.5 | 22.83 | 37.9 | 56.92 |
| B | 15 | 163 | 542 | 33 | 8 | 13.05 | 25.61 | 41.38 |
| B | 16 | 163 | 542 | 33 | 8.5 | 14.5 | 24.29 | 38.15 |
| C | 17 | 68 | 320 | 5 | 7 | - | - | - |
| C | 18 | 68 | 320 | 5 | 7.5 | - | - | - |
| C | 19 | 68 | 320 | 5 | 8 | 7.44 | 13.35 | 21.58 |
| C | 20 | 68 | 320 | 5 | 8.5 | 8.53 | 13 | 20.41 |
| C | 21 | 68 | 320 | 33 | 7 | - | - | - |
| C | 22 | 68 | 320 | 33 | 7.5 | 4 | 21.04 | 99.88 |
| C | 23 | 68 | 320 | 33 | 8 | 39.86 | 75.15 | 120.64 |
| C | 24 | 68 | 320 | 33 | 8.5 | 55.65 | 92.83 | 134.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolb, A.; Gebhardt, T.; Dockhorn, T. Struvite Precipitation from Centrate—Identifying the Best Balance Between Effectiveness and Resource Efficiency. Resources 2025, 14, 56. https://doi.org/10.3390/resources14040056
Kolb A, Gebhardt T, Dockhorn T. Struvite Precipitation from Centrate—Identifying the Best Balance Between Effectiveness and Resource Efficiency. Resources. 2025; 14(4):56. https://doi.org/10.3390/resources14040056
Chicago/Turabian StyleKolb, Andreas, Tim Gebhardt, and Thomas Dockhorn. 2025. "Struvite Precipitation from Centrate—Identifying the Best Balance Between Effectiveness and Resource Efficiency" Resources 14, no. 4: 56. https://doi.org/10.3390/resources14040056
APA StyleKolb, A., Gebhardt, T., & Dockhorn, T. (2025). Struvite Precipitation from Centrate—Identifying the Best Balance Between Effectiveness and Resource Efficiency. Resources, 14(4), 56. https://doi.org/10.3390/resources14040056






